Abstract
Diurnal and nocturnal animals differ with respect to the time of day at which the ovulatory surge in luteinizing hormone occurs. In some species this is regulated by the suprachiasmatic nucleus (SCN), the primary circadian clock, via cells that contain vasoactive intestinal polypeptide (VIP) and vasopressin (AVP). Here, we evaluated the hypothesis that chronotype differences in the timing of the luteinizing hormone surge are associated with rhythms in expression of the genes that encode these neuropeptides. Diurnal Arvicanthis niloticus were housed in a 12:12 light:dark cycle and sacrificed at one of 6 times of day (Zeitgeber time 1, 5, 9, 13, 17, 21; ZT 0 = lights-on). In situ hybridization was used to compare levels of vip, avp, and VIP receptor mRNA (vipr2) in the SCN of intact females, ovariectomized females, ovariectomized females given estradiol, and intact males. We found a sex difference in vip rhythms with a peak occurring at ZT 13 in males and ZT 5 in intact females. In all groups avp mRNA rhythms peaked during the day, from ZT 5 to ZT 9, and had a trough in the dark at ZT 21. There was a modest rhythm and sex difference in the pattern of vipr2. Most importantly, the patterns of each of these SCN rhythms relative to the light:dark cycle resembled those seen in nocturnal rodents. Chronotype differences in timing of neuroendocrine events associated with ovulation are thus likely to be generated downstream of the SCN.
Keywords: circadian rhythm, grass rat, VIP receptor
Introduction
In female rodents mating and the preovulatory surge in luteinizing hormone (LH) occur several hours before the active period (Sodersten et al., 1981; de la Iglesia & Schwartz, 2006; Kriegsfeld & Silver, 2006). In nocturnal rats and hamsters this is around the time of lights-off whereas in the diurnal rodent, Arvicanthis niloticus (grass rat) this occurs around lights-on (McElhinny et al., 1999; Mahoney & Smale, 2005). In some nocturnal rodents the circadian time-keeping system regulates these events (Alleva et al., 1971) through the suprachiasmatic nucleus (SCN) (Weigand & Terasawa, 1982), the primary circadian pacemaker in mammals (Klein et al., 1991).
The SCN appears to coordinate the timing of the LH surge at least in part via rhythmic release of vasoactive intestinal polypeptide (VIP) and arginine vasopressin (AVP) (Perreau-Lenz et al., 2004) at synapses with gonadotropin releasing hormone (GnRH) neurons, and perhaps indirectly via projections to neurons containing kisspeptin or gonadotropin-inhibitory peptide (Kriegsfeld & Silver, 2006; Gibson et al., 2008). In rats and hamsters, GnRH neurons, which stimulate the release of LH, are contacted by VIP fibers (van der Beek et al., 1997), and SCN lesions eliminate approximately 80% of these connections (van der Beek et al., 1993). Disrupting VIP production delays and diminishes the amplitude of the LH surge (Harney et al., 1996); (van der Beek et al., 1999), and rhythms in vip mRNA are quite different in females and males, as is the ability to produce a surge (Becu-Villalobos et al., 1997; Krajnak et al., 1998b).
AVP also appears to mediate the circadian influence on LH secretion (Perreau-Lenz et al., 2004). AVP fibers contact GnRH neurons in the medial preoptic area of female rats and hamsters and in the supraoptic nucleus of female cynomolgus monkeys (Thind et al., 1991; Huhman & van der Beek, 1998; van der Beek et al., 1998). SCN lesions in lab rats reduce the number of these contacts (van der Beek et al., 1998) and the proestrous LH surge can be blocked by an intarcerebroventricular injection of an AVP receptor antagonist (Funabashi et al., 1999).
The role of these neuropeptides in the circadian regulation of reproductive events has not been characterized in diurnal species. Here, we used grass rats as a model to evaluate the hypothesis that chronotype differences in the timing of the LH surge arise from differences in temporal patterns of production of vip and/or avp in the SCN by determining whether these patterns (1) differ in female grass rats from those of female lab rats, (2) differ in males, which are unable to produce a surge, and females which do, and (3) are influenced by ovarian secretions essential for the surge to occur. In addition, we evaluated the possibility that vip might act differently within the SCN of these groups of animals by examining mRNA for one of its receptors.
Materials and Methods
Animals
All experiments were performed in compliance with Michigan State University All-University Committee on Animal Use and Care in accordance with the standard in the National Research Council Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used in these experiments. Adult male and female A. niloticus (>60 days) bred from a laboratory colony maintained at Michigan State University were singly housed, kept in a 12:12 light:dark cycle and provided food (Teklad rodent chow 8640, Harlan Industries, Madison WI, USA) and water ad libitum. A red light (< 5 lux) was left on continuously in each animal room. We used 4 groups of animals: adult males, adult intact females, ovariectomized (OVX) females, and ovariectomized females treated with estradiol benzoate capsules. Adult females were reproductively mature. No attempts were made to further assess their reproductive state as these animals, like many rodents, do not exhibit spontaneous estrous cycles. Vaginal smears and ovarian histology suggest that almost all females housed alone are in a continuously diestrous state (McElhinny, 1996). Brains from animals were collected at 6 time points: ZT 1, 5, 9, 13, 17, and 21. Sample sizes are listed in Table 1.
Table 1.
Sample sizes analyzed for each time point, group, and mRNA transcript. VIP= vasoactive intestinal polypeptide, VIPR2 = VIP receptor 2, AVP = arginine vasopressin. OVX = ovariectomized, E=Estradiol treatment.
| ZT | 1 | 5 | 9 | 13 | 17 | 21 | |
|---|---|---|---|---|---|---|---|
| VIP | intact female | 6 | 6 | 6 | 5 | 5 | 5 |
| OVX | 3 | 4 | 4 | 5 | 4 | 5 | |
| OVX+E | 3 | 4 | 4 | 4 | 3 | 3 | |
| Male | 5 | 4 | 4 | 5 | 5 | 4 | |
| VIPR2 | intact female | 6 | 6 | 6 | 5 | 5 | 5 |
| OVX | 3 | 4 | 4 | 5 | 4 | 5 | |
| OVX+E | 3 | 5 | 4 | 4 | 3 | 3 | |
| Male | 5 | 4 | 3 | 4 | 5 | 4 | |
| AVP | intact female | 6 | 6 | 6 | 5 | 5 | 5 |
| OVX | 4 | 4 | 3 | 5 | 4 | 5 | |
| OVX+E | 4 | 5 | 4 | 4 | 3 | 4 | |
| Male | 5 | 4 | 4 | 2 | 5 | 4 |
Females were bilaterally ovariectomized while under isoflurane anesthesia. Incisions were closed with subcutaneous sutures and treated with Nolvasan (Fort Dodge Animal Health, Fort Dodge IA, USA). Following ovariectomy animals were given saline (1 ml 0.9%, subcutaneously) and Buprenex (intramuscularly, Reckitt & Coleman, Richmond VA, USA) to minimize and control pain. Females recovered from surgery for a minimum of 10 days and all attempts were made to control the animal's pain during their recovery. For animals given estradiol capsules, the capsules were soaked in sterile saline overnight, and then soaked in sterile 100% ethanol for 2 hrs just prior to implantation. Females were given 2 subcutaneous silastic capsules containing estradiol benzoate (OVX+E; 180 μg/ml oil; 0.5 mm inner diameter 2.15 mm outer diameter, 20 mm long, Sigma-Aldrich, St. Louis MO, USA). Capsules were implanted at ZT 11 for OVX+E females that were sacrificed at ZT 1, 5, and 9 (after exposure to estradiol for 38, 42, and 46 hrs respectively) and at ZT 0 for estradiol-treated females sacrificed at ZTs 13, 17, and 21 (after exposure to estradiol for 37, 41, and 45 hrs respectively).
At the time of sacrifice, animals were injected with an overdose of pentobarbital (0.7 cc), decapitated, and their brains removed and frozen in an isopentane bath cooled with dry ice. Animals killed during the dark portion of the light:dark cycle were decapitated under red light. All efforts were made to minimize the pain experienced by these animals. Brains were cut into 4 series (12 μm) on a freezing cryostat. Coronal sections were collected from the medial septum to just past the caudal SCN. One tissue series was processed for in situ hybridization for the detection of vip, vipr2, or avp mRNA respectively.
RNA Probes
The probes for vip and vipr2 were prepared by RT-PCR from RNA extracted from whole A. niloticus hypothalamus. For vip we used a forward primer (5′-ACCCGCCTTAGAAAGCAAAT-3′) and reverse primer (5′-TCCTCAATTGCTACCCTTGC-3′) to amplify a fragment of 303 base pairs length of vip products (Genbank accession no. FJ750350). For vipr2 we used forward and reverse primers based on the vipr2 gene sequence for the mouse (Genbank accession no. BC138572.1) to amplify a 536 base pair fragment (Genbank accession no. FJ750351). Fragments were cloned into pCR II-TOPO vector (Invitrogen Carlsbad CA, USA) using the TOPO TA cloning system and following the manufacture's protocol. The amplified fragment was then sequenced to verify its identity and orientation. For vip, the cDNA fragments corresponded to rat (96%), mouse (96%), and Arvicanthis ansorgei (98%) published sequences. For vipr2 the cDNA fragments corresponded to 95% in rat and 96% in mouse. The 232 base pair rat vasopressin probe was a kind gift from Drs. S. Watson, A. Seasholz (University of Michigan, Ann Arbor MI, USA) and S. Bhatnagar (University of Pennsylvania, Philadelphia PA, USA). This probe corresponds to Exon C of the rat vasopressin gene (Genbank accession no. X01637.1). S35-labeled antisense probes were prepared from these templates. Purified linearized plasmid was incubated at 37°C for 1.5 hours in a transcription buffer that included either T7 or SP6 viral RNA polymerase, depending on orientation of inserts, and 35S-labeled UTP and CTP (MP Biomedicals, Solon OH, USA). The resulting probe was treated with DNase (Invitrogen, Carlsbad CA, USA) to prevent further polymerization. Free nucleotides were removed using Micro Bio-Spin Chromatography Columns (Bio-Rad Laboratories, Hercules CA, USA). For controls, the same procedures were done using a probe corresponding to the sense sequence of the respective genes.
In situ Hybridization
At the time of the in situ hybridization procedure, tissue from all animals (i.e. all time points, sexes, and hormonal conditions) was processed identically and in parallel. Slides were thawed and immediately fixed in 4% paraformaldehyde (freshly prepared, in 0.1M phosphate buffer pH7.3, Sigma-Aldrich, St. Louis MO, USA) for 1 hour, washed extensively in 2× saline-sodium citrate buffer, acetylated in 0.1M triethanolamine/0.25% acetic anhydride (Invitrogen Carlsbad CA, USA) for 10 min, and dehydrated through increasing concentrations of ethanol (50-100%). The S35-labeled probe was diluted in hybridization buffer (50% formamide, 10% dextran sulfate, 3× saline-sodium citrate buffer, 50mM sodium phosphate buffer, pH 7.4, 1× Denhardt's solution, 0.1 mg/ml yeast tRNA, and 10mM dithiothreitol; Invitrogen Carlsbad CA, USA) to yield an approximate concentration of 2.5 × 106 counts per million/80μl. Each slide was covered with 80ul of diluted probe and then coverslipped. The slides were then placed in plastic trays lined with filter paper soaked with 50% formamide. The trays were sealed and incubated at 55° Celsius overnight. Post-hybridization, coverslips were floated off in 2× saline-sodium citrate buffer, and slides were rinsed an additional three times in 2× saline-sodium citrate buffer at room temperature. Slides were then incubated in RNase A (200μg/ml; Invitrogen Carlsbad CA, USA) at 37°Celsius (1 hour). Slides were rinsed in decreasing concentrations of saline-sodium citrate buffer (2×, 1×, and 0.5×) at room temperature, and washed in a final stringency of 0.1× saline-sodium citrate buffer at 65°Celsius for 1 hour; cooled to room temperature in dH20, and dehydrated through increasing concentrations of alcohols (50-100%). Slides were exposed to x-ray film to obtain autoradiographs (Kodak Biomax-MR, Kodak, Rochester NY, USA).
Quantification
Autoradiographs of brain sections were captured digitally and used to analyze mRNA levels. For each animal, the hybridization signal was measured bilaterally from two sections representing the mid SCN, using a circular template. The mid SCN was defined using anatomical landmarks as previously described (Smale & Boverhof, 1999). The magnitude of the signal from the radioactive probes in single-labeled sections was determined using Scion Image (NIH, Bethesda MD, USA), which automatically determined signal above background in each brain region. A background value was obtained from the corpus callosum in each section, and only pixels with a mean gray value exceeding the background value by 2.5 standard deviations were included in the analysis. Results are expressed as the mean integrated density of signal pixels divided by the total number of pixels in the selected area that surpassed threshold, which we defined as optical density. All photography and analysis was performed by an experimenter blind to the identity of each slide.
Statistical analysis
We used an Analysis of Variance with time as the independent variable and signal as the dependent variable. A separate analysis was done for each group of animals (Male, intact female, OVX female, OVX+ E female). We used a 2×6 Analysis of Variance to examine the effects of sex (male to intact female) and time on mRNA signal. This analysis enabled us to compare the results from grass rats with those previously reported in lab rats (Krajnak et al., 1998b).We also used a 2×6 Analysis of Variance to determine the effects of estrogen treatment and time on hybridization signal by comparing OVX and OVX+E females. When significant main effects were found (P = 0.05) a Tukey post-hoc analysis was performed. When outliers were identified by the statistical program they were removed from analyses and graphs (SYSTAT v10, Systat Software Inc., Chicago IL, USA).
Results
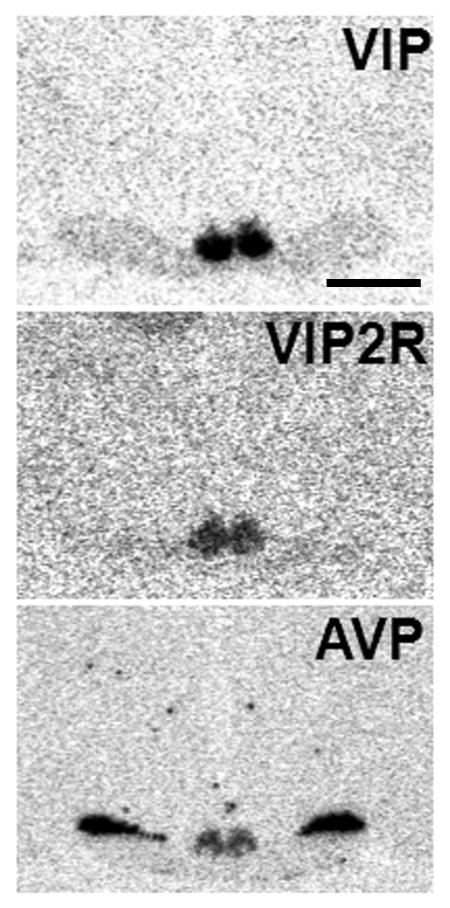
Representative images of radioactive in situ hybridization signals for each mRNA are shown in Figure 1. Hybridization for vip was most visible in the ventral portion of the nucleus while those for vipr2 and avp were concentrated in the medial and dorsal portion of the nucleus. We also saw relatively dark avp mRNA signal within the supraoptic nucleus. No labeling of the SCN was seen in tissue treated with sense control probes.
Figure 1.
Representative autoradiograms of the radioactive in situ hybridization for vip, vipr2 and avp in the SCN of the diurnal grass rat. Scale bar = 10 mm. VIP= vasoactive intestinal polypeptide, VIPR2 = VIP receptor 2, AVP = arginine vasopressin SCN = suprachiasmatic nucleus.
Vip
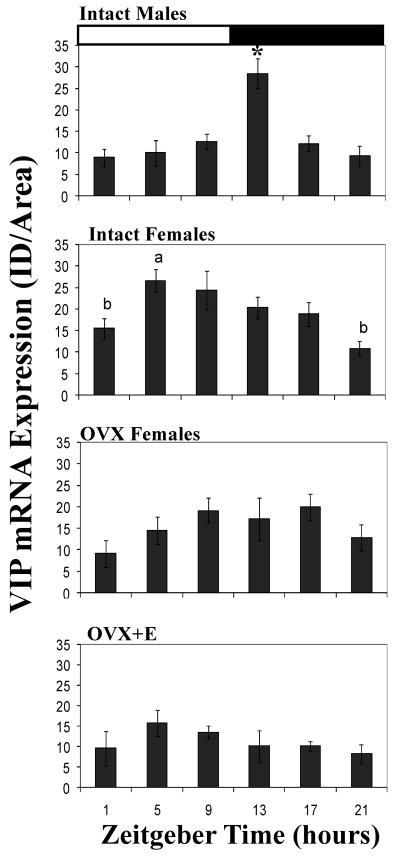
Males and intact females had a daily rhythm in the expression of vip mRNA signal. There was a significant effect of time on the signal of vip mRNA in both males (F5,21=9.4, P = 0.001) and intact females (F5,26=4.9, P = 0.003) (Figure 2). In males, the vip signal peaked just after lights-off (ZT 13) at which point it was significantly higher than at any of the other time points (P = 0.003 or less for all post hoc comparisons). In contrast, in intact females, the peak in signal occurred 8 hrs earlier, at ZT 5. Optical density was significantly higher at this point than at ZT 1 (P = 0.02) or ZT 21 (P = 0.001). When we compared intact females to males we found a significant interaction between time and sex (F5,47=5.39, P = 0.001).
Figure 2.
Daily patterns in the vip mRNA signal in the SCN of males, intact females, ovariectomized (OVX) females, and OVX females given 2 estradiol capsules for 2 days. Values are expressed as mean +standard error. White and black bars indicate the time of lights-on and lights-off respectively. *= significantly greater than all other timepoints (P =0.003). Within each graph, bars with different letters above them are significantly different from one another (P =0.02). See text for specific P values for each comparison. VIP= vasoactive intestinal polypeptide, VIPR2 = VIP receptor 2, AVP = arginine vasopressin SCN = suprachiasmatic nucleus.
OVX+E females did not have a significant rhythm in vip mRNA signal. The effect of time approached significance for OVX females (F5,18=2.7, P = 0.054). However, the 2×6 ANOVA revealed a main effect of hormone treatment such that OVX females had more signal than did OVX+E females (F5,36 =8.8, P = 0.005).
Vipr2
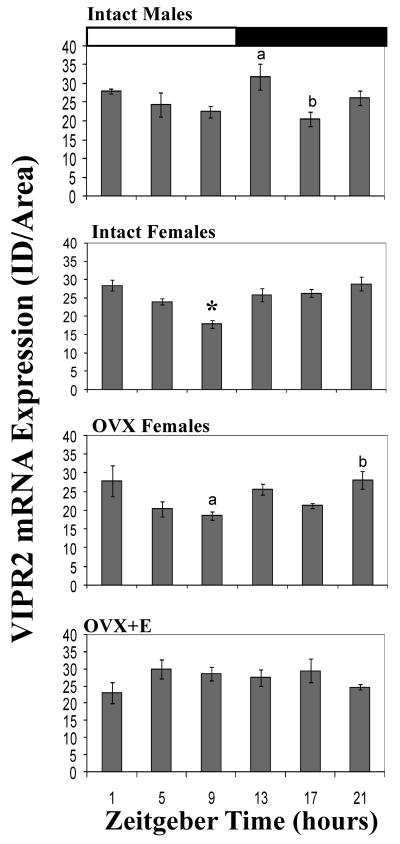
There was a significant effect of time on vipr2 mRNA in males (F5,19=3.25, P = 0.028) and intact females (F5,27=8.56, P = 0.001). In males, the peak of signal at ZT 13 was significantly greater than at ZT 17 (P =0.018). In contrast, in intact females, the signal of vipr2 mRNA at ZT 9 was significantly less than at every other time point (P = 0.03 or less for all comparisons). We found a significant interaction between time of day and sex (male vs intact female) with respect to the pattern of vipr2 signal across the day (F5,46=2.92, P = 0.023).
There was a significant effect of time on vipr2 mRNA in OVX females (F5,18=3.97, P = 0.013, Figure 3). OVX females were similar to intact females in that the vipr2 signal trough measured at ZT 9 was less than that at ZT 21 (P = 0.03). There was no main effect of time on the signal of vipr2 in OVX+E treated females. We found a significant interaction between steroid treatment and time of day when OVX and OVX+E females were compared (F5,36=3.523, P = 0.01). Specifically, OVX+E females had less signal overall when compared to OVX females, and the pattern of vipr2 expression across the day differed between OVX and OVX+E treated females.
Figure 3.
Daily patterns in the vipr2 mRNA signal in the SCN of males, intact females, ovariectomized (OVX) females, and OVX females given 2 estradiol capsules for 2 days. Values are expressed as mean +standard error. Within each graph bars with different letters above them are significantly different from one another (P =0.03). * = significantly less than all other timepoints (P =0.03). See text for specific P values for each comparison. VIP= vasoactive intestinal polypeptide, VIPR2 = VIP receptor 2, AVP = arginine vasopressin SCN = suprachiasmatic nucleus.
Avp
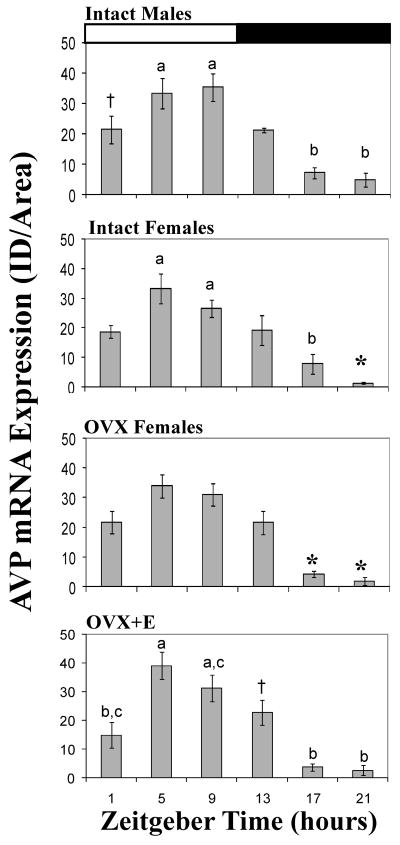
There was a daily rhythm in the expression of avp mRNA in all groups, and the patterns were the same across groups. There was a significant effect of time on avp signal in all groups (males: F5,18=11.36, P =0.001; intact females: F5,26=11.23, P =0.001; OVX females: F5,20=17.54, P =0.001; OVX+E females∷ F5,18=13.29, P =0.001). Post hoc analyses revealed a general pattern with peak signal occurring between ZT 5 and 9, and trough levels occurring at ZT 21 (Figure 4). In males, the levels of avp at ZT 5 and 9 were higher than at ZT 17 and 21 (P = 0.001 for each comparison). Additionally, ZT 1 was higher than ZT 21 (P = 0.05). For intact females the values at ZT 1, 5, 9, and 13 were significantly higher than at ZT 21 (P = 0.008 or less for each comparison). The signal measured at ZT 17 was also significantly lower than that at ZT 5 or 9 (P = 0.003 or less for each). In OVX females the signals of avp mRNA at ZT 1, 5, 9 and 13 were all significantly higher than that measured at either ZT 17 or 21 (P = 0.01 or less for all comparisons). Finally, in OVX+E females post-hoc analyses revealed that the signal of avp mRNA at ZT 5 was significantly greater than that measured at ZT 1, 17, or 21 (P = 0.004 or less for all comparisons). The mRNA levels at ZT 9 were also greater than at ZT 17 or 21 (P = 0.004 for both comparisons). Lastly, signal at ZT 21 was significantly lower than that at ZT 13 (P = 0.021) in these OVX+E females. No significant effect of sex was found between males and intact females nor was there an interaction between sex and time. Similarly, no effect of steroid hormone exposure (E caps) or interaction between treatment and time were found when OVX and OVX+E females were compared.
Figure 4.
Daily patterns in the avp mRNA signal in the SCN of males, intact females, ovariectomized (OVX) females, and OVX females given 2 estradiol capsules for 2 days. Values are expressed as mean +standard error. Within each graph bars with different letters above them are significantly different from one another (P =0.01). †=significantly greater than ZT 21 (P =0.05). *= significantly less than ZT 1, 5, 13, and 19 (P =0.01). See text for specific P values for each comparison. VIP= vasoactive intestinal polypeptide, VIPR2 = VIP receptor 2, AVP = arginine vasopressin SCN = suprachiasmatic nucleus, ZT= zeitgeber time.
Discussion
In nocturnal rodents AVP and VIP appear to influence the timing of physiological rhythms through their oscillatory production and release from cells within the SCN. These effects are likely to be mediated by projections from the SCN to other brain regions, as well as through interactions among SCN cells. Here, we evaluated the hypothesis that differences between day- and night-active species with respect to the timing of the LH surge could be produced by differences in patterns of avp, vip or vipr2 production in the SCN. Specifically, we obtained data on these patterns in diurnal grass rats that could be compared directly with published data on male and female lab rats. Additionally, we compared females that cannot produce an LH surge (OVX) with females that can (OVX + E). Our major findings were that the SCN of A. niloticus have (1) daily rhythms in the expression of vip mRNA that are influenced by hormones and are sexually dimorphic, (2) a robust daily rhythm of avp mRNA that is similar in males and females, and (3) and the phases of all of these rhythms are very similar to those seen in nocturnal species (Krajnak et al., 1998a; b; Kallo et al., 2004).
Daily rhythms in vip mRNA in the SCN
Male and intact female grass rats had daily rhythms in vip gene expression but the patterns of these rhythms were quite different (Fig. 2). In males, the peak occurred just after lights-off (ZT 13), whereas in intact females it occurred during the light portion of the light:dark cycle (ZT 5). It is likely that these patterns are regulated, at least in part, by estradiol secreted by the ovaries. In OVX females given estradiol the pattern of vip expression resembled that of intact females, with the highest levels occurring at ZT 5 (Fig. 2), although in this case the effect of time of day was not statistically significant. In comparison, OVX females had a rhythm with relatively high levels of vip mRNA during the dark portion of the light:dark cycle. Although this rhythm only approached significance (P=0.054), it resembled that of males more than those of females in the other two conditions.
The influence of ovarian hormones observed here provides further support for the hypothesis that VIP signals originating in the SCN are involved in regulation of ovulation in some species. A variety of more general features of SCN function can be modified by the ovarian hormones that are essential for the production of the LH surge in nocturnal species. For example, in ovariectomized female rats, treatment with an estrogen advances the rhythm of the clock gene Per2 mRNA in the SCN (Nakamura et al., 2005) and increases connexin32 mRNA transcripts (Shinohara et al., 2001). Injection of OVX female rats with an estrogen at mid-day or at mid-night leads to significant decreases in Cry2 mRN within the SCN 24 h later (Nakamura et al., 2001). Ovariectomy of female rats reduces expression of the immediate early gene Fos in the SCN, an effect that is restored by treatment with estradiol (Peterfi et al., 2004). These effects of estrogens on gene expression patterns may be mediated through steroid receptor containing cells that project to the SCN (de la Iglesia et al., 1999). These effects may also be mediated by receptors within the SCN itself. Estrogen receptor alpha and beta, and/or their mRNAs, have been detected in the SCN of mice (Vida et al., 2008), rats (Cintra et al., 1986; Su et al., 2001), humans (Kruijver et al., 2003) and the Octodon degus, a diurnal rodent (Hummer, Mahoney and Lee, unpublished observations). Rising levels of estrogen on the afternoon of proestrous may influence the expression of mRNA and peptide rhythms within the SCN, perhaps by directly acting on steroid receptors located in this nucleus. If ovarian steroid hormones alter the expression of SCN genes in a sex-dependent manner, as is the case for vip, this may be one mechanism by which SCN output signals and high estrogen levels coordinate to trigger the preovulatory LH surge
Despite the fact that A. niloticus is a diurnal species, both the daily pattern and the sex difference in vip mRNA expression were similar to those reported for nocturnal lab rats and mice. Male rats have a peak at ZT 14 (Kallo et al., 2004) and in male mice (Mus musculus) the peak occurs at ZT 14.5 (Dardente et al., 2004) whereas in the current study the peak was at ZT 13 in male grass rats. By contrast, intact female A. niloticus had a peak in vip at ZT 5 and significantly lower levels of this transcript at the end of the evening and beginning of the light portion of the light:dark cycle (ZT 21 and 1, Fig.2), a pattern similar to that of female laboratory rats (Krajnak et al., 1998b). Interestingly, the rhythms in vip mRNA expression seen here differ somewhat from that reported for a related diurnal species, Arvicanthis ansorgei, in which the peak occurred at ZT 17 (Dardente et al., 2004). This would suggest that vip mRNA rhythms are quite different in these two closely related diurnal species. However, there are other possible explanations. The two studies differ with respect to the statistical analyses that were used to assess the rhythm patterns (cosinor analysis vs. ANOVA) and the time points sampled. In addition, the report on vip rhythms in A. ansorgei did not indicate the sex of the animals used (Dardente et al., 2004). The vip patterns of males and females are quite different in A. niloticus (current study), as well as in lab rats (Krajnak et al., 1998b) such that combining the data from the two sexes could obscure the patterns.
The similarity between the patterns of vip mRNA rhythms seen here and those reported in rats and mice suggest that this feature of SCN function is unrelated to chronotype. The timing of the LH surge is 12 hours out of phase in A. niloticus and lab rats (McElhinny et al., 1999), yet the time of the daily peak in vip mRNA is similar in females of these two species. These data thus suggest that differences in how targets downstream from the SCN respond to VIP are likely to contribute to differences in neuroendocrine rhythms of nocturnal rodents and grass rats.
Daily rhythms in vipr2 mRNA in the SCN
There was a modest rhythmic pattern of vipr2 mRNA in the SCN of both male and female grass rats (Fig. 3). In males the peak occurred immediately after lights-off (ZT 13). This pattern resembles that seen in male laboratory rats in which vipr2 levels decrease across the day, then peak at the time of lights-off (Shinohara et al., 1999; Kallo et al., 2004). In laboratory rats, however, vipr2 mRNA remains elevated throughout the night (ZT14-24) whereas in grass rats, it declined to a trough at ZT 17 before returning to high levels again at ZT 21. This finding was unanticipated as species differences in rhythm patterns within the SCN are not common (reviewed in (Smale et al., 2003), and it raises the question of whether vipr2 is functionally related to, or may even contribute to, the differences in behavioral or physiological rhythms expressed in males of these two species. In the SCN of intact female A. niloticus, vipr2 expression was lower at ZT 9 than at any other time point and otherwise did not change across the day (Fig. 3). We do not know if this is unusual as patterns of this mRNA transcript have not been characterized in females of any other species. Thus there may be a modest rhythm and sex difference in vipr2 expression, but these daily expression patterns need to be more fully characterized in addition to determining rhythmic expression of this receptor is functionally related to the ability to mount an LH surge.
Daily rhythms in avp mRNA in the SCN
Male and female A. niloticus had robust rhythms in avp mRNA expression across the light:dark cycle. In all four treatment groups, levels of transcript were high during the day (between ZT 5 and 9) and were relatively low in the late evening between ZT 17 and 21. This pattern is fundamentally similar to those of nocturnal lab rats (Krajnak et al., 1998b; Kallo et al., 2004), Siberian hamsters (Duncan et al., 1995), Syrian hamster (Duncan et al., 2001), mice, and another diurnal grass rat, A. ansorgei (Dardente et al., 2004). We did not detect any differences in the pattern of avp expression between groups of animals. However, the avp mRNA rhythm produced within the SCN could still influence the timing of the LH surge in female grass rats, as it appears to do in lab rats (Palm et al., 1999; 2001).
Conclusions
The current data reveal that rhythms in vip and avp mRNAs within the SCN are fundamentally similar in diurnal grass rats and nocturnal murid rodents, reinforcing the view that the SCN have more similarities than differences across species, even those whose temporal niche is very different. Indeed, the SCN of A. niloticus also resembles those of nocturnal rodents with respect to rhythms in expression of Fos (Schwartz et al., 2004) and clock genes (Ramanathan et al., 2006) as well as the SCN output signal prokineticin 2 (Lambert et al., 2005). Here, we have taken this a step further by showing that two additional output pathways, those involving vip and avp, are the same in A. niloticus as in nocturnal rodents. In the case of vip, the phase of the mRNA rhythm is similar in diurnal and nocturnal species even though the rhythms are different in males and females. Taken together, these data support the view that differences between diurnal and nocturnal species arise from differences in responsiveness to SCN signals. A recent elegant study of A. ansorgei has provided direct evidence that this is the case for rhythms in secretion of glucocorticoids (Kalsbeek et al., 2008). In the future, it will be important to determine whether these conclusions apply more generally to nocturnal and diurnal species in other taxonomic groups, as well as how differences in responsiveness to SCN signals might come about.
Acknowledgments
The authors would like to thank Ethan Ebner and Rebecca Demo for their help with the cloning the A. niloticus genes, Drs. Seema Bhatnagar, Audrey Seasholtz, and Stan Watson for providing the vasopressin clone. We also thank Nicole Timm, Anna Baumgras and Jessica Koch for technical assistance. This research was supported by NIH grant MH69518 (TL), NSF IBN-013097(LS), NIMH R01MH53433 (LS) and NIH T32 HD 07048 (MMM).
Abbreviations
- AVP
arginine vasopressin
- E
estradiol
- GnRH
Gonadotropin releasing hormone
- LH
luteinizing hormone
- OVX
ovariectomized
- SCN
suprachiasmatic nucleus
- VIP
vasoactive intestinal polypeptide
- VIPR2
VIP receptor 2
- ZT
Zeitgeber Time
References
- Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology. 1971;88:1368–1379. doi: 10.1210/endo-88-6-1368. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol. 1997;17:699–715. doi: 10.1023/A:1022542221535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Fuxe K, Harfstrand A, Agnati LF, Miller LS, Greene JL, Gustafsson JA. On the Cellular-Localization and Distribution of Estrogen- Receptors in the Rat Telencephalon and Diencephalon Using Monoclonal-Antibodies to Human Estrogen-Receptor. Neurochem Int. 1986;8:587–595. doi: 10.1016/0197-0186(86)90196-8. [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-a-immunoreactive neurones project to the suprachiasmatic nucleus of the female syrian hamster. J Neuroendocrinol. 1999;11:481–490. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Cheng X, Heller KS. Photoperiodic exposure and time of day modulate the expression of arginine vasopressin mRNA and vasoactive intestinal peptide mRNA in the suprachiasmatic nuclei of Siberian hamsters. Brain Res Mol Brain Res. 1995;32:181–186. doi: 10.1016/0169-328x(95)00072-z. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett. 1999;260:37–40. doi: 10.1016/s0304-3940(98)00940-9. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Huhman KL, van der Beek EM. Peptidergic innervation of gonadotropin releasing hormone (GnRH) neurons in female Syrian hamsters. Society for Neuroscience City. 22:451.413. Year. [Google Scholar]
- Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pevet P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci. 2008;27:818–827. doi: 10.1111/j.1460-9568.2008.06057.x. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. Oxford University Press; New York: 1991. [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nucleus of female rats. J Neuroscience. 1998a;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998b;139:4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-beta distribution in the human hypothalamus: Similarities and differences with ERalpha distribution. J Comp Neurol. 2003;466:251–277. doi: 10.1002/cne.10899. [DOI] [PubMed] [Google Scholar]
- Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Smale L. A daily rhythm in mating behavior in a diurnal murid rodent Arvicanthis niloticus. Horm Behav. 2005;47:8–13. doi: 10.1016/j.yhbeh.2004.07.006. [DOI] [PubMed] [Google Scholar]
- McElhinny TL. Reproductive biology and biological rhythms in Arvicanthis niloticus. Department of Zoology. Michigan State University; East Lansing, Michigan: 1996. p. 126. [Google Scholar]
- McElhinny TL, Sisk CL, Holekamp KE, Smale L. A morning surge in plasma luteinizing hormone coincides with elevated Fos expression in gonadotropin-releasing hormone-immunoreactive neurons in the diurnal rodent, Arvicanthis niloticus. Biol Reprod. 1999;61:1115–1122. doi: 10.1095/biolreprod61.4.1115. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–630. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Shinohara K, Funabashi T, Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res. 2001;41:251–255. doi: 10.1016/s0168-0102(01)00285-1. [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93:659–666. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 2001;901:109–116. doi: 10.1016/s0006-8993(01)02309-5. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Pevet P, Buijs RM, Kalsbeek A. The biological clock: the bodyguard of temporal homeostasis. Chronobiol Int. 2004;21:1–25. doi: 10.1081/cbi-120027984. [DOI] [PubMed] [Google Scholar]
- Peterfi Z, Churchill L, Hajdu I, Obal F, Jr, Krueger JM, Parducz A. Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience. 2004;124:695–707. doi: 10.1016/j.neuroscience.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Martinez GS, Schwartz MD, Smale L. Temporal and spatial distribution of immunoreactive PER1 and PER2 proteins in the suprachiasmatic nucleus and peri-suprachiasmatic region of the diurnal grass rat (Arvicanthis niloticus) Brain Res. 2006;1073-1074:348–358. doi: 10.1016/j.brainres.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience. 2004;127:13–23. doi: 10.1016/j.neuroscience.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63:262–267. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Nakamura TJ, Kimura F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett. 2001;309:37–40. doi: 10.1016/s0304-3940(01)02022-5. [DOI] [PubMed] [Google Scholar]
- Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. J Comp Neurol. 1999;403:190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Eneroth P, Hansen S. Neuroendocrine control of daily rhythms in rat reproductive behavior. In: Fuxe K, Wetterberg L, Gustafsson JA, editors. Steroid Hormonal Regulation of Brain. Pergamon; New York: 1981. pp. 301–315. [Google Scholar]
- Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor -alpha and -beta immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. NeuroReport. 2001;12:1955–1959. doi: 10.1097/00001756-200107030-00036. [DOI] [PubMed] [Google Scholar]
- Thind KK, Boggan JE, Goldsmith PC. Interactions between vasopressin- and gonadotropin-releasing-hormone- containing neuroendocrine neurons in the monkey supraoptic nucleus. Neuroendocrinology. 1991;53:287–297. doi: 10.1159/000125731. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Horvath TL, Weigant VM, van den Hurk R, Buijs R. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotrophin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. Journal of Comparative Neurology. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Palm IF, Horvath TL, Kastelijn J, Wiegant VM. Gender specific apposition of SCN-derived vasopressin containing axons on GnRH neurons in the preoptic area of adult rats. Society for Neuroscience City. 24:545.511. Year. [Google Scholar]
- van der Beek EM, Swarts HJ, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology. 1999;69:227–237. doi: 10.1159/000054423. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotropin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20:1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- Weigand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]