Abstract
Although the platinum-based anticancer drugs cisplatin, carboplatin, and oxaliplatin have similar DNA-binding properties, only oxaliplatin is active against colorectal tumors. The mechanisms for this tumor specificity of platinum-based compounds are poorly understood but could be related to differences in uptake. This study shows that the human organic cation transporters (OCT) 1 and 2 (SLC22A1 and SLC22A2) markedly increase oxaliplatin, but not cisplatin or carboplatin, accumulation and cytotoxicity in transfected cells, indicating that oxaliplatin is an excellent substrate of these transporters. The cytotoxicity of oxaliplatin was greater than that of cisplatin in six colon cancer cell lines [mean ± SE of IC50 in the six cell lines, 3.9 ± 1.4 μmol/L (oxaliplatin) versus 11 ± 2.0 μmol/L (cisplatin)] but was reduced by an OCT inhibitor, cimetidine, to a level similar to, or even lower than that of, cisplatin (29 ± 11 μmol/L for oxaliplatin versus 19 ± 4.3 μmol/L for cisplatin). Structure-activity studies indicated that organic functionalities on nonleaving groups coordinated to platinum are critical for selective uptake by OCTs. These results indicate that OCT1 and OCT2 are major determinants of the anticancer activity of oxaliplatin and may contribute to its antitumor specificity. They also strongly suggest that expression of OCTs in tumors should be investigated as markers for selecting specific platinum-based therapies in individual patients. The development of new anticancer drugs, specifically targeted to OCTs, represents a novel strategy for targeted drug therapy. The results of the present structure-activity studies indicate specific tactics for realizing this goal.
Introduction
Platinum-based drugs are among the most active anticancer agents, and cisplatin represents one of the three most widely used cancer chemotherapeutics (1). Although cisplatin is effective against several solid tumors, especially testicular and ovarian cancer, its clinical use is limited because of its toxic effects as well as the intrinsic and acquired resistance of some tumors to this drug (2). To overcome these limitations, platinum analogues with lower toxicity and greater activity in cisplatin-resistant tumors have been developed and tested, resulting in the approval of carboplatin and oxaliplatin in the United States (see Fig. 1). Carboplatin has the advantage of being less nephrotoxic, but its cross-resistance with cisplatin limits its application in otherwise cisplatin-treatable diseases (2). Oxaliplatin, however, exhibits a different anticancer spectrum from that of cisplatin (3, 4). It has been approved as the first-line or second-line therapy in combination with 5-fluorouracil (5-FU)/leucovorin for advanced colorectal cancer, for which cisplatin and carboplatin are essentially inactive (5). In spite of their distinct antitumor specificities, cisplatin and oxaliplatin, as well as other platinum compounds, share similar mechanisms of action. In particular, their cytotoxicity arises primarily from covalent binding to DNA after aquation to form monoaqua and diaqua complexes (6, 7). This chemistry initiates a series of biochemical cascades, eventually leading to cell death (6, 8).
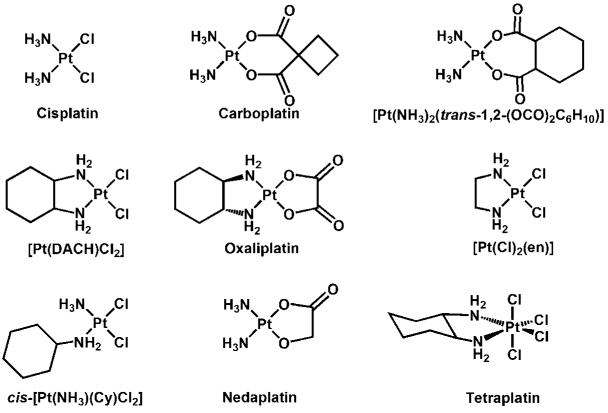
Figure 1.
Chemical structures of platinum compounds.
Because cisplatin and oxaliplatin target similar DNA sites for binding and form similar types of DNA adducts (9-13), mainly 1,2-intrastrand and 1,3-intrastrand cross-links involving purine nucleotides, the mechanisms responsible for their distinct tumor specificities may involve events other than their interaction with and binding to DNA. Studies aiming to identify such mechanisms have focused largely on the cellular processing of cisplatin-DNA and oxaliplatin-DNA adducts (14, 15). However, differences in the mechanism(s) controlling the cellular uptake and efflux of these platinum compounds, although rarely investigated, could also be important because reduced intracellular accumulation is the most common observation in cisplatin-resistant cells (16).
Recent studies suggest a direct involvement of the human copper influx transporter Ctr1 in the cellular uptake of cisplatin, carboplatin, and oxaliplatin to a varying extent (17). Studies in tumor cell lines suggest, however, that Ctr1 may not affect the formation and corresponding cytotoxicity of cisplatin-DNA adducts (18). The human copper efflux transporters ATP7B and ATP7A also recognize these platinum compounds, and their elevated expression has been associated with cisplatin resistance (16). The importance of these interactions in modulating the differential activity and tumor specificity of the platinum compounds is currently unknown.
The organic cation transporters (OCT) 1 [solute carrier 22A1 (SLC22A1)], 2 (SLC22A2), and 3 (SLC22A3) are in the class of plasma membrane transporters belonging to the SLC22A family (19, 20). The OCTs mediate intracellular uptake of a broad range of structurally diverse organic cations with molecular masses generally lower than 400 Da (19, 20). Substrates of OCTs include endogenous compounds, such as choline, creatinine, and monoamine neurotransmitters, and a variety of xenobiotics, such as tetraethylammonium (TEA; a prototypic organic cation), 1-methyl-4-phenylpyridinium (MPP+; a neurotoxin), and clinically used drugs, such as metformin, cimetidine, and amantadine (19). In humans, OCT1 is primarily expressed in the liver (20-22) and less so in the intestine (23), whereas OCT2 is predominantly expressed in the kidney (20, 22). OCT3 is expressed in many tissues, including placenta, heart, liver, and skeletal muscle (24, 25). The expression of the OCTs has also been detected in several human cancer cell lines (26). The interaction of cisplatin with human OCTs has been investigated, and the results are discordant (27, 28). Previous studies suggest that cisplatin is not a substrate of human OCT1 or OCT2 (27), whereas more recent work indicates that the drug interacts with human and rat OCT2 but not OCT1 (28, 29). It is not known whether oxaliplatin or carboplatin interacts with these transporters, however, or whether such interactions contribute to their cytotoxicities and differential tumor specificities.
The goals of the present study were to characterize the interaction of cisplatin, carboplatin, and oxaliplatin with human OCT1, OCT2, and OCT3, to determine whether interactions with OCTs contribute to the differential antitumor specificity of oxaliplatin versus cisplatin, and to understand in a broader context the underlying chemical principles that determine these differences. Our data indicate that OCT1 and OCT2 play a critical role in mediating the uptake and consequent cytotoxicity of oxaliplatin but not cisplatin or carboplatin. Structure-activity relationship studies suggest that the 1,2-diaminocyclohexane (DACH) moiety of oxaliplatin is an important pharmacophore for its interaction with the OCTs and that an organic component on the nonleaving portion of the platinum complexes is essential. Finally, our experiments suggest that interactions with OCT1 and OCT2 are likely to be important contributors to the sensitivity of colorectal cancer to oxaliplatin.
Materials and Methods
Drugs and Reagents
Cisplatin, carboplatin, oxaliplatin, cimetidine, disopyramide, MPP+, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Solutions of carboplatin (10 mmol/L) and oxaliplatin (5 mmol/L) were freshly prepared in water. A solution of cisplatin (2 mmol/L) was made in PBS. These stock solutions were immediately aliquoted, stored frozen at −20°C, and discarded 1 month after preparation. [Methyl-3H]MPP+ was from Perkin-Elmer (Boston, MA), and TEA bromide [ethyl-1-14C] was from American Radiolabeled Chemicals (St. Louis, MO). Hygromycin B and G418 were from Invitrogen (Carlsbad, CA). The cell culture media DMEM, RPMI 1640, and fetal bovine serum (FBS) were from the Cell Culture Facility of the University of California at San Francisco (San Francisco, CA).
Cell Lines and Transfection
Madin-Darby canine kidney (MDCK) cells stably transfected with the full-length human OCT1 cDNA (MDCK-hOCT1) and with the empty vector (MDCK-MOCK) were established previously in our laboratory (30). Human embryonic kidney (HEK) 293 cells transfected with pcDNA5/FRT vector (Invitrogen) containing the full-length human OCT2 cDNA (HEKhOCT2) and with the empty vector (HEK-MOCK) were established using LipofectAMINE 2000 (Invitrogen) per manufacturer's instructions. The stable clones were selected with 75 μg/mL hygromycin B. HEK 293 cells transfected with pcDNA3 vector containing the full-length human OCT3 cDNA (HEK-hOCT3) and with the empty vector (HEK-MOCK) were also established using LipofectAMINE 2000. The stable clones were selected with 600 μg/mL G418. The pcDNA3 vector containing the full-length human OCT3 cDNA was kindly provided by Dr. Bonisch (Institute of Pharmacology and Toxicology, University of Bonn, Bonn, Germany). All the colon cancer cell lines (LS180, SW620, DLD, HCT116, HT20, and RKO) used in the present study were from the American Type Culture Collection (Manassas, VA).
Cell Culture
The culture medium for stably transfected MDCK and HEK 293 cells is DMEM supplemented with 10% FBS and 100 units/mL penicillin, 100 μg/mL streptomycin, (Invitrogen), and with the respective selection antibiotics. The culture medium for all the colon cancer cell lines is RPMI 1640 containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. All cell lines were grown at 37°C in a humidified atmosphere with 5% CO2/95% air.
Drug Sensitivity Assay
The cytotoxicity of the platinum compounds was measured by the MTT assay in 96-well plates at a predetermined cell density. After overnight incubation, the platinum compounds with or without an OCT inhibitor (cimetidine or disopyramide) were then added to the culture medium to give the indicated final concentrations. After drug exposure, the drugcontaining medium was replaced with fresh, drug-free medium and the incubation was continued for a total of 72 hours starting from the addition of platinum compounds. MTT assays were done as described previously (31). The IC50 values were obtained by fitting F, the percentage of the maximal cell growth at different drug concentrations, to the equation using WinNonlin (Pharsight, Mountain View, CA). The maximal cell growth was the cell growth in the medium without any platinum compounds; C is the concentration of the platinum compound and γ is the slope factor.
Cellular Uptake of TEA or MPP+
MDCK or HEK 293 cells were incubated in PBS buffer containing 10 μmol/L [14C]TEA or 2 μmol/L [3H]MPP+ with or without a specified OCT inhibitor. The uptake was done at room temperature for 2 minutes ([14C]TEA uptake) or 5 minutes ([3H]MPP+). Aliquots of cell lysates were used for scintillation counting and bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) to determine the uptake.
Cellular Accumulation of Platinum
The cellular accumulation of platinum was determined as described previously (18) with some modifications. Briefly, the cells were incubated in the culture medium containing the indicated platinum compounds with or without an OCT inhibitor at 37°C in 5% CO2 for 2 hours unless specified. After incubation, cells were washed with ice-cold PBS, harvested, and pelleted by centrifugation at 400 × g and at 4°C for 15 minutes. The cell pellets were dissolved in 70% nitric acid at 65°C for at least 2.5 hours. Distilled water containing 10 ppb of iridium (Sigma) and 0.1% Triton X-100 was added to the samples to dilute nitric acid to 7%. The platinum content was measured by inductively coupled plasma mass spectrometry (MS) in the Analytical Facility at the University of California at Santa Cruz (Santa Cruz, CA). Cell lysates from a set of identical cultures were used for BCA protein assay.
Platinum-DNA Adduct Formation
The platinum content associated with genomic DNA was determined as described previously (32) with some modifications. Briefly, the cells were incubated in the culture medium containing the specified platinum compounds with or without an OCT inhibitor at 37°C in 5% CO2 for 2 hours (or 25 minutes as specified). In some experiments, phosphate buffer [PB; 1.06 mmol/L KH2PO4, 2.97 mmol/L Na2HPO4 (pH 7.4)] containing 155 mmol/L NaCl (PB-Cl buffer) or 103 mmol/L Na2SO4 (PB-SO4 buffer) was used instead of the culture medium as specified. After incubation, the cells were washed with ice-cold PBS, scraped, and pelleted. Genomic DNA was isolated from the cell pellets using Wizard Genomic DNA Purification kit (Promega, Madison, WI) following the manufacturer's instructions. The genomic DNA prepared from two different aliquots of the supernatant (after protein precipitation) was used for platinum (as described above) and DNA content determination, respectively. DNA content was measured by absorption spectroscopy at 260 nm.
RNA Isolation
Total RNA was isolated from cultured cells (70-80% confluent) using an RNeasy Mini kit (Qiagen, Valencia, CA) following the manufacturer's instructions. Samples of tumor and normal colon mucosa were collected from colon cancer resection from the Department of Surgery, Queen Mary Hospital, University of Hong Kong (Pokfulam, Hong Kong, Republic of China). Tissues were frozen in liquid nitrogen within 0.5 hour after they were resected. Total RNA was extracted using Trizol (Invitrogen). This study was approved by the Ethics Committee of the University of Hong Kong and the Internal Review Board of University of California at San Francisco.
Reverse Transcription-PCR
Reverse transcription-PCR (RT-PCR) was done by standard methods. Sense and antisense primers for the PCRs are listed in Supplementary Table S1. All sets of primers were designed to anneal with sequences in different exons of the genes. Real-time PCR was carried out using Taqman Universal Master Mix (Applied Biosystems, Foster City, CA). Primer and probe sets for each gene were Assays-on-Demand purchased from Applied Biosystems. Reactions were run on an ABI Prism 7700, and cycling conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. The expression of specific transcripts relative to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control is reported as the level relative to the expression in a colon cancer sample “T10,” which has some degree of both OCT1 and OCT2 expression.
Synthesis of Platinum Analogues
Potassium tetrachloroplatinate(II) was a gift from Engelhard Corp. (Iselin, NJ), and the starting materials cisplatin and potassium amminetrichloroplatinate(II) were synthesized as reported (33, 34). 1H nuclear magnetic resonance (NMR) spectra were acquired on a Varian 300 MHz spectrometer. Fourier transform-IR (FT-IR) spectra were measured on an Avatar 380 FT-IR (Thermo Nicolet, Waltham, MA). Electrospray ionization-MS (ESI-MS) spectra were obtained on an Agilent Technologies 1100 Series liquid chromatography/MS instrument (Palo Alto, CA). Previously reported procedures were used to prepare [Pt(en)Cl2] (33), cis-[Pt(NH3)(Cy)Cl2] (34), where Cy is cyclohexylamine, and [Pt(R,R-DACH)Cl2] (35). The [Pt(S,S-DACH)Cl2] and [Pt(S,S-DACH)oxalate] complexes were synthesized as described (36). FT-IR and 1H NMR spectra of all compounds matched literature spectra.
Preparation of [Pt(NH3)2(trans-1,2-(OCO)2C6H10)]
The compound was prepared as described for the Pt-DACH derivative (37). Solubility problems, similar to those reported for the DACH compound, prevented analysis by NMR spectroscopy. IR (KBr, cm−1) 3266 (sh), 2920 (s), 2850 (s), 1618 (s), 1556 (sh), 1384 (vs), 1279 (w), 1222 (m), 1111 (w), 1030 (w), 772 (w), 719 (w), 588 (b). ESI-MS: [M+H]+ = 400.2 a.m.u. (observed) and 400.3 a.m.u. (calculated).
Preparation of [Pt(R,R-DACH)(H2O)2]2+
[Pt(R,R-DACH)Cl2] was dissolved in distilled water (200 μmol/L) and incubated with silver nitrate (400 μmol/L) in the dark for 10 hours. [Pt(R,R-DACH)(H2O)2]2+ was obtained by filtering the reaction mixture to remove the silver chloride precipitate.
Statistical Analysis
The differences between the mean values were analyzed for significance using Student's t test. Ps < 0.05 were considered statistically significant.
Results
OCT Expression and Function in Stably Transfected Cell Lines
The expression and function of human OCTs in the stably transfected cells were confirmed by RT-PCR and by examining the uptake of the model OCT substrates (TEA for OCT1 and OCT2 and MPP+ for OCT3). The expression of the mRNA transcripts of OCT1, OCT2, and OCT3 and uptake of model compounds were clearly much higher in OCT-transfected cells compared with empty vector–transfected control counterparts (MOCK cells; Supplementary Figs. S1 and S2). OCT inhibitors [disopyramide (120 μmol/L) for OCT1 and cimetidine (1.5 mmol/L) for OCT2 and OCT3] substantially decreased the uptake of the model compounds in the OCT-transfected cells (P < 0.001; Supplementary Fig. S2).
Effect of OCTs on the Cytotoxicity of Cisplatin, Carboplatin, and Oxaliplatin
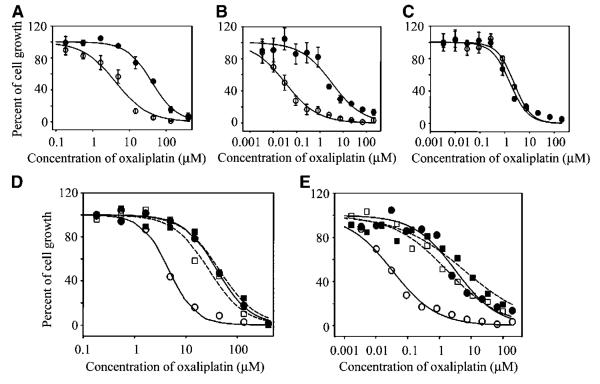
The IC50 values of oxaliplatin in MDCK-MOCK cells after different times (7, 24, and 72 hours) of drug exposure were all significantly higher than those in MDCK-hOCT1 cells. Resistance factors, defined as the ratio of the IC50 value in MOCK cells to that in the corresponding OCT-transfected cells, ranged from 5.7 to 8.5 (P < 0.01 or 0.001; Table 1A; Fig. 2A). In contrast, the IC50 values of both cisplatin and carboplatin were similar in MDCK-hOCT1 and in the MDCK-MOCK cells with resistance factor values close to unity (P > 0.05; Table 1A). Furthermore, coincubation with a known OCT1 inhibitor, disopyramide (150 μmol/L), substantially increased the IC50 value of oxaliplatin in MDCK-hOCT1 (control versus disopyramide treated, 3.8 ± 1.6 μmol/L versus 23 ± 11 μmol/L) by 6-fold (P < 0.05) with little effect in MDCK-MOCK (control versus disopyramide treated, 30 ± 9.3 μmol/L versus 32 ± 13 μmol/L; P > 0.05) tested in parallel (Fig. 2D). Disopyramide itself did not manifest any cytotoxicity up to a concentration of 400 μmol/L under the same test conditions (data not shown). These results indicate that OCT1 enhances the cytotoxicity of oxaliplatin but not that of cisplatin or carboplatin. A similar pattern of observations was obtained in human OCT2-transfected cells, but the increase in oxaliplatin cytotoxicity was much more pronounced (Fig. 2B). The IC50 values of oxaliplatin after different times (7, 24, and 72 hours) of exposure were all markedly greater in HEK-MOCK cells than in HEK-OCT2 cells with resistance factor values ranging from 48 to 77 (P < 0.05 to P < 0.001; Table 1B; Fig. 2B). However, the IC50 values of cisplatin and carboplatin were only slightly higher in HEK-MOCK cells than in HEK-OCT2 cells with resistance factor values ~2 after 7-hour drug exposure (Table 1B). Coincubation with an OCT inhibitor, cimetidine (1.5 mmol/L), dramatically increased the oxaliplatin IC50 (control versus cimetidine treated, 0.039 ± 0.025 μmol/L versus 2.8 ± 1.6 μmol/L) by 72-fold (P < 0.05) in HEK-hOCT2 cells, with only a 3.2-fold increase in HEK-MOCK cells (control versus cimetidine treated, 3.0 ± 1.5 μmol/L versus 9.5 ± 3.0 μmol/L; P < 0.05; Fig. 2E). Cimetidine itself did not exhibit cytotoxicity up to a concentration of 5 mmol/L under the same test conditions (data not shown). These results indicate that OCT2 markedly enhances the cytotoxicity of oxaliplatin with only slight effects on the cytotoxicities of cisplatin and carboplatin. In contrast to OCT1 and OCT2, overexpression of human OCT3 did not affect the cytotoxicity of any of the platinum drugs (Table 1C; Fig. 2C).
Table 1.
Drug sensitivity of cisplatin, carboplatin, and oxaliplatin in the OCT-transfected cells
| A. Cytotoxicity, expressed as IC50, of the platinum drugs in MDCK-MOCK and MDCK-hOCT1 cells | ||||
|---|---|---|---|---|
| Platinum drugs | Drug exposure time (h) | MDCK-MOCK (μmol/L) | MDCK-hOCT1 (μmol/L) | Resistance factor |
| Cisplatin | 7 | 20 ± 7.6 | 15 ± 2.8 | 1.3 |
| Carboplatin | 7 | 260 ± 86 | 230 ± 86 | 1.1 |
| Oxaliplatin | 7 | 33 ± 9.1 | 3.9 ± 1.3 | 8.5* |
| Oxaliplatin | 24 | 14 ± 5.6 | 1.8 ± 0.58 | 8.0† |
| Oxaliplatin | 72 | 9.6 ± 1.8 | 1.7 ± 0.27 | 5.7* |
| B. Cytotoxicity, expressed as IC50, of the platinum drugs in HEK-MOCK and HEK-hOCT2 cells | ||||
| Platinum drugs | Drug exposure time (h) | HEK-MOCK (μmol/L) | HEK-hOCT2 (μmol/L) | Resistance factor |
| Cisplatin | 7 | 2.9 ± 0.23 | 1.3 ± 0.18 | 2.2* |
| Carboplatin | 7 | 110 ± 46 | 62 ± 46 | 1.8 |
| Oxaliplatin | 7 | 3.0 ± 1.5 | 0.039 ± 0.025 | 77† |
| Oxaliplatin | 24 | 1.5 ± 0.69 | 0.020 ± 0.0010 | 74‡ |
| Oxaliplatin | 72 | 0.93 ± 0.056 | 0.019 ± 0.0040 | 48* |
| C. Cytotoxicity, expressed as IC50, of the platinum drugs in HEK-MOCK and HEK-hOCT3 cells | ||||
| Platinum drugs | Drug exposure time (h) | HEK-MOCK (μmol/L) | HEK-hOCT3 (μmol/L) | Resistance factor |
| Cisplatin | 7 | 2.8 ± 0.90 | 2.4 ± 0.71 | 1.2 |
| Carboplatin | 7 | 85 ± 9.7 | 48 ± 23 | 1.8 |
| Oxaliplatin | 7 | 1.5 ± 0.28 | 2.2 ± 0.41 | 0.66 |
| Oxaliplatin | 24 | 0.47 ± 0.050 | 0.62 ± 0.19 | 0.75 |
| Oxaliplatin | 72 | 0.47 ± 0.12 | 0.69 ± 0.19 | 0.68 |
NOTE: The IC50 values (μmol/L) in human OCT1-transfected (A), OCT2-transfected (B), and OCT3-transfected (C) cell lines were determined in parallel with those in the corresponding MOCK cells using MTT assay as described in Materials and Methods. The seeding densities for MDCK and HEK 293 cells were 5,000 and 12,000 cells per well, respectively. Data are expressed as mean ± SD of three to six independent experiments with each done in quadruplicate. The resistance factor was defined as the ratio of the mean IC50 value in the MOCK cells to that in the OCT-transfected cells.
P < 0.001.
P < 0.01.
P < 0.05.
Figure 2.
Cytotoxicity of oxaliplatin in cells stably transfected with human OCTs. The cytotoxicity of oxaliplatin (7 hours of drug exposure) in OCT1-transfected (A), OCT2-transfected (B), and OCT3-transfected (C) cells (○) and in the corresponding MOCK cells (●) was determined as described in Materials and Methods. In addition, the cytotoxicity of oxaliplatin in OCT1-transfected (D) and OCT2-transfected (E) cells (○ and □) and in the corresponding empty vector–transfected cells (MOCK cells; ● and ■) in the presence (□ and ■) or absence (○ and ●) of an OCT inhibitor (disopyramide for OCT1 and cimetidine for OCT2) was also simultaneously determined in a similar fashion. When the OCT inhibitors were used, disopyramide (150 μmol/L) or cimetidine (1.5 mmol/L) was added to the incubation medium immediately before the addition of oxaliplatin. Lines, predicted data obtained by fitting the observed data using WinNonlin. Data from a typical experiment. Three to six independent experiments were done, and similar results were obtained. For clarity, the bars (SD) in (D) and (E) were eliminated.
Platinum Accumulation Rates in Cells after Exposure to Cisplatin, Carboplatin, and Oxaliplatin
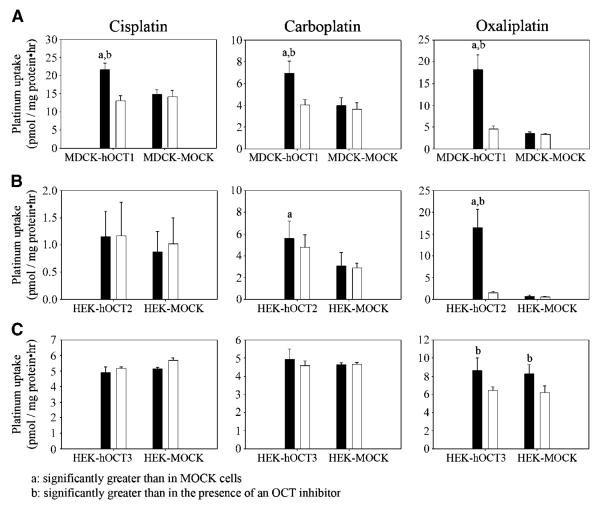
The cellular platinum accumulation rate after 2 hours of exposure to oxaliplatin (6 μmol/L) in MDCK-hOCT1 cells [18.2 ± 3.40 pmol/(mg protein-hour)] was 5.6-fold (P < 0.001) of that in MDCK-MOCK cells [3.60 ± 0.267 pmol/(mg protein-hour); (Fig. 3A)]. Coincubation with disopyramide (150 μmol/L) resulted in a 3.96-fold decrease in the rate of platinum accumulation in MDCK-hOCT1 cells [control versus disopyramide treated, 18.2 ± 3.40 pmol/(mg protein-hour) versus 4.59 ± 0.650 pmol/(mg protein-hour); P < 0.001] with little effect in MDCK-MOCK cells [control versus disopyramide treated, 3.60 ± 0.267 pmol/(mg protein-hour) versus 3.32 ± 0.254 pmol/(mg protein-hour); P > 0.05; Fig. 3A]. However, the cellular accumulation rates of platinum after 2-hour exposure to cisplatin (6 μmol/L) or carboplatin (20 μmol/L) in MDCK-hOCT1 cells [cisplatin, 21.6 ± 1.81 pmol/(mg protein-hour); carboplatin, 6.93 ± 1.14 pmol/(mg protein-hour)] were only slightly higher (<2-fold; P < 0.05) than those in MDCK-MOCK cells [cisplatin, 14.8 ± 1.37 pmol/(mg protein-hour); carboplatin, 3.97 ± 0.720 pmol/(mg protein-hour); Fig. 3A]. Coincubation of disopyramide (150 μmol/L) only produced a small decrease in the platinum accumulation rates after exposure of MDCK-hOCT1 cells to either cisplatin or carboplatin (<2-fold) with little effect in MDCK-MOCK cells (Fig. 3A). These results indicate that human OCT1 contributes substantially to the uptake of oxaliplatin but much less to the uptake of cisplatin and carboplatin in OCT1-transfected cells. The platinum accumulation rate in HEK-hOCT2 [16.5 ± 4.18 pmol/(mg protein-hour)] was markedly higher (23.9-fold; P < 0.001) than that in HEK-MOCK cells and was substantially reduced in the presence of cimetidine [control versus cimetidine, 16.5 ± 4.18 pmol/(mg protein-hour) versus 1.49 ± 0.348 pmol/(mg protein-hour)] after 2-hour exposure to oxaliplatin (0.3 μmol/L; Fig. 3B). However, the cellular accumulation rate of platinum in HEK-hOCT2 cells after 2-hour exposure to cisplatin (0.3 μmol/L) or carboplatin [10 μmol/L; cisplatin, 1.16 ± 0.464 pmol/(mg protein-hour); carboplatin, 5.59 ± 1.61 pmol/(mg protein-hour)] was only slightly higher (P > 0.05 for cisplatin; P < 0.05 for carboplatin) than that in HEK-MOCK cells. Coincubation with cimetidine (1.5 mmol/L) could not produce a significant decrease in platinum accumulation rate after exposure of HEK-hOCT2 to either cisplatin or carboplatin (Fig. 3B). These results indicate that OCT2 plays a critical role in the uptake of oxaliplatin in the transfected cells with a much lower effect on the uptake of cisplatin or carboplatin. In contrast to OCT1 and OCT2, OCT3 overexpression did not affect the uptake of any of these platinum drugs (Fig. 3C).
Figure 3.
Cellular accumulation rates of platinum after 2-hour exposure to cisplatin, carboplatin, and oxaliplatin. The cellular accumulation rates of platinum in OCT1-transfected (A), OCT2-transfected (B), and OCT3-transfected (C) cells and in the corresponding MOCK cells after incubation with cisplatin, carboplatin, and oxaliplatin in the presence (white columns) or absence (black columns) of an OCT inhibitor (disopyramide for OCT1 and cimetidine for OCT2 and OCT3) were determined as described in Materials and Methods. Briefly, MDCK cells (A) were incubated in the antibiotic-free medium containing cisplatin (6 μmol/L), carboplatin (20 μmol/L), or oxaliplatin (6 μmol/L) at 37°C and 5% CO2 for 2 hours. For the inhibitor studies, the incubation medium also contained disopyramide (150 μmol/L). B, HEK 293 cells were incubated in the antibiotic-free medium containing cisplatin (0.3 μmol/L), carboplatin (10 μmol/L), or oxaliplatin (0.3 μmol/L) at 37°C and 5% CO2 for 2 hours. For the inhibitor studies, the incubation medium also contained cimetidine (1.5 mmol/L). C, the study was done similarly as in (B), except that the concentrations of cisplatin, carboplatin, and oxaliplatin in the incubation medium were 2, 10, and 2 μmol/L, respectively. Columns, mean of six measurements for OCT1 and OCT2 and of three measurements for OCT3; bars, SD.
Platinum-DNA Adduct Formation after 2-Hour Exposure to Oxaliplatin
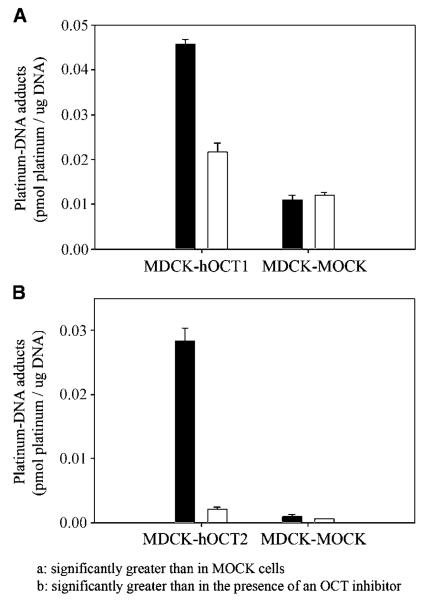
To determine whether the oxaliplatin taken up by cells via the human OCT1 and OCT2 transporters was available for DNA binding, we also measured platinum-DNA adduct formation after a 2-hour exposure to oxaliplatin (Fig. 4). The platinum-DNA adduct level in MDCK-hOCT1 cells [0.0457 ± 0.0011 pmol/μg DNA, ratio of bound platinum atoms per nucleotide (rb) = 1.51 ± 0.04 × 10−5] was 4.15-fold greater (P < 0.001) than that in MDCK-MOCK cells (0.0110 ± 0.0010 pmol/μg DNA, rb = 3.63 ± 0.33 × 10−6) after exposure to oxaliplatin (Fig. 4A). Coincubation with disopyramide (150 μmol/L) significantly decreased (2.11-fold; P < 0.001) platinum-DNA adduct formation in MDCK-hOCT1 cells (control versus disopyramide treated, 0.0457 ± 0.0011 pmol/μg DNA, rb = 1.51 ± 0.04 × 10−5 versus 0.0217 ± 0.0019 pmol/μg DNA, rb = 7.16 ± 0.63 10−6) with no effect in MDCK-MOCK cells. The platinum-DNA adduct level in HEK-hOCT2 cells (0.0284 ± 0.0020 pmol/μg DNA, rb = 9.37 ± 0.66 × 10−6) was 28.8-fold higher (P < 0.001) than that in HEK-MOCK after exposure to oxaliplatin (Fig. 4B) and was markedly reduced by cimetidine (0.00216 ± 0.00031 pmol/μg DNA, rb = 9.37 ± 0.66 × 10−6 versus 7.13 ± 1.02 × 10−7). Cimetidine produced only a small decrease (1.7-fold; P < 0.05) in HEK-MOCK cells.
Figure 4.
Platinum-DNA adduct formation after 2-hour exposure to oxaliplatin. The content of platinum bound to DNA after 2-hour exposure to oxaliplatin in the presence (white columns) or absence (black columns) of an OCT inhibitor (disopyramide for OCT1 and cimetidine for OCT2) was determined as described in Materials and Methods. Briefly, MDCK cells (A) were incubated in the antibiotic-free medium containing oxaliplatin (10 μmol/L) with or without disopyramide (150 μmol/L). B, HEK 293 cells were incubated in the antibiotic-free medium containing oxaliplatin (0.6 μmol/L) with or without cimetidine (1.5 mmol/L). After incubation at 37°C and 5% CO2 for 2 hours, the cells were washed and pelleted. The platinum content associated with genomic DNA was determined and normalized for DNA content. Columns, mean of typical experiment done in triplicate; bars, SD. Two independent experiments were conducted, and similar results were obtained.
Structure-Activity Relationships for Platinum-OCT Interactions
To investigate the structure-activity relationship for platinum-OCT interactions, the drug sensitivities (IC50) and resistance factor values of nine platinum complexes (including R,R-isomers and S,S-isomers for two compounds; Fig. 1) in both OCT-transfected cells and the corresponding MOCK cells were determined (Table 2A and B): the higher the resistance factor values indicates greater recognition by the OCT.
Table 2.
Drug sensitivity of platinum compounds
| A. Drug sensitivity of structurally diverse platinum complexes in OCT1-transfected cells | ||||||
| Platinum complexes | MDCK-MOCK (μmol/L) | MDCK-hOCT1 (μmol/L) | Resistance factor | |||
| Cisplatin | 6.3 ± 0.74 | 3.6 ± 0.30 | 1.7* | |||
| Carboplatin | 260 ± 86 | 230 ± 86 | 1.1 | |||
| [Pt(NH3)2(trans-1,2-(OCO)2C6H10)] | 21 ± 2.9 | 11 ± 2.7 | 2.0† | |||
| [Pt(en)Cl2] | 33 ± 12 | 10 ± 4.8 | 3.3 * | |||
| cis-[Pt(NH3)(Cy)Cl2] | 1.4 ± 0.15 | 0.16 ± 0.030 | 9.0† | |||
| Oxaliplatin | 11 ± 3.7 | 0.48 ± 0.19 | 22† | |||
| [Pt(S,S-DACH)oxalato] | 30 ± 14 | 1.4 ± 1.2 | 21† | |||
| [Pt(R,R-DACH)Cl2] | 15 ± 3.2 | 0.65 ± 0.26 | 23† | |||
| [Pt(S,S-DACH)Cl2] | 16 ± 3.7 | 0.57 ± 0.18 | 28† | |||
| B. Drug sensitivity of structurally diverse platinum complexes in OCT2-transfected cells | ||||||
| Platinum complexes | HEK-MOCK (μmol/L) | HEK-hOCT2 (μmol/L) | Resistance factor | |||
| Cisplatin | 2.6 ± 0.52 | 1.2 ± 0.54 | 2.1* | |||
| Carboplatin | 110 ± 46 | 62 ± 46 | 1.8 | |||
| [Pt(NH3)2(trans-1,2-(OCO)2C6H10)] | 19 ± 5.7 | 9.9 ± 2.8 | 1.9* | |||
| [Pt(en)Cl2] | 6.6 ± 1.5 | 1.1 ± 0.42 | 6.0† | |||
| cis-[Pt(NH3)(Cy)Cl2] | 0.22 ± 0.043 | 0.020 ± 0.0065 | 11† | |||
| Oxaliplatin | 4.1 ± 1.69 | 0.11 ± 0.020 | 37† | |||
| [Pt(S,S-DACH)oxalato] | 9.0 ± 1.7 | 0.27 ± 0.062 | 33† | |||
| [Pt(R,R-DACH)Cl2] | 2.1 ± 0.28 | 0.074 ± 0.026 | 28† | |||
| [Pt(S,S-DACH)Cl2] | 4.5 ± 0.71 | 0.14 ± 0.041 | 33† | |||
| C. The sensitivity of the colon cancer cell lines to oxaliplatin and cisplatin in the presence or absence of cimetidine | ||||||
| Cell lines | Oxaliplatin |
Cisplatin |
||||
| Control | Cimetidine treated | Resistance factor |
Control | Cimetidine treated |
Resistance factor |
|
| HCT116‡ | 2.4 ± 1.4 | 19 ± 6.2 | 7.9† | 5.4 ± 1.3 | 10 ± 3.2 | 1.9* |
| HT29‡ | 4.6 ± 1.4 | 52 ± 19 | 11† | 12. ± 3.9 | 31 ± 11 | 2.5* |
| RKO‡ | 1.6 ± 0.56 | 9.7 ± 2.7 | 5.9† | 8.6 ± 2.4 | 13 ± 4.4 | 1.5 |
| SW620‡ | 2.8 ± 1.0 | 14 ± 2.8 | 5.0† | 13 ± 2.0 | 22 ± 4.9 | 1.8* |
| LS180‡ | 1.3 ± 0.41 | 8.4 ± 2.8 | 6.4† | 5.7 ± 1.8 | 8.3 ± 3.4 | 1.4 |
| DLD | 11 ± 6.0 | 71 ± 13 | 6.7† | 18 ± 7.6 | 32 ± 10 | 1.7§ |
NOTE: The IC50 values (μmol/L) of all the platinum complexes, except for carboplatin, after 7 hours of drug exposure were determined in parallel using a MTT assay as described in Materials and Methods (A and B). The data for carboplatin in (A) and (B) were taken from Table 1A and B, respectively, and were not determined simultaneously with the other compounds. The resistance factor was defined as the ratio of the mean IC50 value in the MOCK cells to that in the OCT-transfected cells. The IC50 values (μmol/L) of oxaliplatin and cisplatin in the colon cancer cell lines (7 hours of drug exposure) were determined in the presence or absence (control) of cimetidine (1.5 mmol/L) in parallel (C). The cell seeding density was 6,000, 8,000, 6,000, 15,000, 12,000, and 4,000 cells per well for HCT116, HT29, RKO, SW620, LS180, and DLD cells, respectively. When cimetidine (1.5 mmol/L) was used, it was added to the wells immediately before the addition of the platinum drugs. The resistance factor was defined as the ratio of the mean IC50 value in the presence to that in the absence of cimetidine. All the data are expressed as mean ± SD of six measurements, and each measurement was done in quadruplicate.
P < 0.01.
P < 0.001.
The IC50 value of oxaliplatin is significantly lower than that of cisplatin in the absence of cimetidine.
P < 0.05.
Platinum-OCT1 interaction
(a) Nature of the nonleaving group(s): resistance factor values <2 were obtained for platinum complexes with diamine nonleaving groups, including cisplatin, carboplatin, and [Pt(NH3)2(trans-1,2-(OCO)2C6H10)], indicating that platinum compounds with this purely inorganic nonleaving unit are poorly recognized by OCT1 (Table 2A). However, when the nonleaving group(s) contained an organic component, as in [Pt(en)Cl2], which has two methylene groups between the amine functionalities, the resistance factor value increased to 3.3. Moreover, with increasing size of the organic component of the nonleaving group(s), the interaction of a platinum compound with OCT1 increased. For example, the platinum compounds cis-[Pt(NH3)(Cy)Cl2], the R,R-isomers and S,S-isomers of oxaliplatin and [Pt(DACH)Cl2], which all have a 6-C cyclohexyl moiety as part of their nonleaving group, had high resistance factor values (9.0-28; Table 2A). Therefore, it seems that the composition of the nonleaving group(s) of a platinum compound is an important determinant of its interaction with OCT1. Lastly, different isomers of the DACH-substituted platinum complexes seem to interact similarly with OCT1. The R,R-isomers and S,S-isomers of oxaliplatin (R,R versus S,S, 22 versus 21) and [Pt(DACH)Cl2] (R,R versus S,S, 23 versus 28) have similar resistance factor values (Table 2A). (b) Nature of the leaving group(s): changes in the leaving group did not substantially alter the resistance factor values of platinum complexes. For example, all the DACH compounds (R,R-isomers and S,S-isomers of oxaliplatin and [Pt(DACH)Cl2]) had similar resistance factor values (21-28; Table 2A), although the leaving group of oxaliplatin (oxalate) is very different from that of [Pt(DACH)Cl2] (chloride; Table 2A). In addition, cisplatin, carboplatin, and [Pt(NH3)2(trans-1,2-(OCO)2C6H10)], all of which have different leaving groups but identical nonleaving groups, had similar resistance factor values (1.1-2.0; Table 2A). Moreover, a cyclohexane ring, when present in the nonleaving group(s) of a platinum complex, such as in those DACH compounds, markedly increases OCT1 interaction (resistance factor, 21-28) compared with diamine ligands (resistance factor, 1.1-2.0). However, when the cyclohexane ring was incorporated into the leaving group, as in [Pt(NH3)2(trans-1,2-(OCO)2C6H10)], it had no effect on the OCT1 interaction, the resistance factor value of [Pt(NH3)2(trans-1,2-(OCO)2C6H10)] being 2.0 (Table 2A).
Platinum-OCT2 interaction
The structure-activity relationship for platinum-OCT1 interactions determined above also applies to platinum-OCT2 interactions because similar patterns of resistance factor values were obtained in OCT2-overexpressing cells (Table 2B) as those in OCT1-overexpressing cells (Table 2A) for these platinum compounds.
Identification of the Chemical Form of Oxaliplatin That Is the Substrate(s) of OCT1
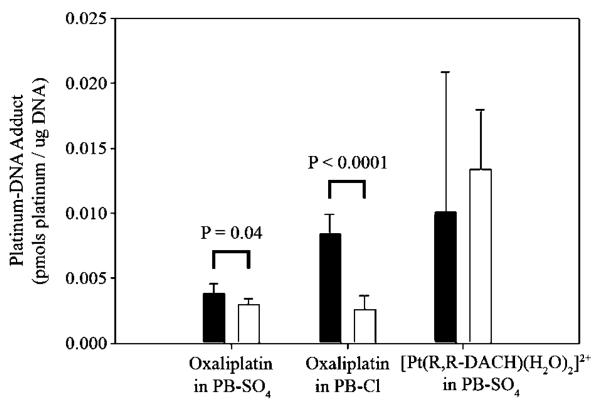
Multiple chemical species exist in equilibrium when platinum complexes are dissolved in an aqueous solution containing high concentrations of chloride ion (38, 39). Therefore, identification of the chemical species that are taken up by OCT1 would contribute to our understanding of the structure-activity relationship of platinum-OCT1 interactions. In chloride-containing media, such as plasma [[Cl−], ~103 mmol/L (39)] and our cell culture medium, the oxalate leaving group of oxaliplatin can be replaced by chloride, resulting in [Pt(R,R-DACH)Cl2]. The latter can be further aquated to form the monocationic [Pt(R,R-DACH)(H2O)Cl]+ and dicationic [Pt(R,R-DACH)(H2O)2]2+ species (40). The monoaqua and diaqua cations are the active forms of oxaliplatin, which bind to DNA. Considering the general properties of OCT substrates, which are positively charged small organic compounds, it is likely that the monoaqua and/or diaqua chemical species, having one or two positive charges, are the chemical forms taken up by OCT1.
To investigate experimentally the oxaliplatin-derived species taken up by OCT1, we first measured the platinum-DNA adduct formation in both MDCK-hOCT1 and MDCK-MOCK cells after incubation with oxaliplatin (20 μmol/L) in chloride-free buffer (PB-SO4). In this buffer, oxaliplatin should remain predominantly intact because the affinity of sulfate for platinum(II) is much lower than that of chloride (39). Displacement of the oxalate group by water will be a relatively slow process. In addition, we used short incubation times (25 minutes) to minimize conversion of oxaliplatin to intermediate aquated species. Under these conditions, the Pt-DNA adduct level in MDCK-hOCT1 cells (0.00384 ± 0.000765 pmol/μg DNA, rb = 1.29 ± 0.258 × 10−6) was only slightly higher (P = 0.04) than that in MDCK-MOCK cells (0.00297 ± 0.000435 pmol/μg DNA, rb = 1.00 ± 0.146 10−6; Fig. 5), suggesting that unmodified oxaliplatin may be not an OCT1 substrate. Secondly, to determine whether an aquated form of oxaliplatin was taken up by OCT1, we measured platinum-DNA adduct formation after incubation with oxaliplatin (20 μmol/L) in the chloride-containing buffer PB-Cl for 25 minutes. Under these conditions, it is likely that conversion to the monochloro/monoaqua cation will occur, with displacement of the oxalate ligand. The DNA-associated platinum level was substantially higher (3.27-fold; P < 0.0001) in MDCK-hOCT1 cells (0.00838 ± 0.00157 pmol/μg DNA, rb = 2.82 ± 0.529 10−6) than that in MDCK-MOCK cells (0.00256 ± 0.00109 pmol/μg DNA, rb = 0.862 ± 0.367 10−6; Fig. 5), consistent with this expectation. We also determined platinum-DNA adduct formation after direct incubation with the diaqua compound [Pt(R,R-DACH)(H2O)2]2+ (1 μmol/L) in the PB-SO4 buffer for 25 minutes. Under these conditions, the platinum complex will be a mixture of diaqua (83%) and aqua/hydroxo (17%) species. Here, the percentage was calculated based on the pKa values of 6.14 and 7.56 for the diaqua and aqua/hydroxo forms of oxaliplatin, respectively (41), and the pH value of 7.4 for the incubation buffer. We assumed no coordination of sulfate ion to platinum. The DNA-associated platinum level in MDCK-hOCT1 cells (0.0100 ± 0.0108 pmol/μg DNA, rb = 3.37 ± 3.63 10−6) was similar (P > 0.05) to that in MDCK-MOCK cells (0.0134 ± 0.00458 pmol/μg DNA, rb = 4.51 ± 1.54 10−6; Fig. 5), suggesting that the diaqua form is not an OCT1 substrate. Whether the aqua/hydroxo form, which carries one positive charge, can be taken up by OCT1 remains unclear. Taken together, these studies suggest that a monoaquated form of oxaliplatin, either the chloro or hydroxo species, both of which carry one positive charge, is the actual substrate of OCT1.
Figure 5.
Platinum-DNA adduct formation after incubation with oxaliplatin or [Pt(R,R-DACH)(H2O)2]2+ in PB-Cl or PB-SO4 buffer. MDCK cells were incubated with oxaliplatin (20 μmol/L) or [Pt(R,R-DACH)(H2O)2]2+ (1 μmol/L) in PB-Cl or PB-SO4 buffer at 37°C and 5% CO2 for 25 minutes. Oxaliplatin was freshly prepared and added to PB-SO4 buffer immediately and to PB-Cl buffer at least 0.5 hour before cell incubation. Columns, mean of six measurements; bars, SD.
Expression of OCT1 and OCT2 in Colon Cancer Cell Lines and Tissue Samples
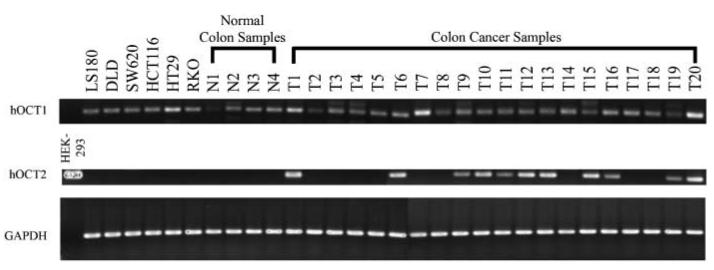
Because oxaliplatin is currently approved for advanced colon cancer therapy, we determined the expression of OCT1 and OCT2 in colon cancer cell lines and tumor samples. As shown in Fig. 6 and Supplementary Table S2, expression of OCT1 mRNA was detected in the six colon cancer cell lines tested in this study (LS180, DLD, SW620, HCT116, HT29, and RKO) and expression levels showed no substantial differences among these cell lines. Four normal colon tissue samples and 20 colon tumor samples exhibited variable OCT1 expression levels. OCT2 was not detected in any of the cell lines or in the normal colon tissue samples; however, 11 of the 20 tumor samples showed significant OCT2 expression (Fig. 6; Supplementary Table S2).
Figure 6.
Expression of OCT1 and OCT2 in colon cancer cell lines and colon tissue samples. Total RNA was isolated from colon cancer cells and normal or cancerous colon tissues. The expression of OCT1 and OCT2 in these samples was detected by RT-PCR as described in Materials and Methods. Forty and 30 cycles were used for amplifying OCTs and GAPDH, respectively.
The Effect of an OCT Inhibitor, Cimetidine, on Drug Sensitivity of Cisplatin and Oxaliplatin in Colon Cancer Cell Lines
To evaluate the potential role of OCT1 in the cytotoxicity of oxaliplatin and to determine whether OCT1 contributes to the differences in activities of cisplatin and oxaliplatin, we determined the sensitivities (IC50) of the colon cancer cells to both oxaliplatin and cisplatin in the presence or absence of an OCT inhibitor, cimetidine (1.5 mmol/L). The resistance factor due to the presence of cimetidine was defined as the ratio of the IC50 value in the presence of cimetidine to that in its absence. As shown in Table 2C, the sensitivity of cells to oxaliplatin was higher (lower IC50) than to cisplatin in each of the tested colon cancer lines in the absence of cimetidine [control, the mean ± SE of IC50 in the six cell lines, 3.9 ± 1.4 μmol/L (oxaliplatin) versus 11 ± 2.0 μmol/L (cisplatin)]. However, in the presence of cimetidine, sensitivity to oxaliplatin was substantially decreased in each of the cell lines [resistance factor values ranged from 5.0 to 11 (P < 0.001)], resulting in IC50 values comparable with, or even higher than, those of cisplatin [mean ± SE of IC50 in the six cell lines, 29 ± 11 μmol/L (oxaliplatin) versus 19 ± 4.3 μmol/L (cisplatin)]. The effect of cimetidine on cisplatin sensitivity was small (range of resistance factor values, 1.4-2.5; Table 2C).
Discussion
The striking activity of cisplatin in an otherwise fatal disease, testicular cancer, has been established by 30 years of clinical experience. However, acquired and intrinsic resistance limits its application to a relatively narrow range of tumor types. To broaden the anticancer spectrum of this platinum agent, thousands of structural analogues have been tested. Cisplatin analogues with two amine ligands, such as carboplatin and nedaplatin (approved in Japan), are cross-resistant with cisplatin (42). Analogues with different ligands display more diverse activity profiles (3). Notably, oxaliplatin, with DACH in place of the two amine ligands, in combination with 5-FU/leucovorin, produced response rates twice that of 5-FU/leucovorin regimens alone in the treatment of colorectal cancer (43), against which cisplatin is inactive (5). Efforts to understand the differences in oxaliplatin versus cisplatin antitumor activity have focused mainly on the cellular processing of cisplatin-DNA and oxaliplatin-DNA adducts (14, 15, 44). Defects in mismatch repair cause modest to moderate resistance to cisplatin but not to oxaliplatin (44, 45). Differences in the mechanism(s) controlling cellular uptake and efflux of these platinum compounds, although rarely investigated, may also contribute to their disparate activities considering the nature of their chemical structures.
In the present study, we observed that the influx transporters OCT1 and OCT2 play a critical role in the cellular uptake and consequent cytotoxicity of oxaliplatin (Table 1; Fig. 2). In contrast, the two transporters were relatively unimportant in mediating the uptake and cytotoxicity of cisplatin and carboplatin (Table 1). Overexpression of OCT1 and, more strikingly, OCT2 in transfected cells not only increased the rate of cellular platinum accumulation but also elevated the level of platinum-DNA adducts after oxaliplatin exposure (Figs. 3 and 4). These effects were blocked by known OCT inhibitors. The data strongly suggest that oxaliplatin is an excellent substrate of human OCT1 and OCT2, and the cellular uptake of platinum mediated by these transporters has ready access to the key pharmacologic target (DNA). These results are in contrast to platinum uptake mediated by human Ctr1, which seems to sequester the drug in some intracellular compartment, rendering it inaccessible to the pharmacologic target (18). It should be noted that a slight or modest increase in cisplatin and carboplatin uptake (Fig. 3A and B) was observed in MDCK-hOCT1 and HEK-hOCT2 cells compared with the corresponding MOCK cells, suggesting that cisplatin and carboplatin may be very weak substrates of human OCT1 and OCT2. A more significant interaction of cisplatin with OCT2 was obtained in a previous report (28) possibly due to higher OCT2 expression levels in the transfected cells used in that study. In contrast to the present observation, this work (28) concluded that oxaliplatin does not interact with human OCT2 based on the observation that oxaliplatin at 100 μmol/L could not inhibit the initial uptake of 4-[4-(dimethylamino)styryl]-N-methylpyridinium (a substrate of OCT2) in the OCT2-transfected HEK 293 cells. As discussed below, we showed that the chemical forms of oxaliplatin that actually interact with OCTs are most likely monoaquated species carrying one positive charge. These charged species represent only a minor fraction of all the species formed when oxaliplatin is dissolved in culture medium or PBS. Therefore, the failure to observe an oxaliplatin-OCT2 interaction in the previous study (28) may have been because the concentration(s) of charged species was too low to inhibit OCT2. Alternatively, oxaliplatin may interact with a binding site on OCT2 that is distinct from the N-methylpyridinium site; therefore, competitive inhibition may not have occurred.
It is noteworthy that expression of OCT1 or OCT2, even at low levels, may play a significant role in the cytotoxicity of oxaliplatin. We consistently observed a >3-fold increase (3.18-fold) in the IC50 value of oxaliplatin (Fig. 2E), but not of cisplatin or carboplatin (data not shown), in HEK-MOCK cells in the presence of the OCT inhibitor cimetidine. This result is most likely due to the inhibition of intrinsic OCT1 and/or OCT2 activity in HEK 293 cells by the OCT inhibitor. Both transporters were detected in HEK-MOCK cells in PCR studies using a cycle number of 40 (data not shown). Furthermore, cimetidine consistently produced a significant decrease in the cellular uptake of oxaliplatin, but not of cisplatin or carboplatin, in HEK-MOCK and HEK-hOCT3 cells (oxaliplatin is not a substrate of OCT3; Fig. 3B and C). The possibility that cimetidine reacts with the platinum compounds and inactivates them was checked by in vitro studies, which revealed no binding (data not shown). Moreover, this explanation is unlikely to be of primary importance because we would have expected to observe similar effects of cimetidine on the cellular uptake and cytotoxicity of cisplatin and carboplatin. Taken together, the data suggest that even low levels of expression of OCT1 and OCT2 play a significant role in sensitizing cells to oxaliplatin.
Structure-activity relationship studies revealed that the nature of the amine ligand bound to platinum is important for interaction with OCTs, with an organic component being required for effective interaction. On the other hand, the structure of the leaving ligand seems to be unimportant. Our work suggests that a monoaqua derivative of oxaliplatin, specifically the monoaqua/monochloride species and not a divalent diaqua complex, is likely to be the preferred substrate of OCT1 (Fig. 5). These results are probably applicable to OCT2 as well, and they are consistent with previous work showing that OCTs interact with small molecular weight monovalent organic cations (19). These studies establish a basis for the design of additional platinum complexes to facilitate the discovery of an even more detailed structure-activity relationship, which could be used to predict and optimize cellular internalization through the OCTs. We anticipate the potential to target platinum complexes for therapy against tumors that express OCT1 and OCT2.
Our structure-activity relationship studies further suggest that OCTs do not play a major role in determining the cytotoxicity of platinum compounds with two ammine ligands, such as cisplatin, carboplatin, and nedaplatin. In contrast, OCTs may be important for mediating cytotoxicity of platinum compounds with organic amine ligands (Table 2A and B). Cell lines that are resistant to cisplatin are cross-resistant to the bis(ammine) complexes carboplatin and nedaplatin but not to the DACH compounds oxaliplatin and tetraplatin, which share a similar activity profile (3, 42). The contrasting activity profiles of these compounds parallel the differences in their interaction with OCTs, suggesting that interactions with OCT1 and OCT2 may explain, at least in part, disparities in the activities and tumor specificities of platinum complexes.
It is likely that the activity of oxaliplatin in colorectal cancer can be explained, at least in part, by the selective uptake via OCTs. In this study, we detected OCT1 expression in all 20 human colon cancer tissue samples and OCT2 expression in 11 of 20 tissue samples (Fig. 6; Supplementary Table S2). Similar levels of OCT1 were also detected in the six tested human colon cancer cell lines, although OCT2 was not observable (Fig. 6; Supplementary Table S2). However, both OCT1 and OCT2 expressions have been detected in another human colon cancer cell line, Caco-2 (23, 26). The marked differences in OCT2 expression among these tumor samples do not seem to be related to gene amplification or differences in methylation of CpG rich sequences in the promoter region.4 As has been observed previously (3), sensitivity to oxaliplatin was greater than to cisplatin in each of the six colon cancer cell lines (Table 2C). The higher activity of oxaliplatin compared with that of cisplatin in these colon cancer cells is probably a consequence of the selective uptake of oxaliplatin mediated by the intrinsic OCT1 in these cells because similar activities of oxaliplatin and cisplatin were observed in these cells when OCTs were blocked by cimetidine (Table 2C).
Based on the expression of OCT1 and OCT2 in the colon cancer tissue samples and the OCT-dependent activity of oxaliplatin in the cell lines, it is reasonable to speculate that these transporters are important determinants of oxaliplatin activity in colorectal cancer. In addition, it is possible that variable expression of OCTs, especially OCT2, may account for the variability in response to oxaliplatin treatment. Further studies are required to determine whether expression levels of OCT1 and OCT2 may be used as markers for the rational selection of oxaliplatin-based versus irinotecan-based or other combination therapies for treatment of individuals with colorectal cancer. Such selection is now primarily based on side effect profiles or clinical experience (46). Oxaliplatin-based therapy may be a better choice for patients with high levels of OCT1 and OCT2 in their tumor samples. In addition, genotyping for nonfunctional and reduced function polymorphisms of OCT1 and OCT2 may be incorporated in the decision-making process (30, 47).
Currently, platinum-based therapies are used in the treatment of a variety of tumors, including testicular cancer, ovarian cancer, small cell lung cancer, and head and neck cancers (42). In these therapies, cisplatin is often the drug of choice because other platinum compounds, such as oxaliplatin, are not superior. However, our studies suggest that when OCT1 or OCT2 is expressed in the tumor, oxaliplatin may be a better choice. Our studies also suggest that, in addition to efflux transporters (48), influx transporters may play a significant role in determining tumor sensitivity/resistance to anticancer agents (49). Recently, OCT1 and OCT2 expression has been observed in several human cancer cell lines (26), suggesting that these transporters may be expressed in the corresponding tumors. The results of this study clearly suggest the need for further investigations to determine whether expression of OCTs can provide a basis for the rational selection of platinum-based therapies.
Acknowledgments
Grant support: NIH grants GM36780 and GM61390, National Cancer Institute grant CA 34992, and National Science Foundation Graduate Research Fellowship (K.S. Lovejoy).
We thank Drs. S.Y. Leung and S.T. Yuen (University of Hong Kong) for providing the colon cancer samples.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Unpublished data.
References
- 1.Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 2.Weiss RB, Christian MC. New cisplatin analogues in development. A review. Drugs. 1993;46:360–77. doi: 10.2165/00003495-199346030-00003. [DOI] [PubMed] [Google Scholar]
- 3.Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–65. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 4.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;10:1053–71. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 5.Misset JL, Bleiberg H, Sutherland W, Bekradda M, Cvitkovic E. Oxaliplatin clinical activity: a review. Crit Rev Oncol Hematol. 2000;35:75–93. doi: 10.1016/s1040-8428(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 6.Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780:167–80. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 7.Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci. 1995;20:435–9. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 9.Woynarowski JM, Chapman WG, Napier C, Herzig MC, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol. 1998;54:770–7. doi: 10.1124/mol.54.5.770. [DOI] [PubMed] [Google Scholar]
- 10.Page JD, Husain I, Sancar A, Chaney SG. Effect of the diaminocyclohexane carrier ligand on platinum adduct formation, repair, and lethality. Biochemistry. 1990;29:1016–24. doi: 10.1021/bi00456a026. [DOI] [PubMed] [Google Scholar]
- 11.Jennerwein MM, Eastman A, Khokhar A. Characterization of adducts produced in DNA by isomeric 1,2-diaminocyclohexaneplatinum(II) complexes. Chem Biol Interact. 1989;70:39–49. doi: 10.1016/0009-2797(89)90061-6. [DOI] [PubMed] [Google Scholar]
- 12.Takahara PM, Rosenzweig AC, Frederick CA, Lippard SJ. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature. 1995;377:649–52. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- 13.Spingler B, Whittington DA, Lippard SJ. 2.4 Å crystal structure of an oxaliplatin 1,2-d(GpG) intrastrand cross-link in a DNA dodecamer duplex. Inorg Chem. 2001;40:5596–602. doi: 10.1021/ic010790t. [DOI] [PubMed] [Google Scholar]
- 14.Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol Hematol. 2005;53:3–11. doi: 10.1016/j.critrevonc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Vaisman A, Lim SE, Patrick SM, et al. Effect of DNA polymerases and high mobility group protein 1 on the carrier ligand specificity for translesion synthesis past platinum-DNA adducts. Biochemistry. 1999;38:11026–39. doi: 10.1021/bi9909187. [DOI] [PubMed] [Google Scholar]
- 16.Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol. 2005;53:13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- 18.Holzer AK, Samimi G, Katano K, et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2004;66:817–23. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- 19.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 20.Wright SH. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol Appl Pharmacol. 2005;204:309–19. doi: 10.1016/j.taap.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol. 1997;51:913–21. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 22.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–81. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 23.Müller J, Lips KS, Metzner L, Neubert RHH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–60. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Verhaagh S, Schweifer N, Barlow DP, Zwart R. Cloning of the mouse and human solute carrier 22a3 (Slc22a3/SLC22A3) identifies a conserved cluster of three organic cation transporters on mouse chromosome 17 and human 6q26-27. Genomics. 1999;55:209–18. doi: 10.1006/geno.1998.5639. [DOI] [PubMed] [Google Scholar]
- 25.Gründemann D, Schechinger B, Rappold GA, Schömig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–51. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 26.Hayer-Zillgen M, Brüss M, Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2, and hOCT3. Br J Pharmacol. 2002;136:829–36. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briz O, Serrano MA, Rebollo N, et al. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–60. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- 28.Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–84. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–31. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Shu Y, Leabman MK, Feng B, et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A. 2003;100:5902–7. doi: 10.1073/pnas.0730858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 32.Samimi G, Safaei R, Katano K, et al. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res. 2004;10:4661–9. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- 33.Dhara SC. A rapid method for the synthesis of cis-[Pt(NH3)2Cl2] Indian J Chem. 1970;8:193–4. [Google Scholar]
- 34.Giandomenico CM, Abrams MJ, Murrer BA, et al. Carboxylation of kinetically inert platinum(IV) hydroxy complexes. An entree into orally active platinum(IV) antitumor agents. Inorg Chem. 1995;34:1015–21. doi: 10.1021/ic00109a004. [DOI] [PubMed] [Google Scholar]
- 35.Hoeschele JD, Farrell N, Turner WR, Rithner CD. Synthesis and characterization of diastereomeric (substituted iminodiacetato)(1,2-diaminocyclohexane)platinum(II) complexes. Inorg Chem. 1988;27:4106–13. [Google Scholar]
- 36.Kidani Y, Inagaki K. Antitumor activity of 1,2-diaminocyclohexane-platinum complexes against Sarcoma-180 ascites form. J Med Chem. 1978;21:1315–8. doi: 10.1021/jm00210a029. [DOI] [PubMed] [Google Scholar]
- 37.Al-Allaf TAK, Rashan LJ, Steinborn D, Merzweiler K, Wagner C. Platinum(II) and palladium(II) complexes analogous to oxaliplatin with different cyclohexyldicarboxylate isomeric anions and their in vitro antitumour activity. Structural elucidation of [Pt(C2O4)(cis-dach)] Transit Met Chem. 2003;28:717–21. [Google Scholar]
- 38.Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol. 2002;42:317–25. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 39.Howe-Grant ME, Lippard SJ. Aqueous platinum(II) chemistry; binding to biological molecules. Met Ions Biol Syst. 1980;11:63–125. [Google Scholar]
- 40.Di Francesco AM, Ruggiero A, Riccardi R. Cellular and molecular aspects of drugs of the future: oxaliplatin. Cell Mol Life Sci. 2002;59:1914–27. doi: 10.1007/PL00012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill DS, Rosenberg B. Syntheses, kinetics, and mechanism of formation of polynuclear hydroxo-bridged complexes of (trans-1,2-diaminocyclohexane)-platinum(II) J Am Chem Soc. 1982;104:4598–604. [Google Scholar]
- 42.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–34. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 43.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553–60. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 44.Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 45.Fink D, Zheng H, Nebel S, et al. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res. 1997;57:1841–5. [PubMed] [Google Scholar]
- 46.Goldberg RM. Advances in the treatment of meta-static colorectal cancer. Oncologist. 2005;10:40–8. doi: 10.1634/theoncologist.10-90003-40. [DOI] [PubMed] [Google Scholar]
- 47.Leabman MK, Huang CC, Kawamoto M, et al. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics. 2002;12:395–405. doi: 10.1097/00008571-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Szakacs G, Annereau JP, Lababidi S, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–37. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y, Anderle P, Bussey KJ, et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]