Abstract
Hair follicle stem cells (HfSCs) play crucial roles in hair follicle morphogenesis and hair cycling. These stem cells are self-renewable and have the multi-lineage potential to generate epidermis, sebaceous glands, and hair follicle. The separation and identification of hair follicle stem cells are important for further research in stem cell biology. In this study, we report on the successful enrichment of rat hair follicle stem cells through vario magnetic activated cell sorting (Vario MACS) and the biological characteristics of the stem cells. We chose the HfSCs positive surface markers CD34, α6-integrin and the negative marker CD71 to design four isolation strategies: positive selection with single marker of CD34, positive selection with single marker of α6-integrin, CD71 depletion followed by CD34 positive selection, and CD71 depletion followed by α6-integrin positive selection. The results of flow cytometry analysis showed that all four strategies had ideal effects. Specifically, we conducted a series of researches on HfSCs characterized by their high level of CD34, termed CD34bri cells, and low to undetectable expression of CD34, termed CD34dim cells. CD34bri cells had greater proliferative potential and higher colony-forming ability than CD34dim cells. Furthermore, CD34bri cells had some typical characteristics as progenitor cells, such as large nucleus, obvious nucleolus, large nuclear:cytoplasmic ratio and few cytoplasmic organelles. Our findings clearly demonstrated that HfSCs with high purity and viability could be successfully enriched with Vario MACS.
Keywords: hair follicle stem cell, stem cell purification, enrichment, surface markers
I. Introduction
The multipotent hair follicle stem cells (HfSCs) are responsible for the regeneration and reparation of epidermis, sebaceous gland and hair follicle [26, 29]. Hair follicle stem cells are self-renewable: when a stem cell divides, it generates another stem cell and a transient amplifying (TA) cell, which will replenish cell types in tissue after proliferation and differentiation [13]. Investigation of hair follicle stem cells is important and promising, in terms of its great application value in hair regenerative medicine and its prospective function as a model system where stem cell reprogramming, plasticity and characterization of niches can be studied [30].
It is evident that hair follicle stem cells are located in the bulge region of the outer root sheath of hair follicle [2, 4, 7, 19, 29, 32]: label-retaining cells (LRC) could only be found in the bulge region of hair follicle after mice were injected with tritiated thymidine (3H-TdR) and the label chased for an additional four weeks. These LRC cells, with slow-cycling nature, were relatively undifferentiated ultrastructurally [4]. Similar results were obtained when using 5-bromo-2-deoxyuridine (BrdU) and 3H-TdR double-labeling and chased for a much longer period [29]. Hair follicle stem cells could be divided into basal and suprabasal populations through fluorescence-activated cell sorting (FACS) method. These two distinct cell groups remained quiescent within niche and could be stimulated to self-renew and terminally differentiate into all lineages of epidermis and hair in vitro [2].
By purifying and isolating hair follicle stem cells, it will be easier to eliminate the influence brought from other cells to HfSCs. This feature will benefit the further research on biological characteristics of our target cells, genes, and signaling pathway involved in stem cell fate definition. Furthermore, successful collection of a stem cell group with similar properties is prerequisite to cell line establishment. Convincing stem cell markers are crucial for cell isolation. Recently, the most compelling positive markers for murine hair follicle stem cells are Keratin 15, β1-integrin, α6-integrin and CD34 [2, 5, 18, 20, 31], while the main negative marker is CD71 [23]. Currently, adherence separation and immunity separation are the two main techniques for isolating hair follicle stem cells. Magnetic activated cell sorting (MACS) belongs to immunity separation and it has many advantages over other cell sorting methods, such as high efficiency, high cell viability and ease of operation [3, 11]. Nowadays, MACS techniques have been widely used in cell biology, clinical diagnostics, environment protection, and food security area [3, 6, 10], especially in nucleic acid and protein separation, cell fast isolation and tumor cells depletion [8, 34]. Meanwhile, MACS techniques are also popular in hematological system cell sorting [14].
Our study utilized HfSCs surface markers to design single labeling and double labeling separation strategies, and then effectively isolated and enriched rat hair follicle stem cells combined using Vario MACS technology. We demonstrated that the isolated stem cells were progenitor cells with high viability and reproductive activity.
II. Materials and Methods
Preparation of cell suspensions from bulge region of rat hair follicles
Nascent SD rats 7–8 days of age (Animal Centre, Third Military Medical School, Chongqing, China) were bred according to the institution guidelines and used in all our experiments.
Vibrissa skin tissues (8 rats each experiment) were washed with D-Hank’s balanced salt solution three times. Hair follicle and connective tissue sheath were first separated with injector needle and then incubated with 0.25% Dispase II (Roche, Basel, Switzerland) for 20 min at 37°C. After washing three times with D-Hank’s, hair follicles were separated from connective tissue sheath. The bulge region of hair follicles (about 1/3 from the top of the shaft) was cut and incubated with 0.25% trypsin (Invitrogen Corp., Burlington, Canada) at 37°C for 5 min, and then neutralized by serum (Hyclone, Beijing, China). After filtering the cell suspension through 50 µm nylon mesh filters, bulge cells were collected by centrifuging 250 g for 3 min and resuspending in buffer. The buffer was PBS with 2 mM EDTA (Amresco, Solon, OH, USA) and 0.5% bovine serum albumin (Sigma, Mainland, China).
Selected hair follicle stem cell with Vario MACS
Four isolation strategies were devised to enrich hair follicle stem cells: positive selection with single marker of CD34, positive selection with single marker of α6-integrin, CD71 depletion followed by CD34 positive selection, and CD71 depletion followed by α6-integrin positive selection. In our strategies, all the incubations were conducted at 4°C for 20 min. For CD34 single positive selection, bulge cells were firstly stained with PE-conjugated anti-rat CD34 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), washed with buffer, and incubated with anti-PE MicroBeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). After the cell suspension went through a MACS MS separation column (Miltenyi Biotec GmbH) which was placed in Vario MACS (Miltenyi Biotec GmbH), CD34 positive cells were enriched.
For α6 positive separation, bulge cells were incubated with anti-rat α6 monoclonal antibody (Serotec, Oxford, UK), washed, and then followed by the Goat anti mouse IgG FITC (Serotec, Oxford, UK) incubation. Next, the cells were incubated with anti-FITC MicroBeads (Miltenyi Biotec GmbH) and positive selection was performed.
As for CD71 and CD34 compound separation, bulge cells were firstly stained with FITC-conjugated anti-rat CD71 monoclonal antibody (Serotec, Oxford, UK), washed, and then incubated with anti-FITC MicroBeads (Miltenyi Biotec GmbH). CD71 negative cells were collected after the depletion experiments and followed by staining with the PE-conjugated anti-rat CD34 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing and incubating with anti-PE microbeads (Miltenyi Biotec GmbH) and positive selection, CD71 negative-CD34 positive cells were obtained. CD71 negative-α6 positive cells were selected by a similar selection procedure.
Cell culture and cell growth curve
The separated CD34bri cells and C34dim cells were planted into 48-well plates at a density of 5×103/well. Cells were cultured with K-SFM medium (Invitrogen, Carlsbad, CA, USA) in a 37°C, 5% CO2 tissue-culture incubator. After 24 hours in culture, three wells were trypsinized and counted at day 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11 days, respectively. The growth curve was plotted from the mean cell number at each time point.
Immunocytochemistry
CD34bri cells and C34dim cells cultured for 5 days were washed three times with PBS, fixed with 4% polyoxymethylene in PBS for 10 min, and then washed three times with PBS. To block peroxidase and nonspecific antibody binding, cells were incubated with 3% H2O2 at room temperature for 15 min, and followed by incubation with goat serum for 20 min at room temperature. After removing the serum, the cells were stained with primary antibodies overnight at 4°C. Primary antibodies and dilutions used were anti-α6-integrin monoclonal (1:150, Serotec), anti-β1-integrin monoclonal (1:100, Boster) and anti-CD34 monoclonal (1:100, Santa Cruz). PBS took the place of the primary antibody to set a control. Cells were washed three times for 5 min with PBS and incubated with a second antibody for 30 min at 37°C, washed, and then incubated with a third antibody and detected with a DAB kit. Cells with brown-yellow color in cytoplasm and cell membrane were considered to be positive cells.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
To observe cells with SEM (FEI Company, Hillsboro, OR, USA), hair follicle bulge cells primary cultured for 7 days, cells digested directly from bulge region, CD34bri cells and CD34dim cells were uniformly added on cover slips coated with polylysine. Twenty min later, the cover slips were washed with PBS, fixed with 2.5% glutaraldehyde and 1% osmic acid, dehydrated with graded alcohol, and then immersed in isoamyl acetate, dried and vacuum coating was performed.
For TEM (FEI Company) observation, CD34bri cells and CD34dim cells were fixed with 2.5% glutaraldehyde and 1% osmic acid, dehydrated with acetone, embedded with ethoxyline resin (Chenguang, Sichuan, China) and ultrathin sections were prepared.
FACS analysis
FACS analysis of the selected cells utilized the same antibodies described above for Vario MACS isolation. Bulge cells that did not stain with antibody were used as control. Sorted cells were analyzed with a FACSCalibur flow cytometer equipped with CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA) for 10,000–15,000 events.
Statistical analysis
All date were analyzed using SPSS (Windows version 10.0) software package. Results were expressed as mean±SEM and were representative of at least three separate experiments. P<0.05 was used to determine the statistical significance of the data.
III. Results
Enrichment of rat hair follicle stem cells by Vario MACS and the isolation efficiencies
In order to evaluate the isolation efficiencies and screen the best strategy, hair follicle stem cells were enriched from cell suspension digested from rat vibrissa follicle bulge region with MACS technology and immunofluorescence staining was conducted. Then, fluorescence intensities were detected by flow cytometry to identify our target cells and estimate the isolation efficiencies. For single marker isolation experiments, bulge region cell suspension without antibody staining was used as control, and more than 97% of the cells were negative cells (Fig. 1a, 1c). The positive rates of CD34bri cells and α6bri cells were 81.24% (Fig. 1b) and 100.00% (Fig. 1d), respectively. Although α6-integrin is known to be highly expressed by hair follicle stem cells, it is surprising that its positive rate in our enriched cells was as high as 100%. In an attempt to enrich purer hair follicle stem cells, we also performed double markers separation selections. Before staining with antibodies, a portion of bulge cells was taken out and set as the control (Fig. 1e, 1g). As expected, in CD34 and CD71 compound separation, most of the sorted cells expressed high levels of CD34 and low levels of CD71 (termed CD34briCD71dim), representing 82.54% of the total isolated cells. Ideal results could be also obtained when isolating stem cells with α6 and CD71 antibodies. The percentage of α6briCD71dim cells in isolated cells was 89.24%, a little higher than CD34briCD71dim cells. These data revealed that all four strategies had ideal effects.
Fig. 1.
Flow cytometric analysis of follicle stem cells separated by Vario MACS. (a–d) Single-color flow cytometric analysis of the selected follicle stem cells. X axis represents fluorescence intensity, and Y axis represents cell number. (a) Bulge region cell suspension without antibody staining was used as control. (b) CD34bri cells were isolated by Vario MACS. (c) Control for α6 separation. (d) α6bri cells were isolated by Vario MACS. (e–h) Two-color flow cytometric analysis of follicle stem cells selected by Vario MACS. Cells were divided into four subpopulations: a PEbri FITCdim cells; b PEbri FITCbri cells; c PEdim FITCdim cells; and d PEdim FITCbri cells. (e) Control. (f) CD71 and CD34 was detected with FITC (X axis) and with PE (Y axis). The majority of the isolated cells were CD34briCD71dim cells, characterized by high level expression of CD34 and low level of CD71. No CD34dimCD71bri cells were detected in our experiments. (g) Control for α6 and CD71 combined separation; (h) The α6briCD71dim cells were the main subpopulation of the separated cells.
The biological characteristics of CD34bri hair follicle stem cells
To compare the viability and proliferative capacity of the isolated cells, CD34bri cells and CD34dim cells were cultured and the growth curve was plotted. Cells adhered to the culture plastic within 24 hours after inoculation. Two days later, CD34bri cells began to form small colonies and the cells were round and small, with uniform morphology (Fig. 2a). As for CD34dim cells, more dead cells were found in this population and colonies were smaller than CD34bri cells (Fig. 2b). When cultured for five days, colonies of CD34bri cells reached a relatively large size, with more tight junction between cells (Fig. 2c). In contrast, CD34dim cells were hardly able to form large colonies and cells appeared to have different morphology (Fig. 2d). According to the cell growth curve, cells grew slowly in the first three days after inoculation, and began to proliferate quickly from the fourth day. From the fourth day, CD34bri cells grew much more quickly than CD34dim cells (P<0.05). At approximately the tenth day, CD34bri cells reached the peak of proliferation, two days later than CD34dim cells (Fig. 2e).
Fig. 2.
Culture of CD34bri cells and CD34dim cells with K-SFM medium and the cell growth curve. ×100 magnification. (a) CD34bri cells cultured for 2 days. (b) CD34dim cells cultured for 2 days. (c) CD34bri cells cultured for 5 days. (d) CD34dim cells cultured for 5 days. (e) CD34bri cells and C34dim cells were selected by Vario MACS and cultured in 48-well plates at a density of 5×103/well with K-SFM medium. After 24 hours in culture, three wells were trypsinized and counted everyday during an 11-day period. The growth curve was projected from the mean cell number at each time point.
Subsequently, we detected the expression of positive markers of hair follicle stem cells in our cultured cells. CD34, α6-integrin and β1-integrin were expressed in cytoplasm and cell membrane, and expression in CD34bri cells was much stronger than in CD34dim cells (Fig. 3). CD34 only had weak expression in CD34dim cells (Fig. 3f). Our results demonstrated that CD34bri cells had greater proliferation potential and higher colony-forming ability than CD34dim cells, representing the high levels of hair follicle stem cell markers that were expressed in our positive cells.
Fig. 3.
Identification of follicle stem cells separated by Vario MACS and cultured for 5 days. ×200 magnification. (a) Expression of α6-integrin in CD34bri cells. (b) Expression of β1-integrin in CD34bri cells. (c) Expression of CD34 in CD34bri cells. (d) Expression of α6-integrin in CD34dim cells. (e) Expression of β1-integrin in CD34dim cells. (f) Expression of CD34 in CD34dim cells.
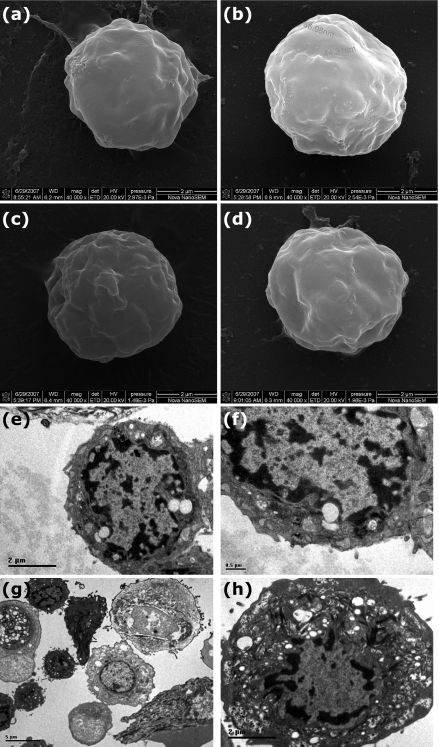
In addition, SEM and TEM were employed to observe the morphology and ultrastructures of these cells. SEM indicated that the CD34bri follicle stem cells were round in shape and with some MicroBeads attached to the cell membrane (Fig. 4b). In contrast, no MicroBeads adhered to the other three kinds of cells: cells digested directly from follicle bulge region, CD34dim cells, and follicle bulge cells primarily cultured for 7 days (Fig. 4a, 4c, 4d). TEM revealed that CD34bri cells had some typical characteristics as progenitor cells, such as big nucleus, obvious nucleolus, large nuclear:cytoplasmic ratio and few organelles in cytoplasm (Fig. 4e, 4f). Compare with CD34bri cells, CD34dim cells had smaller nuclear:cytoplasmic ratio (Fig. 4h). When observing CD34dim cells population with lower magnification, we found various cells types were contained in this cell group (Fig. 4g).
Fig. 4.
Observations of cells by SEM and TEM. (a–d) SEM images of cells. ×40000. (a) Cells digested directly from follicle bulge region. (b) MicroBeads could be clearly observed on CD34bri cell membrane selected by Vario MACS. (c) CD34dim cells selected by Vario MACS. (d) Follicle bulge cells cultured for 7 days. (e–h) TEM images of CD34bri cells and CD34dim cells. (e) CD34bri cells selected by Vario MACS. Bar=2 µm. (f) High magnification image of Figure e. Bar=0.5 µm. (g) Low magnification image of CD34dim cells. Bar=5 µm. (h) CD34dim cells selected by Vario MACS. Bar=2 µm.
IV. Discussion
Murine hair follicle stem cells inhabit the bulge area, a region situated below the opening of the sebaceous duct, that is approximately the attachment site of the arrector pili muscle and marks the bottom of the permanent portion of the follicle during cycling. More recently, other multipotent or unipotent stem cells have been identified in or immediately adjacent to the hair follicle epithelium, such as Nestin+ cells [1, 12], melanocyte stem cells [21, 22] and mesenchymal hair follicle stem cells [15]. Due to the location of these progenitor cells populations, the bulge region serves as a stem cell repository. It provides an ideal model system for the study of stem cell interaction; at the same time, it forms an obstacle to investigating the single stem cell type.
In this study, we devised and implemented four strategies to isolate HfSCs from the bulge region with Vario MACS. Thereafter, we characterized the selected CD34bri hair follicle stem cells and CD34dim cells. We cultured the isolated CD34bri cells and CD34dim cells, detected CD34, α6-integrin, β1-integrin in the two types of cells, and employed SEM and TEM to observe the morphology and ultrastructure of these cells.
The results of flow cytometry analysis showed that all the four strategies we used had ideal effects. Surprisingly, in the α6 positive selection, all the isolated cells highly expressed this marker. This may due to several reasons. First, only bulge regions of hair follicles were used to prepare cell suspension for isolation, and the density of stem cells in the suspension was relative high. Second, the anti-α6 antibody we utilized was without fluorescence, and the cells must be incubated with a secondary reagent before being detected for fluorescence with flow cytometry. This indirect labeling may result in non-specific binding and lead to high positive ratio ultimately. Third, it is possible that no single positive marker could efficiently identify stem cells from their progenies [16]. Therefore, double labeling was significant if we want to enrich purer hair follicle stem cells.
Tani et al. [28] demonstrated that combined used of α6 and CD71 could distinguish keratinocyte stem cells (KSCs) from TA cells. Consistently, Webb et al. [33] also proved that α6briCD71dim cells exhibited several stem cell characteristics, such as high clonogenic capacity and lacking the differentiation marker K10. In light of their studies, we implemented α6 and CD71 compound selection to separate hair follicle stem cells with Vario MACS. We also revealed that the combined use of CD34 and CD71 could successfully enrich HfSCs. Moreover, the CD34 and CD71 compound selection was superior to α6 and CD71 compound strategy in our experiments, considering the facility and precision of the direct labeling effect of the former strategy and the similar isolation efficiencies of the two methods.
Although double labeling could enrich high frequency HfSCs, these cells would suffer lower viability, as a result of the relatively complex protocol of compound selection. Thus, we chose CD34bri cells in our attempts to characterize HfSCs after comparing the viability and purity of cells selected by our four strategies. Trempus et al. [31] demonstrated that CD34 is a specific marker of bulge cell keratinocytes in the cutaneous epithelium. CD34 expression colocalized with both slowly cycling (label retaining) cells and keratin 15 expression. Our studies revealed that CD34bri cells had some typical characteristics of stem cell. Firstly, CD34bri cells had higher clonality and proliferative potential (from the fourth day, P<0.05), showing a longer growth period when cultured in compared with CD34dim cells. Furthermore, CD34bri cells could form larger colonies within ten days in culture. Secondly, the cultured CD34bri cells highly expressed hair follicle stem cell markers CD34, α6-integrin and β1-integrin. Thirdly, CD34bri cells were small and immature, with high nuclear:cytoplasmic ratio and few organelles in cytoplasm.
In conclusion, we have demonstrated here that Vario MACS is a favourable method to identify and separate viable hair follicle stem cells and the transient amplifying cells. The separation and purification of HfSCs will facilitate the application of these multipotent cells in tissue engineering and regeneration medicine. For example, HfSCs enriched from a patient’s own hair follicle can be exploited for the preparation of skin equivalents to treat burn victims, promoting the healing of leg ulcers, or regenerating new hair follicle for psilotic patients [9, 17, 24, 25, 27]. The capability to isolate HfSCs with high viability and purity is the prerequisite for these further applications.
V. Acknowledgments
This work was supported in part by the grants of the National Nature Science Foundation of China [Nos. 30671888], the Innovation and Attracting Talents Program for Colleges and University (“111 project”), the Key Natural Science Foundation of Scientific Committee of Chongqing of China [Nos. 2004796] and the “973” Project Foundation [Nos. 2005CB522703].
VI. References
- 1.Amoh Y., Li L., Yang M., Moossa A. R., Katsuoka K., Penman S., Hoffman R. M. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc. Natl. Acad. Sci. U S A. 2004;101:13291–13295. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanpain C., Lowry W., Geoghegan A., Polak L., Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Busch R., Cesar D., Higuera-Alhino D., Gee T., Hellerstein M. K., McCune J. M. Isolation of peripheral blood CD4(+) T cells using RosetteSep and MACS for studies of DNA turnover by deuterium labeling. J. Immunol. Methods. 2004;286:97–109. doi: 10.1016/j.jim.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J. Invest. Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 6.Deng M. Q., Lam K. M., Cliver D. O. Immnunomagnetic separation of Cryptosporidium parvum oocysts using MACS MicroBeads and high gradient separation columns. J. Microbiol. Methods. 2000;40:11–17. doi: 10.1016/s0167-7012(99)00127-x. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 8.Georgieva J., Milling A., Orfanos C. E., Geilen C. C. Magnetic bead RT-PCR: establishment of a new methods for detecting circulating melanoma cells. Melanoma Res. 2002;12:309–317. doi: 10.1097/00008390-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ghazizadeh S., Taichman L. B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goon P. K., Boos C. J., Stonelake P. S., Blann A. D., Lip G. Y. Detection and quantification of mature circulating endothelial cells using flow cytometry and immunomagnetic beads: a methodological comparison. Thromb. Haemost. 2006;96:45–52. doi: 10.1160/TH06-04-0185. [DOI] [PubMed] [Google Scholar]
- 11.Heng B. C., Cao T. Immunoliposome-mediated delivery of neomycin phosphotransferase for the lineage-specific selection of differentiated/committed stem cell progenies: potential advantages over transfection with marker genes, fluorescence-activated and magnetic affinity cell-sorting. Med. Hypotheses. 2005;65:334–336. doi: 10.1016/j.mehy.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman R. M. The pluripotency of hair follicle stem cells. Cell Cycle. 2006;5:232–233. doi: 10.4161/cc.5.3.2397. [DOI] [PubMed] [Google Scholar]
- 13.Jones P. H., Watt F. M. Separation of human epidermal stem cells form transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T., Minamiguchi H., Wang J., Kaneko H., Nakagawa H., Fujii H., Sonoda Y. Impaired stem cells function of CD34+ cells selected by two different immunomagnetic beads systems. Leukemia. 2004;18:566–574. doi: 10.1038/sj.leu.2403211. [DOI] [PubMed] [Google Scholar]
- 15.Lako M., Armstrong L., Cairns P. M., Harris S., Hole N., Jahoda C. A. B. Hair follicle dermal cells repopulate the mouse haematopoietic system. J. Cell Sci. 2002;115:3967–3974. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- 16.Li A., Simmons P. J., Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. U S A. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Z., Ghazizadeh S. Host immune responses in ex vivo approaches to cutaneous gene therapy targeted to keratinocytes. Exp. Dermatol. 2005;14:727–735. doi: 10.1111/j.1600-0625.2005.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D. R., Yang E. N., Lee S. T. A review: the location, molecular characterisation and multi-potency of hair follicle epidermal stem cells. Ann. Acad. Med. Singapore. 2004;33:784–788. [PubMed] [Google Scholar]
- 19.Morris R. J., Potten C. S. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Morris R. J., Liu Y., Marles L., Yang Z. X., Trempus C., Li S. L., Lin J. S., Sawicki J. A., Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura E. K., Jordan S. A., Oshima H., Yoshida H., Osawa M., Moriyama M., Jackson I. J., Barrandon Y., Miyachi Y., Nishikawa S. I. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura E. K., Granter S. R., Fisher D. E. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 23.Ohyama M., Terunuma A., Tock C. L., Radonovich M. F., Pise-Masison C. A., Hopping S. B., Brady J. N., Udey M. C., Vogel J. C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paus R. Therapeutic strategies for treating hair loss. Drug Discov. Today Ther. Strateg. 2006;341:491–497. [Google Scholar]
- 25.Richardson G. D., Arnott E. C., Whitehouse C. J., Lawrence C. M., Reynolds A. J., Hole N., Jahoda C. A. Plasticity of rodent and human hair follicle dermal cells: implications for cell therapy and tissue engineering. J. Investig. Dermatol. Symp. Proc. 2005;10:180–183. doi: 10.1111/j.1087-0024.2005.10101.x. [DOI] [PubMed] [Google Scholar]
- 26.Roh C., Tao Q., Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol. Genomics. 2004;19:207–217. doi: 10.1152/physiolgenomics.00134.2004. [DOI] [PubMed] [Google Scholar]
- 27.Stenn K. S., Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr. Opin. Biotechnol. 2005;16:493–497. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Tani H., Morris R. J., Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. U S A. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor G., Lehrer M. S., Jensen P. J., Sun T. T., Lavker P. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 30.Tiede S., Kloepper J. E., Bodò E., Tiwari S., Kruse C., Paus R. Hair follicle stem cells: Walking the maze. Eur. J. Cell Biol. 2007;86:355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Trempus C. S., Morris R. J., Bortner C. D., Cotsarelis G., Faircloth R. S., Reece J. M., Tennant R. W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 32.Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W. E., Rendl M., Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb A., Li A., Kaur P. Location and phenotype of human adult keratinocyte stem cells of skin. Differentiation. 2004;72:387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- 34.Weihrauch M. R., Skibowski E., Draube A., Geller A., Tesch H., Diehl V., Bohlen H. Immunomagnetic enrichment and detection of isolation tumor cells in bone marrow of patients with epithelial malignancies. Clin. Exp. Metastasis. 2002;19:617–621. doi: 10.1023/a:1020988227349. [DOI] [PubMed] [Google Scholar]