Abstract
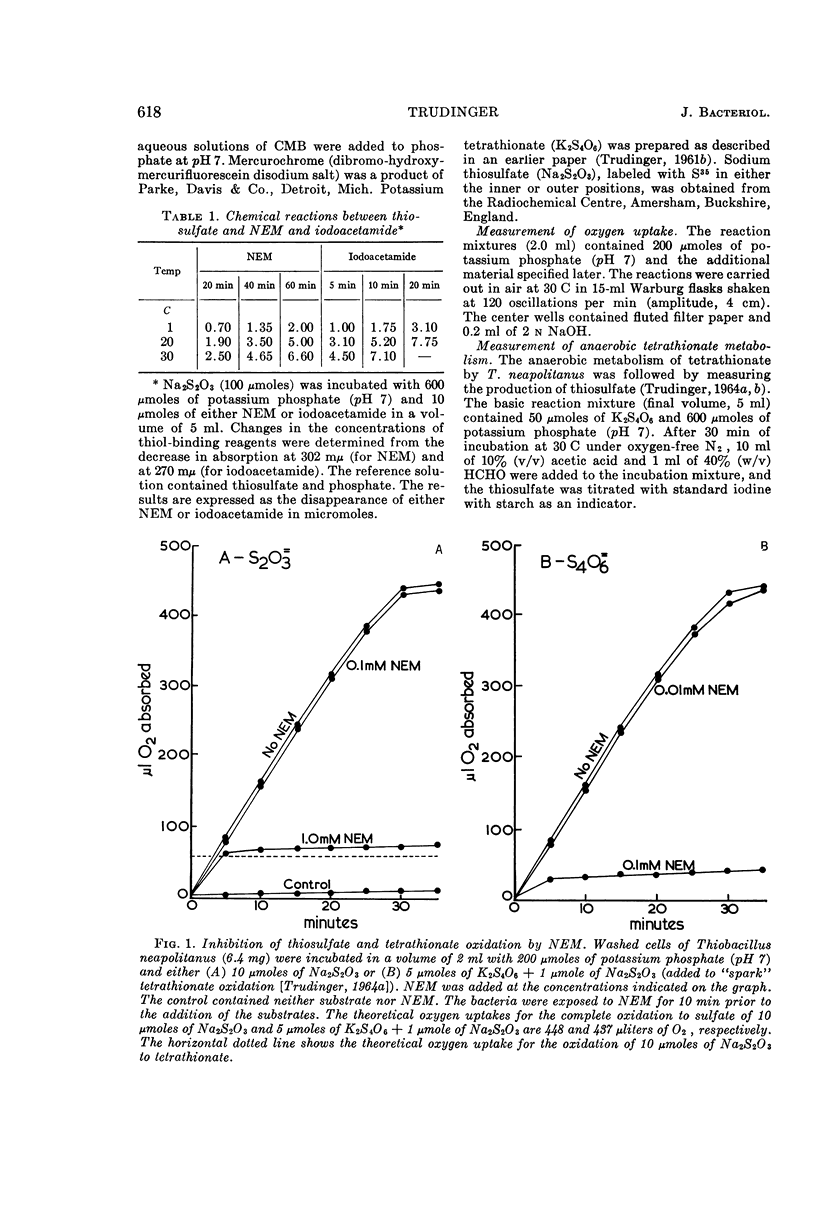
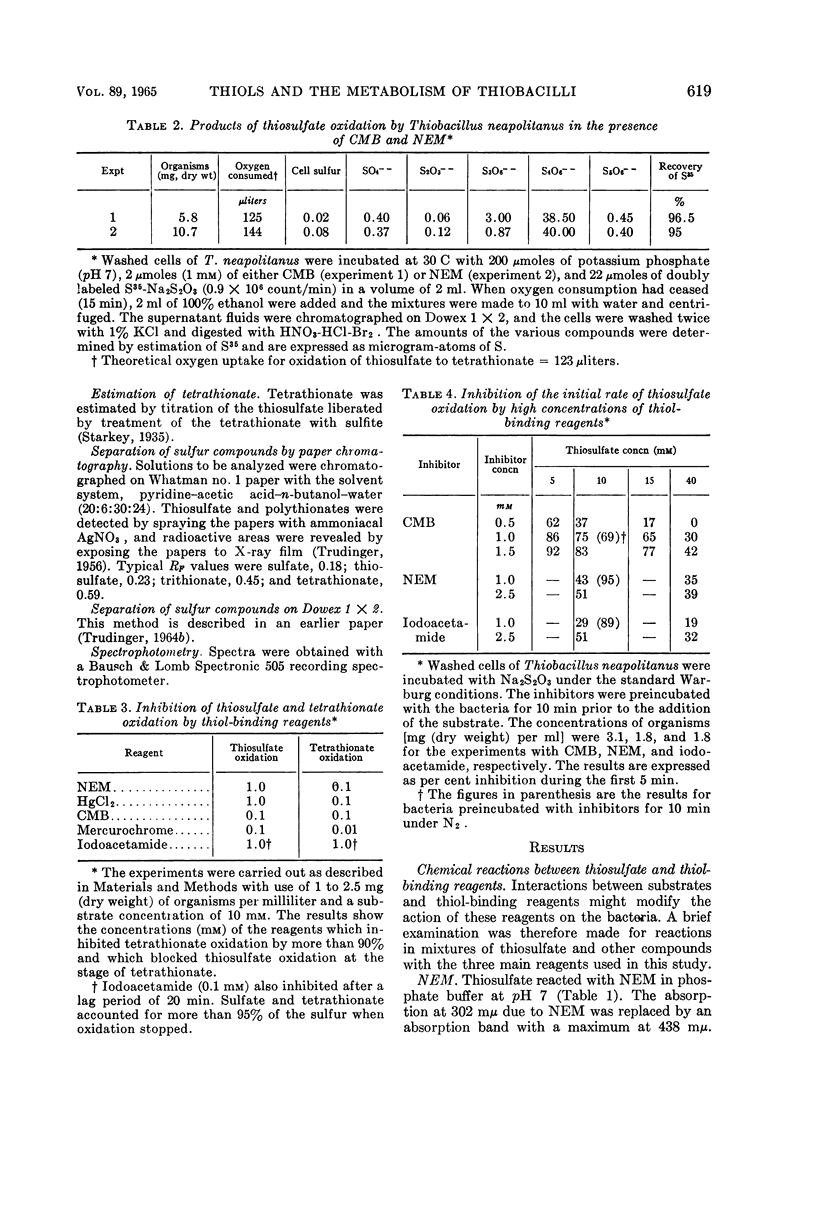
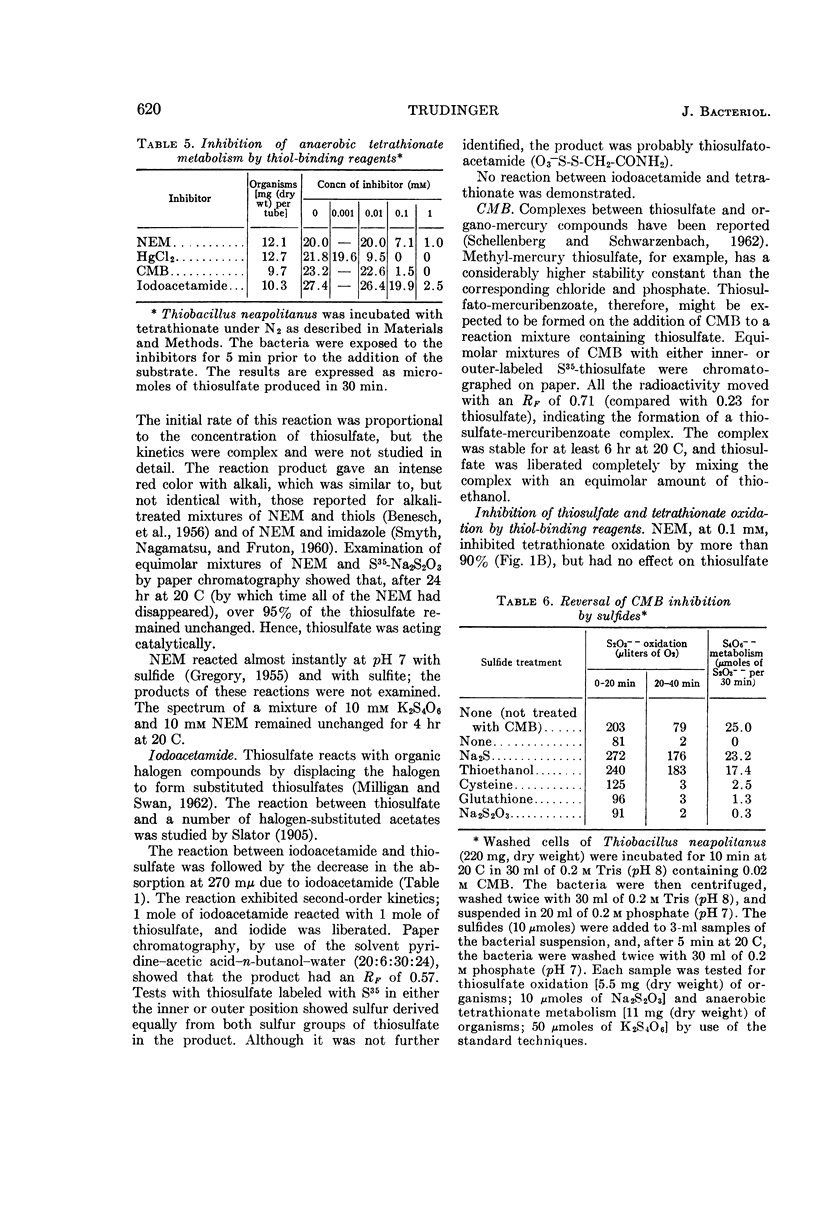
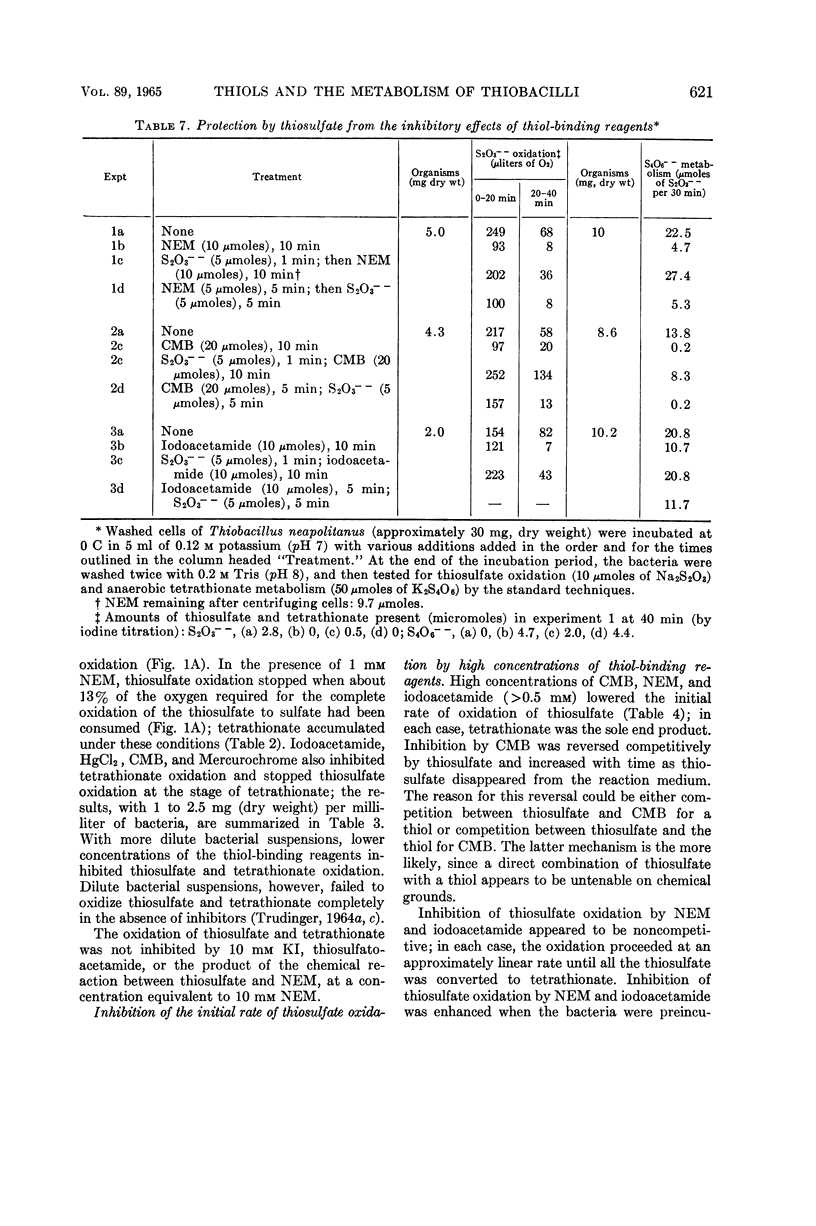
Trudinger, P. A. (Division of Plant Industry, Canberra, Australia). Effect of thiol-binding reagents on the metabolism of thiosulfate and tetrathionate by Thiobacillus neapolitanus. J. Bacteriol. 89:617–625. 1965.—Iodoacetamide, N-ethyl maleimide (NEM), p-chloromercuribenzoate (CMB), Mercurochrome, and HgCl2 inhibited the oxidation of thiosulfate to sulfate by Thiobacillus neapolitanus; tetrathionate accumulated under these conditions. High concentrations of the thiol-binding reagents lowered the rate of oxidation of thiosulfate to tetrathionate; inhibition by CMB was reversed by high concentrations of thiosulfate. Relatively low concentrations of the thiol-binding reagents completely inhibited the oxidation and anaerobic metabolism of tetrathionate. Similar reagents had no effect on a soluble thiosulfate-oxidizing enzyme. Inhibition by thiol-binding reagents was overcome by washing the bacteria with Na2S or thioethanol after their exposure to the inhibitors. Under some conditions, the addition of thiosulfate or tetrathionate to bacterial suspensions before the addition of the thiol-binding reagents prevented the inhibition of thiosulfate and tetrathionate metabolism by these reagents. Thiosulfate catalyzed a rapid chemical breakdown of NEM and reacted with iodoacetamide. A complex between thiosulfate and mercuribenzoate was demonstrated. Three types of thiol group appear to be associated with the metabolism of thiosulfate and tetrathionate; one of these types may be located at the bacterial cell membrane. The results are consistent with the hypothesis that thiols (or disulfide groups) are binding sites for the substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R., BENESCH R. E., GUTCHO M., LAUFER L. New color test for thiols and thiolesters. Science. 1956 Jun 1;123(3205):981–982. doi: 10.1126/science.123.3205.981. [DOI] [PubMed] [Google Scholar]
- BLACK S. THE BIOCHEMISTRY OF SULFUR-CONTAINING COMPOUNDS. Annu Rev Biochem. 1963;32:399–418. doi: 10.1146/annurev.bi.32.070163.002151. [DOI] [PubMed] [Google Scholar]
- KAJI A., McELROY W. D. Mechanism of hydrogen sulfide formation from thiosulfate. J Bacteriol. 1959 May;77(5):630–637. doi: 10.1128/jb.77.5.630-637.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES H. Energy metabolism in chemolithotropic bacteria. Annu Rev Microbiol. 1960;14:83–98. doi: 10.1146/annurev.mi.14.100160.000503. [DOI] [PubMed] [Google Scholar]
- OSTROWSKI W., KRAWCZYK A. Biochemia samozywnych bakterii siarkowych. IV. Badania nad przemiana siarki w Thiobacillus thioparus przy zastosowaniu 35S. Acta Biochim Pol. 1957;4(4):249–265. [PubMed] [Google Scholar]
- PARKER C. D., PRISK J. The oxidation of inorganic compounds of sulphur by various sulphur bacteria. J Gen Microbiol. 1953 Jun;8(3):344–364. doi: 10.1099/00221287-8-3-344. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D. ADENOSINE 5'-PHOSPHOSULFATE AS AN INTERMEDIATE IN THE OXIDATION OF THIOSULFATE BY THIOBACILLUS THIOPARUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1053–1057. doi: 10.1073/pnas.46.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZCZEPKOWSKI T. W. Reactions of thiosulphate with cysteine. Nature. 1958 Oct 4;182(4640):934–935. doi: 10.1038/182934a0. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Werkman C. H. GLUTATHIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS. Proc Natl Acad Sci U S A. 1959 Feb;45(2):239–244. doi: 10.1073/pnas.45.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Fixation of carbon dioxide by extracts of the strict autotroph Thiobacillus denitrificans. Biochem J. 1956 Oct;64(2):274–286. doi: 10.1042/bj0640274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 1. Fractionation of bacterial extracts and properties of cytochromes. Biochem J. 1961 Apr;78:673–680. doi: 10.1042/bj0780673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 2. Thiosulphate-oxidizing enzyme. Biochem J. 1961 Apr;78:680–686. doi: 10.1042/bj0780680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. The effects of thiosulphate and oxygen concentration on tetrathionate oxidation by Thiobacillus X and T. thioparus. Biochem J. 1964 Mar;90(3):640–646. doi: 10.1042/bj0900640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., TRUDINGER P. A. Symposium on autotrophy. V. Carbon dioxide fixation and substrate oxidation in the chemosynthetic sulfur and hydrogen bacteria. Bacteriol Rev. 1962 Jun;26:168–175. [PMC free article] [PubMed] [Google Scholar]


