Abstract
Lymphopenia is frequent in advanced cancers and predicts the toxicity of chemotherapy. Its impact on relapse and survival is uncertain. Its prognostic value for survival was analyzed in 3 databases of previously reported prospective multicenter studies: 1) FEC chemotherapy in metastatic breast carcinoma; 2) CYVADIC in advanced soft-tissue sarcoma (EORTC-STBSG 62791); 3) prospective, consecutive phase III studies of aggressive diffuse large-cell non-Hodgkin’s lymphomas conducted at Bérard center between 1987 and 1993. Univariate and multivariate analyses of prognostic factors for survival were performed. The incidence of lymphopenia <1000/μL before treatment was constant among series: 25%, 24%, 27% respectively. Lymphopenia was significantly more frequent (p<0.05) in metastatic breast cancer patients with performance status (PS)>1, non-Hodgkin’s lymphoma patients with international prognostic index (IPI)>0, and advanced soft-tissue sarcoma and metastatic breast cancer patients with bone metastases. In univariate analysis, lymphopenia <1000/μL significantly correlated to overall survival in patients with metastatic breast cancer (median 10 vs. 14 months, p <0.0001), advanced soft-tissue sarcoma (median 5 vs. 10 months, p <0.01), and non-Hodgkin lymphoma (median 11 vs. 94 months, p <0.0001). In multivariate analysis (Cox model), lymphopenia was an independent prognostic factor for overall survival in metastatic breast cancer (RR: 1.8; 95%CI 1.3–2.4) along with liver metastases and PS; in advanced soft-tissue sarcoma (RR: 1.46; 95%CI 1.0–2.1) along with liver metastases, lung metastases and PS; and in non-Hodgkin’s lymphoma (RR: 1.48; 95%CI 1.03–2.1) along with IPI. Our findings demonstrate that lymphopenia is an independent prognostic factor for overall and progression-free survival in several cancers.
INTRODUCTION
Factors related to 1) disease extent and dissemination (stage, tumor size, tumor markers), 2) patient characteristics (age, gender, associated co-morbidities, performance status) and 3) treatment (quality of surgery, radiotherapy, type of chemotherapy) have prognostic significance for survival from cancer. Long-term survival (e.g. >5 years) is generally observed in patients with favorable prognostic factors in all categories. 5–10% of patients with metastatic soft tissue sarcoma or metastatic breast cancer, and 50% of NHL patients are still alive 10 years after the diagnosis of metastasis.1–6
Previously published studies have shown that lymphopenia is frequently observed in patients with advanced cancers and is a powerful predictor of chemotherapy-induced toxicity, in addition to patient characteristics, disease characteristics, biological parameters and previous treatments. Lymphopenia has also been found associated with an increased risk of febrile neutropenia,7–11 thrombocytopenia requiring platelet transfusion,12 severe anaemia requiring red cell transfusion in adults and children,13–14 and early death.15 All subsets of lymphocytes are altered in lymphopenic patients, while CD4+ and, to a lesser extent, CD56+ lymphopenia have been found to be the most powerful predictors of toxicity.16
In the present study, we retrospectively investigated the prognostic value of lymphopenia for overall survival and progression-free survival in 3 prospectively collected series: 1) untreated aggressive non-Hodgkin’s lymphoma (NHL) patients receiving first-line CHOP or a CHOP-derived regimen, 2) hormone-resistant metastatic breast carcinoma (MBC) patients receiving first-line FEC chemotherapy, 3) untreated advanced soft tissue sarcomas (ASTS) patients treated in the 62761 trial of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with the CYVADIC regimen.
MATERIALS AND METHODS
OBJECTIVES
The study aimed to determine the prognostic value of lymphopenia for progression-free and overall survival in patients treated with chemotherapy for metastatic breast cancer, non–Hodgkin’s lymphoma and advanced sarcoma.
PATIENTS
Non Hodgkin’s lymphomas
We analyzed the data from non-pre-treated, human immunodeficiency virus (HIV) negative patients with intermediate or high-grade NHL who were included in 2 prospective multi-center phase III trials of the GELA (Groupe d’ Etude des Lymphomes de l’Adulte) at Léon Bérard cancer center between 1986 and 1997. All patients were treated with the CHOP or ACVBP regimens.
Metastatic breast carcinomas
The second series included non-pre-treated patients with metastatic breast cancer included in the prospective multicenter phase III trial ERASME, who received first-line chemotherapy with FEC at Léon Bérard cancer center between 1986 and 1990.17
Advanced soft tissue Sarcomas
The third series involved non-pre-treated patients with metastatic soft tissue sarcoma included in the prospective multicenter phase III trial of the EORTC Soft Tissue and Bone Sarcoma Group (trial 62791).18
PROGNOSTIC FACTORS
Previously validated prognostic factors for overall survival had been collected for the 3 series of patients. For non Hodgkin’s lymphoma, prognostic factors were those of the International Prognosis Index (IPI);19 for metastatic breast cancer, they included age at diagnosis, menopausal status, presence or absence of hormone receptors, metastasis-free interval, site of metastasis, number of metastatic sites, previous adjuvant treatments;1 for soft tissue sarcoma the factors were age over 60 years, gender, histological subtype, site of metastasis, histological grade.3,4 In all patients, lymphocyte counts immediately before initiation of systemic treatment had been prospectively recorded in the CRF of the different trials and were available for analysis. Lymphopenia was defined as a lymphocyte count below 1500/μL, but the relevant threshold value in this series was found to be 1000/μL.
STATISTICS
Survival analysis
overall survival was defined as the time from treatment initiation (or from date of diagnosis for NHL patients) to the date of death or the date of last follow-up for patients alive at last contact. Progression-free survival was defined as the time from treatment initiation (or from date of diagnosis for NHL patients) to the date of disease progression or death, or to the date of last follow-up for patients alive at last contact. Survival distributions were estimated by the Kaplan-Meier method20.
Univariate analysis
to evaluate the relationship between survival and all biological and/or clinical factors known to be relevant in each disease, potential prognostic factors were included in univariate Cox proportional hazard regression models. The risk factors most commonly used in previous studies (e.g. PS>1) were dichotomized. Lymphopenia was also included in the models as a dichotomous variable (<1000/μL vs. ≥1000/μL). These categories were defined by first determining the quartiles of the distribution of lymphocyte counts for each tumor type. The overall survival distributions of these quartiles were further examined using the Kaplan-Meier method. The conclusion was that the threshold corresponding to the lower quartile (close to 1000/μL for each tumor type) was the most discriminative parameter to predict overall survival in the three tumor types studied. Candidate prognostic factors with a 0.05 level of significance in univariate analysis were then selected for inclusion in the multivariate analysis.
Multivariate analysis
Independent prognostic variables of overall survival and progression-free survival were respectively identified by a Cox regression analysis using a backward selection procedure21,22. The add-value of lymphopenia in each model where it was found to be an independent prognostic factor was evaluated using a likelihood ratio test (LRT): likelihood scores of the model evaluated with and without lymphopenia were compared, considering that lower likelihood scores indicate better fitting models23.
All statistical analyses were performed using SPSS12.1® and SAS® v.9.1 (Cary, NC, USA).
RESULTS
PATIENT CHARACTERISTICS
The characteristics of patients with non–Hodgkin’s lymphoma, metastatic breast cancer, and metastatic sarcoma are given in Tables 1 to 3, respectively. In total, 322 NHL, 287 MBC and 193 ASTS patients were analyzed.
Table 1.
Non-Hodgkin Lymphoma (N=322)
| Overall survival |
|||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis** | ||||
| Patients’ characteristics | No (%) | Median(months) | p value | HR(95%CI) | p value |
| Age (years) | 322 | ||||
| <60 | 160 (50) | >165 | <0.0001 | - | NS |
| ≥60 | 162 (50) | 21 | |||
|
| |||||
| Gender | 322 | ||||
| Male | 187 (58) | 30 | NS | - | NS |
| Female | 135 (42) | 69 | |||
|
| |||||
| Ann Arbor stage | 321 | ||||
| I | 30 (9) | > 123 | |||
| II | 107 (33) | 112 | <0.0001 | - | NS |
| III | 37 (12) | 39 | |||
| IV | 147(46) | 18 | |||
|
| |||||
| PS (ECOG) | 286 | ||||
| 0–1 | 199 (70) | 112 | <0.0001 | - | NS |
| >1 | 87 (30) | 12 | |||
|
| |||||
| B symptoms | 277 | ||||
| No | 227 (82) | 78 | 0.002 | - | NS |
| Yes | 50 (18) | 11 | |||
|
| |||||
| Extra nodal involv. | 322 | ||||
| 0–1 | 311 (97) | 52 | NS | - | NS |
| >1 | 11 (3) | 14 | |||
|
| |||||
| Serum LDH level | 314 | ||||
| ≤ normal | 115 (37) | 94 | 0.02 | - | NS |
| > normal | 199 (63) | 29 | |||
|
| |||||
| Bone Marrow involv. | 321 | ||||
| No | 277 (86) | 56 | |||
| Yes | 44 (14) | 16 | NS | - | NS |
|
| |||||
| β2 microglob. level | 252 | ||||
| ≤ normal | 207 (82) | 89 | <0.0001 | - | NS |
| > normal | 45 (18) | 11 | |||
|
| |||||
| IPI | 279 | ||||
| 0–1* | 96 (34) | >165 | |||
| 2 | 106 (38) | 94 | <0.0001 | 1.99 (1.3–3.1) | <0.0001 |
| 3 | 47 (17) | 30 | 3.22 (1.9–5.4) | ||
| 4–5 | 30 (11) | 4 | 7.25 (4.2–12.6) | ||
|
| |||||
| Lymphocytes (/μL) | 322 | ||||
| ≥ 1000* | 234 (73) | 94 | 0.04 | ||
| <1000 | 88 (27) | 11 | <0.0001 | 1.48 (1.03–2.1) | |
Reference modality;
the final model was performed on 279 patients
Table 3.
Advanced soft tissue sarcoma (N=193)
| Overall survival |
|||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis** | ||||
| Patients’ characteristics | No (%) | Median(months) | p value | HR(95%CI) | p value |
| Age (years) | 192 | ||||
| <60 | 149 (78) | 9 | NS | - | NS |
| ≥60 | 43 (22) | 8 | |||
|
| |||||
| Gender | 192 | ||||
| Male | 100 (52) | 8 | NS | - | NS |
| Female | 92 (48) | 9 | |||
|
| |||||
| Grade | 145 | ||||
| I | 11 (8) | 15 | NS | - | NS |
| II | 28 (19) | 8 | |||
| III | 106 (73) | 9 | |||
|
| |||||
| Karnofsky Index | 184 | ||||
| 100-80* | 111 (60) | 12 | <0.0001 | <0.0001 | |
| <80 | 73 (40) | 4 | 2.33 (1.7–3.3) | ||
|
| |||||
| Liver metastases | 173 | ||||
| No* | 142 (82) | 9 | 0.02 | 0.002 | |
| Yes | 31 (18) | 7 | 2.03 (1.3–3.1) | ||
|
| |||||
| Bone metastases | 183 | ||||
| No | 159 (87) | 9 | NS | - | NS |
| Yes | 24 (13) | 8 | |||
|
| |||||
| Lung metastases | 189 | ||||
| No* | 80 (42) | 9 | 0.02 | 0.03 | |
| Yes | 109 (58) | 9 | 1.49 (1.05–2.1) | ||
|
| |||||
| Histotype | 153 | ||||
| Leiomyosarcoma | 41 (27) | 11 | |||
| MFH | 30 (20) | 8 | |||
| Synovialosarcoma | 5 (3) | 24 | 0.01*** | - | - |
| Liposarcoma | 15 (10) | 16 | |||
| Fibrosarcoma | 18 (12) | 10 | |||
| Other | 44 (29) | 7 | |||
|
| |||||
| Lymphocytes (/μL) | 193 | ||||
| ≥ 1000* | 147 (76) | 10 | 0.006 | 0.05 | |
| <1000 | 46 (24) | 5 | 1.46 (1.0–2.1) | ||
Reference modality;
the final model was performed on 162 patients;
Not included in the multivariate analysis since interpretation is difficult.
The incidence of lymphopenia was remarkably similar among the studied patient populations, i.e. 27%, 25%, and 24% respectively (Table 4).
Table 4.
Incidence of lymphopenia in the 3 groups of patients
| N (%) Lymphocyte count (/μL) | ||||
|---|---|---|---|---|
| <400 | [400–700] | ]700–1000[ | ≥ 1000 | |
| Non-Hodgkin’s lymphoma (n=322) | 9 (3) | 45 (14) | 34 (11) | 234 (73) |
| Metastatic breast carcinoma (n=279) | 10 (4) | 23 (8) | 38 (14) | 208 (75) |
| Advanced soft-tissue sarcoma (n=193) | 6 (3) | 18 (9) | 22 (11) | 147 (76) |
In patients with NHL, lymphopenia <1000/μL was more frequently associated with women (36% vs. 21% p=0.002), age >60 years (33% vs. 22% p=0.03), increased pre-treatment levels of serum beta 2 microglobulin (49% vs. 24%, p=0.0007), clinical B symptoms (42% vs. 24%, p=0.009), LDH level above normal (34% vs. 16%, p=0.0005), higher international prognostic index (p=0.03), or hemoglobin levels <12g/dL (38% vs. 19%, p=0.0002), but was not correlated to clinical stage or bone marrow involvement. In MBC patients, lymphopenia <1000/μL was more frequently associated with PS>1 (34% vs. 20%, p=0.01), post-menopausal status (28% vs. 6%, p=0.01), bone marrow involvement (67% vs. 24%, p=0.009), bone metastasis (29% vs. 18%, p=0.04) or more than one metastatic site (30% vs. 18%, p=0.03), but not with age. In sarcoma patients, lymphopenia was correlated only to the presence of bone metastases (46% vs. 21%, p=0.01) but not to age, gender, histologic grade of the primary tumor or liver metastases.
OVERALL SURVIVAL
Non Hodgkin’s lymphoma
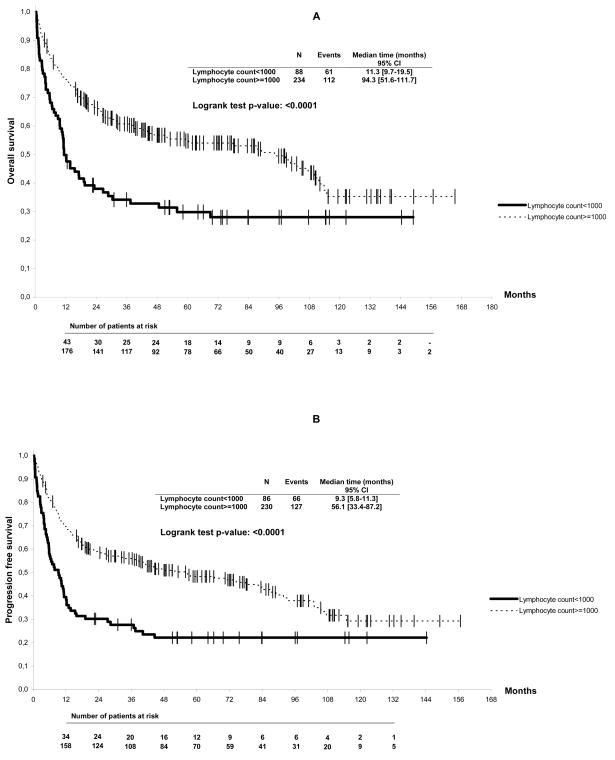
In the univariate analysis, age over 60 years, PS >1, presence of B symptoms, Ann Arbor stage ≥III, serum LDH level above normal, β2 microglobulin level above normal, IPI score (Table 1, p< 0.05), and baseline lymphocyte count <1000/μL were found to be correlated to overall survival (Figure 1A). In the multivariate analysis, only the IPI score (p<0.0001) and lymphocyte counts below 1000/μL (RR 1.48, [95% CI, 1.03 – 2.1], p=0.04) were found to be independently correlated to overall survival (Table 1). Results of the LRT indicated that this multivariate model fitted the data significantly better than the same model without lymphopenia (p=0.05). Lymphopenia was found significantly correlated to progression-free survival, with a median interval of 9 vs. 56 months (p<0.0001) (Figure 1B). Using the Cox model, the IPI score (p<0.0001) and lymphopenia (RR 1.71, [95% CI, 1.2 – 2.4], p=0.002) were independently correlated to progression-free survival. Results of the LRT indicated a better fitting model when including lymphopenia (p=0.003).
Figure 1.
Overall (A) and progression-free (B) survival of non-Hodgkin’s lymphoma patients according to baseline lymphocyte counts
Metastatic breast cancer
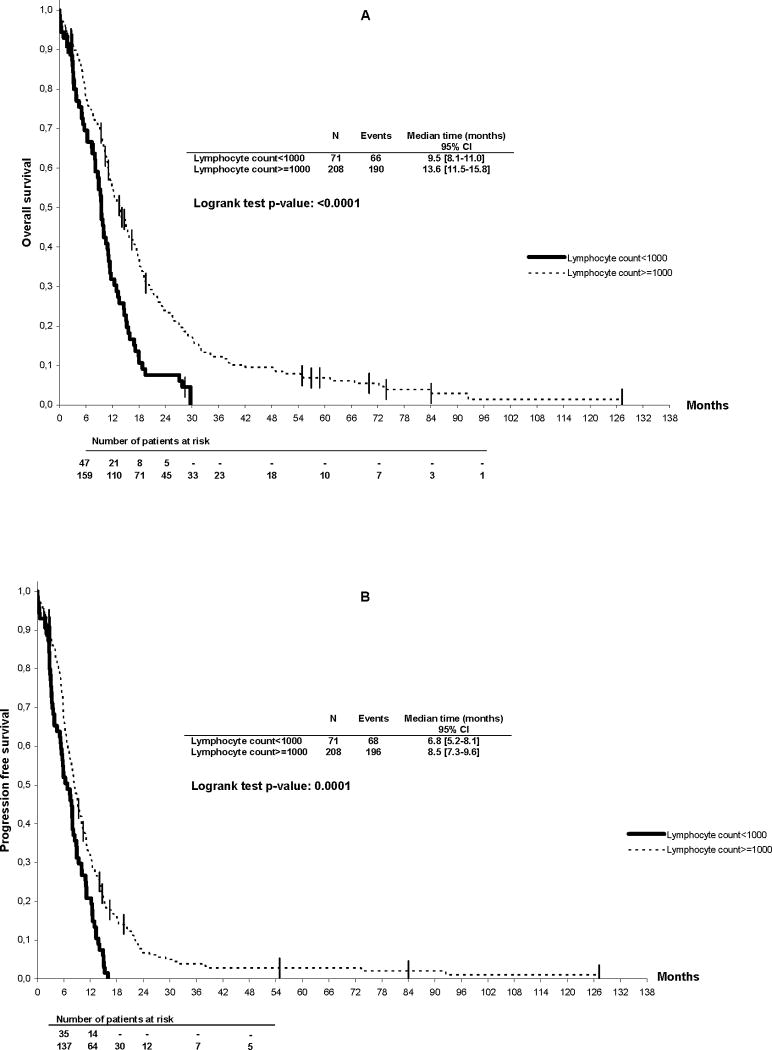
In the univariate analysis, PS >1, presence of liver metastases, number of metastatic sites >1, presence of bone marrow involvement and lymphocyte count <1000/μL were correlated to overall survival (Table 2). Figure 2 shows the overall survival of MBC patients according to lymphocyte count. Median survival was significantly better for patients with lymphocyte counts ≥1000/μL as compared to <1000/μL (14 vs. 10 months, p< 0.0001). In the multivariate analysis, only performance status (RR 1.99, [95% CI, 1.5 – 2.6], p<0.0001), presence of liver metastases (RR 1.85, [95% CI, 1.4 – 2.4], p<0.0001) and lymphocyte count (RR 1.8, [95% CI, 1.3 – 2.4] p=0.0002) were found to be correlated to survival. Progression-free survival was also significantly shorter in patients with lymphocyte counts <1000/μL as compared to ≥1000/μL (7 vs. 9 months, p= 0.0001) (Figure 2B). Using the Cox model, PS (RR 1.6, [95% CI, 1.2 – 2.1], p=0.0004), presence of liver metastases (RR 1.67, [95% CI, 1.3 – 2.2], p<0.0001) and lymphocyte count (RR 1.48, [95% CI, 1.1 – 2.0] p=0.01) were found to be correlated to progression-free survival. For both multivariate models, results of the LRT showed an improved goodness of fit of the models compared to the same models without lymphopenia (p<0.0001).
Table 2.
Breast cancer (N=287)
| Overall survival |
|||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis** | ||||
| Patients’ characteristics | No (%) | Median(months) | p value | HR(95%CI) | p value |
| Age (years) | 286 | ||||
| <60 | 193 (67) | 11 | NS | - | NS |
| ≥ 60 | 93 (33) | 13 | |||
|
| |||||
| Hormone receptor | 181 | ||||
| No | 60 (33) | 10 | NS | - | NS |
| Yes | 121 (67) | 12 | |||
|
| |||||
| SBR grade | 108 | ||||
| I | 22 (20) | 13 | NS | - | NS |
| II | 60 (56) | 12 | |||
| III | 26 (24) | 14 | |||
|
| |||||
| PS (ECOG) | 265 | ||||
| 0–1* | 160 (60) | 15 | <0.0001 | <0.0001 | |
| >1 | 105 (40) | 8 | 1.99 (1.5–2.6) | ||
|
| |||||
| Liver metastases | 279 | ||||
| No* | 147 (53) | 16 | <0.0001 | <0.0001 | |
| Yes | 132 (47) | 10 | 1.85 (1.4–2.4) | ||
|
| |||||
| Bone metastases | 279 | ||||
| No | 101 (36) | 13 | NS | - | NS |
| Yes | 178 (64) | 11 | |||
|
| |||||
| Skin metastases | 279 | ||||
| No | 263 (94) | 12 | NS | - | NS |
| Yes | 16 (6) | 16 | |||
|
| |||||
| Bone Marrow involv. | 279 | ||||
| No | 269 (94) | 12 | 0.03 | - | NS |
| Yes | 10 (6) | 11 | |||
|
| |||||
| Nb metastatic sites | 281 | ||||
| 1 | 100 (36) | 17 | 0.0002 | - | NS |
| >1 | 181 (64) | 11 | |||
|
| |||||
| Lymphocytes (/μL) | 279 | ||||
| ≥ 1000* | 208 (75) | 14 | <0.0001 | 0.0002 | |
| <1000 | 71 (25) | 10 | 1.8 (1.3–2.4) | ||
Reference modality;
the final model was performed on 257 patients
Figure 2.
Overall (A) and progression-free survival (B) of hormone-resistant metastatic breast cancer patients according to baseline lymphocyte counts
Soft tissue sarcoma
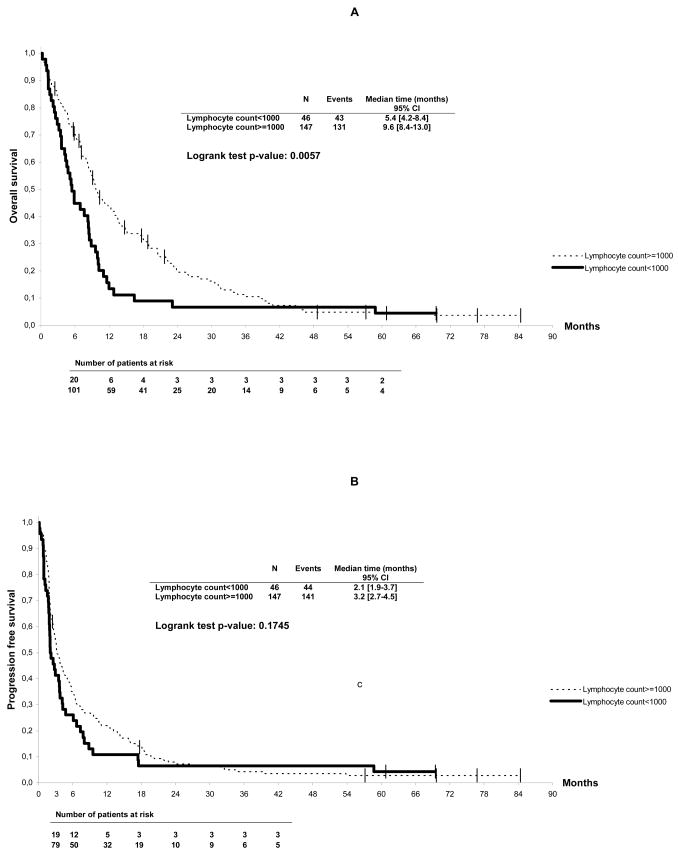
In the univariate analysis, Karnofsky Index < 80, presence of liver and/or lung metastases, histotype and lymphocyte count <1000/μL were correlated to overall survival (Table 3). Figure 3 shows the overall survival of ASTS patients according to pre-treatment lymphocyte count. Median survival was significantly better for patients with lymphocyte counts ≥1000/μL as compared to <1000/μL (10 vs. 5 months, p=0.006). In the multivariate analysis, Karnofsky Index <80 (RR 2.33, [95 % CI, 1.7 – 3.3], p<0.0001), presence of liver metastases (RR 2.03, [95% CI, 1.3 – 3.1], p=0.002), presence of lung metastases (RR 1.49, [95% CI, 1.05 – 2.1], p=0.03) and lymphocyte count (RR 1.46, [95% CI, 1.0 – 2.1], p=0.05) were found to be independently correlated to overall survival (Table 3). Results of the LRT showed that this multivariate model fitted the data significantly better than the same model without lymphopenia (p=0.05). In the univariate analysis, progression-free survival was not significantly different between the 2 groups, even though the median interval was shorter for lymphopenic patients (2 vs. 3 months, p=0.17). In the multivariate analysis, only Karnofsky Index <80 (RR 1.97, [95% CI, 1.3–2.9], p=0.0007), presence of liver metastases (RR 1.91 [95% CI, 1.1–3.2], p=0.01) and tumor grade >2 (RR 1.58, [95% CI, 1.04–2.4], p=0.03) were found correlated to progression-free survival.
Figure 3.
Overall (A) and progression-free survival (B) of advanced soft tissue sarcoma patients according to baseline lymphocyte counts
DISCUSSION
Several different parameters may influence survival in cancer patients: variables related to the characteristics of the tumor (tumor size, stage, biological characteristics including molecular alterations), those related to the characteristics of the patient (age, gender, co-morbidities), and those directly related to the nature and quality of treatment. Some of these prognostic factors, such as age, clinical stage and performance status, are common across tumor types and represent the basis of treatment decision for health care providers, as well as the major inclusion criteria for entering patients into clinical trials.
In the present study, we identified lymphopenia as a simple prognostic factor for overall survival shared by the three tumor types studied: metastatic breast carcinoma, advanced sarcoma, and non-Hodgkin’s lymphoma. Lymphopenia prior to initiation of systemic treatment was found to independently correlate to overall survival in all patients, and to progression-free survival in MBC and NHL patients. We and others have previously reported the prognostic impact of lymphopenia on hematological toxicity from chemotherapy, especially for neutropenia, severe thrombocytopenia, severe anemia requiring red cell transfusion and early death after chemotherapy.7,12–15,24 Most previous studies have used a threshold level of 700 lymphocytes per μL which has been identified as the most discriminative predictive value for hematological toxicities, although a cut-off value of 1000/μL retains predictive value for toxicity (not shown). The biological significance of this threshold remains unclear: it may select a larger proportion of patients with CD4 counts <450/μL, later identified as the most discriminative factor to predict toxicity.16
In the present study, the threshold of 1000/μL was found to be more discriminative to predict overall survival in the three tumor types studied. The first interesting observation was that the frequency of lymphopenia <1000/μL in all three cancer patient populations was very similar, with 24 to 27% of the patients presenting a lymphocyte count less than 1000/μL before any systemic treatment. Interestingly, the incidence of lymphopenia <1000/μL was lower in patients with localized breast cancer or sarcoma, with a rate of 3–5% for the series of patients treated in our institution (data not shown). Lymphopenia was found correlated with performance status, as well as with specific prognostic factors for NHL (beta 2 microglobulin, B symptoms), breast cancer (bone and bone marrow involvement, number of metastatic sites, menopausal status), and sarcoma (bone metastases). These observations strongly show that lymphopenia is related to tumor cell mass, metastatic sites and paraneoplastic inflammatory syndrome, but also to host characteristics (menopausal status or age).
Although the actual mechanisms of the association between low lymphocyte count and poor prognosis is unclear, the different possibilities are as follows: (i) the low lymphocyte count may be associated with a preexisting immunosuppressed condition, suggesting that the host tends to have an inadequate immunological reaction; (ii) the low lymphocyte count may be a consequence of lympholytic cytokines produced by the lymphoma cells, and such lymphoma may itself be resistant; or (iii) a combination of both or other factors. Effectively, the mechanisms of lymphopenia in cancer patients remain unclear and are probably multifactorial. The three series studied included only previously untreated patients; therefore lymphopenia was not due to exposure to cytotoxic agents. Lymphopenia may have resulted from a destruction of lymphocytes elicited by the tumor25,26 and/or an altered homeostasis of lymphocyte pools in cancer patients.27 In support of the first hypothesis, it has been reported that the lymphocytes of cancer patients undergo activation-induced death in vivo and that pro-apoptotic ligands such as FasL or TNFβ are produced in vivo in cancer patients.25,28–30 Physiological lymphocyte homeostasis and maintenance of lymphocyte subset pools in adult patients are dependent on the presence and function of dendritic cells.27 The differentiation of dendritic cells is impaired by the overproduction of numerous cytokines and mediators such as IL-6, PGE2, IL-10, TGFβ, which are produced within the tumor environment and are released in the blood stream in breast carcinoma, lymphoma and other tumors.31 The correlations between lymphopenia and inflammatory B symptoms as well as serum IL-6, IL-10 and TNF levels in breast carcinoma, lymphoma and other neoplastic diseases are consistent with this hypothesis.32–38
Bone marrow involvement and the presence of bone metastases are likely contributing factors in some patients, since they were found correlated to lymphopenia in our breast cancer series. Cachexia associated with tumor progression may also contribute to decreased lymphocyte counts, although weight loss and hypoalbuminemia were observed in less than 40% of our lymphopenic breast and lymphoma patients for whom this information was available (data not shown). Partial correlation between lymphopenia and hypoalbuminemia has also been reported in a recent series of metastatic carcinomas with unknown primary.39
Several of the prognostic parameters previously reported in the literature for each tumor type were found to be correlated to overall survival: liver metastases, number of metastatic sites and PS in breast carcinoma; IPI score 40 in NHL; histotype, liver metastases and PS in sarcomas.1–6,17–19 In the 3 populations, the prognostic value of lymphopenia was found to be independent of these factors in the multivariate analysis using the Cox model. Moreover, we also showed that the 3 models better fitted the data when they included lymphopenia as a prognostic factor. Lymphopenia had been previously found correlated to overall survival. In a study published in 1970, Riesco reported a significant positive correlation between cancer curability and the total number of peripheral lymphocytes in miscellaneous cancer patients (n = 589), notably those with localized cervix and breast cancers, with a threshold level of 1000/μL similar to the threshold identified in our study.41 Ownby et al analyzed recurrences in a series of patients with breast cancer: patients with preoperative lymphocyte counts less than or equal to 1500/mm3 and/or eosinophil counts less than 55/mm3 had a significantly higher risk of recurrent disease than those with normal or high levels of eosinophils and/or lymphocytes.42 In Hodgkin’s disease and diffuse large B cell lymphomas, lymphopenia has been reported to be correlated to overall survival.40,43,44 We previously reported that lymphopenia is not restricted to a specific lymphocyte subset and involves the CD4, CD8, CD19, CD56 cell compartments, even though correlation to patient outcome is mainly associated with CD4 and CD56 depletion.16 In 1999, Ayoub et al also reported a series of 238 newly diagnosed patients with Hodgkin’s disease and showed a correlation between quantitative changes of B, T, and natural killer cells and patients’ clinical characteristics: white blood cell counts were higher in patients with advanced disease, while peripheral blood lymphocytes and CD4, CD8, and CD3−/CD56+/CD16+ subsets were decreased in advanced stages.43
Lymphopenia may not only be a parameter correlated to survival but also a biological mechanism stimulating tumor progression, both in NHL where immune suppression is clearly involved in tumor progression and etiology, and in other tumor types, including melanoma and head and neck carcinoma25,26,45 In addition to the increased risk of death due to treatment toxicity,15 the poor outcome observed in lymphopenic patients may also result from the loss of an anti-tumor specific immune response.46 The other fundamental question about the results presented here and previous studies8,12,15 is the link between drug toxicity, lower dose intensity of treatment (due to toxicity) or spontaneous poor prognosis. Unfortunately this question remains unclear because data on treatment intensity and chemotherapy were not available from the datasets used in the present study. It will be very important to explore this point in further studies.
Determining whether lymphopenia represents a cause as well as a consequence of tumor progression will be of importance in the perspective of the correction of lymphopenia using lymphocyte growth factors such as IL-7.47–50 Indeed, exogenous recombinant IL-7 can enhance T-cell recovery following lymphocyte depletion.40,41 This cytokine, like IL-2, is being developed therapeutically as an immunorestorative agent in lymphopenic patients and deserves to be tested in a clinical setting.47–49 In melanoma patients, a partial correction of lymphopenia has been observed following tumor antigen vaccination in those patients in whom tumor control had been achieved.25 Of note, we observed a normalization or correction of lymphocyte counts after complete remission only in lymphoma patients; lymphocyte counts dropped again in the 10 patients who subsequently relapsed (not shown). Not surprisingly, normalization could not be observed in breast carcinoma series where complete remission was not achieved. In conclusion, our results show that, in addition to its predictive value for hematological toxicity and early death, lymphopenia is a general prognostic factor for overall survival in patients with different types of cancers. The final question is how to use these findings in routine practice. Firstly, although the prognostic value of lymphocyte counts is now well established for NLH, their add-value for other cancers (sarcoma, MBC, but also lung cancer, etc) needs to be confirmed by others. Secondly, only the results of randomized clinical trials comparing homogeneous groups of patients treated or not with potential correctors of lymphopenia (anti CTLA4 antibody, IL-7 LGF, etc.) could modify clinical practice and thereby permit to use lymphopenia for decision making in routine practice. Currently, we plan to evaluate this prognostic factor in other solid tumors (ovarian carcinoma, lung cancer)51 and also to explore the mechanisms of such lymphopenia.
The understanding of mechanisms involved in lymphopenia and of its consequences on patient outcome may facilitate the development of corrective measures with the aim of reducing treatment toxicity and improving patient survival.
Acknowledgments
This publication was supported by grants number 5U10 CA011488-38 through 5U10 CA011488-38 from the National Cancer Institute (Bethesda, Maryland, USA) and by the Fonds Cancer (FOCA) from Belgium. Its content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute. The study was also supported in part by the Comités de l’Ain, du Rhone et de la Drome de la Ligue contre le Cancer, and by the Conticanet Network of Excellence (FP6-018806). The authors would like to thank M-Dominique Reynaud for editing assistance.
Footnotes
Previous presentations: Presented in part at the annual meeting of ASCO, San Francisco, May 2001.
References
- 1.Hortobagyi GN, Kris MG. Expanding horizons: an update on the use of docetaxel in non-small cell lung, ovarian, and breast cancers. Semin Oncol. 2002;29:1–3. doi: 10.1053/sonc.2002.34253. [DOI] [PubMed] [Google Scholar]
- 2.Crown J, Dieras V, Kaufmann M, et al. Chemotherapy for metastatic breast cancer-report of a European expert panel. Lancet Oncol. 2002;3:719–27. doi: 10.1016/s1470-2045(02)00927-0. [DOI] [PubMed] [Google Scholar]
- 3.van Glabbeke M, Van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens - a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–7. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 4.Blay JY, van Glabbeke M, Verweij J, et al. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer. 2003;39:64–9. doi: 10.1016/s0959-8049(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B. Standard treatment of advanced-stage diffuse large B-cell lymphoma. Semin Hematol. 2006;43:213–20. doi: 10.1053/j.seminhematol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol. 5:341–53. doi: 10.1016/S1470-2045(04)01490-1. [DOI] [PubMed] [Google Scholar]
- 7.Ray-Coquard I, Borg C, Bachelot T, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003;88:181–6. doi: 10.1038/sj.bjc.6600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blay JY, Chauvin F, Le Cesne A, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J Clin Oncol. 1996;14:636–43. doi: 10.1200/JCO.1996.14.2.636. [DOI] [PubMed] [Google Scholar]
- 9.Choi CW, Sung HJ, Park KH, et al. Early lymphopenia as a risk factor for chemotherapy-induced febrile neutropenia. Am J Hematol. 2003;73:263–6. doi: 10.1002/ajh.10363. [DOI] [PubMed] [Google Scholar]
- 10.Oguz A, Karadeniz C, Ckitak EC, Cil V. Which one is a risk factor for chemotherapy-induced febrile neutropenia in childhood solid tumors: early lymphopenia or monocytopenia? Pediatr Hematol Oncol. 2006;23:143–51. doi: 10.1080/08880010500457673. [DOI] [PubMed] [Google Scholar]
- 11.Alexandre J, Rey E, Girre V, et al. Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol. 2007;18:168–72. doi: 10.1093/annonc/mdl321. [DOI] [PubMed] [Google Scholar]
- 12.Blay JY, Le Cesne A, Mermet C, et al. A risk model for thrombocytopenia requiring platelet transfusion after cytotoxic chemotherapy. Blood. 1998;92:405–10. [PubMed] [Google Scholar]
- 13.Marec-Berard P, Blay JY, Schell M, et al. Risk model predictive of severe anemia requiring RBC transfusion after chemotherapy in pediatric solid tumor patients. J Clin Oncol. 2003;21:4235–8. doi: 10.1200/JCO.2003.09.121. [DOI] [PubMed] [Google Scholar]
- 14.Ray-Coquard I, Le Cesne A, Rubio MT, et al. Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group. J Clin Oncol. 1999;17:2840–6. doi: 10.1200/JCO.1999.17.9.2840. [DOI] [PubMed] [Google Scholar]
- 15.Ray-Coquard I, Ghesquieres H, Bachelot T, et al. Identification of patients at risk for early death after conventional chemotherapy in solid tumours and lymphomas. Br J Cancer. 2001;85:816–22. doi: 10.1054/bjoc.2001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borg C, Ray-Coquard I, Philip I, et al. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer. 2004;101:2675–80. doi: 10.1002/cncr.20688. [DOI] [PubMed] [Google Scholar]
- 17.Chauvin F, Magnet M, Lasset C, et al. [Prognostic factors in the response of a first line chemotherapy in advanced breast cancer] Bull Cancer. 1990;77:941–7. [PubMed] [Google Scholar]
- 18.Pinedo HM, Bramwell VH, Mouridsen HT, et al. Cyvadic in advanced soft tissue sarcoma: a randomized study comparing two schedules. A study of the EORTC Soft Tissue and Bone Sarcoma Group. Cancer. 1984;53:1825–32. doi: 10.1002/1097-0142(19840501)53:9<1825::aid-cncr2820530904>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:437–81. [Google Scholar]
- 21.Dyer AR. A method for combining results from several prospective epidemiologic studies. Stat Med. 1986;5:303–17. doi: 10.1002/sim.4780050403. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, George SL. The bootstrap and identification of prognostic factors via Cox’s proportional hazards regression model. Stat Med. 1985;4:39–46. doi: 10.1002/sim.4780040107. [DOI] [PubMed] [Google Scholar]
- 23.Dahm PF, Gail MH, Rosenberg PS, Pee D. Determining the value of additional surrogate exposure data for improving the estimate of an odds ratio. Stat med. 1995;14:2581–98. doi: 10.1002/sim.4780142307. [DOI] [PubMed] [Google Scholar]
- 24.Blay JY, Ray-Coquard I, Mermet C, et al. A multicentric prospective study of prognostic factors for febrile neutropenia after chemotherapy in general and cancer hospitals. J Clin Oncol. 1997;16:56a. (suppl; abstr) [Google Scholar]
- 25.Saito T, Kuss I, Dworacki G, et al. Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin Cancer Res. 1999;5:1263–73. [PubMed] [Google Scholar]
- 26.Dworacki G, Meidenbauer N, Kuss I, et al. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947s–57s. [PubMed] [Google Scholar]
- 27.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 28.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion? Nat Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–22. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 30.Voorzanger N, Touitou R, Garcia E, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non- Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–505. [PubMed] [Google Scholar]
- 31.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 32.Blay JY, Farcet JP, Lavaud A, et al. Serum concentrations of cytokines in patients with Hodgkin’s disease. Eur J Cancer. 1994;30A:321–4. doi: 10.1016/0959-8049(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 33.Casasnovas RO, Mounier N, Brice P, et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin’s lymphoma: a study from the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:1732–40. doi: 10.1200/JCO.2006.08.1331. [DOI] [PubMed] [Google Scholar]
- 34.Bachelot T, Ratel D, Menetrier-Caux C, et al. Autoantibodies to endostatin in patients with breast cancer: correlation to endostatin levels and clinical outcome. Br J Cancer. 2006;94:1066–70. doi: 10.1038/sj.bjc.6603037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blay JY, Negrier S, Combaret V, et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992;52:3317–22. [PubMed] [Google Scholar]
- 36.Seymour JF, Talpaz M, Cabanillas F, et al. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13:575–82. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- 37.Negrier S, Perol D, Menetrier-Caux C, et al. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6--from the Groupe Francais d’Immunotherapie. J Clin Oncol. 2004;22:2371–8. doi: 10.1200/JCO.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 38.Thomachot MC, Driss-Vermare N, Massacrier C, et al. Breast carcinoma cells promote the differentiation of CD34+ progenitors towards 2 different subpopulations of dendritic cells with CD1a(high)CD86(−)Langerin- and CD1a(+)CD86(+)Langerin+ phenotypes. Int J Cancer. 2004;110:710–20. doi: 10.1002/ijc.20146. [DOI] [PubMed] [Google Scholar]
- 39.Seve P, Ray-Coquard I, Trillet-Lenoir V, et al. Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer. 2006;107:2698–705. doi: 10.1002/cncr.22300. [DOI] [PubMed] [Google Scholar]
- 40.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 41.Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970;25:135–40. doi: 10.1002/1097-0142(197001)25:1<135::aid-cncr2820250120>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52:126–30. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 43.Ayoub JP, Palmer JL, Huh Y, et al. Therapeutic and prognostic implications of peripheral blood lymphopenia in patients with Hodgkin’s disease. Leuk Lymphoma. 1999;34:519–27. doi: 10.3109/10428199909058479. [DOI] [PubMed] [Google Scholar]
- 44.Plonquet A, Haioun C, Jais JP, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2–3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18:1209–15. doi: 10.1093/annonc/mdm110. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62. [PubMed] [Google Scholar]
- 46.Mazur G, Bogunia-Kubik K, Wrobel T, et al. TGF-beta1 gene polymorphisms influence the course of the disease in non-Hodgkin’s lymphoma patients. Cytokine. 2006;33:145–9. doi: 10.1016/j.cyto.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–9. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7R alpha expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–62. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fry TJ, Moniuszko M, Creekmore S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–9. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 51.Penel N, Delord JP, Bonneterre ME, Bachelot T, et al. Development and validation of a model that predicts early death among cancer patients participating in phase I clinical trials investigating cytotoxics. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9224-x. (in press) [DOI] [PubMed] [Google Scholar]