Abstract
The neurons in the trigeminal ganglion (TG) are surrounded by satellite glial cells (SGCs), which passively support the function of the neurons, but little is known about the interactions between SGCs and TG neurons after peripheral nerve injury. To examine the effect of nerve injury on SGCs, we investigated the activation of SGCs after neuronal damage due to the extraction of the upper molars in rats. Three, 7, and 10 days after extraction, animals were fixed and the TG was removed. Cryosections of the ganglia were immunostained with antibodies against glial fibrillary acidic protein (GFAP), a marker of activated SGCs, and ATF3, a marker of damaged neurons. After tooth extraction, the number of ATF3-immunoreactive (IR) neurons enclosed by GFAP-IR SGCs had increased in a time-dependent manner in the maxillary nerve region of the TG. Although ATF3-IR neurons were not detected in the mandibular nerve region, the number of GFAP-IR SGCs increased in both the maxillary and mandibular nerve regions. Our results suggest that peripheral nerve injury affects the activation of TG neurons and the SGCs around the injured neurons. Moreover, our data suggest the existence of a neuronal interaction between maxillary and mandibular neurons via SGC activation.
Keywords: satellite glial cells, trigeminal ganglion, neurons, glial fibrillary acidic protein
I. Introduction
Neuropathic pain caused by peripheral nerve injury is a common occurrence after tooth extraction [3, 26], but the mechanism underlying neuropathic pain is unclear. In response to oral nociceptive stimulation, the neurons of the trigeminal ganglion (TG) produce various neuropeptides, which are secreted retrogradely [28, 30] and modulate peripheral inflammation [8]. The TG consists of neuronal cells and two types of glial cells: satellite glial cells (SGCs) and Schwann cells [19]. SGCs, the support cells that surround neuronal cell bodies in the peripheral ganglia, carry numerous neuroactive molecules such as adenosine triphosphate (ATP) and bradykinin. They also receive signals from other cells and respond to changes in their environment. Therefore, glial cells directly influence neuronal activity by controlling the microenvironment in the ganglion [12, 20].
Neuropathic pain in the central nervous system (CNS) has been extensively investigated. In response to damage or inflammation, astrocytes release several inflammatory and immune mediators [2]. After peripheral nerve injury, glial fibrillary acidic protein (GFAP) immunoreactivity was found to increase in CNS astrocytes [7]. Additionally, neuron-glia interactions have been shown to be involved in all stages of inflammation and pain associated with several CNS diseases [27]. These findings indicate that glial cells are involved in neuropathic pain.
On the other hand, satellite glial cells have been suggested to play a role in neuropathic pain in the peripheral nervous system. A recent study revealed structural changes and an increase in GFAP immunoreactivity among the SGCs of the dorsal root ganglia after nerve axotomy [11, 29]. However, the role of neuron-glial cell communication in the TG after nerve injury is not well understood.
In this study, we examined the effect of nerve injury on the activity of SGCs in the TG by investigating the relationship between neuronal cell injury and SGC activation in the rat TG after upper molar extraction.
II. Materials and Methods
Animals
All experimental protocols involving rats were reviewed and approved by the Animal Care Committee of Kyushu Dental College. Twenty-four male Sprague-Dawley rats weighing 200–250 g each were used. The animals were acclimatized for at least 1 week prior to the start of the experiment.
Surgical procedures
The rats were anesthetized with an intramuscular injection of ketamine (70 mg/kg; Daiichi Pharmaceutical, Tokyo, Japan) and xylazine (13 mg/kg; Bayer, Tokyo, Japan). After sweeping up around the teeth, the right maxillary first and second molars were carefully extracted using dental forceps. After extraction, the rats were fed powdered food. Untreated animals were used as a control group.
Tissue preparation
At 3, 7, and 10 days post-extraction, the rats were anesthetized with diethylether and perfused transcardially with 4% paraformaldehyde in 0.2 M phosphate buffer (pH 7.4) containing 0.2% picric acid. The TGs were then removed and post-fixed in the same fixative overnight.
Immunohistochemistry
TGs were rapidly frozen then cut into sections 6 µm in thickness using a cryostat (Leica Instruments GmbH, Wetzlar, Germany). Double-labeling for GFAP and glutamine synthetase (GS), a satellite glial cell marker; protein-gene product 9.5 (PGP-9.5), a neuron marker; or activating transcription factor 3 (ATF-3), a marker of damaged neurons, was carried out as follows. First, the sections were preincubated in 0.1 M phosphate-buffered saline (PBS, pH 7.4) with 1% normal goat serum (ICN Pharmaceuticals, Aurora, OH, USA) for 30 min at room temperature. The sections were then incubated with mouse monoclonal antibodies against GFAP (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hr at 37°C. Negative control sections were incubated in diluent buffer alone. After rinsing with 0.1 M PBS, the sections were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:400; Molecular Probes, Eugene, OR, USA) for 2 hr at 37°C. After rinsing with 0.1 M PBS, the sections were incubated with rabbit polyclonal antibodies against GS (1:2,000; Sigma, St. Louis, MO, USA), guinea pig polyclonal antibodies against PGP-9.5 (1:500; Neuromics, Minneapolis, MN, USA), or rabbit polyclonal antibodies against ATF-3 (1:200; Santa Cruz Biotechnology) for 2 hr at 37°C, washed in PBS, and incubated with tetramethylrhodamine-5-isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG (1:400; Molecular Probes) or TRITC-conjugated goat anti-guinea pig IgG (1:400; Molecular Probes) for 2 hr at 37°C. Finally, the sections were washed in 0.1 M PBS and covered with coverslips.
Cell counts
Images were acquired under a fluorescence microscope (Olympus Optical, Tokyo, Japan) equipped with a CoolSNAP CCD camera (RS Photometrics, Tucson, AZ, USA) at 10×. Sections obtained from the TGs of three separate animals were analyzed. Images were obtained from three to four nonoverlapping areas within two randomly chosen sections of each TG, which allowed us to evaluate more than 75% of the cells in a given section. The images were obtained at a standard exposure time across the slides. The images were analyzed quantitatively using NIH ImageJ software (NIH, Bethesda, MD, USA). Positive SGCs were identified based on a pixel intensity value over the threshold (background pixel intensity ± 5 SD). Individual neurons were selected by means of the freehand outline tool in ImageJ on a pen tablet (XP-8060A; Active, Taipei, Taiwan). The outlined neurons were saved, and measurements were taken in the neuron area.
The total number of neurons enclosed by immunopositive SGCs in the maxillary nerve region was determined for each section. Only those neurons with visible nuclei were counted.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine the ratio of neurons with GFAP-immunoreactive (IR) SGCs, followed by individual post hoc comparisons (Scheffé).
III. Results
GFAP- and GS-IR SGCs
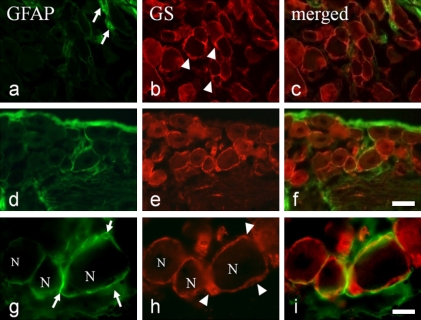
In the maxillary nerve region of the TG, GS-IR SGCs were distributed around the TG neurons (Fig. 1b, e, and h). In the controls, only a few GFAP-IR SGCs were localized around the TG neurons (Fig. 1a and c). At 7 days after extraction, a strong GFAP signal was observed around some TG neurons (Fig. 1d and g). Double-staining for GS and GFAP confirmed that each TG neuron was surrounded by a pair of elongated GFAP-IR SGCs (Fig. 1f and i). Control specimens that were incubated in the absence of primary antibody were found to have no specific staining (data not shown).
Fig. 1.
GFAP- and GS-immunoreactive (IR) satellite cells in the trigeminal ganglion (TG). (a, d, and g) GFAP- and (b, e, and h) GS-IR satellite cells in control rat TG (a–c) and at 7 days after tooth extraction (d–i). (c, f, and i) are merged images of (a, d, and g) and (b, e, and h). (g and h) show a higher magnification view of (d and e). Arrows: GFAP-IR satellite cells; arrowheads: GS-IR satellite cells, N: TG neuron. Bars=30 µm (f) and 10 µm (i).
GFAP-IR SGCs and PGP-9.5-IR neurons
Temporal changes in the GFAP-IR SGCs around PGP-9.5-IR neurons were examined in the maxillary nerve region of the TG. In the uninjured controls, only a few PGP-9.5-IR neurons were surrounded by GFAP-IR SGCs (Fig. 2a–c).
Fig. 2.
GFAP-immunoreactive (IR) satellite cells and PGP-9.5-IR neurons in the trigeminal ganglion. GFAP-immunopositive (IP) satellite cells (a, d, g, and j) and PGP-9.5-IP neurons (b, e, h, and k) in control rats (a and b) and in rats at 3 (d and e), 7 (g and h), and 10 days (j and k) after tooth extraction. (c, f, i, and l) are merged images of (a, d, g, and j) and (b, e, h, and k). Arrows indicate GFAP-IP satellite cells. Bar=30 µm.
Three days after extraction, some PGP-9.5-IR neurons were incompletely surrounded by GFAP-IR SGCs (Fig. 2d–f). By day 7, the GFAP signal became stronger and some PGP-9.5-IR neurons were surrounded by GFAP-IR SGCs (Fig. 2g–i). On day 10, intense GFAP signals were observed around the PGP-9.5-IR neurons (Fig. 2j–l).
Since GFAP is expressed in satellite glial cells without stimulation, we examined the proportion of PGP9.5-IR neurons in the TG surrounded by GFAP-IR SGCs (GFAP/PGP-9.5 neurons) by double-staining for GFAP and PGP9.5. After extraction, the proportion of GFAP/PGP-9.5 neurons had significantly increased compared to the controls in a time-dependent manner (control 11.0%; day 3, 28.7%; day 7, 33.1%; and day 10, 49.8%) (Fig. 3a). Furthermore, the neurons surrounded by GFAP-IR SGCs were mainly small (<500 µm2) and medium (500–1,200 µm2) in size (Fig. 3b).
Fig. 3.
The ratio of PGP-9.5-positive neurons surrounded by GFAP-positive satellite cells in the trigeminal ganglion. (a) The ratio of neurons surrounded by GFAP-immunopositive (IP) satellite cells per PGP-9.5-positive neuron in the maxillary nerve region between 3 and 10 days after extraction. Mean±SD. Significant differences from control (*p<0.05, **p<0.01) (b) The ratio of small (<500 µm2), medium (500–1,200 µm2), and large (>1,200 µm2) neurons surrounded by GFAP-IP satellite cells per PGP-9.5-positive neuron. Mean±SD.
GFAP-IR SGCs and ATF3-IR neurons
Next, the temporal changes in the GFAP-IR SGCs around ATF3-IR neurons were examined in the maxillary and mandibular nerve regions of the TG.
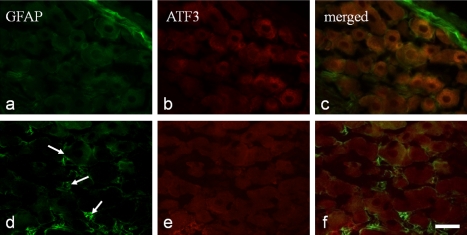
In the maxillary nerve region, no ATF3-IR neurons were observed among the uninjured neurons, while a few GFAP-IR SGCs were identified (Fig. 4a–c). After tooth extraction, some nuclear ATF3-IR neurons appeared in the maxillary nerve region of the TG (Fig. 4m–o). On day 3 after extraction, GFAP-IR SGCs were distributed around the ATF3-negative neurons (Fig. 4d–f). On days 7 and 10, the number of GFAP-IR SGCs increased, and ATF3-IR neurons surrounded by GFAP-IR SGCs were detected (Fig. 4g–l).
Fig. 4.
GFAP-immunoreactive (IR) satellite cells and ATF3-IR neurons in the trigeminal ganglion. GFAP-immunopositive (IP) satellite cells (a, d, g, j, and m) and ATF3-IP neurons (b, e, h, k, and n) in control rats (a and b) and in rats 3 (d and e), 7 (g and h), and 10 days (j and k) after tooth extraction. GFAP-IP satellite cells (m) and ATF-3-IR neurons (n) were observed in low magnification in rat 7 days after tooth extraction. (c, f, i, l, and o) are merged images of (a, d, g, j, and m) and (b, e, h, k, and n). Arrows indicate ATP-IP nuclei. Bar=30 µm in (a–l) and 200 µm in (m–o).
In the mandibular nerve region, no ATF3 immunoreactivity was observed in either the uninjured neurons or in the neurons 7 days after extraction (Fig. 5b and e). Among the controls, no GFAP-IR SGCs were identified (Fig. 5a–c), although GFAP-IR SGCs were detected around the ATF3-negative neurons 7 days after extraction (Fig. 5d–f).
Fig. 5.
GFAP-immunoreactive (IR) satellite cells and ATF3-IR neurons in the mandibular nerve region of the trigeminal ganglion. GFAP-immunopositive (IP) satellite cells (a and d) and ATF3-IP neurons (b and e) in control rats (a and b) and in rats 3 and 7 days (d and e) after tooth extraction. (c and f) are merged images of (a and d). No ATP-IP nuclei were located in the mandibular nerve region. Arrows indicate GFAP-IP satellite cells. Bar=30 µm.
IV. Discussion
Our results demonstrate that the extraction of the upper molars increased the number of GFAP-IR SGCs around the neurons in the maxillary nerve region. Although the neurons in the mandibular nerve region were uninjured, an increased number of GFAP-IR SGCs was also noted. These findings strongly suggest that injured neurons affect local SGC activation, whereas SGCs located at a distance from injured neurons are activated by the SGCs around the injured neurons.
SGCs are laminar and surround the neuronal cell bodies in the TG. Even in the control rats, some flat SGCs surrounding the neurons were immunopositive for GFAP, which indicates that SGCs express GFAP at a low level under resting conditions. This finding is consistent with that of Ajima et al. [1], who reported that GFAP-positive satellite cells formed a ring around several normal TG neurons.
Previous studies on the up-regulation of GFAP in response to nerve injury have shown either a rapid (4–6 hr) or delayed (≥3 days) response [4, 24]. In this study, we found an increase in the number of GFAP-positive SGCs in the TG more than 3 days after tooth extraction. Similarly, the up-regulation of GFAP in the TG was noted 2 days after the injection of complete Freund’s adjuvant into the whisker pad area in rats [25]. We also found that the number of neurons surrounded by GFAP-IR SGCs in the maxillary nerve region was elevated compared to the control value by approximately three- and fivefold, respectively, 3 and 10 days after extraction. A similar previous study showed that an inferior alveolar nerve crush increased the number of GFAP-IR SGCs by approximately 30-fold, compared to that of the controls [6]. This difference in the ratio of GFAP-positive SGCs may have been due to differences in the number of injured neural fibers between the tooth extraction and inferior alveolar nerve crush.
Most of the neurons surrounded by GFAP-IR SGCs were small (<500 µm2) or medium (500–1,200 µm2) in size, indicating that they were largely C neurons rather than Aδ neurons [16]. However, a previous study reported increased GFAP immunoreactivity in satellite cells surrounding neurons of various sizes, including large ones, in the ganglia of paclitaxel-treated rats [14]. Furthermore, the number of SGCs per neuron was found to increase in proportion to the neuron’s volume [17, 18]. Whether SGC sheath formation is cell type-specific or not requires further study.
Since SGCs are found in close proximity to the neurons, SGCs and neurons are thought to form a functional unit with neutral chemical communication occurring between the two cell types. After nerve injury, GFAP-IR SGCs were localized around both the injured and uninjured neurons [21, 22]. SGCs communicate with adjacent neurons or other SGCs through gap junctions [5] and by the secretion of various chemical mediators [12]. In this study, ATF3-IR neurons appeared 3 days after tooth extraction; however, GFAP-IR SGCs were distributed not only around the ATF3-IR neurons but also around ATF3-immunonegative neurons. Since satellite cell GFAP immunoreactivity is site-specific and injury-related [23], the differences in distribution between GFAP-IR satellite cells and ATF3-IR neurons may be due to a time lag in expression between GFAP and ATF3. That is, after tooth extraction, nociceptic stimulation reaches the neural body and is rapidly transported to neighboring SGCs where it stimulates the expression of GFAP, after which the injured neurons begin to express ATF3.
In this study, GFAP-IR SGCs were distributed close to and away from the injured ATF3-IR neurons. Moreover, GFAP-IR SGCs were observed in the neurons of the mandibular region, which was undamaged. A previous study revealed contralateral neuropathic pain following hemilateral nerve injury [13]. The activation of SGCs far away from the injured neuron might be related to neuropathic pain. In addition, SCGs may play a role in communication between the maxillary and mandibular nerve regions in the TG. Previously, we found an interaction between the maxillary and mandibular nerve regions in the TG [10]. Because the neurons in sensory ganglia have neither dendrites nor synapses, neurotransmitters may be involved in the communication between the cells [12]. One candidate transmitter is ATP, which binds P2X3, a receptor that is associated with primary nociceptive sensory neurons. Taken together, these findings suggest that cross-excitation occurs via non-synaptic neurotransmitters such as ATP from SCGs. Although we found no direct evidence of cross-excitation in the TG, the synchronized activation of the SCGs in the maxillary and mandibular nerve regions of the TG strongly suggests the existence of cross-excitation via a non-synaptic neurotransmission system. On the other hand, some growth factors such as fibroblast growth factors (FGFs) were suggested to be involved in the activation of glial cells and regeneration of damaged neuron after nerve injury [15]. In the peripheral nervous system previous study reported that FGF-2 immunoreactive SGCs were increased after the axotomy, and that FGF-2 may react through the FGF receptor-1 [9]. Further investigations would be needed to clarify which molecules or growth factors are associated with the interaction between TG neurons and SGCs.
In conclusion, nerve injury caused by extraction of the maxillary molars in rats induced the activation of SCGs around the injured neurons. SCG activation then spread to uninjured neurons in the maxillary nerve region, as well as to the mandibular nerve region. Our data strongly suggest the existence of a neuronal interaction between maxillary and mandibular neurons via SGC activation.
V. Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, to T. Goto (15591941) and K. K. Gunjigake (19791592).
VI. References
- 1.Ajima H., Kawano Y., Takagi R., Aita M., Gomi H., Byers M. R., Maeda T. The exact expression of glial fibrillary acidic protein (GFAP) in trigeminal ganglion and dental pulp. Arch. Histol. Cytol. 2001;64:503–511. doi: 10.1679/aohc.64.503. [DOI] [PubMed] [Google Scholar]
- 2.Aldskogius H., Kozlova E. N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 3.Berge T. I. Incidence of chronic neuropathic pain subsequent to surgical removal of impacted third molars. Acta Odontol. Scand. 2002;60:108–112. doi: 10.1080/000163502753509518. [DOI] [PubMed] [Google Scholar]
- 4.Canady K. S., Ali-Osman F., Rubel E. W. Extracellular potassium influences DNA and protein syntheses and glial fibrillary acidic protein expression in cultured glial cells. Glia. 1990;3:368–374. doi: 10.1002/glia.440030508. [DOI] [PubMed] [Google Scholar]
- 5.Cherkas P. S., Huang T-Y., Pannicke T., Tal M., Reichenbach A., Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Chudler E. H., Anderson L. C., Byers M. R. Trigeminal ganglion neuronal activity and glial fibrillary acidic protein immunoreactivity after inferior alveolar nerve crush in the adult rat. Pain. 1997;73:141–149. doi: 10.1016/S0304-3959(97)00088-2. [DOI] [PubMed] [Google Scholar]
- 7.Eddleston M., Mucke L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallicchio M., Rosa A. C., Benetti E., Collino M., Dianzani C., Fantozzi R. Substance P-induced cyclooxygenase-2 expression in human umbilical vein endothelial cells. Br. J. Pharmacol. 2006;147:681–689. doi: 10.1038/sj.bjp.0706660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grothe C., Meisinger C., Hertenstein A., Kurz H., Wewetzer K. Expression of fibroblast growth factor receptor 1 messenger RNAs in spinal ganglia and sciatic nerve: regulation after peripheral nerve lesion. Neuroscience. 1997;76:123–135. doi: 10.1016/s0306-4522(96)00355-7. [DOI] [PubMed] [Google Scholar]
- 10.Gunjigake K. K., Goto T., Nakao K., Konoo T., Kobayashi S., Yamaguchi K. Correlation between the appearance of neuropeptides in the rat trigeminal ganglion and reinnervation of the healing root socket after tooth extraction. Acta Histochem. Cytochem. 2006;39:69–77. doi: 10.1267/ahc.05057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanani M., Huang T. Y., Cherkas P. S., Ledda M., Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 12.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Hatashita S., Sekiguchi M., Kobayashi H., Konno S., Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine. 2008;33:1344–1351. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Andrade J. M., Peters C. M., Mejia N. A., Ghilardi J. R., Kuskowski M. A., Mantyh P. W. Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci. Lett. 2006;405:62–67. doi: 10.1016/j.neulet.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Koshinaga M., Sanon H. R., Whittemore S. R. Altered acidic and basic fibroblast growth factor expression following spinal cord injury. Exp. Neurol. 1993;120:32–48. doi: 10.1006/exnr.1993.1038. [DOI] [PubMed] [Google Scholar]
- 16.Lawson S. N., Waddell P. J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledda M., De Palo S., Pannese E. Ratios between number of neuroglial cells and number and volume of nerve cells in the spinal ganglia of two species of reptiles and three species of mammals. Tissue Cell. 2004;36:55–62. doi: 10.1016/j.tice.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Pannese E. The satellite cells of the sensory ganglia. Adv. Anat. Embryol. Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 19.Pannese E. Perikaryal surface specializations of neurons in sensory ganglia. Int. Rev. Cytol. 2002;220:1–34. doi: 10.1016/s0074-7696(02)20002-9. [DOI] [PubMed] [Google Scholar]
- 20.Pannese E., Ledda M., Cherkas P. S., Huang T. Y., Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat. Embryol. 2003;206:337–347. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- 21.Peters C. M., Jimenez-Andrade J. M., Kuskowski M. A., Ghilardi J. R., Mantyh P. W. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters C. M., Jimenez-Andrade J. M., Jonas B. M., Sevcik M. A., Koewler N. J., Ghilardi J. R., Wong G. Y., Mantyh P. W. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 2007;203:42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson J. L., Byers M. R. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp. Neurol. 1995;131:11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 24.Steward O., Torre E. R., Tomasulo R., Lothman E. Neuronal activity up-regulates astroglial gene expression. Proc. Natl. Acad. Sci. U S A. 1991;88:6819–6823. doi: 10.1073/pnas.88.15.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda M., Tanimoto T., Kadoi J., Nasu M., Takahashi M., Kitagawa J., Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Tay A. B., Zuniga J. R. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int. J. Oral Maxillofac. Surg. 2007;36:922–927. doi: 10.1016/j.ijom.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Watkins L. R., Maier S. F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 28.Weissner W., Winterson B. J., Stuart-Tilley A., Devor M., Bove G. M. Time course of substance P expression in dorsal root ganglia following complete spinal nerve transection. J. Comp. Neurol. 2006;497:78–87. doi: 10.1002/cne.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodham P., Anderson P. N., Nadim W., Turmaine M. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci. Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L. F., Wang R., Xu Y. Z., Yi X. N., Zhang J. W., Zeng Z. C. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 2008;1187:20–32. doi: 10.1016/j.brainres.2007.10.044. [DOI] [PubMed] [Google Scholar]