Abstract
The elastic system fibers comprise oxytalan, elaunin and elastic fibers, which differ in their relative microfibril and elastin content. Human periodontal ligaments (PDL) contain only oxytalan fibers (pure microfibrils) among them. Since fibulin-5 regulates the organization of elastic fibers to link the fibers to cells, we hypothesized that fibulin-5 may contribute to the formation of oxytalan fibers. We used siRNA for fibulin-5 in PDL cell culture to examine the extracellular deposition of fibrillin-1 and -2, which are the major components of microfibrils. Fibulin-5 was labeled on microfibrils positive for fibrillin-1 and -2. Fibulin-5 suppression reduced the level of fibrillin-1 and -2 deposition to 60% of the control level. These results suggest that fibulin-5 may control the formation of oxytalan fibers, and play a role in the homeostasis of oxytalan fibers.
Keywords: fibrillin, fibulin-5, periodontal ligaments, oxytalan fiber, microfibrils
I. Introduction
The elastic system fibers that give tissue flexibility and extensibility have two components: an amorphous core of cross-linked elastin and a peripheral mantle of microfibrils [12]. Elastic fiber formation is thought to begin with the formation of a scaffold of microfibrils on which the elastin is deposited. Depending on the relative proportions of microfibrils and elastin, elastic system fibers can be classified into three types: oxytalan, elaunin and elastic fibers. Interestingly, in the periodontal ligaments (PDL), only oxytalan fibers, which are pure microfibrils, are identified, whereas all three types of fibers are present in the gingiva [5, 17]. In PDL, oxytalan fibers are thought to have some functional roles, such as supporting of vascular orientation and regulation of vascular flow [18]. In our series of studies [20], we have demonstrated that human PDL fibroblasts express fibrillin-1 and -2 (the major components of oxytalan fibers) without expression of tropoelastin (precursor of mature elastin). By PDL fibroblast culture, we clearly showed that pure oxytalan fibers are formed in cell/matrix layers. Furthermore, activated matrix metalloproteinase-2 controls the degradation of fibrillin-2 to maintain a precise amount of oxytalan fibers in PDL [25]. However, precisely how oxytalan fibers develop remains unclear.
Fibulins are extracellular glycoproteins, which are associated with elastic fibers [1, 19]. They function as molecular bridges, thus contributing to assembly and organization of the extracellular matrix. Fibulin families comprise six glycoproteins, which are characterized by a C-terminal fibulin-type globular domain preceded by cbEGF (calcium-binding epidermal growth factor)-like modules. Among them, fibulin-5, a 55-kDa glycoprotein, has been reported to have a relationship with elastic system fibers [16, 27]. Fibulin-5 can interact with both fibrillin-1 and tropoelastin [7, 8], and we have reported that the fibulin-5 gene is controlled by the tropoelastin gene [23]. Moreover, experiments using genetically targeted mice have shown that fibulin-5 is necessary for the biogenesis of elastic fibers [30]. Therefore, fibulin-5 is thought to be an adaptor for binding tropoelastin to microfibrils for the formation of elastic fibers [10].
On the other hand, fibulin-5 expression is known to be distributed even in elastin-free tissues [19]. However, fibulin-5 has been assumed to play a role in elastic fiber formation. We previously showed that human PDL fibroblasts express not only fibrillins but also fibulin-5 [23]. Therefore, based on the fact that fibulin-5 has the ability to bind to fibrillin-1 [8], we hypothesized that fibulin-5 may contribute to the formation of microfibrils in PDL. In the present study we used a culture model of pure microfibrils to investigate the role of fibulin-5 in the development of microfibrils, and found that fibulin-5 co-localizes with microfibrils and controls the biogenesis of microfibril bundles.
II. Material and Methods
Cells and culture
The protocol for these experiments was reviewed and approved by the Fukuoka Dental College Research Ethics Committee, and informed consent was obtained from the tissue donors.
PDL fibroblasts were isolated from three different donors and cultured, as described previously [20]. Briefly, connective tissues were obtained surgically from the PDL molar teeth extracted for orthodontic reasons. After washing in phosphate-buffered saline (PBS) supplemented with 100 units/ml penicillin and 100 µg/ml streptomycin (Roche Diagnostics, Mannheim, Germany), the PDL tissues were cut into small pieces, plated in petri dishes, and incubated in Minimum Essential Medium (MEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% newborn calf serum (NCS; Invitrogen) at 37°C in humidified air containing 5% CO2. When the outgrowth of the cells reached confluence, they were harvested with 0.025% trypsin (Invitrogen) in PBS, and transferred to plastic culture dishes at a 1:4 split ratio. For experiments, the cells were trypsinized and seeded at 1×106 cells/ml per 35-mm culture dish (Corning Incorporated, Corning, NY, USA) in MEM supplemented with 10% NCS, 100 units/ml penicillin and 100 µg/ml streptomycin.
Immunoprecipitation
The cell culture medium was harvested and mixed with proteinase inhibitor cocktail containing 5 mM ethylenediaminetetraacetic acid, 50 µM N-ethylmaleimide, and 50 µM phenylmethylsulfonyl fluoride. The medium of the PDL fibroblast culture was incubated with anti-human fibrillin-1 polyclonal antibody (Elastin Products Co., Owensville, MO, USA) or anti-human fibulin-5 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in the presence of 2% bovine serum albumin (BSA, fraction V; Sigma, St. Louis, MO, USA) for 2 hr at 4°C. Protein G-agarose beads (Pierce Biotechnology, Inc., Rockford, IL, USA), pre-washed with MEM containing 2% BSA, were then added to the sample-antibody mixture and incubated for 1 hr at 4°C. Then the beads were collected by centrifugation and washed five times extensively with MEM, changing the tube for the last spin. The immunoprecipitated protein retained by the washed beads was then eluted with a gel electrophoresis loading buffer (1% sodium dodecyl sulfate, 10 mM Tris, 5% glycerol, and 25% dithiothreitol, pH 7.0), and subjected to 4–12% polyacrylamide gel electrophoresis for western blotting.
Small interference RNA (siRNA) design and transient transfection
siRNA for human fibulin-5 (Accession # NM_006329) was designed and synthesized by Hokkaido System Science Co., Ltd. (Sapporo, Japan). The synthesized siRNA corresponded to the 1165–1190 coding region of fibulin-5. The siRNA sequence for fibulin-5 was: sense 5'-GGCAGAGAAUUUUACAUGCGGCAAAAG-3', antisense 3'-UACCGUCUCUUAAAAUGUACGCCGUUU-5. Negative control (scrambled order) was: sense 5'-CGGUCUAGACUAGCGAGAUAAUAGAAG-3', antisense 3'-UAGCCAGAUCUGAUCGCUCUAUUAUCU-5'. A sequence of the negative control was designed as a randomized sequence of the 1165–1190 coding region of fibulin-5. BLAST searches of the database indicated that this siRNA is specific for fibulin-5, and has no homology with other proteins. Transfection was performed at 7 and 10 days of culture continuously. The siRNA was transfected into PDL fibroblasts using the X-treme GENE siRNA transfection reagent (Roche). First, 237.5 µl of OptiMEM medium/dish (Invitrogen) and 12.5 µl of the transfection reagent were preincubated for 10 min at room temperature. During this time, 748 µl of OptiMEM medium was mixed with 2 µl of 100 µM siRNA. The two mixtures were then combined and incubated for 20 min at room temperature to allow complex formation. The entire mixture was added to the cells in one dish, resulting in a final concentration of 200 nM for the siRNAs. After 12 hr of incubation, the transfection medium was replaced with fresh complete medium (MEM with 10% NCS). Mock transfection of cultures with the transfection reagent alone was used as a control. PDL fibroblasts were transfected twice with the siRNA duplex (0, 50 and 200 nM), with a 72-hr interval, and harvested at 14 days.
Immunofluorescence
At 14 days of culture, PDL fibroblasts were fixed in ice-cold 1% paraformaldehyde in PBS for 15 min. The culture dishes were rinsed with PBS, and treated with 6 M guanidine-HCl, 20 mM Tris, and 50 mM dithiothreitol (pH 8.0) for 15 min. This treatment is effective to be exposed an epitope of the cross-linked proteins. Nonspecific immunoreactivity was blocked with 1% bovine serum albumin in PBS for 1 hr at room temperature. The cell layers were then incubated for 2 hr at room temperature with the appropriate primary antibodies (polyclonal goat antibody against fibulin-5 diluted 1:2000; Santa Cruz, and rabbit antibody against fibrillin-1 and -2 diluted 1:2000; Elastin Products Co.). Controls included the use of preimmune normal goat or rabbit IgG for the primary antibody incubation. We had already confirmed the specificity of the fibrillin-1 and fibrillin-2 antibodies as reported previously [20]. After rinsing in PBS, the cells were incubated with Alexa Fluor® 568-labeled donkey anti-goat IgG1 antibody or Alexa Fluor® 488 or 568-labeled goat anti-rabbit Ig (H+L) antibody (Invitrogen), diluted 1:2000 with blocking buffer, for 1 hr at room temperature. In some cases, SYTOX® Green (Molecular Probes) was added at 100 nM for nuclear staining. Following a rinse in PBS, immunoreactivity was observed using a confocal microscope (MRC-1024; Bio-Rad, Hemel Hempstead, UK). For double immunostaining, the cells were reacted sequentially with two different primary antibodies against fibulin-5, fibrillin-1 and -2 at 1:2000 dilutions. Then the cells were reacted with two secondary antibodies (Alexa Fluor® 488-labeled goat anti-rabbit Ig (H+L) and Alexa Fluor® 568-labeled donkey anti-goat IgG1) in a 1:1 mixture.
Western blot analysis
At 14 days of culture, the cell/matrix samples were prepared as described previously [16]. For detection of fibrillin-1 and fibrillin-2, we partly separated matrix and cellular proteins from the cell/matrix samples as described previously [17].
The immunoprecipitates or the proteins (5 µg) were subjected to electrophoresis on 4–12% NuPAGE Bis-Tris gel (Invitrogen) for western blot analysis, as described previously [17]. A549 cell (human lung carcinoma) lysates were used as the positive control for fibulin-5, which was purchased from Santa Cruz Biotechnology (Santa Cruz). The monoclonal fibrillin-1 antibody for the detection of fibrillin-1 in the immunoprecipitates against fibrillin-1 was purchased from NeoMarkers (Fremont, CA, USA). The primary antibodies used were those against fibulin-5, fibrillin-1 and -2 at 1:5000 dilution, and β-actin at 1:2000 dilution. Prestained molecular weight markers (Invitrogen) run on each blot. Densitometric analysis of the signals was performed using the Image J program (National Institutes of Health, Bethesda, MD, USA) after finding the linear portion by sequential dilution of the proteins. Small variations in protein loading were corrected by normalization relative to the intensity of the corresponding band of β-actin. Each value presented is expressed as the mean±standard deviation (SD) and all quantitative results represent at least three independent analyses. The unpaired Student’s t-test was used for analyzing differences between experimental groups [6].
Northern blot analysis
Total RNA was prepared from the cultured PDL fibroblasts at 14 days using an RNeasy Mini Kit (Qiagen, Hilden, Germany). One microgram of RNA was subjected to northern blot analysis, which was performed as described previously [21]. The probes for recognition of human fibrillin-1 and -2 were generated as described previously [22]. The RNA probe for β-actin was from Roche Molecular Biochemicals. Densitometric analysis of the signals was also performed using the Image J program. Small variations in RNA loading were corrected by normalization relative to the intensity of the corresponding band of β-actin probe.
III. Results
Fibulin-5 localization on microfibrils
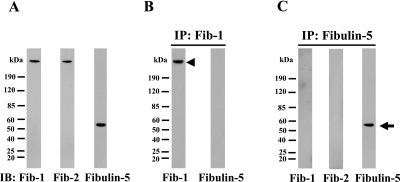
We first investigated the specificities of the fibrillin-1, fibrillin-2 and fibulin-5 antibodies. We had already confirmed the specificities of the fibrillin-1 and -2 antibodies as reported previously [20]. Figure 1A shows the results of western blotting carried out on PDL culture media using the three antibodies. For fibrillin-1 and -2, each antibody labels a single band at 350 kDa, whereas the fibulin-5 antibody labels a single band at 55 kDa. To confirm that each of the antibodies recognizes a distinct protein, we carried out immunoprecipitation with fibrillin-1 antibody and fibulin-5 antibody from PDL culture media. The immunoprecipitated materials were then analyzed by western blotting with each of the three antibodies. It is clear from Figure 1B that immunoprecipitates with fibrillin-1 antibody were reasonably recognized by fibrillin-1 antibody, and that the fibulin-5 anybody did not cross-react with the protein immunoprecipitated by fibrillin-1 antibody. Similarly, immunoprecipitates with fibulin-5 antibody were not recognized by the fibrillin-1 and fibrillin-2 antibodies (Fig. 1C). Therefore we confirmed the specificity of the three antibodies.
Fig. 1.
Characterization of anti-fibrillin-1, fibrillin-2 and fibulin-5 antibodies. (A) Immunoblotting of PDL culture medium against anti-fibrillin-1 (left lane), anti-fibrillin-2 (middle lane) and anti-fibulin-5 (right lane) antibodies. Fibrilin-1, fibrillin-2 and fibulin-5 antibodies interact with an estimated single protein. (B) Immunoblotting against anti-fibrillin-1 (left lane) and anti-fibulin-5 (right lane) antibodies following immunoprecipitation of PDL culture medium using anti-fibrillin-1 antibody. The immunoprecipitates were reasonably recognized by fibrillin-1 antibody (arrowhead), and the fibulin-5 antibody did not cross-react with the immunoprecipitates. (C) Immunoblotting against anti-fibrillin-1 (left lane), anti-fibrillin-2 (middle lane) and anti-fibulin-5 (right lane) antibodies following immunoprecipitation using anti-fibulin-5 antibody. The immunoprecipitates were reasonably recognized by fibulin-5 antibody (arrow), and neither of the other two antibodies cross-reacted with the immunoprecipitates.
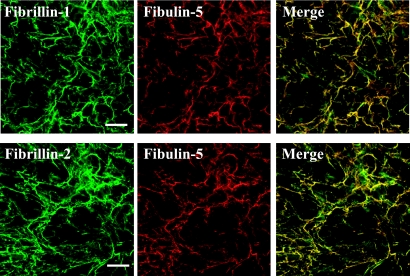
We next examined whether fibulin-5 was localized on microfibrils (Fig. 2). The positive staining by fibrillin-1 and fibrillin-2 were microfibril, which were observed as networks of fiber patterns. Fibulin-5 was labeled on fibrillins-1- and -2-immunolabeled microfibrils of human PDL fibroblasts cultured for 14 days. Control immune serum produced no labeling (not shown). The results show that fibulin-5 was co-localized with microfibrils.
Fig. 2.
Immunolocalization of fibulin-5 to microfibrils. Double immunofluorescence of fibrillin-1/fibulin-5 and fibrillin-2/fibulin-5 in PDL fibroblasts cultures. Human PDL fibroblasts were cultured for 14 days, then simultaneously labeled with fibrillin-1 (green), fibulin-5 (red), and superimposition of both labels (upper panels). Similarly, the cells were labeled with fibrillin-2 (green), fibulin-5 (red) and superimposition (lower panels). Bar=100 µm.
Fibulin-5 siRNA inhibits deposition of its protein
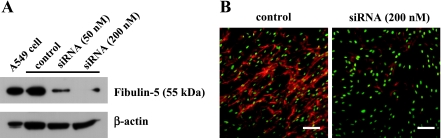
Next, in order to investigate the function of fibulin-5, we used siRNA to suppress fibulin-5 expression. Densitometric analysis based on the western blot showed that when 200 nM siRNA was used for transfection, the siRNA effectively reduced the level of fibulin-5 deposition to more than 90% of that of the control (vehicle only) in the PDL cell/matrix layers at 14 days (Fig. 3A). In contrast, scrambled siRNA had no effect on fibulin-5 deposition, and no difference from the control was evident (data not shown), proving that this siRNA was specific for fibulin-5.
Fig. 3.
Fibulin-5 siRNA suppresses deposition of its protein. PDL fibroblasts were cultured for 7 days, and then transiently transfected with 0, 50, 200 nM fibulin-5 siRNA for another 7 days. (A) cell/matrix lysates (5 µg) were analyzed by western blotting. Mock-transfected (vehicle only) culture was used as a control (second lane). A549 cell lysates were used as fibulin-5-positive controls (first lane). β-actin was used as an internal control in each lane. (B) PDL fibroblasts were immunolabeled with anti-fibulin-5 antibody (red). SYTOX® Green was used for nuclear staining (green). Bar=100 µm.
The immunolabeling data also supported the data from western blotting. With 200 nM siRNA-transfected cells, fibulin-5 staining was diminished in comparison with the control cells (Fig. 3B).
Decrease of microfibril assembly by knock-down of fibulin-5 expression
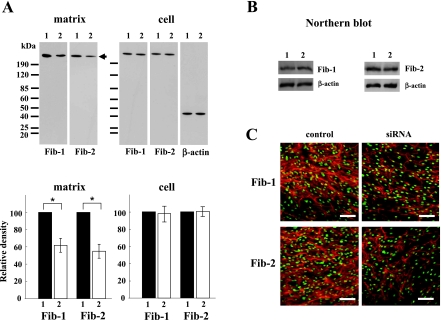
To investigate the effect of fibulin-5 on microfibril assembly, we transfected PDL fibroblasts with 200 nM fibulin-5 siRNA. In matrix proteins at 14 days, densitometric analysis of the western blot results identified a significant decrease of fibrillin-1 and -2 to 62±8% and 55±8% of the control, respectively. As expected, transfection with fibulin-5 siRNA did not affect the amounts in cellular proteins (Fig. 4A) and gene expression (Fig. 4B) of fibrillin-1 and -2. Furthermore, immunostaining of fibrillin-1 and -2 showed less intense labeling after transfection of 200 nM fibulin-5 siRNA (Fig. 4C).
Fig. 4.
Immunodetection of fibrillin-1 and fibrillin-2 in cell/matrix samples from PDL fibroblasts. PDL fibroblasts were transiently mock-transfected (lane 1), or transiently transfected with 200 nM siRNAs for fibulin-5 (lane 2). (A) Western blots of matrix and cellular samples of PDL fibroblasts cultured for 14 days. Equal amounts of proteins (5 µg) were separated by SDS-PAGE and transferred to Immobilon-P membranes. The blots were probed with anti-fibrillin-1 and anti-fibrillin-2 antibodies. Each antibody recognized a 350-kDa band (arrow). Densitometric analysis of changes in the levels of matrix and cell samples of fibrillins-1 and -2 was conducted using Image J software, and the value for each control sample were arbitrarily assigned a value of 100. The results are representative of three independent experiments. Data are presented as the mean±standard deviation (*p<0.05). (B) Northern blots of RNA samples (1 µg) extracted from PDL fibroblasts at 14 days. (C) Immunofluorescence staining was performed with anti-fibrillin-1 antibody (upper panels) and anti-fibrillin-2 antibody (lower panels). SYTOX® Green was used for nuclear staining (green). Bar=100 µm.
IV. Discussion
In the present study using the RNA interference technique, we have demonstrated for the first time that fibulin-5 has an effect on the amounts of extracellular deposition of fibrillin-1 and -2. Previously, exogenous fibulin-5 [7, 10] as well as endogenous fibulinb-5 [11] was shown to be localized on fibrillin-1-positive fibers, and their molecular interactions have been demonstrated [8]. Fibrillins-1 and -2 are the principle structural components of microfibrils [12], and co-assemble in the same microfibril fibers [4, 13]. Many researchers have focused on microfibrils as a template for tropoelastin deposition. In general, it has been believed that fibrillins-1 and -2 have overlapping function for elastic fiber formation [3]. Fibrillin-2 is expressed earlier in development than fibrillin-1 in elastin-rich tissues, and its expression is correlated with elastic fiber formation [29]. In accordance with these findings, we have demonstrated that gene expression of fibrillin-2 is correlated with that of tropoelastin [22], and that fibrillin-2 is essential for elastic fiber formation [24]. However, there are few experimental data on the mechanism of pure microfibril development.
Oxytalan fibers, which consist of collections of microfibrils, were first described in PDL [9]. In the PDL tissues, the microfibrils as well as collagen fibers are important matrix components for maintaining PDL homeostasis. In our culture system, PDL fibroblasts express abundant fibrillin-1 and -2 without tropoelastin, which reflects the situation in vivo. We have demonstrated the synthesis [20] and degradation [25] of fibrillins in PDL fibroblasts culture. The fibrillin-2 is crucial for microfibril formation [2]. However, the mechanism of microfibril development remains unclear, as described above. Fibulin-5 can bind to fibrillin-1 and tropoelastin [8]. In the present study, we showed that fibulin-5 was colocalized with fibrillin-2 as well as with fibrillin-1. This result is noteworthy in view of the paucity of information on the relationship between fibulin-5 and pure microfibrils. We speculated that fibulin-5 functions as not only a mediator between microfibrils and tropoelastin but also in the biogenesis of pure microfibrils. In this study, suppression of fibulin-5 inhibited microfibril deposition by about 40% (fibrillins-1 and -2 were inhibited to almost the same degree). The reason that decreases of deposition occur, despite the absence of any difference in its gene expression and secretion, is thought to be due to either protein degradation or regulation by outside molecules. We could not detect degradation products of fibrillins in the culture media and cell lysates (data not shown). Therefore, it is thought to that fibulin-5 contributes microfibril deposition directly or indirectly. Moreover, fibulin-5 binds to microfibril-associated molecule, such as lysyl oxidase-like 1 [14], EMILIN-1 [28] and so on. Therefore, further study is necessary to identify the molecule related with microfibril assembly.
Fibulin-5 is an integrin (αvβ3, αvβ5, α5β1, α4β1) ligand [15, 16]. PDL fibroblasts express αvβ3 integrins at least, and the αvβ3 integrin controls microfibril assembly [26]. Therefore, fibulin-5 on the cell surface may play a role in gathering the microfibrils located at the cell periphery.
In summary, we have demonstrated that fibulin-5 is colocalized with microfibrils, and may regulate microfibril formation. Our findings may provide new insights into the homeostasis of oxytalan fibers.
V. Acknowledgments
This work was supported by the Advanced Science Research Center and by Grants-in-Aid for Scientific Research (No. 19592391) from the Ministry of Education, Science, Sports and Culture of Japan.
VI. References
- 1.Argraves W. S., Greene L. M., Cooley M. A., Gallagher W. M. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artega-Solis E., Gayraud B., Lee S. Y., Shum L., Sakai L., Ramirez F. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carta L., Pereira L., Arteaga-Solis E., Lee-Arteaga S. Y., Lenart B., Starcher B., Merkel C. A., Sukoyan M., Kerkis A., Hazeki N., Keene D. R., Sakai L. Y., Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charbonneau N. L., Dzamba B. J., Ono R. N., Keene D. R., Corson G. M., Reinhardt D. P., Sakai L. Y. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J. Biol. Chem. 2003;278:2740–2749. doi: 10.1074/jbc.M209201200. [DOI] [PubMed] [Google Scholar]
- 5.Chavrier C., Hartmann D. J., Couble M. L., Herbage D. Distribution and organization of the elastic system fibres in healthy human gingiva. Ultrastructural and immunohistochemical study. Histochemistry. 1988;89:47–52. doi: 10.1007/BF00496583. [DOI] [PubMed] [Google Scholar]
- 6.Du Z., Han F., Shi Y. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Hitochem. Cytochem. 2008;41:89–95. doi: 10.1267/ahc.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Hallous E., Sasaki T., Hubmacher D., Getie M., Tiedemann K., Brinckmann J., Batge B., Davis E. C., Reinhardt D. P. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J. Biol. Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- 8.Freeman L. J., Lomas A., Hodson N., Sherratt M. J., Mellody K. T., Weiss A. S., Shuttleworth A., Kielty C. M. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fullmer H. M., Lillie R. D. The oxytalan fiber: a previously undescribed connective tissue fiber. J. Histochem. Cytochem. 1958;6:425–430. doi: 10.1177/6.6.425. [DOI] [PubMed] [Google Scholar]
- 10.Hirai M., Ohbayashi T., Horiguchi M., Okawa K., Hagiwara A., Chien K. R., Kita T., Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J. Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q., Loeys B. L., Coucke P. J., De Paepe A., Mecham R. P., Choi J., Davis E. C., Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum. Mol. Genet. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 12.Kielty C. M., Sherratt M. J., Shuttleworth C. A. Elastic fibres. J. Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 13.Lin G., Tiedemann K., Vollbrandt T., Peters H., Batge B., Brinckmann J., Reinhardt D. P. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J. Biol. Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Zhao Y., Gao J., Pawlyk B., Starcher B., Spencer J. A., Yanagisawa H., Zuo J., Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 15.Lomas A. C., Mellody K. T., Freeman L. J., Bax D. V., Shuttleworth C. A., Kielty C. M. Fibulin-5 binds human smooth-muscle cells through α5β1 and α4β1 integrins, but does not support receptor activation. Biochem. J. 2007;405:417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T., Lozano P. R., Ikeda Y., Iwanaga Y., Hinek A., Minamisawa S., Cheng C. F., Kobuke K., Dalton N., Takada Y., Tashiro K., Ross J., Jr., Honjo T., Chien K. R. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 17.Sculean A., Donos N., Windisch P., Reich E., Gera I., Brecx M., Karring T. Presence of oxytalan fibers in human regenerated periodontal ligament. J. Clin. Periodontol. 1999;26:318–321. doi: 10.1034/j.1600-051x.1999.260510.x. [DOI] [PubMed] [Google Scholar]
- 18.Sims M. R. Oxytalan-vascular relationships observed in histologic examination of the periodontal ligaments of man and mouse. Arch. Oral Biol. 1975;20:713–716. doi: 10.1016/0003-9969(75)90040-0. [DOI] [PubMed] [Google Scholar]
- 19.Timpl R., Sasaki T., Kostka G., Chu M. L. Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 2003;4:479–489. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruga E., Irie K., Sakakura Y., Yajima T. Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J. Periodontal Res. 2002;37:23–28. doi: 10.1034/j.1600-0765.2002.00662.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruga E., Irie K., Sakakura Y., Yajima T. Tropoelastin expression by periodontal fibroblasts. J. Dent. Res. 2002;81:198–202. [PubMed] [Google Scholar]
- 22.Tsuruga E., Irie K., Yajima T. Gene expression and accumulation of fibrillin-1, fibrillin-2, and tropoelastin in cultured periodontal fibroblasts. J. Dent. Res. 2002;81:771–775. doi: 10.1177/0810771. [DOI] [PubMed] [Google Scholar]
- 23.Tsuruga E., Yajima T., Irie K. Induction of fibulin-5 gene is regulated by tropoelastin gene, and correlated with tropoelastin accumulation in vitro. Int. J. Biochem. Cell Biol. 2004;36:395–400. doi: 10.1016/s1357-2725(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsuruga E., Yajima T., Irie K. Microfibril-associated glycoprotein-1 and fibrillin-2 are associated with tropoelastin deposition in vitro. Int. J. Biochem. Cell Biol. 2005;37:120–129. doi: 10.1016/j.biocel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Tsuruga E., Irie K., Yajima T. Fibrillin-2 degradation by matrix metalloproteinase-2 in periodontium. J. Dent. Res. 2007;86:352–356. doi: 10.1177/154405910708600410. [DOI] [PubMed] [Google Scholar]
- 26.Tsuruga E., Sato A., Ueki T., Nakashima K., Nakatomi Y., Ishikawa H., Yajima T., Sawa Y. Integrin αvβ3 regulates microfibril assembly in human periodontal ligament cells. Tissue Cell. 2009;41:85–89. doi: 10.1016/j.tice.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Yanagisawa H., Davis E. C., Starcher B. C., Ouchi T., Yanagisawa M., Richardson J. A., Olson E. N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 28.Zanetti M., Braghetta P., Sabatelli P., Mura I., Doliana R., Colombatti A., Volpin D., Bonaldo P., Bressan G. M. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell. Biol. 2004;24:638–650. doi: 10.1128/MCB.24.2.638-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Apfelroth S. D., Hu W., Davis E. C., Sanguineti C., Bonadio J., Mecham R. P., Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J. Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Q., Choi J., Rouleau L., Leask R. L., Richardson J. A., Davis E. C., Yanagisawa H. Normal wound healing in mice deficient for fibulin-5, an elastin binding protein essential for dermal elastic fiber assembly. J. Invest. Dermatol. 2006;126:2707–2714. doi: 10.1038/sj.jid.5700501. [DOI] [PubMed] [Google Scholar]