Abstract
Native myocardium consists of several cell types, of which approximately one-third are myocytes and most of the nonmyocytes are fibroblasts. By analogy with monolayer culture in which fibroblasts were removed to prevent overgrowth, early attempts to engineer myocardium utilized cell populations enriched for cardiac myocytes (CMs; ~80–90% of total cells). We hypothesized that the pre-treatment of synthetic elastomeric scaffolds with cardiac fibroblasts (CFs) will enhance the functional assembly of the engineered cardiac constructs by creating an environment supportive of cardiomyocyte attachment and function. Cells isolated from neonatal rat ventricles were prepared to form three distinct populations: rapidly plating cells identified as CFs, slowly plating cells identified as CMs, and unseparated initial population of cells (US). The cell fractions (3 × 106 cells total) were seeded into poly(glycerol sebacate) scaffolds (highly porous discs, 5 mm in diameter × 2-mm thick) using Matrigel™, either separately (CM or CF), concurrently (US), or sequentially (CF pre-treatment followed by CM culture, CF + CM), and cultured in spinner flasks. The CF + CM group had the highest amplitude of contraction and the lowest excitation threshold, superior DNA content, and higher glucose consumption rate. The CF + CM group exhibited compact 100- to 200-μm thick layers of elongated myocytes aligned in parallel over layers of collagen-producing fibroblasts, while US and CM groups exhibited scattered and poorly elongated myocytes. The sequential co-culture of CF and CM on a synthetic elastomer scaffold thus created an environment supportive of cardiomyocyte attachment, differentiation, and contractile function, presumably due to scaffold conditioning by cultured fibroblasts. When implanted over the infarcted myocardium in a nude rat model, cell-free poly(glycerol sebacate) remained at the ventricular wall after 2 weeks of in vivo, and was vascularized.
Keywords: cardiac tissue engineering, myocardium, fibroblast, cardiomyocyte, collagen, scaffold
INTRODUCTION
Cardiac myocytes (CMs) represent approximately one-third of the number of all cells in the myocardium, and are responsible for synchronous contractions of the ventricles.1 Most of the nonmyocytes are cardiac fibroblasts (CFs), which contribute to the structural, biochemical, mechanical, and electrical properties of the myocardium and can affect adjacent cardiomyocytes by secretion of regulatory molecules and extracellular matrix (ECM) components, and by the coupling of gap junctions.2–6 The myocardial ECM consists of a fibrillar collagen network, with predominant collagen types I and III, a basement membrane, proteoglycans, glycosaminoglycans, and a variety of bioactive molecules.7 The composition of the ECM is regulated by a crosstalk between CFs and cardiomyoyctes.2 Recent studies demonstrated that CFs are electrically coupled with cardiomyocytes and can propagate electrical stimuli over 100-μm distances.3–6
Since fibroblasts rapidly overgrow myocytes in monolayer cultures, they are routinely removed prior to cell culture using pre-plating. Pre-plating is a technique that relies on faster attachment rates of fibroblasts compared to cardiomyocytes, such that after 1 h of pre-plating, most of the adherent cells are fibroblasts and the cell suspension is enriched for cardiomyocytes.8 By analogy with these monolayer cultures, attempts to engineer cardiac tissue, including our previous studies, typically involved the use of cell populations enriched for cardiomyocytes by pre-plating.9–13 The engineered constructs exhibited markers of cardiac differentiation and were able to propagate electrical signals over millimeter distances.9,10,12 The velocity of signal propagation increased with an increase in the fraction of myocytes in the cell preparation.10 Zimmermann et al.13 recognized the importance of the presence of multiple cell types for the in vitro cultivation of heart tissue in their cultivation system with cyclic stretch. In separate studies Akhyari et al.14 detected the presence of a newly synthesized collagen network in tissue constructs based on passage 3 human pediatric heart cells, suggesting that fibroblasts may play a role in the remodeling of engineered cardiac constructs in vitro.
Based on the evidence collected in these previous studies, we hypothesized that scaffold pretreatment with CFs prior to seeding of CMs can enhance functional assembly of the engineered cardiac constructs by creating an environment supportive of cardiomyocyte attachment, differentiation, and contractility. To test this hypothesis, neonatal rat heart cells were prepared to form three distinct cell populations: rapidly plating cells identified as CFs, slowly plating cells identified as CMs, and the unseparated initial population of cells (US). The cells were cultured for 11 days on porous scaffolds made of poly(glycerol sebacate) (PGS). Four experimental groups were studied: (1) CF applied to the scaffolds separately, (2) CM applied to the scaffolds separately, (3) the two cell types applied to the scaffold concurrently (US), or (4) the two cell types applied to the scaffold sequentially (CF pre-treatment followed by CM culture, CF + CM).
The porous PGS scaffold was selected as an excellent candidate scaffold for cardiac tissue engineering because it is a mechanically stable, yet flexible, bioresorbable elastomer.15–17 In the current study we also demonstrated the feasibility of implantation of the PGS scaffold in a rat model of myocardial infarction. Experimental data supported our hypothesis that the presence of CF and the sequential mode of their application in cell co-culture improve the structural and contractile properties of engineered cardiac tissue.
METHODS
Cell preparations
Cardiomyocytes were obtained from 1- to 2-day old neonatal Sprague Dawley rats (Charles River) as previously described9 and according to procedures approved by the Institute’s Committee on Animal Care. In brief, ventricles were quartered, trypsinized overnight at 4°C in a 0.06% (w/v) solution of trypsin in Hank’s balanced salt solution (HBSS, Gibco), and subjected to a series of digestions (8 min, 37°C, 75 rpm) in 0.1% (w/v) solution of collagenase type II in HBSS. The cell suspension from digestions three to five were collected and labeled US cells. To separate myocytes and fibroblasts, the cell suspension was centrifuged (750 rpm, 5 min) and the pellet was resuspended in Dulbecco’s modified eagle’s medium (Gibco) containing 4.5 g/L glucose, 10% FBS, 10 mM HEPES, 2 mM L-glutamine, and 100 U/mL penicillin. The cells from the pellet were preplated in T75 flasks for a 75-min period. Cells that remained unattached were labeled enriched CMs, whereas the attached cells were labeled CFs.3
Construct preparation
Porous PGS scaffolds were prepared by porogen leaching as previously described16,17 and die-punched into discs measuring 5 mm in diameter × 2-mm thick. Scaffolds were sterilized and prewetted at room temperature by sequential treatment in the following solutions: ethanol (70% for 12 h and then 95% for 4 h and then 100% for 1 h), phosphate buffered saline (1 h), and fetal bovine serum (100% for 1 h).
The US and CM cell fractions were used for scaffold seeding immediately after isolation. The CF fraction was propagated in monolayers for 3–7 days prior to seeding onto the scaffolds. The four experimental groups (CF + CM-11, US-11, CM-11, and CF-5 or CF-11) were established as follows (Table I). Briefly, we used our previously developed rapid inoculation procedure in which cells were seeded into the scaffold encapsulated in Matrigel®.18 This ensures that the cells are evenly distributed in the scaffold volume and retained in the pores following Matrigel gelation at 37°C. Scaffold pre-treatment with fibroblasts was preformed in orbitally mixed six-well plates for 5 days. Following Matrigel seeding of cardiomyocytes, the constructs were cultivated in spinner flasks for an additional 6 days. The details of procedures for all of our experimental groups are outlined later.
TABLE I.
Experimental Design
| Group | Pre-treatment (Days 1–5; Orbitally Mixed Six-Well Plates) | Cultivation (Days 6–11; Spinner Flasks) |
|---|---|---|
| CF-5 (−11) | 1 × 106 fibroblasts | No cells added |
| CM-11 | No cells added | 3 × 106 enriched cardiomyocytes |
| US-11 | No cells added | 3 × 106 unseparated cells |
| CF + CM-11 | 1 × 106 fibroblasts | 2 × 106 enriched cardiomyocytes |
Four experimental groups were studied: (1) CF-scaffolds seeded with cardiac fibroblasts, (2) CM-scaffolds seeded with enriched cardiomyocytes, (3) US-scaffolds seeded with an unseparated cell population, and (4) CF + CM, scaffolds pre-treated with cardiac fibroblasts followed by addition of enriched cardiomyocytes. For pre-treatment, PGS-based constructs were cultivated in orbitally mixed six-well plates for 5 days. Subsequently, the constructs were cultivated for additional 6 days in spinner flasks.
In the CF group, on culture day 1, 1 × 106 CF were suspended in 10 μL cold Matrigel, seeded on a scaffold, placed in a well plate, and cultured for 5 days with orbital mixing in six-well plates (one construct and 5 mL media per 35-mm well, 25 rpm). On culture day 6, constructs were removed from the dish, threaded on needles suspended in spinner flasks (six CF constructs and 60 mL media per flask), and cultured as in the CF + CM group until culture day 11. Constructs analyzed after 5 days in culture are named CF-5 and those analyzed after 11 days of culture are named CF-11.
In the CM-11 and US-11 groups, on culture day 1, cell-free scaffolds were placed in six-well plates (one scaffold and 5 mL media per 35-mm well, 25 rpm) and maintained in the incubator for 5 days. On culture day 6, scaffolds were seeded with either 3 × 106 CM or 3 × 106 US in 10 μL cold Matrigel, threaded on needles suspended in spinner flasks (six CM or US constructs and 60 mL media per flask), and cultured for total of 11 days.
In the CF + CM group, on culture day 1, 1 × 106 CF were suspended in 10 μL cold Matrigel (BD), seeded on a scaffold, placed in a 35-mm well of the six-well plate, and cultured for 5 days with orbital mixing (one construct and 5 mL media per well, 25 rpm). Then, on culture day 6, constructs were removed from the well plates, reseeded with an additional 2 × 106 CM in 10 μL cold Matrigel, threaded on needles, and suspended in a spinner flask (six constructs and 60 mL media per flask).9 The flask was cultured statically for 24 h and then with magnetic stirring at 30 rpm until culture day 11.
Culture media were completely replaced every 3 days. Constructs were harvested on culture day 11 from all four groups, and also on culture day 5 from the CF group.
Scanning electron microscopy
Postfabrication, the scaffolds were assessed using an environmental scanning electron microscope (Philips/FEI XL30 FEG-SEM).
Properties of the initial cell populations
The fractions of CM and CF in the initial cell preparations obtained from neonatal rat heart ventricles (CM, US, CF) were assessed by fluorescence-activated cell sorting (FACS). The following cell suspensions were subjected to FACS: US cell suspension immediately after collagenase digests; the unattached cell suspension after pre-plating (i.e. CM fraction); the cells that attached following pre-plating (i.e. CF fraction) were propagated for 3–7 days, trypsinized to obtain cell suspension, and then subjected to FACS. The cell suspensions were fixed and permeabilized with the solution of acetone and methanol (3:2) at −20 °C and antibody-stained. To identify CM, cells were suspended in 5% FBS in PBS (106 cells/mL), incubated with mouse anti-sarcomeric α-actin (clone 5C5, 1:100, Sigma) for 30 min on ice, rinsed, and incubated with fluorescein-conjugated horse anti-mouse IgG for an additional 30 min on ice (1:400, Vector Laboratories).
To identify CF, the cells were labeled with Cy 3 conjugated mouse anti-vimentin (clone V9, 1:200, Sigma) for 30 min on ice. The fluorescence was read on FACScan (Becton Dickinson). Unlabeled cells and cells labeled only with the secondary antibody served as controls. To confirm the identity of CM, independent studies were done using mouse anti-troponin I (Clone 23C6, 1:100, Biodesign) as a marker. Fractions of the troponin-I labeled cells were similar to the fractions of sarcomeric α-actin labeled cells (data not shown). Cell types identified by FACS represented 74, 93, and 81% of the total cells in the US-0, CF-0, and CM-0 groups, respectively.
Biochemical and metabolic assessment
Medium samples were taken on culture days 3, 5, and 11 of culture and analyzed for glucose and lactate using a glucose and L-lactate analyzer model 2300 STAT plus (Yellow Spring Instrument9) and for lactate dehydrogenase levels (LDH) using a commercially available kit (LDH-L, Chiron Diagnostics5).
Contractile response
Constructs sampled on culture day 11 were analyzed with respect to excitation threshold (ET, minimum stimulation voltage required for synchronous contractions), maximum capture rate (MCR, maximum beating frequency), and amplitude of contraction (fractional area change) by electrical field stimulation (square pulses, 2 ms, 1 Hz) as previously described19 at room temperature. A total of nine constructs were analyzed in the CM and CF + CM groups, and 10 constructs in the US group as described in detail in Supplemental Information. Average amplitude of contraction (i.e., average fractional area change measured during a 1- to 5-min-long contractile sequence) and contraction profile (i.e., time history of fractional area change obtained measured during a single, 800-ms contractile cycle) were determined using image analysis software (Scion Image).
Histology and immunofluorescence
Histological analyses were done on constructs (n = 4–5 per group) sampled on culture day 11 from all four groups and on culture day 5 for the CF group (CF-5). Sections were stained with hematoxylin and eosin for general evaluation and Masson’s Trichrome for collagen. Sections were immunofluorescently stained for markers of CM (troponin I, sarcomeric α-actin, connexin-43)19 and markers of CF (vimentin and prolyl-4-hydroxylase)17 according to a protocol described in Supplemental Information. Neonatal rat heart tissue and bovine articulate cartilage served as positive and negative control, respectively.
The structural organization and relative fractions of CM and CF in cultured tissue constructs were assessed by semiquantitative analysis of histological samples double-stained for α-sarcomeric actin and vimentin. Sections were stained with actin, blocked with 10% NHS, and incubated with mouse Cy3 conjugated anti-vimetin, and evaluated using a fluorescent microscope (Axioplan, Zeiss) and Open Lab software. The number of actin-labeled cells (presumed to be CM) and vimentin-labeled cells (presumed to be CF) were quantified by three independent observers at a 400× magnification (n = 1–4 photomicrographs per section, n = 2–5 sections per group).
DNA and protein
Constructs sampled on culture day 11 from all four groups and on day 5 from the CF group (CF-5) were analyzed with respect to wet weight and total amounts of DNA and total protein as previously described.9,19 In brief, constructs (n = 3 per group) were weighed, frozen in liquid nitrogen, homogenized in a buffer (1N NH4OH, 2% Triton X-100) using a Mini-bead beater, centrifuged, and stored at −80°C. For DNA assay, the homogenate was incubated at 37°C for 10 min, diluted with 10 mM Tris-1 mM EDTA buffer containing 100 mM NaCl (1:20), centrifuged at 2500g for 30 min, and the supernatant was used to measure DNA content fluorometrically by Hoescht dye binding9 and total protein using a commercially available kit (Bio-Rad).9,10
Implantation studies
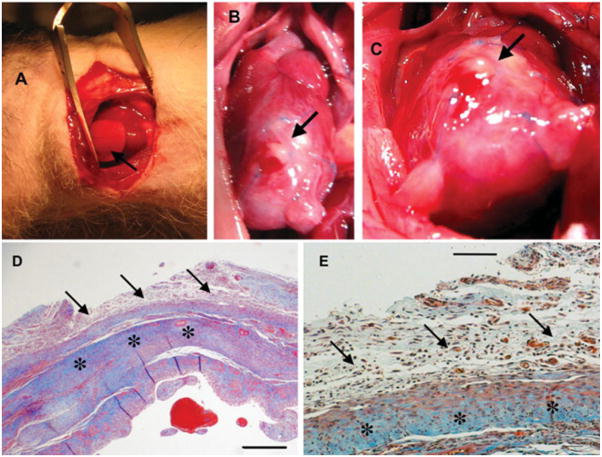
The scaffold was tested in a nude rat model in the setting of infarction secondary to chronic total coronary occlusion as described in detail in Supplemental Information. Briefly, the left anterior descending coronary artery (LAD) was exposed through a left thoracotomy. A prolene suture was passed around the LAD and tied. Following ischemia, identified by the pale/white color of the infarcted region, a cell-free scaffold (1 cm in diameter × 1.5-mm thick) was sutured onto the infarct bed [Fig. 7(A)]. Implants were harvested after 2 weeks.
Figure 7.
Interactions between the scaffold and the in vivo environment: implantation in a rat heart infarction model. (A) Implantation of the elastomer scaffold in a nude rat after induction of myocardial infarction by occlusion of the left anterior descending coronary artery. The scaffold (1 cm diameter × 1.5-mm thick disc) was sutured over the entire infarct bed (arrow). (B,C) Macroscopic view of the area at 2 weeks following implantation. (D) Cross-sectional view of the graft–host interface at 2 weeks (Mason’s trichrome staining, collagen stains blue). Note excellent integration between the graft (arrows) and host (stars). (E) Higher magnification view of image D. Note the formation of multiple blood vessels within the graft, which were connected to the native circulation as evidenced by the presence of intraluminal red blood cells. Scale bars: 0.5 mm (D), 100 μm (E). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Statistical analysis
Results obtained for different initial cell populations [Fig. 1] and different 11-day construct groups [Figs. 1, 2, and 3] were compared using Tukey’s test followed by one-way ANOVA for pairwise comparisons. Results obtained using different culture systems and durations (Table II) were compared using Dunn’s post hoc test followed by one way ANOVA. p < 0.05 was considered significant. Normality and equality of variance were tested for all data sets.
Figure 1.
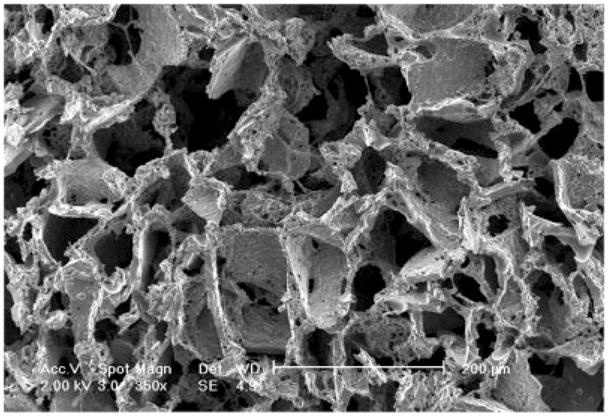
Scanning electron micrograph of the PGS scaffold used for cardiac tissue engineering. The scaffold, obtained by salt leaching procedure, exhibited extensive interconnected macropores of 75–150 μm in diameter.
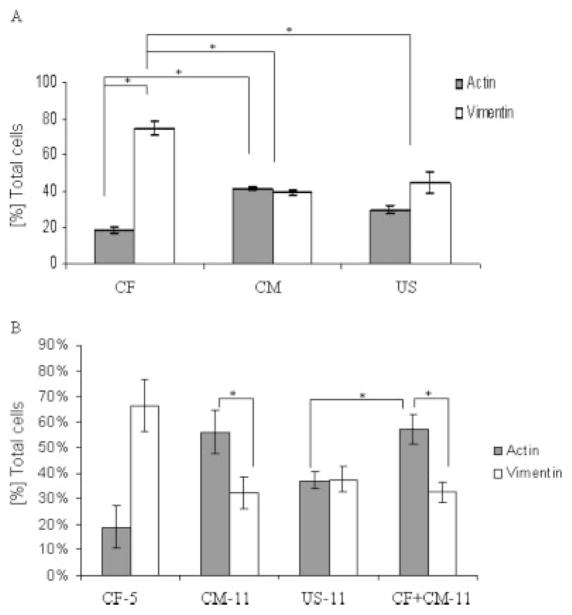
Figure 2.
Composition of the initial cell populations and tissue constructs at days 5 and 11 of culture. (A) Percentages of myocytes and fibroblasts in initial cell populations as determined by FACS for sarcomeric α-actin and vimentin. (B) Percentages of fibroblasts and myocytes in 5-day and 11-day constructs as determined by immunofluorescent staining for sarcomeric α-actin and vimentin. Data are Ave ± SE (n = 2–16). *Significantly different (p < 0.05) by Tukey’s test and one-way ANOVA.
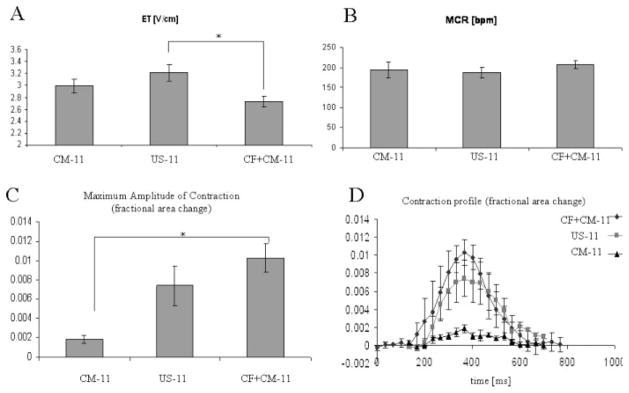
Figure 3.
Contractile properties of constructs at day 11 of culture. (A) Excitation threshold (ET, volts/cm). (B) Maximum capture rate (MCR, beats per minute). (C) Average value of the maximum amplitude of contraction (fractional area change). (D) Contraction profile (time history of fractional area change during a single contraction cycle). Data are Ave ± SE (n = 9–10). *Significantly different by Tukey’s test and one-way ANOVA.
TABLE II.
Metabolic Properties of the Constructs During the Culture
| Group | Glucose Consumption Rate (μmol/construct/h) | Lactate/Glucose (mol/mol) | LDH (mU/construct/h) |
|---|---|---|---|
| CF-5 | 0.11 ± 0.01 | 2.43 ± 0.21 | 0.29 ± 0.04 |
| CM-11 | 0.08 ± 0.02 | 1.67 ± 0.40 | 1.73 ± 0.61* |
| US-11 | 0.11 ± 0.03 | 1.62 ± 0.17 | 2.30 ± 0.55* |
| CF + CM-11 | 0.18 ± 0.06 | 1.70 ± 0.22 | 1.54 ± 0.51 |
Data are Ave ± SE (n = 3–12).
Significantly higher than CF-5 by Dunn’s test.
RESULTS
The PGS scaffold used in the described cardiac tissue engineering studies was prepared by a salt leaching procedure and was thoroughly characterized in previous studies.16 Scanning electron microscopy (Fig. 1) indicated the presence of interconnected macropores in the range from 75 to 150 μm as well as micropores in the range of 5–20 μm. The macroporous structure is supportive of cardiac tissue assembly. Our previously used collagen sponge scaffolds also exhibited macroporous structure, with a pore diameter in the range of 100–200 μm.20
Relative fractions of cardiomyocytes and CFs in initial cell populations are compared in Figure 2(A). The US cell population from neonatal heart ventricles had ~35% α-actin positive cells (identified as cardiomyocytes) and ~45% vimentin positive cells (identified as CFs), and the difference between the two fractions was not statistically significant. The CF group, obtained by expanding the cells attached to the tissue culture plastic during the pre-plating, consisted of ~75% cells expressing vimentin (identified as CFs) and a relatively small fraction of cells (6–18%) expressing α-actin (identified as cardiomyocytes). The cells that remained unattached after one pre-plating had equal fractions of cells expressing cardiac markers (41–45% CM) and cells expressing vimentin (~40% CF). The ratio of cardiomyocytes to CFs in US group was 0.67 while for the CM group it was 1.05 indicating enrichment.
Relative fractions of CM and CF in constructs from the CM-11, US-11, and CF + CM-11 groups are compared in Figure 2(B). The CM-11 and CF + CM-11 groups had significantly higher fractions of cardiomyocytes than CFs, whereas the US-11 group had similar numbers of cardiomyocytes and CFs, and relatively fewer cardiomyocytes than the CF + CM-11 group [Fig. 2(B)]. Interestingly, CF-5 constructs closely resembled the initial CF cell population with respect to relative fractions of myocytes and fibroblasts [compare CF-5 in Fig. 2(B) with CF in Fig. 2(A)]. Moreover, in vitro cultivation of heart cells on 3D scaffolds yielded an increased fraction of myocytes in the CM group [compare CM-11 in Fig. 2(B) with CM in Fig. 2(A)], and maintained the fraction of myocytes in the US and CF groups [compare corresponding groups in Fig. 2(A,B)].
Glucose consumption rate per construct (Table II) was comparable for the US and CM groups and it was higher in the CF + CM group. In order to compare glucose consumption rates in 11-day constructs, we utilized a conversion factor of 12 pg DNA/cell and DNA content per construct determined at the end of cultivation.17 Glucose consumption rates, expressed in micromoles of glucose consumed/106 cells/h were 0.24 for CF-11, 0.09 for US-11, and 0.08 for CM-11 and 0.19 for CF + CM-11), and were in the order of those reported previously.21,22 The molar ratio of lactate produced to glucose consumed (L/G), an index of anaerobic metabolism, was higher in well plate (~2.43, CF group days 1–5) than in spinner flask cultures (CM, US, and CF + CM groups, days 6–11), consistent with the bioreactor-enhanced oxygen transport at construct surfaces and the limitations of oxygen supply in the construct interior (Table II). The LDH release, an index of cell damage, was lowest in well plate cultures of the CF group (culture days 0–5), relatively higher in spinner flask cultures of the CF + CM group (culture days 6–11), and the highest values in spinner flasks cultures of the CM and US groups (Table II).
We were able to reproducibly induce a synchronous contractile response to electrical field stimulation in the CM-11, US-11 and CF + CM-11 constructs, whereas only occasional contractions of individual cell clusters were observed in the CF-5 and CF-11 constructs. The CF + CM-11 constructs had significantly lower ETs than the US-11 constructs [Fig. 3(A)], and significantly higher amplitudes of contraction and contraction profiles than the CM-11 constructs [Fig. 3(C,D)]. MCRs were comparable in all groups [Fig. 3(B)].
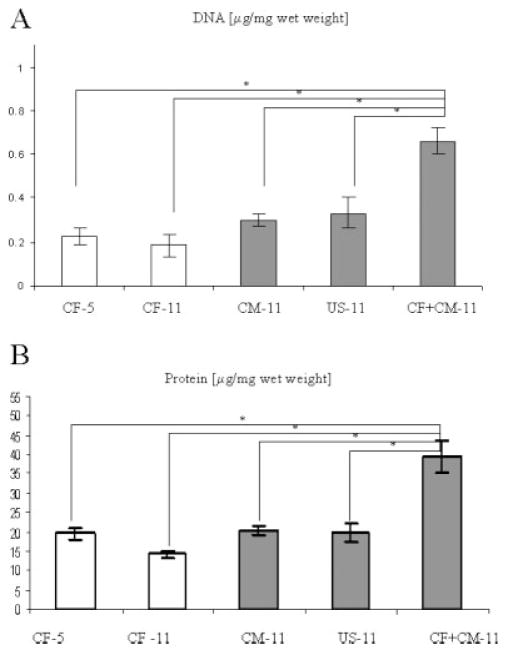
Total DNA content per unit wet weight of CF + CM-11 group was significantly higher compared to the all other groups (CF-5, CF-11, CM-11, US-11), and comparable to that measured for native neonatal ventricle9 [Fig. 4(A)]. The total cell numbers at the end of cultivation were comparable for the US-11, CM-11, and CF + CM-11 groups (~106 cells); CF-5 and CF-11 group had ~0.5 × 106 cells. Construct wet weights were as follows: 26 ± 3 mg for CF-5, 28 ± 3 for CF-11, 39 ± 2 for CM-11, 43 ± 5 for CM-11, and 17 ± 4 for CF + CM-11. Protein content was twice as high in the CF + CM-11 group that in the other groups [Fig. 4(B)] and comparable to that measured for the native ventricle.9 All other groups had protein content inferior to that present in the native ventricles.
Figure 4.
Construct size and composition at days 5 and 11 of culture. (A) Cellularity index (μg DNA/mg wet weight). (B) Protein content (μg protein/mg wet weight). Data are Ave ± SE (n = 3). *Significantly different (p < 0.05) by Tukey’s test and one-way ANOVA.
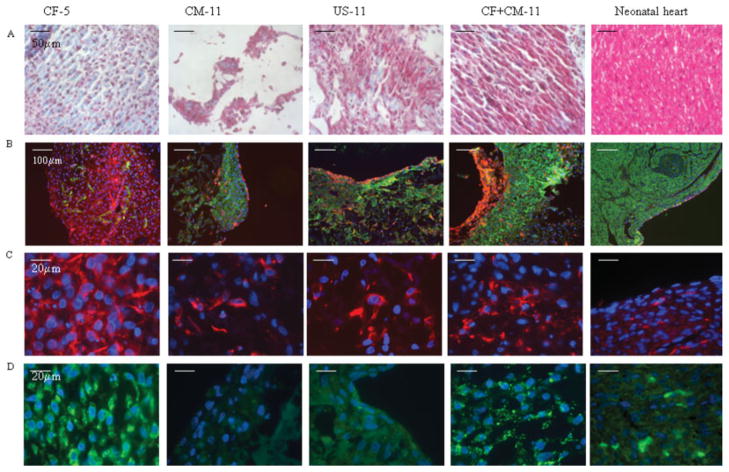
Effects of the initial cell population(s) and the timing of cell seeding on 3D scaffolds on the histological appearance of tissue engineered constructs are shown in (Figs. 5 and 6). No difference between CF-5 and CF-11 groups could be observed and therefore representative images of the former group are shown. Masson’s trichrome stain [Fig. 5(A)] revealed round-to-oval cell morphologies in the CF-5 and CM-11 groups, whereas many cells appeared elongated and aligned in parallel layers in the US-11 and CF + CM-11 groups. Trichrome [Fig. 5(A)] also showed that the cells were interspersed in a collagenous ECM in the CF-5, US-11, and CF + CM-11 groups, but not in the CM-11 group.
Figure 5.
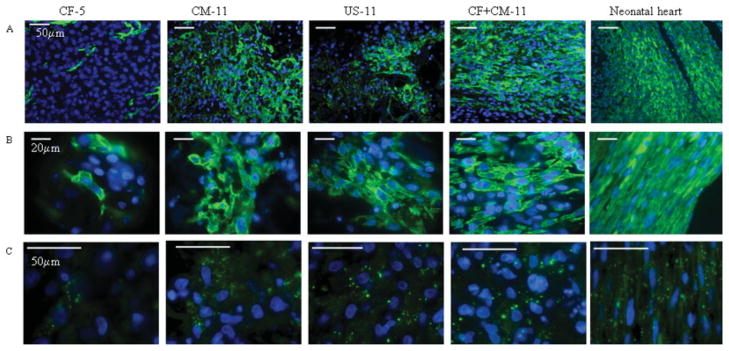
Differential effects of cell populations on PGS-based construct (A,B) general appearance, (C,D) fibroblast markers, (A) Masson’s trichrome stains cytoplasm and muscle pink and collagen blue, (B) vimentin/α-actin co-stain CF red and CM green, (C) vimentin stains CF red, (D) prolyl-4-hydroxylase, involved in collagen synthesis, stained green (B,D) DAPI stains nuclei blue. All panels represent face sections within the first 100 μm of the construct surface. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 6.
Presence of cardiac markers in the PGS-based constructs: (A,B) cardiac Tn-I stains CM green, (C) connexin-43, a gap junctional protein is stained green, (A–C) DAPI stains nuclei blue. Scale bars: (A,F) 50 μm; (B) 100 μm; (C,D,E) 20 μm. All panels represent face sections within the first 100 μm of the construct surface. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The cardiomyocytes and CFs were colocalized within samples by costaining with sarcomeric α-actin and vimentin, respectively [Fig. 5(B)]. Costaining revealed that a 50- to 200-μm-thick fibrous layer was present at the outer surfaces of constructs from all groups, and occasionally present at the interface between the construct and the stainless steel needle in the spinner flask. Costaining [Fig. 5(B)] also revealed large regions containing densely packed cardiomyocytes in the CF + CM-11 and CM-11 groups, more diffusely distributed cardiomyocytes in the US-11 group, and few scattered cardiomyocytes in the CF-5 group. Vimentin [Fig. 5(C)] and prolyl-4-hydroxylase [Fig. 5(D)] stains, respectively, showed that fibroblasts and collagen synthesis were present to a high degree in the CF-5 and CF + CM-11 groups, to an intermediate degree in the US-11 group, and to a minimal degree in the CM-11 group.
The distribution, morphology, and interconnectivity of cardiomyocytes were examined by immunofluorescence staining for cardiac Tn-I [Fig. 6(A,B)] and the gap junctional protein Cx-43 [Fig. 6(C)]. In the CF-5 group, Tn-I positive cells were sparse and Cx-43 staining was almost undetectable, whereas in the CM-11 group, Tn-I positive cells with round to “starlike” morphologies were present, and Cx-43 staining was diffusely present (Fig. 6). In the US-11 and CF + CM-11 groups, Tn-I positive cells were increasingly elongated and Cx-43 staining was strongly positive and characteristically punctuate (Fig. 6). In the US-11 group, short domains of Tn-I positive cells that were elongated and aligned were interrupted by domains of cells that did not stain for Tn-I, whereas the CF + CM-11 group exhibited longer, uninterrupted domains of elongated, Tn-I positive cells (Fig. 6). The identities of Tn-I and Cx-43 were confirmed by Western blotting (data not shown).
There was no infection or other adverse reaction to scaffold implantation in the nude rat model of myocardial infarction. The elastomer scaffold was easy to suture, without tears or breakdown of the material. At the time of explant [2-week time point, Fig. 7(B,C)], the scaffold was partially degraded but in place and it appeared integrated with the host ventricle. The presence of collagen in the host myocardium [blue staining, Fig. 7(D)] is a result of the myocardial infarction and subsequent remodeling process. Fibrous adhesions were formed between the site of implantation and chest wall, which is considered normal at the 2-week time point. The implant was cellularized, indicative of the invasion of the host cells into the initially acellular scaffold, and contained new blood vessels. Red blood cells were visible in some of the vessels, indicating connection to the host vasculature [Fig. 7(E)].
DISCUSSION
In recent studies17,23 we explored if the use of channeled scaffolds and medium perfusion resulted in thick and compact cardiac constructs containing a physiologic density of viable and differentiated cells. In those studies, we started to use a new, synthetic scaffold made of porous PGS, because collagen could not be processed into channeled scaffolds. Even the use of the lowest energy laser for the creation of channel arrays resulted in damage of collagen. In contrast, the PGS was easily processed into porous scaffolds with arrays of channels. The PGS scaffolds maintained their favorable structural and mechanical properties over long times in culture, whereas collagen scaffolds became difficult to handle and tended to collapse in perfusion flow. In addition, the PGS scaffolds were more mechanically stable than the collagen scaffolds according to the published elastic moduli (~6 kPa for PGS16 versus ~0.3 kPa for collagen sponge24). Yet, the cultivation of enriched cardiomyocytes on porous PGS scaffolds resulted in cardiac tissue with contractile properties inferior to constructs based on collagen sponges. Pre-treatment with CFs was required to achieve properties similar to the constructs based on enriched cardiomyocytes and collagen scaffolds.17
We undertook the present study to thoroughly investigate the interaction of the PGS scaffold with CF and cardiomyocyte subpopulations under the conditions of in vitro culture. We also documented the interactions between the scaffold and cardiac cells in a rigorous in vivo model of cardiac implantation. PGS scaffolds were implanted onto ischemic myocardium, following infarction created by LAD ligation. This is a difficult surgical model and one that ultimately addresses the envisioned use of the PGS scaffold. Moreover, application of the PGS scaffold in a heart implantation model has not been reported previously. Current work documents the feasibility of the PGS scaffold implantation, whereas our future work will investigate functional improvements of heart function upon implantation of the PGS-based constructs in a nude rat model of myocardial infarction. The presence of multiple cell types is one of the hallmarks of the healthy native tissue and one of the key requirements for tissue engineering of functional cardiac constructs.
After a single pre-plating of dissociated heart cells, CFs represent the majority of the attached cells, whereas cardiomyocytes and endothelial cells remain unattached.25 The majority of the cells in our CF population were CFs, as were 30–40% of the cells in our CM and US populations [Fig. 2(A)]. In contrast to the previously held notion that CFs should be depleted by pre-plating lest they overgrow the cardiomyocytes in engineered cardiac tissues,10 we showed that the 3D cultivation of mixed populations of CFs and cardiomyocytes was associated with an increase in the fraction of myocytes in the CM-11 group, and maintenance of the initial fractions of myocytes in the CF-5 and US-11 groups (Fig. 2).
It was demonstrated previously that microtopography affects fibroblast proliferation, such that the rate was slower for fibroblasts cultivated on surfaces with arrays of microscaled pegs.26 It is possible that the microarchitecture of PGS scaffold prevented the overgrowth of fibroblasts in this system. These findings are consistent with improved contractile function of engineered cardiac tissue based on mixed cell populations relative to cardiomyocyte-enriched populations.27 In addition, the values in Figure 2 do not add to 100%, since there are other cell types (e.g., endothelial cells and smooth muscle cells) that are present in native heart tissue. Yet, these other cell types are present at a low fraction (less than 4% as demonstrated in Ref. 27), thus they were not considered in this study.
A synchronous contractile response to electrical pacing (Fig. 3) was reproducibly induced in CM-11, US-11, and CF + CM-11 constructs that were based on a PGS scaffold with interconnected 75- to 150-μm pores, high porosity of ~90%, and a compressive modulus of ~5 kPa.16 In previous studies, constructs based on mechanically robust composite scaffolds, including a knitted fabric and fibrin,28 could not be electrically induced to contract, whereas constructs based on weaker materials, including collagen gel,29 nonwoven polyglycolic acid mesh,10 and collagen sponges alone14 or combined with Matrigel,19 did exhibit contractile responses. In the present study, PGS-based constructs exhibited lower MCRs and contractile amplitudes than we previously measured for collagen-Matrigel,19 which may be attributed to a relatively higher modulus of the PGS. Further studies are needed to improve our understanding of how scaffold biomechanical properties can impact construct electromechanical properties.
As expected, the groups that contained CFs in addition to CMs (US-11, CF + CM-11) had a more robust contractile response than those containing mostly CMs (CM-11). Consistently, the highest amplitude of contraction was observed in the CF + CM-11 group [Fig. 3(C,D)]. MCR was comparable for constructs from all groups [Fig. 3(B)], and it was lower than the values reported for similar constructs previously,21 most likely due the higher modulus of PGS compared to collagen sponge and as discussed earlier. Consistent with the previous reports, CM-11 group had lower ET than the US-11 group [Fig. 3(A)]. Unexpectedly, the CF + CM-11 group, in addition to all other properties, also had the lowest ET among the groups. One possible explanation for these observations is that the sequential application of CF and CM improved the connections of cardiomyocytes within the constructs beyond levels measured for either the US-11 group or the CM-11 group.
The superior contractile properties in the CF + CM-11 group—a combination of the lowest ET and the highest amplitude of contractions—had the biochemical and morphological basis. The DNA content of CF + CM-11 group, which was significantly higher [Fig. 4(A)] than in any other group and comparable to that of the native heart, was a likely contributor to the decrease in the ET. Higher construct cellularity was previously correlated to the lower ET.21 High amounts of Cx 43 identified in the immunostaining for CM-11 and CF + CM-11 groups are further consistent with the lower ET [Fig. 6(C)]. The release of LDH as a measure of cell damage was the highest in the US group (Table II), a finding consistent with the low cellularity and high ET observed in this group.
The protein content [Fig. 4(B)] and metabolic activity (Table II) were almost twice as high as high in the CF + CM-11 group as in any other group, thereby enabling the cells to more easily develop active force necessary for the high amplitude of construct contraction. However, the ratio of protein/DNA was not significantly different for CM-11, US-11, and CF + CM-11 groups. Glucose consumption rate, a measure of metabolic activity, correlated well with the amplitude of contraction (the lowest in the CM-11 group, the highest in the CM + CF-11 group). The lowest tissue wet weight in the CF + CM-11 group is consistent with the high metabolic rate and good contractile properties, which may create an environment supportive of faster scaffold degradation.
The amplitude of contractions correlated well with the spatial orientation of the myocytes. In the CF + CM-11 group, the cells expressing cardiac markers [Figs. 5(B) and 6], troponin I, and sarcomeric α-actin were aligned in parallel in compact regions resembling the myofibers in the native myocardium, allowing for a synchronous and vigorous contractile response. In the US-11 group, parallel alignment of the myocytes was observed only in some isolated regions while myocytes in the CM-11 group lacked specific orientation, yielding low amplitude of contraction. In our recent study we were able to achieve parallel orientation and elongation of cardiomyocytes cultured on collagen sponges by subjecting the cultured constructs to electrical field stimulation.19 We thus expect that electrical field stimulation of constructs from the CF + CM-11 group will further enhance structural and functional construct properties.
During normal development of the neonatal heart, CFs proliferate and secrete collagenous ECM; in the normal adult heart the CFs are involved in the maintenance of myocardial tissue structure; in the diseased heart CFs are implicated in pathological remodeling and scar-formation.5,30 The histological appearance of constructs in the US-11 and CF + CM-11 groups (Fig. 6) was similar in some respects to developing heart with respect to the combined presence of cardiomyocytes that were partially aligned and elongated and CFs capable of depositing a collagenous ECM.
The overwhelming difference between the CF + CM-11 group and the CM-11 group was that CM-11 group contained almost no cells expressing prolyl-4-hydroxylase [Fig. 5(D)], indicating that the fibroblasts present in the CM-11 constructs were not involved in the deposition of collagen, a key component of the ECM. In the US-11 group, prolyl-4-hydroxylase cells were sparse, while the CF + CM-11 group had a considerable fraction of prolyl-4-hyrdoxylase cells scattered among the myocytes. In all groups, vimentin-positive cells (identified as CFs) were lining the interface between the construct and culture medium [Fig. 5(B,C)], thereby protecting the myocytes from the hydrodynamic shear associated with the convective flow in spinner flask. However, in the CF + CM-11 group, the fibroblasts in the tissue interior were actively involved in scaffold remodeling and deposition of ECM components. Constructs based on fibroblasts alone (CF-5) exhibited the presence of deposited collagen [blue, Fig. 6(A)]. Thick layers of deposited collagen were observed in the CF + CM-11 group at the periphery of the scaffold that was exposed to shear stress due to flow. Constructs based on US cells (US-11) or enriched myocytes alone (CM-11) contained small patches of cardiomyocytes and showed little evidence of collagen deposition [Fig. 6(A)].
Collagen deposition is likely required for remodeling of synthetic scaffolds in cardiac tissue engineering. ECM deposition has only recently been addressed in cardiac tissue engineering studies.14,28 One explanation for the improved functional assembly in the CF + CM-11 group is that the CFs first adhered to the PGS scaffold and then created a favorable environmental milieu for subsequent cardiomyocyte attachment and the assembly of contractile engineered heart tissue. In particular, scaffold pre-treatment in the CF + CM-11 group provided a feeder-layer of CFs that secreted collagen and possibly also other regulatory molecules that supported myocardial tissue assembly as compared to the US-11 and CM-11 groups, in which CF and CM cell fractions were seeded on unconditioned scaffolds.
Vimentin, which was used as a fibroblast marker in our study, is expressed in both the fibroblasts and endothelial cells of the heart.31 Most cells used for scaffold pre-treatment were indeed fibroblasts, based on the isolation procedure which involved pre-plating and significantly different morphology of fibroblasts (star shape) compared to endothelial cells (cobble-stone). It was demonstrated previously25 that after one preplate, fibroblasts contribute to the majority of the attached cells while myocytes and endothelial cells remain unattached. Similar protocols for fibroblast isolation based on faster attachment and proliferation3 as well as identification of fibroblasts by the use of vimentin32 were reported previously.
Vimentin-positive CFs were observed at the interface between the construct and culture medium in constructs from all groups [Fig. 5(B)], implying that one role of the CFs in our model system may have been to protect the shear-sensitive cardiomyocytes within the turbulently mixed spinner flasks. Vimentin-positive CFs were also observed deep within the construct, that is in regions where cardiomyocyte survival is expected to be reduced due to diffusional gradients of oxygen transport,33 implying that in our model, as in native myocardium,5 the CFs provided a structural support for the CMs.
The presented data suggest that the presence of fibroblasts and/or the extracellular components they secrete significantly improved the properties of the engineered heart tissue. When seeded at lower density during scaffold pre-treatment, CFs had enough time to recover from the isolation procedure, and to remodel the polymeric scaffold by depositing components of ECM and secreting soluble factors. When myocytes were subsequently added, the scaffold was already conditioned to provide an environment similar to that of the native ventricle and supportive of the tissue assembly. It appears that the myocytes and fibroblasts seeded at the high density simultaneously (US group) did not receive appropriate cues to engage in scaffold remodeling and tissue assembly, perhaps due to the initial competition for oxygen. These results are in line with recently published data indicating that the 3D cultivation of cardiomyocytes on preformed networks of endothelial cells improved myocyte survival compared to either concurrent culture or culture of myocytes alone.34 Similar to our findings, concurrent culture of fibroblasts and myocytes did not improve myocyte survival and assembly in previous studies.34
Taken together, our results suggest that the pre-treatment of a synthetic PGS scaffold with CFs in conjunction with CFs-derived molecular components significantly improved the properties of the engineered heart tissue. Surgical implantation of the PGS scaffold in a rat MI model is feasible and will be explored thoroughly in future long-term studies.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Aeronautics and Space Administration; contract grant number: NNJ04HC72G
Contract grant sponsor: Poitras Fellowship
Contract grant sponsor: NIH; contract grant numbers: P41 EB002520, R01 HL076485
Footnotes
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1549-3296/suppmat.
References
- 1.Nag AC. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 2.Sussman MA, McCulloch A, Borg TK. Dance band on the Titanic: Biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;91:888–898. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- 3.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 4.Rudy Y. Conductive bridges in cardiac tissue: A beneficial role or an arrhythmogenic substrate? Circ Res. 2004;94:709–711. doi: 10.1161/01.RES.0000125647.56687.D3. [DOI] [PubMed] [Google Scholar]
- 5.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Kohl P. Heterogeneous cell coupling in the heart: An electro-physiological role for fibroblasts. Circ Res. 2003;93:381–383. doi: 10.1161/01.RES.0000091364.90121.0C. [DOI] [PubMed] [Google Scholar]
- 7.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- 8.Wang JX, Fan J, Cheung F, Laschinger C, Seth A, McCulloch C. Alpha-smooth muscle actin (SMA) is required for mechanical force-induced p38 activation. FASEB J. 2004;18:C39. doi: 10.1074/jbc.M410819200. [DOI] [PubMed] [Google Scholar]
- 9.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G. Cardiac tissue engineering: Cell seeding, cultivation parameters and tissue construct characterization. Biotechnol Bioeng. 1999;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: Toward an in vitro model for electrophysiological studies. Am J Physiol Heart Circ Physiol. 1999;277:H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 11.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 12.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: Molecular, structural and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Nehuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 14.Akhyari P, Fedak PWM, Weisel RD, Lee TYJ, Verma S, Mickle DAG, Li RK. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106(12 Suppl 1):I137–I142. [PubMed] [Google Scholar]
- 15.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–925. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 17.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 18.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High density seeding of myocyte cells for tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 19.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, Vunjak-Novakovic G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol Bioeng. 2004;88:379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 21.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 22.Casey TM, Arthur PG. Hibernation in noncontracting mammalian cardiomyocytes. Circulation. 2000;102:3124–3129. doi: 10.1161/01.cir.102.25.3124. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 24.Borene ML, Barocas VH, Hubel A. Mechanical and cellular changes during compaction of a collagen-sponge-based corneal stromal equivalent. Ann Biomed Eng. 2004;32:274–283. doi: 10.1023/b:abme.0000012747.97620.3a. [DOI] [PubMed] [Google Scholar]
- 25.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1 + cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J Physiol Cell Physiol. 2003;285:C171–C182. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 27.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(1 Suppl):I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 28.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 2005;11:1122–1132. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 29.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Woil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart model system. FASEB J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 30.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: Friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 31.Speiser B, Weihrauch D, Riess CF, Schaper J. The extracellular-matrix in human cardiac tissue, Part II: Vimentin, laminin, and fibronectin. Cardioscience. 1992;3:41–49. [PubMed] [Google Scholar]
- 32.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 33.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 34.Narmoneva DA, Vukmirovic R, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization in engineered cardiac tissue. Circulation. 2003;108:165. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.