Abstract
Normal mouse marrow cells were stimulated by stem cell factor (SCF) to form dispersed or multicentric blast colonies containing progenitor cells committed to various hematopoietic lineages. Combination of the eosinophil-specific regulator interleukin 5 with SCF increased the frequency of colonies containing eosinophil-committed progenitor cells with multicentric but not dispersed blast colonies. Combination of thrombopoietin with SCF increased the frequency of colonies containing megakaryocyte-committed progenitor cells with both types of blast colony. Neither interleukin 5 nor thrombopoietin significantly altered the number or total cell content of blast colonies or progenitor cell numbers in blast colonies from those stimulated by SCF alone. No correlation was observed between total progenitor cell content and the presence or absence of either eosinophil or megakaryocyte progenitors in either type of blast colony. The data argue against a random process as being responsible for the formation of particular committed progenitor cells or the possibility that lineage-specific regulators merely enhance survival of such committed progenitor cells formed in developing blast colonies.
During the continuing process of hematopoiesis (blood cell formation), multipotential hematopoietic stem cells generate progeny populations with progressively reduced differentiative and proliferative potential, culminating in the production of specialized mature cells in each of the eight major lineages of blood cells (1). A key question in this complex sequence of events is the manner in which multipotentiality is progressively restricted in progeny cells—whether this occurs on a wholly random basis or can be influenced by the action of extrinsic regulatory molecules.
The opportunities for analyzing this question experimentally are somewhat restricted because events need to be analyzed on a clonal basis in which the progeny of individual cells can be examined. Earlier studies using relatively mature bipotential granulocyte-macrophage progenitor cells suggested that an initial action of the colony-stimulating factors (CSFs)—granulocyte-macrophage-CSF (GM-CSF) or macrophage-CSF (M-CSF)—could alter the frequency of granulocyte- or macrophage-committed progeny produced by such cells (2). On the other hand, clonal analyses using less mature, multipotential blast colony-forming cells, surviving pretreatment with 5-fluorouracil, indicated that the pattern of differentiation commitment exhibited by the progeny appeared compatible with a random commitment process (3–6).
Colony formation in vitro by murine preprogenitor hematopoietic cells (blast colony-forming cells) (7) can be achieved by using stimulation by stem cell factor (SCF, kit-ligand) (8) and such colonies can be shown to contain a high proportion of lineage-committed progenitor cells (colony-forming cells) demonstrable by recloning individual blast colonies (9).
Earlier studies using this system documented that addition of Multi-CSF [interleukin 3 (IL-3)] to SCF resulted most evidently in the development of an increased frequency of blast colonies containing eosinophil-committed progenitors (9). The present experiments have extended such studies by using two regulatory factors with a highly lineage-restricted action, at least as assessed by their action on committed progenitor cells. These were thrombopoietin (TPO) with a selective action on megakaryocytic lineage cells (10) and IL-5 with a proliferative action restricted to eosinophil lineage cells (11). The question posed was whether the addition of either factor to SCF during blast colony formation would result in an increased frequency of developing blast colonies containing megakaryocyte- or eosinophil-committed progenitor cells? If so, could the results be attributed to induced commitment or merely to enhanced survival of committed progenitor cells that had been generated by some random process?
MATERIALS AND METHODS
Mice.
Mice used were 3-month-old C57BL males and females that had been bred and housed under specific pathogen-free conditions.
Cultures.
All cultures were prepared in 35-mm Petri dishes using 1 ml of DMEM with a final concentration of 20% newborn calf serum and 0.3% agar (Difco). Primary cultures were prepared containing 50,000 well-dispersed bone marrow cells and colony formation was stimulated by inclusion in the medium of 0.1 ml mouse tonicity PBS containing 100 ng purified recombinant murine SCF with or without 50 ng purified recombinant murine TPO or 10 ng purified recombinant murine IL-5. These recombinant proteins were produced and purified in this laboratory as described (12–14). Using a unit scale of 50 units/ml to denote a concentration stimulating the formation of 50% of maximal colony numbers, the specific activity of each recombinant product approximated 108 units/mg protein.
The cultures were allowed to gel at room temperature then incubated for 7 days at 37°C in a fully humidified atmosphere of 10% CO2 in air. Colony formation (clones > 50 cells) was scored initially in the unstained cultures at ×35 using an Olympus dissection microscope (SZIII). For full analysis, cultures were then fixed with 1 ml of 2.5% glutaraldehyde and, after 4 hr, the intact cultures were floated onto glass slides. After drying, the cultures were stained in sequence for acetyl cholinesterase (to identify megakaryocytes), Luxol fast blue (to identify eosinophils), and hematoxylin (to identify granulocytic and macrophage cells) (15). The stained cultures were mounted with coverslips by using DePeX mounting medium (BDH), then all colonies in each culture were analyzed at ×160 and ×320.
Recloning of Blast Colonies.
After 7 days of incubation, sequential probable blast colonies (usually three to five per culture) were identified that were not overlapping other colonies and, by using a fine pipette, individual intact colonies were transferred to 8 ml of agar medium. Colony cells were dispersed by pipetting and the cell suspension recultured in three pairs of 1-ml secondary cultures containing one or other of the following purified murine stimuli: 10 ng of GM-CSF (Schering), 10 ng of IL-3 (PeproTech, Rocky Hill, NJ), 10 ng of M-CSF, 100 ng of SCF, 100 ng of SCF plus 50 ng of TPO, 50 ng of TPO, or 10 ng of IL-5 (12–14). After 7 days of incubation, the secondary cultures were scored, then stained and analyzed as above. The numbers of progenitor cells of each type in a recloned colony were calculated from the mean number present in duplicate cultures after stimulation by a stimulus, or stimulus combination, that resulted in the formation of the highest numbers of that particular colony type. For the enumeration of eosinophil progenitor cells, secondary cultures were stimulated by IL-3, GM-CSF, or IL-5 and for enumeration of megakaryocyte progenitor cells by IL-3 plus TPO or IL-3. In many cases, M-CSF was used as the third stimulus to establish the maximum frequency of macrophage-committed progenitor cells.
Statistical Analyses.
Analyses of the statistical differences between data from different sets of blast colonies were performed by using the χ2 method.
RESULTS
As documented previously (8, 9), and shown by representative data in Table 1, in cultures of murine marrow cells, SCF stimulated the formation of blast colonies together with larger numbers of granulocytic colonies and small numbers of granulocyte-macrophage and macrophage colonies. TPO stimulated the formation exclusively of megakaryocytic colonies usually containing only mature megakaryocytes and IL-5 stimulated the exclusive formation of eosinophil colonies.
Table 1.
Colony formation stimulated by SCF, TPO, or IL-5
| Stimulus | Mean number of colonies
|
|||||
|---|---|---|---|---|---|---|
| Bl | G | GM | M | Eo | Meg | |
| SCF | 10 | 47 | 2 | 3 | – | – |
| TPO | – | – | – | – | – | 5 |
| IL-5 | – | – | – | – | 9 | – |
| SCF + TPO | 10 | 44 | 3 | 2 | – | 13 |
| SCF + IL-5 | 9 | 48 | 3 | 2 | 13 | – |
Figures are mean colony counts in duplicate 7-day cultures of 50,000 C57BL bone marrow cells stimulated by 100 ng of SCF, 50 ng of TPO, 10 ng of IL-5, or the latter factors combined with SCF, Bl, blast cell; G, granulocytic; GM, granulocyte–macrophage; M, macrophage; Eo, eosinophilic; Meg, megakaryocytic colonies.
Analyses revealed that SCF-stimulated colonies composed exclusively or predominantly of blast cells after 7 days of incubation were of three morphological types: irregularly shaped collections of loosely dispersed cells, multicentric colonies whose individual subcolonies were of irregular shape and size, and single compact, roughly spherical, colonies. Mean data from five experiments indicated that the relative frequencies of these were: dispersed, 19%; multicentric, 61%; and compact, 20%.
Central to the validity of data from blast colony recloning experiments is the accuracy with which blast colonies can be identified in unstained cultures. Because fixation and staining of intact cultures does not grossly alter the overall shape of hemopoietic colonies, stained cultures were able to be examined under the optical conditions (×35) used for selecting blast colonies for recloning. Candidate blast colonies were identified and marked, then the accuracy of identification was verified by examination at ×360. Analysis of cultures from eight separate experiments revealed that dispersed colonies could be identified with 100% accuracy, multicentric colonies with 90% accuracy (being confused sometimes with immature, multicentric, granulocytic colonies) and that single compact blast colonies could not be distinguished from immature granulocytic colonies with >50% accuracy. All recloning studies were therefore restricted to sequentially sampled dispersed and multicentric colonies.
Dispersed and multicentric blast colonies were of widely variable size in any one culture and overall colony size varied somewhat from one experiment to another. The mean number of cells in pooled dispersed colonies ranged from 350 to 1,300 and in pooled multicentric colonies from 1,760 to 6,900. Both colony types were identifiable as early as after 3 days of incubation and therefore appeared to initiate equally early in the culture period. Mapping analysis of the fate of dispersed colonies present at 5 or 7 days of incubation indicated that most evolved into multicentric colonies but with coronae of dispersed cells, because maturation occurred progressively in most colonies with extended culture. The slower growth rates and differing migratory behavior of cells in the two types of colony suggested that the two colony types might be generated by distinct subsets of preprogenitor cells and recloning data were analyzed separately. Selection of colonies for recloning was skewed to provide data from adequate numbers of dispersed colonies.
As shown in Tables 1 and 2, the addition of neither TPO nor IL-5 had a consistent effect on the number of blast colonies developing or on mean cell numbers in these colonies. The data suggested that these two variables could probably be discounted as likely to have had a major impact on data from recloning experiments.
Table 2.
Influence of thrombopoietin or interleukin-5 on the number or mean cell content of blast colonies stimulated by stem cell factor
| Experiment number | Stimulus
|
||
|---|---|---|---|
| SCF | SCF + TPO | SCF + IL-5 | |
| 1 | 13* | 18 | 15 |
| 2 | 25* | 25 | |
| 3 | 24* | 20 | |
| 4 | 14* | 17 | |
| 5 | 13* | 14 | |
| 6 | 12* | 11 | |
| 7 | 21* | 22 | |
| 1 | |||
| Dispersed | 1,310† | 810 | 1,170 |
| Multicentric | 6,220† | 6,680 | 6,960 |
| 2 | |||
| Dispersed | 250† | 440 | – |
| Multicentric | 1,760† | 2,470 | – |
Cultures of 50,000 C57BL bone marrow cells were stimulated for 7 days by 100 ng of SCF with or without 50 ng of TPO or 10 ng of IL-5. Data shown are mean blast colony counts from duplicate cultures or mean blast colony cell counts from pools of 10–30 colonies of each type.
*Blast colony number.
Mean cells per blast colony.
Progenitor Cells in SCF-Stimulated Blast Colonies.
Secondary culture of resuspended 7-day colonies stimulated by SCF revealed without exception the presence of lineage-committed progenitor cells in the colonies. The most frequent were macrophage progenitors (best detected by using M-CSF) with granulocytic or granulocyte-macrophage progenitors usually being present, but in lower numbers. Where eosinophil or megakaryocyte progenitors were present, these were in much lower numbers. For the detection of eosinophil progenitors, IL-3 GM-CSF and IL-5 were approximately equally effective stimuli. For the detection of megakaryocyte progenitors, IL-3 was usually effective, with the addition of TPO not usually improving detection efficiency. In only 8 of 150 (5%) recloned blast colonies were blast colony-forming cells detected, indicating that the level of self-renewal able to be exhibited by preprogenitor cells was low, when stimulated by SCF.
Effect of IL-5 on Eosinophil Progenitor Content of SCF-Stimulated Blast Colonies.
In stained cultures stimulated by SCF plus IL-5, no green-colored eosinophils were observed in any dispersed or multicentric blast colonies.
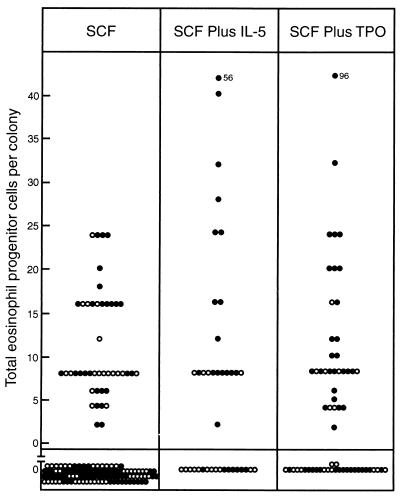
Fig. 1 shows the observed content of eosinophil progenitor cells in 149 sequential SCF-stimulated blast colonies and 39 blast colonies stimulated by a combination of SCF plus IL-5. Only 30% of multicentric and 29% of dispersed blast colonies stimulated by SCF contained eosinophil progenitor cells. In multicentric blast colonies stimulated by SCF plus IL-5 the percentage of colonies containing eosinophil progenitors was increased to 71%, a significant increase (χ2 = 11.6, P < 0.01). In contrast, in dispersed colonies stimulated by SCF plus IL-5, only 27% contained eosinophil progenitor cells, not differing from dispersed colonies stimulated by SCF alone. Where eosinophil progenitors were present in colonies initiated either by SCF or SCF plus IL-5, IL-5 was an equally effective proliferative stimulus for these cells from both multicentric and dispersed colonies. Addition of IL-5 to SCF did not consistently increase the number of eosinophil progenitors in positive colonies. In blast colonies initiated by SCF plus IL-5 there was no increase in the frequency of the uncommon bipotential, granulocyte-eosinophil, type of progenitor cell.
Figure 1.
Calculated total content of eosinophil progenitor cells in individual dispersed (○) and multicentric (•) 7-day blast cell colonies stimulated to develop by 100 ng of SCF alone or in combination with 10 ng of IL-5 or 50 ng of TPO.
Effect of TPO on Megakaryocyte Progenitor Cell Content of SCF-Stimulated Blast Colonies.
In stained cultures stimulated by SCF, no megakaryocytes were observed in blast colonies and in cultures stimulated by SCF plus TPO, megakaryocytic cells at varying stages of maturation were observed only occasionally in dispersed or multicentric blast colonies (9 of 194 colonies analyzed).
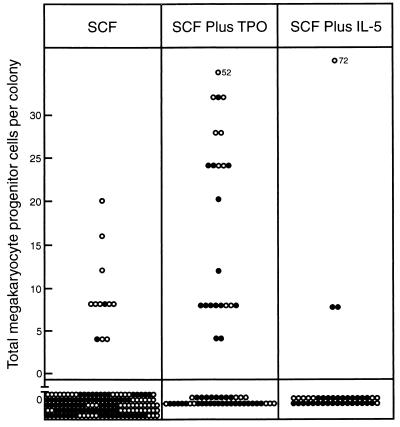
Fig. 2 shows an analysis of the megakaryocyte progenitor cell content of 150 SCF-stimulated blast colonies and of 62 blast colonies stimulated by SCF plus TPO. Only 2% of multicentric and 16% of dispersed blast colonies stimulated by SCF contained megakaryocytic progenitors. However, in multicentric blast colonies stimulated by SCF plus TPO 33% contained megakaryocyte progenitors (χ2 = 21.7, P < 0.01) and dispersed colonies also more frequently contained such progenitor cells (47% χ2 = 6.5, 0.01 < P < 0.02). There was a trend for increased numbers of megakaryocyte progenitor cells to be present in positive colonies stimulated by SCF plus TPO although there was a wide variation in actual cell numbers between different colonies. The majority of megakaryocytic progenitors in these colonies formed small colonies composed exclusively of mature megakaryocytes. It was remarkable that TPO, used as a single stimulus in secondary cultures of blast colonies, consistently failed to stimulate the formation of any megakaryocyte colonies, even with cells from those colonies in which the use of IL-3 showed megakaryocyte progenitors to be present. Finally, no secondary megakaryocytic colonies were observed that contained maturing cells of other lineages, despite the use of stimuli in the secondary cultures that should have been able to reveal such colonies.
Figure 2.
Calculated total content of megakaryocyte progenitor cells in individual dispersed (○) or multicentric (•) 7-day blast colonies stimulated to develop by 100 ng of SCF alone or in combination with 50 ng of TPO or 10 ng of IL-5.
Anomalous Actions of TPO and IL-5.
In Figs. 1 and 2, data are also presented from an analysis of whether IL-5 had an anomalous action resulting in an increased proportion of blast colonies with megakaryocyte progenitors and, conversely, whether TPO influenced colony content of eosinophil progenitor cells.
As shown in Fig. 2 only 8% of colonies stimulated to develop by SCF plus IL-5 contained megakaryocyte progenitor cells, a content not differing from the situation in colonies stimulated to develop by SCF alone.
In contrast, Fig. 1 shows that addition of TPO to SCF led to the presence of eosinophil progenitor cells in 60% of multicentric colonies, a significant increase over the content in colonies stimulated by SCF alone (χ2 = 10.0, P < 0.01). However, only 37% of dispersed colonies stimulated by SCF plus TPO contained eosinophil progenitors, a content not differing from dispersed colonies stimulated by SCF alone.
In murine bone marrow cultures, initial stimulation for 3–4 days by SCF, but not TPO, moderately enhanced the survival of IL-5-responsive eosinophil progenitor cells. Initial stimulation by SCF plus TPO did not further enhance the survival of these cells. Initial stimulation of marrow cultures by IL-5 for 3–4 days did not enhance the survival of TPO-responsive megakaryocyte progenitor cells (data not shown).
Total Progenitor Cell Content vs. Presence of Eosinophil- or Megakaryocyte-Committed Progenitor Cells.
Did the addition of a lineage-specific regulator stimulate the formation of blast colonies with an increased content of total progenitor cells? Related to this, did colonies containing eosinophil- or megakaryocyte-committed progenitors have a higher overall content of progenitor cells than other colonies? An analysis of these questions is given in Figs. 3 and 4. In the recloning experiments, M-CSF had not always been used in the secondary cultures. Overall progenitor cell numbers that include calculations from M-CSF-stimulated cultures are higher than those based on GM-CSF- or IL-3-stimulated cultures. Accordingly, the data have been presented separately in these figures and cross-comparisons need to be made by using the appropriate sets of data.
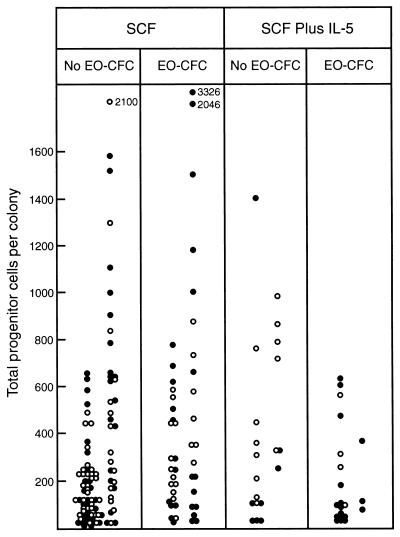
Figure 3.
Total progenitor cells in dispersed (○) or multicentric (•) 7-day blast colonies stimulated to develop by 100 ng of SCF alone or in combination with 10 ng of IL-5, grouped according to whether the colonies did or did not contain eosinophil progenitor cells (EO-CFC). Within each panel, total progenitor cells are grouped according to whether M-CSF was used as a stimulus in the secondary cultures (Right columns) or was not (Left columns).
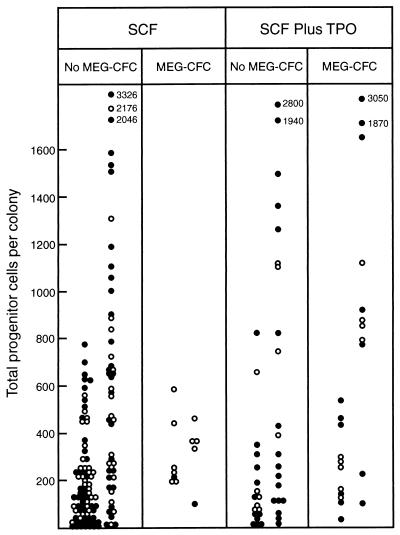
Figure 4.
Total progenitor cells in dispersed (○) or multicentric (•) 7-day blast colonies stimulated to develop by 100 ng of SCF alone or in combination with 50 ng of TPO, grouped according to whether the colonies did or did not contain megakaryocyte progenitor cells (MEG-CFC). Within each panel, total progenitor cells are grouped according to whether M-CSF was used as a stimulus in the secondary cultures (Right columns) or was not (Left columns).
Fig. 3 shows the data with respect to colonies developing with or without added IL-5. SCF-stimulated blast colonies containing eosinophil progenitor cells did have slightly higher overall progenitor cell numbers than those that did not contain eosinophil progenitors. This was only the case where M-CSF had not been used in the secondary cultures and even then the difference was slight (χ2 = 6.4, 0.01 < P < 0.02). Blast colonies stimulated by SCF plus IL-5 did not have higher progenitor cell numbers than those stimulated by SCF alone. Furthermore, SCF plus IL-5-stimulated colonies containing eosinophil progenitor cells did not have higher progenitor cell numbers than those that did not contain eosinophil progenitors— if anything, the opposite was the case.
Fig. 4 shows the comparable data on overall progenitor cell numbers in blast colonies with or without megakaryocyte progenitor cells. With SCF-stimulated blast colonies there was no evidence that megakaryocyte progenitors occurred preferentially in colonies with high overall progenitor cell numbers. Blast colonies stimulated by SCF plus TPO did not exhibit a higher content of progenitor cells although, differing from the situation with eosinophil progenitors, colonies stimulated by SCF plus TPO and containing megakaryocyte progenitor cells did appear to include a subset with higher overall progenitor cell numbers than the corresponding colonies stimulated by SCF alone.
In total, the data in Figs. 3 and 4 indicated that the presence of either eosinophil- or megakaryocyte-committed progenitors did not correlate overall with total progenitor cell numbers, that neither IL-5 nor TPO resulted in an overall increase in progenitor cells in blast colonies and that neither factor influenced the frequency of colonies containing relevant committed progenitor cells simply by increasing total progenitor cell numbers.
DISCUSSION
The formation of committed progenitor cells by hematopoietic stem or blast colony-forming cells is commonly described as occurring on a random basis (3–6). However, what was actually observed in the experiments used to reach this conclusion was the asymmetrical production by blast colony-forming cells of daughter cells with varying combinations of differentiation potential. If committed progeny are indeed generated on a random basis in this situation, it would be logical to expect that a correlation should exist between the total number of progenitor cells in a blast colony and the occurrence in the colony of progenitor cells that are committed to a particular lineage. In fact, what was observed in the present analysis of blast colonies stimulated to develop by SCF was that total progenitor cell numbers did not correlate with the presence of cells committed either to the eosinophil or megakaryocytic lineage. The present data therefore argue against the hypothesis of a simple random process as the basis for the formation of committed progeny. Blast colonies were markedly heterogeneous in their size and progenitor cell content and may well be heterogeneous in their capacity to generate one or other type of committed progenitor cell.
Addition of TPO or IL-5 did not significantly alter the size range or range of progenitor cell numbers of blast colonies stimulated to develop by SCF acting alone, but addition of TPO increased the frequency of colonies containing megakaryocyte-committed progenitor cells and addition of IL-5 the frequency of colonies containing eosinophil-committed progeny. In neither case was the absolute number of such committed progenitor cells significantly increased compared with numbers in those positive colonies arising after stimulation by SCF alone.
There are two possible interpretations of what was observed. The added lineage-specific factor may have increased survival of appropriate cells that had been generated on some other basis in the colonies. Alternatively, the added factor may have induced or facilitated commitment of some of the cells within a blast colony to a particular lineage. There are three features of the present data that are of potential relevance in considering these possibilities. (i) TPO, when acting alone, proved unable to stimulate the proliferation of committed megakaryocytic progenitor cells present in blast colonies. (ii) IL-5 was an effective proliferative stimulus for eosinophil progenitor cells arising in both dispersed and multicentric blast colonies but was able to increase the frequency of eosinophil progenitor cells only in multicentric and not in dispersed colonies. (iii) TPO had no proliferative or survival-enhancing action on eosinophil progenitor cells, yet TPO increased the frequency of blast colonies containing eosinophil progenitor cells.
All three features argue against the simple interpretation that TPO or IL-5 acted merely to support the survival of relevant committed progenitor cells that had been generated by some other process in developing blast colonies. The data seem more consistent with the possibility that these agents influenced the actual commitment process.
No information exists on the presence or absence of receptors for TPO or IL-5 on blast colony-forming cells and no studies appear to have been performed on the possible action of IL-5 on hematopoietic stem or multipotential progenitor cells. Neither agent is capable of stimulating blast colony formation in vitro, when acting alone on murine marrow cells, although TPO has been reported to stimulate blast colony formation by human cells (16). Studies on mice with inactivation of the gene encoding TPO or its receptor (mpl) (17, 18) indicate clearly that TPO strongly influences the numbers of stem cells, preprogenitor cells and progenitor cells of multiple lineages in vivo. When acting as a cofactor in vitro, TPO can enhance progenitor cell formation by more primitive cells (19). The possibility exists therefore that TPO, and possibly also IL-5, might have direct actions on preprogenitor cells of the type responding by blast colony formation when stimulated by SCF. In view of the evident heterogeneity of blast colony-forming cells, such an action might well be confined to only certain subsets of these cells.
The present data extend earlier information that combination of G-CSF or IL-6 with SCF did not perturb the differentiation pattern of progenitor cells in developing blast colonies (9, 20) but that combination with IL-3 increased the frequency of colonies containing committed eosinophil progenitor cells and combination with GM-CSF had a less marked action on the frequency of granulocyte-committed progenitor cells (9). In total, these observations suggest that extrinsic hematopoietic regulators can influence the generation by preprogenitor cells of particular types of committed progenitor cells, although the observed changes are quantitatively small when compared with the dominant background pattern of differentiation commitment occurring in these blast colonies.
In the development of the embryo and the first hematopoietic cells in the embryo, commitment has been established as being regulated by factors extrinsic to the cell, whether these represent cell position effects or gradients of inductive stimuli (21). It is reasonable to anticipate that this principle might continue to apply during differentiation commitment in adult hematopoietic cells. The interest of the present observations is that they provide evidence that extrinsic agents may be able to alter patterns of differentiation commitment in multipotential hematopoietic cells. This makes it reasonable to search for other agents, not merely hematopoietic growth factors but also agents influencing transcription of key nuclear transcription factors, that might influence in a more prominent manner the process of differentiation commitment.
Acknowledgments
The author is indebted to the careful technical assistance of Sandra Mifsud and Angela d’Amico throughout these studies. This work was supported by the Carden Fellowship Fund of the Anti-Cancer Council of Victoria, the National Health and Medical Research Council, Canberra, the AMRAD Corporation, Melbourne, and National Institutes of Health Grant CA-22556.
ABBREVIATIONS
- SCF
stem cell factor, IL, interleukin, TPO, thrombopoietin
- CSF
colony-stimulating factor
- GM-CSF
granulocyte-macrophage CSF
- M-CSF
macrophage CSF
References
- 1.Metcalf D. Nature (London) 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf D, Burgess A W. J Cell Physiol. 1982;111:275–283. doi: 10.1002/jcp.1041110308. [DOI] [PubMed] [Google Scholar]
- 3.Humphries R K, Eaves A C, Eaves C J. Proc Natl Acad Sci USA. 1981;78:3629–3633. doi: 10.1073/pnas.78.6.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakahata T, Gross A J, Ogawa M. J Cell Physiol. 1982;113:455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa M, Porter P N, Nakahata T. Blood. 1983;61:823–829. [PubMed] [Google Scholar]
- 6.Tsuji K, Nakahata T. J Cell Physiol. 1989;139:647–653. doi: 10.1002/jcp.1041390327. [DOI] [PubMed] [Google Scholar]
- 7.Metcalf D. Leukemia. 1998;12:1–3. doi: 10.1038/sj.leu.2400886. [DOI] [PubMed] [Google Scholar]
- 8.Metcalf D, Nicola N A. Proc Natl Acad Sci USA. 1991;88:6239–6243. doi: 10.1073/pnas.88.14.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf D. Proc Natl Acad Sci USA. 1991;88:11310–11314. doi: 10.1073/pnas.88.24.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushansky K, Broudy V C, Lin N, Jorgensen M J, McCarty J, Fox N, Zucker-Franklin D, Lofton-Day C. Proc Natl Acad Sci USA. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson C. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 12.Nicola N A, Robb L, Metcalf D, Cary D, Drinkwater C C, Begley C G. Blood. 1996;87:2665–2674. [PubMed] [Google Scholar]
- 13.Alexander W S, Roberts A W, Nicola N A, Li R, Metcalf D. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 14.Metcalf D, Willson T, Rossner M, Lock P. Growth Factors. 1994;11:145–152. doi: 10.3109/08977199409001056. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf D. The Hemopoietic Colony Stimulating Factors. Amsterdam: Elsevier; 1984. [Google Scholar]
- 16.Yoshida M, Tsuji K, Ebihara Y, Muraoka K, Tanaka R, Miyazaki H, Nakahata T. Br J Haematol. 1997;98:254–264. doi: 10.1046/j.1365-2141.1997.2283045.x. [DOI] [PubMed] [Google Scholar]
- 17.Kimura S, Roberts A W, Metcalf D, Alexander W S. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carver-Moore K, Broxmeyer H E, Luoh S-M, Cooper S, Peng J, Burstein S A, Moore M W, de Sauvage F J. Blood. 1996;88:803–808. [PubMed] [Google Scholar]
- 19.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Blood. 1996;87:4544–4551. [PubMed] [Google Scholar]
- 20.Metcalf, D. (1993) Stem Cells (Dayton) 11 (Suppl. 2), 1–11. [DOI] [PubMed]
- 21.Zon L I. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]