Abstract
Ten cerebellar patients were compared to ten control subjects on a verbal working memory task in which the phonological similarity of the words to be remembered and their modality of presentation were manipulated. Cerebellar patients demonstrated a reduction of the phonological similarity effect relative to controls. Further, this reduction did not depend systematically upon the presentation modality. These results first document that qualitative differences in verbal working memory may be observed following cerebellar damage, indicating altered cognitive processing, even though behavioral output as measured by the digit span may be within normal limits. However, the results also present problems for the hypothesis that the cerebellar role is specifically associated with articulatory rehearsal as conceptualized in the Baddeley-Hitch model of working memory.
Keywords: cerebellum, verbal working memory, speech perception, language, cognition, short-term memory, dysarthria, aphasia
Researchers in cognitive neuroscience have in many cases looked to converging data from multiple methodologies when investigating a cognitive process of interest. The complementary perspectives of functional neuroimaging and cognitive neuropsychology are often employed in this regard; a strong case can be made for the involvement of a brain region in a given cognitive process when it is both metabolically active when healthy participants engage in the cognitive process, and when damage to this region disrupts the same cognitive process. Although the precise nature of this involvement may prove elusive to characterize, the brain region comes to be regarded as an essential component of the system in question. When the two methodologies of neuroimaging and neuropsychology do not converge, however, even the most basic question of whether a given region is involved in a cognitive process is difficult to address.
One issue that must be kept in mind when making comparisons between data from neuroimaging and data from neuropsychology is that the two methods provide very different kinds of evidence about cognition. Neuroimaging studies have the potential to document qualitative aspects of cognitive processing that may not be evident from the behavioral outcome. In contrast, neuropsychological data – particularly that from standardized batteries that examine for gross impairment across a wide range of cognitive tasks – sometimes do not allow for such an observation. Identical behavioral outcomes may become falsely equated with identical cognitive processes, when this most certainly is not always the case.1 Neuropsychological studies must be designed such that the relevant qualitative as well as quantitative differences in a cognitive process may be observed.
A relative lack of convergence in the neuroimaging and neuropsychological literatures was a part of the motivation for the current study, which investigated the verbal working memory abilities of patients with damage to the cerebellum. As will be discussed below, the cerebellum is one of the most consistently activated regions in neuroimaging studies that employ verbal working memory tasks. However, a large reduction in verbal working memory capacity, for instance as measured by the digit span, is not typically reported in patients with cerebellar disorders. A possible explanation for this discrepancy is that although the verbal working memory system of these patients has been altered in a qualitative way, the altered system is nonetheless capable of producing near-normal behavioral output on standardized neuropsychological tests. For this reason, we wished to design a verbal working memory experiment that would go beyond simple measures of overall capacity and search for qualitative changes following cerebellar damage. The design that we chose not only allowed for the documentation of such a qualitative change in verbal working memory in our patient group, but also allowed us to examine a functional hypothesis, namely that the role of the cerebellum in verbal working memory is specifically articulatory rehearsal.
With this in mind, we shall first briefly review the Baddeley-Hitch multiple component model (e.g., Baddeley & Hitch, 1974; Baddeley, 1986), which is the dominant model of verbal working memory. Then we shall consider the relatively weak evidence from neuropsychology and the much more consistent evidence from functional neuroimaging regarding a cerebellar role in verbal working memory. Finally, we shall consider the ways in which the cerebellum may map onto the components of the Baddeley-Hitch model and motivate the design of the current neuropsychological study.
The Baddeley-Hitch Model
In the Baddeley-Hitch model (e.g., Baddeley, 1986), working memory is divided into three components: a central executive that coordinates information processing in all modalities and two modality-specific systems, a visuospatial sketchpad and a phonological loop. The phonological loop is further divided into two subsystems: a phonological short-term store and an articulatory rehearsal mechanism. The phonological short-term store is thought to be the locus of input-based phonetic representations and an output-based rehearsal process is thought to be required to refresh information in the store.2
A key feature of the Baddeley-Hitch model is that it posits separate phonetic and articulatory representations, rather than arguing that speech is immediately perceived in terms of articulation (i.e., motor theories of speech perception, e.g., Liberman & Mattingly, 1985). The primary psychological evidence for this separation comes from studies that have manipulated phonological similarity, word length, modality, and articulatory suppression. Phonologically similar words are more difficult to remember than phonologically dissimilar words (Conrad, 1964). This phonological similarity effect is believed to reflect conflicts that arise in the phonological short-term store. Additionally, words with many syllables are more difficult to remember than words with fewer syllables (Baddeley et al., 1975). This word length effect is believed to reflect the process of articulatory rehearsal, with increasing rehearsal demands for longer words. In support of this hypothesis, the word length effect disappears when articulation is suppressed, as for example when the participant must count repeatedly from one to three when perceiving and rehearsing the word list (Baddeley et al., 1984). Interestingly, manipulations of input modality can further affect the phonological similarity effect, but not the word length effect under these conditions; articulatory suppression eliminates the word length effect regardless of whether the word list is heard or read, but eliminates the phonological similarity effect only when the words are read. This interaction between modality and articulatory suppression for the phonological similarity effect is the primary empirical basis for hypothesizing a distinction between phonetic and articulatory processing in working memory (see Figure 1). We shall return to this interaction later in the introduction when making predictions for the current study.
Figure 1.
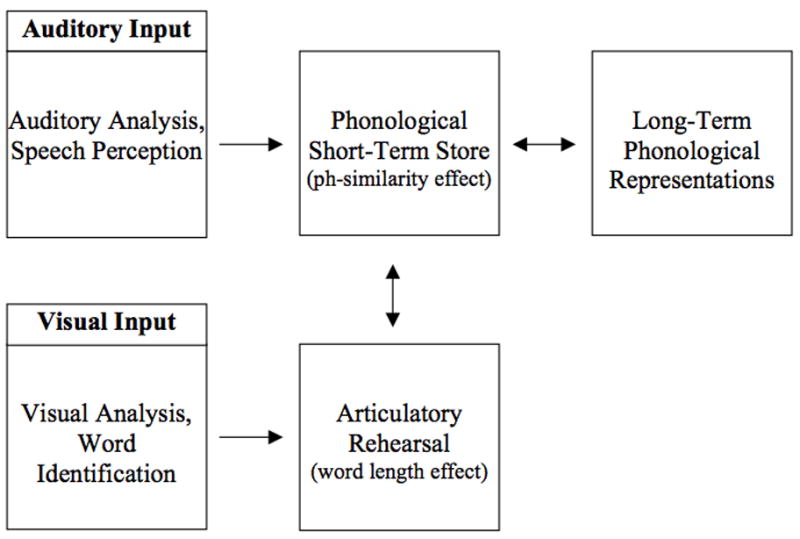
The Baddeley-Hitch model. According to the model, the phonological short-term store is the locus of the phonological similarity effect, and receives spoken language automatically. The articulatory rehearsal mechanism is the locus of the word length effect and is required to recode written language for the phonological short-term store (adapted from Baddeley, Gathercole, and Papagno, 1998).
Neuropsychology of Verbal Working Memory
Neuropsychological studies have provided an important source of evidence in the development of the Baddeley-Hitch model, including dissociations in support of its subdivisions. The typical profile of the “short-term memory (STM) patient” includes a reduced word span despite normal speech perception and production.3 This pattern has been interpreted as a selective disruption of the phonological short-term store. Two of the most studied STM patients were KF (Warrington & Shallice, 1969) and JB (Warrington et al., 1971). KF suffered damage to the left inferior parietal/occipitotemporal region, whereas JB suffered damage to the left middle and superior temporal gyri spreading into the inferior parietal lobe. The area of overlap in these and other STM patients, the inferior parietal lobule (BA 40), has been argued to be critical for phonological short-term storage (Shallice & Vallar, 1990). The literature on the verbal working memory abilities of these cortical patients is extensive, and it is beyond the scope of this paper to review it here. The reader is referred to reviews by Shallice and Vallar (1990) and a more recent review by Vallar and Papagno (2002).
In contrast to the many cases of verbal working memory deficit following damage to the cerebral cortex, such impairments have not typically been reported following damage to the cerebellum. Studies of cerebellar patients that have incorporated the digit span into the neuropsychological battery typically have found scores in the normal range. For instance, Bürk et al. (1999) found that even the demented subset of their German spinocerebellar ataxia (SCA) 2 patients was only slightly lowered (4.8 +/− 1) relative to the controls (6.1 +/− 1), whereas their non-demented SCA2 patients did not differ significantly from controls (5.9 +/− 1.3). Other studies have similarly reported digit spans in the low-normal to normal range with no statistical differences between patients and controls (e.g., Bracke-Tolkmitt et al., 1989; Fiez et al., 1992; Schmahmann & Sherman, 1998; Le Pira et al., 2002; Timmann et al., 2002; Bürk et al., 2003; Globas et al., 2003; Fabbro et al., 2004). Those studies that have reported digit spans to be reduced (e.g., Akshoomoff et al., 1992; Schelhaas et al., 2001), even when significantly reduced relative to controls (e.g., Witt et al., 2002; Ravizza et al., 2004, submitted; Maddox et al., in press), typically report a reduction of only one or two items from the normal range. This stands in contrast to the more profound deficits of cortical STM patients (e.g., 2 or 3 items). Interestingly, more severe digit-span deficits are observed in individuals who suffered cerebellar damage during childhood (Schatz et al., 1998; Steinlin et al., 1999; Scott et al., 2001; Steinlin et al., 2003), emphasizing the difference between damage that disrupts the developmental process and damage acquired as an adult.
One case study does report verbal working memory data that revealed qualitative as well as quantitative changes. Silveri et al. (1998) described an eighteen-year-old Italian patient who underwent surgical removal of the right cerebellar hemisphere. The patient was tested before surgery, three days after the surgery, and again five months later. Before and immediately after the surgery the patient had a reduced digit span of four items forwards and three items backwards. This study was unique in that the authors went beyond the simple digit span and collected data from verbal working memory tasks in which phonological similarity, word length, modality of presentation, and articulatory suppression were manipulated, and thus had the potential to observe some qualitative as well as quantitative changes in verbal working memory.
The patient showed a reduction in the phonological similarity effect that was dependant on the modality of presentation; there was an effect with auditory presentation but not with visual presentation. Interestingly, this patient’s digit span improved to seven when he was tested five months later and the phonological similarity effect for visual presentation was significant. The patient also showed no significant effect of word length in either modality, even when tested 5 months later. This result is difficult to attribute to the patient’s surgery because Silveri et al. also report two control subjects showing the same pattern. However, the interaction between phonological similarity and modality in particular suggests a sparing of the phonological short-term store and an impairment of a component of the articulatory rehearsal mechanism. This conclusion is not clear cut, however, as the patient still showed a significant effect of articulatory suppression, unlike other patients with a proposed selective rehearsal deficit (Vallar et al., 1997).
Neuroimaging of Verbal Working Memory
In contrast to the mixed results from neuropsychology, the cerebellum is one of the most consistently activated regions in neuroimaging studies of verbal working memory, along with a network of cortical regions including the inferior frontal lobe (especially BA 44/45), the supplementary motor area (SMA, medial BA 6), premotor cortex (PMC, lateral BA 6), and the parietal lobe (BA 7/40) (Andreasen et al., 1995; Awh et al., 1996; Chein & Fiez, 2001; Davachi et al., 2001; Fiez et al., 1996; Grasby et al., 1994; Gruber, 2001; Jonides et al., 1998; Paulesu et al., 1993; Petrides et al., 1993; Ravizza et al., 2004; Salmon et al., 1996; Schumacher et al., 1996). For example, Paulesu et al. (1993, Experiment 1) showed their participants a series of six letters in each trial, followed by a probe presented two seconds after the end of the sequence. Participants judged whether each probe was present in the preceding sequence. Activation during this task was compared to a second condition in which Korean characters were used, which the English-speaking participants could not code phonologically. This contrast (Roman letters - Korean characters) revealed significant differences in BA 44, the SMA, BA 40, BA 22/42 (superior temporal), the insula, BA 18 (occipital), and the cerebellum.
The Articulatory Rehearsal Hypothesis
The majority of these neuroimaging studies, like the Silveri et al. (1998) neuropsychological study, hypothesize a cerebellar role in articulatory rehearsal. Paulesu et al. (1993) argued that the cerebellum, in conjunction with the inferior frontal lobe and supplementary motor area, is part of an articulatory rehearsal mechanism, whereas the inferior parietal lobe is the locus of the phonological short-term store. In support of a link to overt speech, Petrides et al. (1993) observed bilateral cerebellar activation when comparing a condition involving more speech output (generating the numbers 1 through 10 in a mixed order) to a condition involving more speech input (monitoring a series generated by the experimenter and providing the missing number). Rehearsal processes were further suggested by a study showing a correlation between the length of the items to be remembered and activation in the cerebellar vermis and hemispheres (Grasby et al., 1994; also see Chein & Fiez, 2001).
The articulatory rehearsal hypothesis is motivated in part by the long-standing connection between the cerebellum and speech output (e.g., Ackermann & Hertrich, 2000). However, overt articulation and the processes used in articulatory rehearsal do not necessarily overlap. A group of dysarthric patients studied by Baddeley and Wilson (1985) did not show any evidence of impairment to the articulatory rehearsal mechanism. Similarly, Bishop and Robson (1989) reported intact articulatory rehearsal in a group of teenagers who were developmentally dysarthric due to cerebral palsy. In contrast, Waters et al. (1992) reported a group of left-hemisphere patients with speech apraxia (a disorder of speech planning rather than implementation), who did show an abnormal pattern of rehearsal effects, as did a group of five Broca’s aphasics studied by Goerlich et al. (1995). Although none of these studies focused on patients with a dysarthria related to cerebellar damage, they do suggest that one cannot simply equate the mechanisms used in covert rehearsal with those of overt speech.
Some of the previously mentioned neuroimaging studies also suggest that articulatory rehearsal may not provide a complete account of cerebellar involvement in verbal working memory. Awh et al. (1996) replicated the cerebellar involvement during verbal working memory tasks. However, a condition involving an articulatory-rehearsal control failed to account for this activity; significant activation in the right cerebellar hemisphere was observed even when a rehearsal control condition was subtracted from their working memory (two-back) task. Assuming that the rehearsal condition in this study was sufficient to mimic the articulatory requirements of the working memory tasks, the result suggests that the cerebellum is doing something in addition to or instead of articulatory rehearsal.
The results of Chein and Fiez (2001) are also problematic for the rehearsal hypothesis. They attempted to separate activations associated with encoding, maintenance, and retrieval. Whereas the dorsolateral and inferior frontal cortex, insula, SMA, and (in some conditions) the inferior parietal lobe remained active throughout the maintenance period, the cerebellum was primarily active during encoding and retrieval. Contrary to the rehearsal hypothesis, no increase in cerebellar activation was observed during maintenance. This suggests that although the cerebellum may play a role in the initial perceptual analysis and/or initial articulatory encoding of the stimuli, it may not be engaged during rehearsal per se.4
Alternative Hypotheses
An alternative hypothesis is that the cerebellum contributes to the phonological short-term store, or to the phonetic analysis that precedes this representation. Although this possibility has not been considered within the verbal working memory literature, a variety of evidence from neuropsychology and neuroimaging in other areas of language is suggestive of cerebellar roles in speech perception and phonological processing. Ackermann and colleagues (1997) showed that a subset of patients with cerebellar atrophy did not perceive a clear phoneme distinction between sounds constructed along a closure time (CLT) continuum between the words Boten and Boden. The deficit has only been found when the cue is predominantly temporal and is not based on aspiration or articulatory events that result in spectral differences, as is typically the case with voice onset time (VOT) (Ackermann et al., 1997; Ivry & Gopal, 1992). Further, Mathiak et al. (2002) found that during a Boten/Boden discrimination task, the left inferior frontal gyrus (in the vicinity of BA 47) and the right cerebellar hemisphere were recruited to a larger degree when the stimuli were constructed using a CLT continuum, the purely temporal distinction, compared to a VOT continuum that also included distinctions based on aspiration (see also Burton et al., 2000). Other work in a variety of lexical retrieval paradigms (e.g., Petersen et al., 1988, 1989; Desmond et al., 1998; Roskies et al., 2001) suggests that the cerebellum is involved in some combination of the semantic and phonological stages of lexical retrieval.5 Developmental work in reading and dyslexia, a disorder considered by many to stem from abnormal phonological processing, has also suggested a cerebellar component (e.g., Nicholson et al., 2001; but see Ramus et al., 2003). Finally, perceptual tasks tapping grammatical morphology have also suggested that cerebellar patients may have difficulty perceiving and encoding morphological markers that are not acoustically salient (Justus, 2004; Justus et al., 2004). Given these links to phonetics and phonology, we also consider the hypothesis that the cerebellum contributes to the phonological short-term store.
A third hypothesis, argued by Desmond et al. (1997; Desmond, 2001), is that the cerebellum compares the contents of the articulatory rehearsal mechanism with the intended action represented by the phonological short-term store, and thus serves as an interface between the phonological short-term store and articulatory rehearsal (Figure 2). In this model, regions within the superior cerebellum (lobules HVI and HVIIA) receive input from frontal areas involved in articulatory rehearsal via the medial pontine nuclei. The inferior cerebellum (lobule HVIIB) receives input from parietal areas involved in the phonological short-term store via the lateral pontine nuclei. Discrepancies between the two are detected when these two pathways converge at the dentate nucleus and this information is fed forward to the frontal lobe via the thalamus. This model was based on a neuroimaging study in which the memory load was manipulated for conditions designed to engage working memory or rehearsal processes alone. Whereas the superior loci were affected by the load manipulation in both working memory and rehearsal conditions, the inferior loci were only affected by the load manipulation in the working memory condition, suggesting that phonological storage and not just rehearsal was essential for their participation (Desmond et al., 1997).
Figure 2.
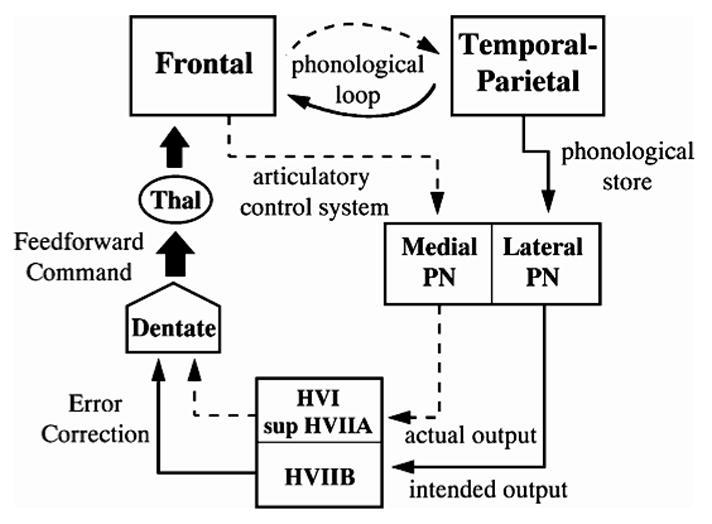
Model of Desmond et al. (1999). According to this model, during each rehearsal cycle a frontal articulatory rehearsal mechanism sends input through the medial pontine nuclei (PN) to the superior cerebellum and a temporal-parietal phonological short-term store sends input through the lateral pontine nuclei to the inferior cerebellum. Discrepancies between the two are fed forward through the dentate nuclei and thalamus back to the frontal lobe.
The Present Study
The relationship between the phonological similarity effect, modality of presentation, and articulatory suppression in verbal working memory studies in normal individuals offers a nontrivial prediction regarding selective damage to the articulatory rehearsal mechanism. In neurologically normal individuals, articulatory suppression is believed to engage the rehearsal mechanism selectively and not the phonological short-term store. Requiring a second articulatory task during a verbal working memory study diminishes the word length effect with both auditory and visual presentation, but diminishes the phonological similarity effect only when presentation is visual (Baddeley et al., 1984). Because of this, the Baddeley-Hitch model claims that spoken language gains access to the phonological short-term store automatically, whereas written language is dependent upon the articulatory rehearsal mechanism to be re-coded phonologically.
The articulatory rehearsal hypothesis predicts that the pattern associated with articulatory suppression should also be found with cerebellar patients: a reduced effect of phonological similarity for word lists presented visually but not aurally. This is the pattern observed in the previously mentioned cerebellar case study reported by Silveri et al. (1998).
In contrast, a single deficit to the phonological short-term store (Hypothesis 2), would predict a different pattern of results. The phonological similarity effect should be diminished with damage to the phonological short-term store with either presentation modality, given that it is the proposed locus of the effect.
Desmond’s interface hypothesis (Hypothesis 3) also makes similar predictions to the rehearsal hypothesis. Despite the fact that the cerebellum receives input from the phonological short-term store in this model, we argue that a reduction of the phonological similarity effect is predicted only when presentation is visual, as in Hypothesis 1. Whereas the rehearsal mechanism in this model would be critically disrupted with damage to the cerebellum, the phonological short-term store would still be intact in its hypothesized temporal-parietal locus. The intact store would continue to input clearer phonological representations for phonologically dissimilar lists compared to phonologically similar lists into the rehearsal mechanism, which then would presumably degrade at an equal rate in the absence of effective rehearsal (thus preserving any initial difference between the two). If the model were altered to include a more direct role for the cerebellum in the phonological short-term store, rather than being downstream from it, then reductions in the auditory modality might be expected as well.
Table 1 lists these three hypotheses and associated predictions. Note that the hypotheses in Table 1 do not speak to the possibility of multiple deficits. In each case the predictions are based on the assumption that all other elements of the working memory system are intact. Thus failing to find a particular pattern in the data predicted by Table 1 argues against a single deficit in each of these components of working memory, rather than arguing that the component in question is intact.
Table 1.
Predictions for the current study
| Hypothesized role for the cerebellum | Prediction: Visual presentation | Prediction: Auditory presentation |
|---|---|---|
| 1) Articulatory rehearsal e.g., Paulesu et al., 1993 |
(−) Reduced similarity effect | (+) Preserved similarity effect |
| 2) Phonological short-term store | (−) Reduced similarity effect | (−) Reduced similarity effect |
| 3) Interface between articulatory rehearsal and phonological short-term store e.g., Desmond et al., 1997 |
(−) Reduced similarity effect | (+) Preserved similarity effect* |
Assuming that the inferior cerebellum is not itself part of the phonological STS.
Additionally, it should be noted that the current study was not designed to distinguish between Hypotheses 1 and 3, which make identical predictions and would have required additional study to tease them apart if the data were consistent with these predictions. To anticipate, the data were not consistent with either of these hypotheses.
Method
Participants
Ten patients with damage to the cerebellum were examined for this experiment: four with bilateral degeneration (B2, B3, B4, and B5), three with focal lesions in the left hemisphere (L2, L3, and L4), and three with focal lesions in the right hemisphere (R1, R2, and R3).6 Ten controls of similar age (mean 67), education (mean 13 years), and handedness (8 right handed) also participated in the experiment. Further details concerning the etiologies, demographics, and test scores of the patients are given in Table 2. The specific regions of cerebellar damage varied from patient to patient and are illustrated in Figure 3.
Table 2.
Cerebellar Patients
| Patient | Hem. | Etiology (at age) | Age | Ed. | Hand. | Sex | Language (n = native) | NART | Verbal Fluency | WAIS-III Digit Span | ICARS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (letter, | category) | (forward, | back., | scaled) | (total, | dysarthria) | |||||||||
| B2 | bilateral | degeneration (c. 30-) | 71 | 12 | right | M | English | 100 | 25 | 47 | 4.5 | 2.5 | 6 | 45 | 4.75 |
| B3 | bilateral | degeneration (c. 61-) | 62 | 20 | right | M | English | 116 | 35 | 45 | 4 | 3.5 | 7 | 17.75 | 3.25 |
| B4 | bilateral | degeneration (c. 20s-) | 62 | 17 | right | M | Spanishn, English (5-) | 111 | 30 | 47 | 4.5 | 2 | 6 | 42.75 | 5.5 |
| B5 | bilateral | SCA3 (c. 27-) | 44 | 13 | right | F | English | -- | 16 | 34 | ~5 | -- | -- | -- | -- |
| L2* | left | tumor (34) | 56 | 11 | mixed | M | English | 87 | 21 | 35 | 4 | 2.5 | 6 | 23.25 | 5 |
| L3* | left | stroke (66) | 78 | 8 | right | M | English | -- | 18 | 33 | ~3 | -- | -- | -- | -- |
| L4 | left | stroke (48) | 52 | 13 | right | M | English | 117 | 51 | 48 | 8 | 4.5 | 13 | 10 | 1.5 |
| R1 | right | stroke (66) | 75 | 18 | right | M | English | 112 | 19 | 48 | 5.5 | 5 | 12 | 34 | 3 |
| R2 | right | tumor (42) | 46 | 18 | left | M | Englishn, German (9-) | 114 | 27 | 50 | 6.5 | 4 | 10 | 32.75 | 3.5 |
| R3 | right | stroke (55) | 65 | 12 | right | M | English | 111 | 61 | 58 | 7.5 | 3 | 11 | 4.25 | 1 |
participated in only the auditory experiment due to poor vision
abbreviations:
NART: National Adult Reading Test (mean 100, estimate of premorbid IQ)
WAIS: Wechsler Adult Intelligence Scale
forward span, number of items able to hold and recall (not the score)
backward span, number of items able to hold and recall backwards (not the score)
WAIS scaled score representing forward and backward span, standardized to age group (mean 10)
ICARS: International Cooperative Ataxia Rating Scale (Trouillas et al., 1997)
overall estimate of ataxic impairment (maximum 100)
dysarthria subscore (maximum 8)
Figure 3.
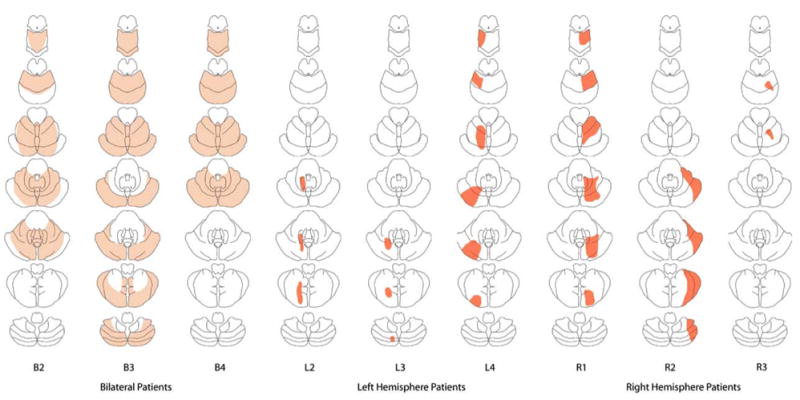
Cerebellar lesions of the patients in Experiments 1 and 2. For each patient, a column of seven horizontal slides through the pons and cerebellum are shown, with the most superior slice at the top. Within each slice, rostral is toward the top and caudal toward the bottom; left is left and right is right. Dark gray indicates a tissue lesion, whereas medium gray indicates tissue degeneration. No scan was available for Patient B5, who is a genetically confirmed case of SCA3 ataxia.
Consistent with the previously mentioned neuropsychological studies, standardized data for eight of the ten cerebellar patients indicated that overall verbal working memory capacity (WAIS-III digit span) was in the low average range for some of the participants (B2, B3, B4, L2), whereas the scores of the other patients were normal (R1, R2, R3, L4). No standardized data on the digit span were available for L3, who is now deceased, and for B5, who is unavailable for further testing. For these two patients, an estimate of forward digit span is given based on performance in the current study.
One might object that, because there was no compelling deficit on the digit span, no further neuropsychological study of the verbal working memory of these patients was motivated. The group detriment in verbal working memory capacity is subtle; only when combined in larger groups have we demonstrated a significant reduction in digit span scores relative to controls, with no effect on WAIS spatial span (Ravizza et al., 2004; Maddox et al., in press). As described earlier, the relatively preserved digit span of these patients, along with the evidence from neuroimaging which consistently documented cerebellar activation during similar tasks, suggested to us that qualitative changes in verbal working memory performance might be observed in these patients even if overall capacity were not significantly reduced.
Experimental Design
The stimuli were 60 monosyllabic English words, representing six vowels and ten initial consonants (Table 3). In the auditory condition, the experimenter read the word lists. In the visual condition, the words were presented on the computer screen. In both conditions, the words were presented at a rate of 1 word per 1.5 seconds. The visual stimuli were printed in the middle of the screen, with the words spanning approximately five degrees of visual angle. After five seconds, the participant was cued by the computer to recall the words orally to the experimenter.
Table 3.
Experimental Stimuli for Generating Phonologically Similar and Dissimilar Lists
| Vowel | ||||||
|---|---|---|---|---|---|---|
| Initial Consonant | /i/ | /e/ | /E/ | /ae/ | /^/ | /I/ |
| /b/ | bead | bathe | bell | back | bus | bin |
| /p/ | peace | pace | peg | pan | pun | pick |
| /d/ | deal | date | deaf | dad | done | dip |
| /t/ | tease | tail | ten | tab | tug | tin |
| /k/ | keen | cage | keg | cat | cut | kiss |
| /s/ | seek | safe | set | sad | sum | sip |
| /f/ | feet | fame | fed | fad | fudge | fit |
| /l/ | leaf | lake | ledge | lag | luck | lid |
| /m/ | meet | maze | men | mass | mud | mill |
| /n/ | need | name | neck | nap | nut | knit |
Word lists were composed of five or six items depending on each individual’s overall ability, as determined by a practice session. We were concerned that testing all participants with the same list length would result in ceiling effects for some participants, thus diminishing the observed size of the phonological similarity effect. Thus, we used five-item lists for all of the participants unless they performed perfectly on multiple five-item lists during the practice session. This occurred in four cases (Patient B3, Patient B5, and two controls). These four individuals were tested with six-item lists.
Half of the lists were constructed using words from the same vowel category (e.g., bead, peace, leaf, tease, deal) to create a phonologically similar list. The other half were constructed using only one word per vowel category (e.g., bead, pace, ledge, tab, dip) to create a phonologically dissimilar list.7 Note that any particular word occurred equally often in both the phonologically similar and dissimilar conditions; thus both conditions were inherently balanced for word frequency, abstractness, and the like. Each block consisted of twelve lists, six phonologically similar and six phonologically dissimilar, and the participants tested with both visual and auditory conditions alternated between the two modalities, doing two blocks of each. The modality order was counterbalanced.
Because the stimuli were real words, as opposed to letters or pseudowords, one might object that participants could have used a semantic strategy to remember the lists. However, this would not undermine the utility of the difference scores representing the phonological similarity effect. Any additional boost in performance resulting from semantic coding would have increased recall in both the phonologically similar and dissimilar lists (and in both the visual and auditory conditions as well), given that every word was equally likely to occur in all conditions. Thus the comparison of performance in different conditions should not be affected by a semantic effect. Further, a semantic strategy was specifically discouraged by the experimenter, who informed the participants that each word would appear in the experiment multiple times and that the best strategy was to mentally rehearse each list.
The use of real words was motivated, on the other hand, for three reasons. First, the dysarthria of some of the patients would have made the coding of errors in the production of letter names or pseudowords extremely unreliable. Secondly, we would have had the additional concern that participants were not perceiving the stimuli correctly, particularly for the aural condition. Finally, unlike the use of letters, our real-word stimulus set allowed for the same words to be used in both phonologically similar and dissimilar lists. For instance, bead was a similar item if combined with peace, leaf, tease, and deal, and was a dissimilar item if combined with pace, ledge, tab, and dip. The pronunciations of the 26 letters do not allow this kind of manipulation, which raises the possibility of any number of confounds between phonologically similar and dissimilar items (e.g., orthographic similarity).
In summary, there were three variables in the experiment: (1) phonological similarity: whether the list words were combined such that all contained the same or different vowels, (2) modality of presentation: auditory or visual, and (3) group: cerebellar patient (bilateral, left, or right) or healthy control.
Results
Auditory Condition
Given that there were two patients who completed only the auditory condition, separate analyses of variance were conducted for the auditory and visual experiments, each with the variables of phonological similarity and group, before combining the data into a larger analysis, which included the variables of phonological similarity, modality, and group.
Figure 4 presents the data for the auditory condition only for both the patients individually and the four groups. The data are presented as the probability of recalling a word when presented in a phonologically dissimilar context (black bars) and when presented in a phonologically similar context (gray bars). Although there was a trend for worse performance in general on the part of the patients, this difference was not significant (F (1,18) = 2.2, p = .16). This was as expected, in part because we hypothesized a qualitative (effect size) rather than quantitative (overall ability) difference, and because the overall difficulty of the task was adjusted to make the task easier on average for the patients. The critical information comes from the difference in performance between the two conditions for each participant.
Figure 4. Phonological Similarity Effects with Auditory Presentation.
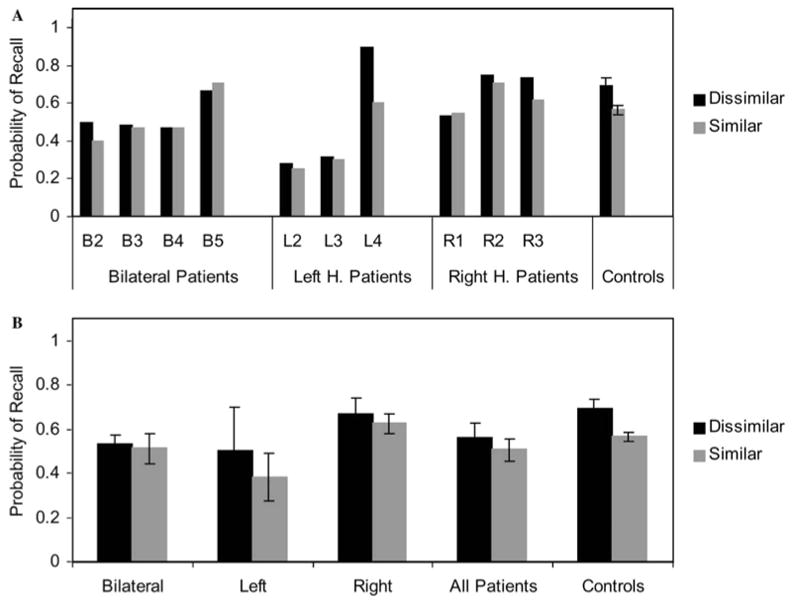
The probability of recalling a word is shown for both phonologically dissimilar contexts (black) and phonologically similar contexts (gray), for the individual patients (A) and the groups (B). These data are for the trials in which words were presented aurally.
The control participants, shown at the right of each plot, showed a significant effect of phonological similarity (t (9) = 4.7, p = .001). The patients demonstrated a good deal of individual variability in the size of the effect. Patient L4, who had the strongest digit span of the group, stood out with the largest effect size. Additionally, patients B2 and R3 showed effects in the same range as the controls. However, the other seven patients demonstrated relatively flat effects, suggesting that phonological dissimilarity did not aid them in their performance of the task. As shown in the lower plot, the patients as a single group showed a trend in the same direction as the controls that was not significant (t (9) = 1.8, p = .10). Comparisons of the patients divided into groups based on laterality also did not show a significant effect of phonological similarity for any of the three groups (bilateral, left, and right, all p > .30). This reduction in the effect for the patients relative to controls would have been strongly supported by an interaction between phonological similarity and group, but this did not reach statistical significance (F (1,18) = 3.0, p = .10).
Visual Condition
Figure 5 presents an analogous plot for the visual condition. Note that patients L2 and L3 could not participate in this condition. Unlike the auditory condition, the patients performed more poorly in general on this task relative to the controls (F (1,16) = 6.0, p = .03). But again, the design of the study emphasizes the difference in the size of the phonological similarity effect for each participant.
Figure 5. Phonological Similarity Effects with Visual Presentation.
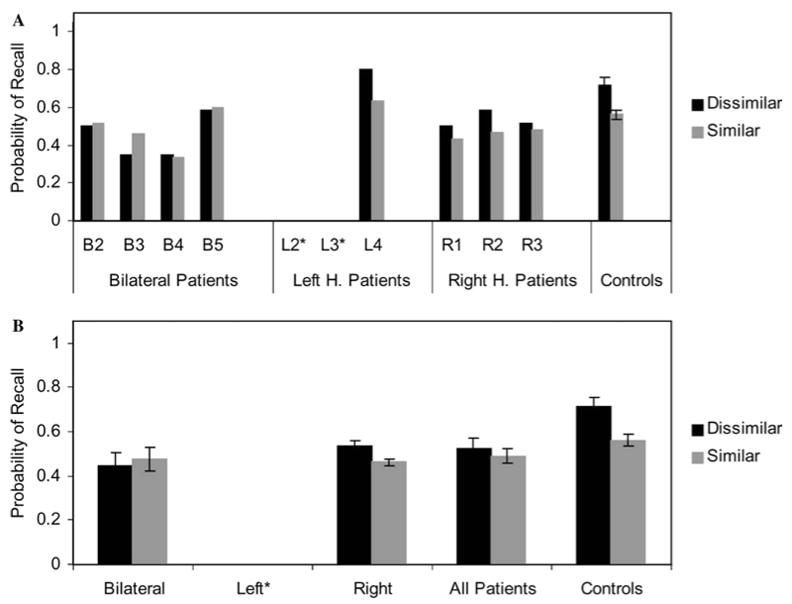
The probability of recalling a word is shown for both phonologically dissimilar contexts (black) and phonologically similar contexts (gray), for the individual patients (A) and the groups (B). These data are for the trials in which words were presented visually. (Only one left hemisphere patient participated in the visual portion of the study, thus no left-hemisphere group data are shown in the lower plot.)
The control participants showed a significant effect of phonological similarity (t (9) = 3.9, p = .004), but as in the auditory condition, the patients were more variable. Patient L4 again stood out as having the largest effect of phonological similarity. The three right hemisphere patients demonstrated relatively weak effects in the predicted direction, whereas patient B3 showed a reverse effect. The remaining three bilateral patients showed relatively flat effects. As shown in the lower plot, the patients as a single group showed a weak trend in the same direction as the controls that was not significant (t (7) = 1.1, p = .32). Comparisons of the patients divided into right hemisphere and bilateral groups also did not show a significant effect of phonological similarity for either (right: t (2) = 3.0, p = .10; bilateral: t (3) = −1.1, p = .34). This reduction in the effect for the patient group as a whole was supported by a significant interaction between phonological similarity and group (F (1,16) = 5.4, p = .03). Paired comparisons indicated that the interaction was only significant for the bilateral patients compared to the controls (F (1,12) = 7.8, p = .02).
Combined Auditory and Visual Analysis
In order to compare the reduction of the phonological similarity effect across the two modalities in a more direct way, a third analysis was conducted on the combined data for the eight patients who participated in both auditory and visual conditions. First, there was a tendency for the patients to do more poorly in the visual condition relative to the auditory condition in general (F (1,7) = 9.8, p = .02), whereas the controls did not differ between the modalities (F (1,9) = .06, p = .82). This difference was supported by a marginally significant interaction between modality and group (F (1,16) = 3.9, p = .07).
Consistent with the individual modality analyses, the overall effect of phonological similarity for both modalities combined (F (1,16) = 18.8, p = .001) was significant for the control participants (F (1,9) = 21.7, p = .001), but not for the patients (F (1,7) = 2.4, p = .16). The interaction between phonological similarity and group was significant (F (1,16) = 4.5, p = .05). Paired comparisons indicated that the interaction was only significant for the bilateral patients compared to the controls (F (1,12) = 8.6, p = .01).
The separate analyses of the auditory and visual condition suggested that the group reduction in the phonological similarity effect was more consistent in the visual condition. However, in the combined analysis there was neither an interaction between phonological similarity and modality (F (1,16) = .02, p = .89; patients only: F (1, 7) = .95, p = .36; controls only F (1, 9) = .67, p = .43) nor a three-way interaction between similarity, modality, and group: F (1,16) = 1.6, p = .23). This suggests that the difference between the patients and controls in the size of the phonological similarity effect was not systematically affected by the modality of presentation.
To help illustrate the phonological similarity effect as a function of modality more clearly, Figure 6 shows the same data from the auditory condition (black bars) and the visual condition (gray bars) as difference scores between the probability of recalling a word within a phonologically dissimilar list and the probability of recalling a word within a phonologically similar list. A value of zero means that the participant was equally successful in recalling words in the two conditions, and thus had no effect of phonological similarity. Positive values mean that phonologically dissimilar words were recalled more successfully than phonologically similar words, the typical phonological similarity effect, whereas negative values mean the reverse. As can be seen in the lower plot, the mean phonological similarity effect for the patient group is smaller for that of the controls in both modalities.
Figure 6. Difference Scores Representing Phonological Similarity Effects in Both Modalities.
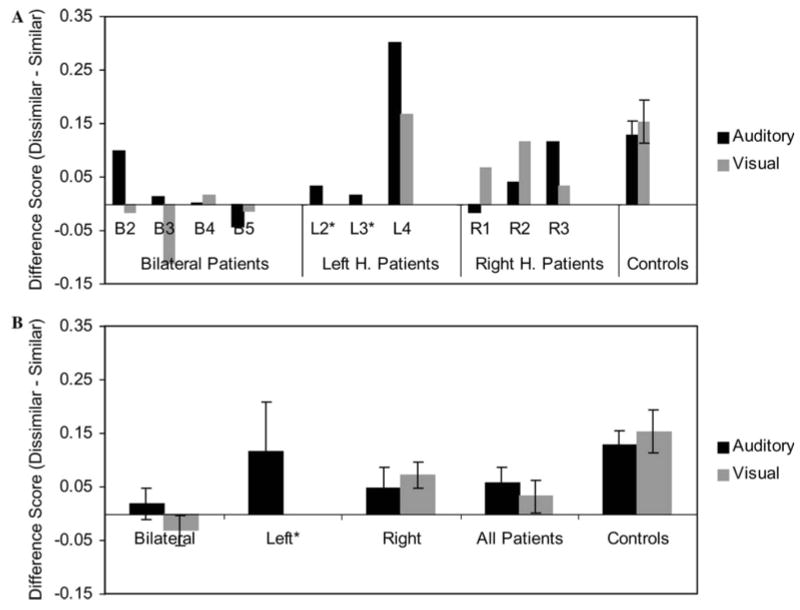
The data from Figures 4 and 5 have been redrawn to show the difference between the recall for words in phonologically similar contexts and similar contexts, for both auditory presentation (gray) and visual presentation (white). (Only one left hemisphere patient participated in the visual portion of the study, thus left-hemisphere group data are shown only for the auditory condition in the lower plot.)
Discussion
Ten cerebellar patients were compared to control subjects on a verbal working memory task in which the phonological similarity of the words to be remembered and their modality of presentation were manipulated. With the exception of one left-hemisphere patient, cerebellar patients demonstrated a reduction of the phonological similarity effect relative to controls in one or both of the modalities. Although separate analyses of each modality suggested that the group reduction of the effect may have been more consistent in the visual modality, examination of the individual scores and group means clearly suggests that a reduction occurred in the auditory condition as well.
Although the group means are also suggestive of a laterality effect, with the right cerebellar patients showing smaller similarity effects than did the left cerebellar patients, this should be taken with caution. Two of the left hemisphere patients (L2 and L3) could participate only in the auditory portion of the experiment (and both showed very flat similarity effects in this condition), whereas the third patient (L4) showed particularly large similarity effects.
A first point of discussion is that these experiments demonstrate a qualitative change in the verbal working memory of patients with damage to the cerebellum. The results suggest a way in which an apparent discrepancy might be resolved between the neuroimaging literature, which has shown consistent involvement of the cerebellum in verbal working memory, and the neuropsychological literature, which has typically demonstrated normal or near-normal digit span scores in cerebellar patients. It may be the case that the cerebellum does contribute to verbal working memory as the neuroimaging data suggest, but that upon cerebellar damage, other neural systems may be able to compensate for the damage by performing the task in a qualitatively different way.
The manipulations of phonological similarity and modality of presentation were designed as a critical test of the most frequently hypothesized role for the cerebellum in verbal working memory, namely that the cerebellum plays a role within the articulatory rehearsal component and not phonological short-term storage (Hypothesis 1 in Table 1). The Baddeley-Hitch model predicts that selective damage to the articulatory rehearsal mechanism should result in a reduced phonological similarity effect only when the modality of presentation is visual. The phonological similarity effect should be preserved with auditory presentation; this is because the Baddeley-Hitch model attributes the phonological similarity effect entirely to the phonological short-term store, which receives speech independently of articulatory rehearsal. Our results do not provide clear support for the articulatory rehearsal hypothesis, as a reduction of the phonological similarity effect was observed in some patients for both auditory (Figure 4) and visual (Figure 5) modalities of presentation. Note that this does not mean that we are arguing that the articulatory rehearsal mechanism is necessarily preserved in cerebellar patients. Rather, a single deficit to articulatory rehearsal does not seem to explain the cerebellar contribution to verbal working memory completely.8
Next consider the hypothesis of Desmond et al. (1997) that the cerebellum serves as the interface between the articulatory rehearsal mechanism and the phonological short-term store (Hypothesis 3). Assuming that the inferior cerebellum is receiving phonological input from the inferior parietal lobe without playing an integral role in analysis or storage, our results are also inconsistent with this account. As with the pure articulation hypothesis, the diminished effect of phonological similarity with auditory presentation would not be expected. However, one point of interest with regard to the Desmond hypothesis relates to the data of patient R3, a right hemisphere patient whose cerebellar damage is the most selective to the superior portions of the hemisphere and patient B2, a bilateral patient with damage also concentrated in the superior portions of the cerebellum (as well as the vermis). Patients R3 and B2 did seem to fit the predictions of an articulatory deficit: they showed a reduction of the phonological similarity effect primarily with visual presentation. With auditory presentation, their effect sizes were similar to the control average. The other patient with damage concentrated in the superior regions of the cerebellum, patient B4, does not fit this pattern; he showed no effect of phonological similarity in either modality.
Finally, consider the hypothesis that the cerebellum is part of the phonological short-term store (Hypothesis 2). When considered in isolation, the current results are the most consistent with this hypothesis, explaining the reduction of the phonological similarity effect in both modalities. The phonological short-term store hypothesis is also consistent with another study on a separate group of eight cerebellar patients who demonstrated preserved word length and articulatory suppression effects (Ravizza et al., submitted, Experiment 4). Such an idea may seem at odds with the connection of the cerebellum with speech dysarthria. However as mentioned previously, work with cortical patients suggest that speech dyspraxia is more likely to be related to articulatory rehearsal problems than is speech dysarthria (Baddeley & Wilson, 1985; Bishop & Robson, 1989; Waters et al., 1992; Goerlich et al., 1995). Further, there is a growing consensus that the cerebellum contributes to components of language other than overt and covert articulation (for reviews see Mariën et al., 2001; Justus & Ivry, 2001), and it is not implausible that its contribution to verbal working memory could relate to phonological short-term storage instead of or in addition to articulatory rehearsal. The current studies add to the case for a non-articulatory role for the cerebellum, and, if one is partial to the Baddeley-Hitch model, suggest consideration of how the cerebellum may be a component of both the phonological and articulatory sides of speech.
Beyond the Baddeley-Hitch Model
All of the discussion thus far has assumed that the algorithmic-level description given by the Baddeley-Hitch model is correct. Although the cognitive neuroscience literature has shown a preference for interpreting studies within this model, it is certainly not the only possibility (e.g., Miyake & Shah, 1999).9 In evaluating the Baddeley-Hitch model, it is important to examine three critical and interrelated assumptions. First, the model posits a clear distinction between phonetic and articulatory representation. Secondly, the model assumes that the phonological similarity effect and the word length effect are the results of capacity limits of the phonological short-term store and the articulatory rehearsal mechanism, respectively. Thirdly, the model argues that spoken language gains initial, automatic representation in the phonological short-term store whereas written language requires articulatory rehearsal to gain access to phonological representations. We discuss each of these in turn.
As mentioned previously, a central position of the Baddeley-Hitch model is that phonetic and articulatory representation are separated, both at the algorithmic level of description and in terms of neural implementation. Other theories have suggested that speech perception inherently involves mapping the speech signal onto the articulatory gestures used by the speaker (i.e., motor theories of speech perception, e.g., Liberman & Mattingly, 1985). These theories would suggest that the division between phonetic, phonological, and articulatory processing is less clear.
The second critical assumption of the Baddeley-Hitch model is that the phonological similarity and word length effects stem from the operations of the phonological short-term store and articulatory rehearsal mechanism, respectively. Even if one acknowledges the separation of the two components, it could be the case that the similarity manipulation affects rehearsal (as phonologically similar words are also similarly articulated) or that the length manipulation affects phonological short-term storage (as there is more phonological information to be represented).
Finally, the interpretation of these experiments also relies on claims concerning how spoken and written language gain access to the verbal working memory system. It could be the case that the analysis of spoken language does require intact articulatory representations, or that written language does not, contrary to the claims of the Baddeley-Hitch model. Either case would change the pattern of predictions made concerning the modality effects that are at the core of our predictions in these studies.
These two final claims of the model – the locus of the two effects and their relationship with presentation modality – were critical to the initial arguments concerning the separation of the phonological short-term store and articulatory rehearsal mechanisms (e.g., Baddeley et al., 1984). Thus questioning any of them relates back to the argument of phonetic-articulatory separation in the model. Perhaps rather than contributing independently to phonetic and articulatory representations, the cerebellum (and other areas) contributes to verbal working memory tasks in ways that do not allow for a clear distinction to be made between the two.
Conclusion
Although cerebellar patients do not consistently present with a profound deficit in verbal working memory as measured by the digit span, neuropsychological tests designed to tap qualitative differences suggest that even in patients whose spans are largely preserved, the verbal working memory system may be altered. Our data suggesting a reduction in the phonological similarity effect for words presented in both the visual and auditory modalities are also of relevance to the functional role often attributed to the cerebellum in neuroimaging studies of verbal working memory; these data suggest that the assignment of the cerebellum to the articulatory side of the Baddeley-Hitch model may be premature, and complement other sources of data suggesting that the cerebellum may play numerous roles in language, including ones that do not relate to articulation.
Acknowledgments
This research was supported by grants from the US National Institutes of Health (T32 GM07048-25 and P01 NS40813). We would like to thank the ten patients who participated in these experiments. We also thank Paul Aparicio, Christina Middleton, and Natalie Marchant for assistance with the patient testing, Jörn Diedrichsen for the patient lesion reconstructions, and Alexandra List for comments on an earlier version of the manuscript. A related paper was presented at the 2001 meeting of the Society for Neuroscience in San Diego, California, and also appeared as the second chapter in the dissertation of T. Justus (2003).
Footnotes
For a similar argument from a developmental cognitive neuroscience perspective on the “preserved” abilities of individuals with Williams Syndrome, see Karmiloff-Smith (1998).
The term phonetic refers to a more perceptual representation than does the term phonological. The determination of voice-onset time (VOT) to distinguish a /t/ from a /d/, for example, is more correctly referred to as a phonetic process, whereas the symbolic representation of the phonemes /t/ or /d/ is more correctly referred to as phonological. Phonetic representations are input-based, while phonological representations are abstract. We attempt to maintain this distinction here, while retaining the connection in the Baddeley-Hitch model between input-based representations and the “phonological” short-term store. (See Phillips et al., 2000; Phillips, 2001, for a discussion of the differences between phonetic and phonological representation). For output-based representations, we use the term articulatory.
Or at least a production deficit that cannot account for the reduction in span. Many of the patients summarized by Shallice and Vallar (1990) showed some degree of anomia and/or paraphasia.
Chein and Fiez (2001) also question the hypothesis that the inferior parietal lobe serves as the locus of the phonological short-term store. See Fiez et al. (1996), Becker et al. (1999), Jonides et al. (1998), Chein et al. (2003), and Ravizza et al. (2004) for discussion of this issue.
Interestingly, lexical retrieval paradigms seem to show the same discrepancy between neuroimaging findings that consistently indicate cerebellar involvement and preserved behavioral outcomes (although not necessarily identical cognitive processes) in cerebellar patients (e.g., Helmuth et al., 1997; Richter et al., 2004).
Patient labels correspond to those used by Justus (2004).
The stimuli in Table 3 also lend themselves to combination by initial consonant, rather than by vowel. We chose a vowel manipulation for this study to be consistent with the majority of previous manipulations of phonological similarity, and because we suspected that explicit strategies might aid in recalling lists that begin with the same consonant. Only one patient (B3) and one control had any awareness of the (vowel-based) phonological similarity manipulation by the end of the experiment.
It should be noted that given our design, the strongest support for the articulatory rehearsal hypothesis would have been provided by a three-way interaction between phonological similarity, modality, and group. Although this interaction did not approach significance (p = .23), the two-way interaction was in fact stronger for the visual modality (p = .03) than for the auditory modality (p = .10). Thus, caution should be used in interpreting this null result. Nevertheless, with the exception of patients R3 and B2, it is difficult to reconcile the individual data with the predictions of the articulatory rehearsal hypothesis.
See Chein et al. (2003) for an interpretation of the neuroimaging working memory literature from the perspective of Cowan’s Embedded-Process Model (1995).
References
- Ackermann H, Gräber S, Hertrich I, Daum I. Categorical speech perception in cerebellar disorders. Brain and Language. 1997;60:323–331. doi: 10.1006/brln.1997.1826. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Hertrich I. The contribution of the cerebellum to speech processing. Journal of Neurolinguistics. 2000;13:95–116. [Google Scholar]
- Akshoomoff NA, Courchesne E, Press GA, Iragui V. Contribution of the cerebellum to neuropsychological functioning: Evidence from a case of cerebellar degenerative disorder. Neuropsychologia. 1992;30:315–328. doi: 10.1016/0028-3932(92)90105-u. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. Short-term and long-term verbal memory: A positron emission tomography study. Proceedings of the National Academy of Sciences USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley A. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. The Psychology of Learning and Motivation. Vol. 8. San Diego, CA: Academic Press; 1974. pp. 47–90. [Google Scholar]
- Baddeley A, Lewis V, Vallar G. Exploring the phonological loop. Quarterly Journal of Experimental Psychology. 1984;36A:233–252. [Google Scholar]
- Baddeley A, Thomson N, Buchanan M. Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior. 1975;14:575–589. [Google Scholar]
- Baddeley A, Wilson B. Phonological coding and short-term memory in patients without speech. Journal of Memory and Language. 1985;24:490–502. [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA. A comment on the functional localization of the phonological storage subsystem of working memory. Brain and Cognition. 1999;41:27–38. doi: 10.1006/brcg.1999.1094. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Robson J. Unimpaired short-term memory and rhyme judgement in congenitally speechless individuals: Implications for the notion of “articulatory coding. Quarterly Journal of Experimental Psychology. 1989;41A:123–140. [Google Scholar]
- Bracke-Tolkmitt R, Linden A, Canavan AGM, Rockstroh B, Scholz E, Wessel K, Diener HC. The cerebellum contributes to mental skills. Behavioral Neuroscience. 1989;103:442–446. [Google Scholar]
- Bürk K, Globas C, Bösch S, Gräber S, Abele M, Brice A, Dichgans J, Daum I, Klockgether T. Cognitive deficits in spinocerebellar ataxia 2. Brain. 1999;122:769–777. doi: 10.1093/brain/122.4.769. [DOI] [PubMed] [Google Scholar]
- Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I, Dichgans J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. Journal of Neurology. 2003;250:207–211. doi: 10.1007/s00415-003-0976-5. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL, Blumstein SE. The role of segmentation in phonological processing: An fMRI investigation. Journal of Cognitive Neuroscience. 2000;12:679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cerebral Cortex. 2001;11:1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Conrad R. Acoustic confusion in immediate memory. British Journal of Psychology. 1964;55:75–84. doi: 10.1111/j.2044-8295.1964.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and Memory: An Integrated Framework. Oxford: Oxford University Press; 1995. [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. Journal of Cognitive Neuroscience. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Desmond JE. Cerebellar involvement in cognitive function: Evidence from neuroimaging. International Review of Psychiatry. 2001;13:283–294. [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro F, Tavano A, Corti S, Bresolin N, De Fabritiis P, Borgatti R. Long-tern neuropsychological deficits after cerebellar infarctions in two young adult twins. Neuropsychologia. 2004;42:536–545. doi: 10.1016/j.neuropsychologia.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globas C, Bösch S, Zühlke C, Daum I, Dichgans J, Bürk K. The cerebellum and cognition: Intellectual function in spinocerebellar ataxia type 6 (SCA6) Journal of Neurology. 2003;250:1482–1487. doi: 10.1007/s00415-003-0258-2. [DOI] [PubMed] [Google Scholar]
- Goerlich C, Daum I, Hertrich I, Ackermann H. Verbal short-term memory and motor speech processes in Broca’s aphasia. Behavioural Neurology. 1995;8:81–91. doi: 10.3233/BEN-1995-8203. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RSJ, Dolan RJ. A graded task approach to the functional mapping of brain areas implicated in auditory-verbal memory. Brain. 1994;117:1271–1282. doi: 10.1093/brain/117.6.1271. [DOI] [PubMed] [Google Scholar]
- Gruber O. Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cerebral Cortex. 2001;11:1047–1055. doi: 10.1093/cercor/11.11.1047. [DOI] [PubMed] [Google Scholar]
- Helmuth LL, Ivry RB, Shimizu N. Preserved performance by cerebellar patients on tests of word generation, discrimination learning, and attention. Learning & Memory. 1997;3:456–474. doi: 10.1101/lm.3.6.456. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Gopal HS. Speech production and perception in patients with cerebellar lesions. In: Meyer DE, Kornblum S, editors. Attention and Performance, Volume XIV: Synergies in Experimental Psychology, Artificial Intelligence, and Cognitive Neuroscience. Cambridge, MA: MIT Press; 1992. pp. 771–802. [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marschuetz C, Willis CR. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus T. Doctoral dissertation. University of California; Berkeley: 2003. Cerebellar contributions to human language: Neuropsychological studies of verbal working memory and grammatical morphology. [Google Scholar]
- Justus T. The cerebellum and English grammatical morphology: Evidence from production, comprehension, and grammaticality judgments. Journal of Cognitive Neuroscience. 2004;16:1115–1130. doi: 10.1162/0898929041920513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus T, Hertrich I, Ackermann H, Bürk K, Ivry RB. Impact of cerebellar damage on grammatical morphology in English and German. Poster presented at the annual meeting of the Cognitive Neuroscience Society; San Francisco, CA. 2004. Apr, [Google Scholar]
- Justus TC, Ivry RB. The cognitive neuropsychology of the cerebellum. International Review of Psychiatry. 2001;13:276–282. [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Le Pira F, Zappalà G, Saponara R, Domina E, Restivo DA, Reggio E, Nicoletti A, Giuffrida S. Cognitive findings in spinocerebellar ataxia type 2: Relationship to genetic and clinical variables. Journal of the Neurological Sciences. 2002;201:53–57. doi: 10.1016/s0022-510x(02)00194-6. [DOI] [PubMed] [Google Scholar]
- Liberman A, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21:1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Aparicio P, Marchant NL, Ivry RB. Rule-based category learning is impaired in patients with Parkinson’s disease but not in patients with cerebellar disorders. Journal of Cognitive Neuroscience. doi: 10.1162/0898929053747630. in press. [DOI] [PubMed] [Google Scholar]
- Mariën P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: A review and a new hypothesis. Brain and Language. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H. Cerebellum and speech perception: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2002;14:902–912. doi: 10.1162/089892902760191126. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron tomographic studies of the processing of single words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans A. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proceedings of the National Academy of Sciences USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Pellathy T, Marantz A, Yellin E, Wexler K, Poeppel D, McGinnis M, Roberts T. Auditory cortex accesses phonological categories: An MEG mismatch study. Journal of Cognitive Neuroscience. 2000;12:1038–1055. doi: 10.1162/08989290051137567. [DOI] [PubMed] [Google Scholar]
- Phillips C. Levels of representation in the electrophysiology of speech perception. Cognitive Science. 2001;25:711–731. [Google Scholar]
- Ramus F, Pidgeon E, Frith U. The relationship between motor control and phonology in dyslexia children. Journal of Child Psychology and Psychiatry. 2003;44:712–722. doi: 10.1111/1469-7610.00157. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Ravizza S, McCormick C, Justus T, Fiez J. Focal cerebellar brain damage produces selective deficits in verbal working memory. Poster presented at the annual meeting of the Cognitive Neuroscience Society; San Francisco, CA. 2004. Apr, [Google Scholar]
- Ravizza S, McCormick C, Justus T, Ivry RB, Fiez J. Focal cerebellar brain damage produces selective deficits in verbal working memory. doi: 10.1093/brain/awh685. submitted. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Richter S, Kaiser O, Hein-Kropp C, Dimitrova A, Gizewski E, Beck A, Aurich V, Ziegler W, Timmann D. Preserved verb generation in patients with cerebellar atrophy. Neuropsychologia. 2004;42:1235–1246. doi: 10.1016/j.neuropsychologia.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain. 1996;119:1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Schatz J, Hale S, Myerson J. Cerebellar contribution to linguistic processing efficiency revealed by focal damage. Journal of the International Neuropsychological Society. 1998;4:491–501. doi: 10.1017/s1355617798455085. [DOI] [PubMed] [Google Scholar]
- Schelhaas HJ, Ippel PF, Hageman G, Sinke RJ, van der Laan EN, Beemer FA. Clinical and genetic analysis of a four-generation family with a distinct autosomal dominant cerebellar ataxia. Journal of Neurology. 2001;248:113–120. doi: 10.1007/s004150170245. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Scott RB, Stoodley CJ, Anslow P, Paul C, Stein JF, Sugden EM, Mitchell CD. Lateralized cognitive deficits in children following cerebellar lesions. Developmental Medicine & Child Neurology. 2001;43:685–691. doi: 10.1017/s0012162201001232. [DOI] [PubMed] [Google Scholar]
- Shallice T, Vallar G. The impairment of auditory-verbal short-term storage. In: Vallar G, Shallice T, editors. Neuropsychological Impairments of Short-Term Memory. Cambridge: Cambridge University Press; 1990. pp. 11–53. [Google Scholar]
- Silveri MC, Di Betta AM, Filippini V, Leggio MG, Molinari M. Verbal short-term store-rehearsal system and the cerebellum: Evidence from a patient with a right cerebellar lesion. Brain. 1998;121:2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Styger M, Boltshauser E. Cognitive impairments in patients with congenital nonprogressive cerebellar ataxia. Neurology. 1999;53:966–973. doi: 10.1212/wnl.53.5.966. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lövblad KO, Ridolfi Lüthy A, Perrig W, Kaufmann F. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain. 2003;126:1998–2008. doi: 10.1093/brain/awg195. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Maschke M, Kolb FP, Böring D, Thilmann AF, Diener HC. Motor deficits cannot explain impaired cognitive associative learning in cerebellar patients. Neuropsychologia. 2002;40:788–800. doi: 10.1016/s0028-3932(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. Journal of the Neurological Sciences. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: Patterns of impairment and neural correlates. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Vallar G, Papagno C. Neuropsychological impairments of verbal short-term memory. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. 2. Chichester, U.K.: John Wiley; 2002. pp. 249–270. [Google Scholar]
- Warrington EK, Shallice T. The selective impairment of auditory verbal short-term memory. Brain. 1969;92:885–896. doi: 10.1093/brain/92.4.885. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Logue V, Pratt RTC. The anatomical localisation of selective impairment of auditory verbal short-term memory. Neuropsychologia. 1971;9:377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- Waters GS, Rochon E, Caplan D. The role of high-level speech planning in rehearsal: Evidence from patients with apraxia of speech. Journal of Memory and Language. 1992;31:54–73. [Google Scholar]
- Witt K, Nühsman A, Deuschl G. Intact artificial grammar learning in patients with cerebellar degeneration and advanced Parkinson’s disease. Neuropsychologia. 2002;40:1534–1540. doi: 10.1016/s0028-3932(02)00027-1. [DOI] [PubMed] [Google Scholar]


