Summary
Toll-like receptors (TLRs) are central mediators of innate antimicrobial and inflammatory responses and play instructive roles in the development of the adaptive immune response. Thus when stimulated by certain agonists, TLRs serve as adjuvant receptors that link innate and adaptive immunity. However, when excessively activated or inadequately controlled during an infection, TLRs may contribute to immunopathology associated with inflammatory diseases, such as periodontitis. Moreover, certain microbial pathogens appear to exploit aspects of TLR signalling in ways that enhance their adaptive fitness. The diverse and important roles played by TLRs suggest that therapeutic manipulation of TLR signalling may have implications in the control of infection, attenuation of inflammation, and the development of vaccine adjuvants for the treatment of periodontitis. Successful application of TLR-based therapeutic modalities in periodontitis would require highly selective and precisely targeted intervention. This would in turn necessitate precise characterization of TLR signalling pathways in response to periodontal pathogens, as well as development of effective and specific agonists or antagonists of TLR function and signalling. This review summarizes the current status of TLR biology as it relates to periodontitis, and evaluates the potential of TLR-based approaches for host-modulation therapy in this oral disease.
Toll-like receptors and potential for periodontal host modulation
Periodontitis can be defined as an infection-driven chronic inflammatory disease that affects the integrity of tooth-supporting tissues (179). Several oral bacteria within the subgingival dental plaque biofilm have been implicated in the initiation and progression of the disease. These include the “red complex” bacteria, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, as well as other organisms, such as Aggregatibacter actinomycetmcomitans, Prevotella intermedia, and Eikenella corrodens (99, 212). Although periodontal bacteria are essential for disease initiation, host susceptibility, as determined by various genetic, environmental, and other risk factors, is also implicated in the etiopathogenesis of periodontitis (65, 121). In fact, it is the host inflammatory reaction to uncontrolled bacterial challenge that primarily mediates tissue damage (9, 120, 219).
The discovery of the Toll-like receptor (TLR) family of pattern recognition receptors in the late 1990s (2) has filled a great mechanistic gap in our understanding of inflammatory mechanisms in infectious diseases such as periodontitis (68, 85, 151, 220). Indeed, at the molecular level, TLRs have emerged as central mediators of innate immunity and inflammation with an instructive role in adaptive immunity and polarization of the T cell responses (73, 175). Although TLRs can induce critical antimicrobial responses (34, 183), it has become apparent that TLR-mediated inflammation constitutes an important link between infection and a number of clinical conditions, such as periodontitis, atherosclerosis, ulcerative colitis, rheumatoid arthritis, and septic shock (5, 29, 33, 120). The potentially diverse roles played by TLRs suggest that therapeutic manipulation of TLR signalling may have implications in the control of infection, attenuation of inflammation, development of vaccine adjuvants, as well as in desensitization to allergens (62, 115, 231) (Table 1). The first three potential applications are relevant to periodontal disease and will concern us here in this review. Prior to that, a short review of existing modulation therapies is necessary for comparative reasons, that is to allow the reader to determine what additional advantages could be offered through TLR-based approaches.
Table 1.
TLR-mediated responses and potential applications for therapeutic immunomodulation*
| TLR-mediated responses |
Applications | Agents | TLRs involved** |
Refs. |
|---|---|---|---|---|
| Antimicrobial molecules (e.g., nitric oxide, β- defensins) |
infection control | agonists | TLR2, TLR4 | (233) |
| Type I interferons | antiviral immunity | agonists | TLR-3, -4, 7-, 8-, and -9 |
(15, 70, 155) |
| Adjuvant activities (e.g., upregulation of costimulatory molecules) |
vaccination | agonists | TLR2, TLR4, TLR9 |
(106, 231, 245) |
| Regulatory effects on antigen-presenting cells or lymphocytes |
skewing of the T cell subsets to the desired type of response |
agonists or antagonists |
TLR2, TLR4, TLR9 |
(60, 64, 73, 137) |
| Inflammatory mediators (e.g., TNF-α, IL-1β) |
chronic inflammatory diseases, septic shock |
antagonists | TLR4, TLR2/1, TLR2/6 |
(43, 140, 141, 154) |
| Evasion or subversion (of TLR signaling) by pathogens |
counteraction of microbial immune evasion/subversion |
agonists or antagonists |
TLR2, TLR4 | (40, 49, 89, 157, 207) |
Examples selected on the basis of their relevance to periodontal host modulation.
Potentially more TLRs are involved; mentioned were only those for which strong evidence exists.
Overview of current host-modulation therapies in periodontitis
Mechanical approaches to reduce the numbers of periodontal pathogens, such as scaling and root planing (SRP), are important for periodontal therapy although often inefficient on their own (63, 76). Several adjunctive therapies for host response modulation have thus been proposed and tested experimentally or clinically. These will be briefly mentioned here, as they have been extensively covered by other more specialized reviews (122, 169, 181, 196, 197).
Tetracyclines received considerable interest when it was discovered that subantimicrobial doses inhibited the activity of matrix metalloproteinases (72). Matrix metalloproteinases are elevated under pathological conditions, partly through TLR activation (135, 190), and mediate periodontal tissue destruction (24). Treatment of periodontitis patients with doxycycline, the most potent tetracycline at inhibiting collagenolytic activity, was shown to be generally beneficial as an adjunct to SRP. Specifically, the SRP/doxycycline combination is effective in terms of clinical attachment gain, reduction in probing depths, and reduction of gingival crevicular fluid levels of certain matrix metalloproteinases compared to SRP/placebo (169, 181, 197). Efforts to improve the clinical benefits of the combined SRP/doxycycline approach continue with modification of the dose and frequency of doxycycline administration (182). It is conceivable that this treatment may confer additional protective effects if supplemented with additional, compatible therapeutic agents that may be identified.
Another approach to modulating the host response in periodontitis involves systemic or topical administration of non-steroidal anti-inflammatory drugs (reviewed in refs. 162, 196). Non-steroidal anti-inflammatory drugs are characterized as non-selective or selective depending on whether they inhibit the activity of both isoforms of cyclooxygenase, i.e., cyclooxygenase -1 and cyclooxygenase-2 or only cyclooxygenase-2, respectively. Cyclooxygenase-2 represents the inducible isoform expressed in inflammatory cells. Both cyclooxygenase-1 and cyclooxygenase-2 utilize arachidonic acid as a substrate and synthesize prostanoids, among which prostaglandin E2 is strongly associated with periodontal tissue destruction (97, 187). Studies in animal models and clinical trials have shown that non-selective non-steroidal anti-inflammatory drugs inhibit alveolar bone resorption, although generally poor results were observed in their ability to influence clinical attachment gains or reductions in probing depth (122, 196). More recently, the use of selective cyclooxygenase-2 inhibitors was shown to inhibit periodontal bone loss in rats (22, 100). Treatment with selective cyclooxygenase-2 inhibitors may avoid certain adverse effects associated with the use of non-selective non-steroidal anti-inflammatory drugs, such as gastrointestinal mucosal damage and renal toxicity (95, 136). However, cyclooxygenase-2 inhibitors are not free of concerns either since they can cause prothrombotic side-effects (238), attributable to reversal of cyclooxygenase-2—dependent attenuation of expression of tissue factor, a protein that activates blood clotting (66). Since some non-steroidal anti-inflammatory drugs (e.g., flurbiprofen) are readily absorbed through the gingivae, topical application of non-steroidal anti-inflammatory drugs may help reduce the risk of systemic adverse effects (196). Nevertheless, additional, long-term studies are required to determine the safety and efficacy of the adjunctive use of non-steroidal anti-inflammatory drugs in the treatment of periodontitis. An alternative approach to regulating inflammation is discussed below.
In the past few years, it has been appreciated that resolution of inflammation is an active process mediated by specific pro-resolution agonists of endogenous (host) origin (202). Such agonists include small lipid molecules, such as lipoxins and resolvins, which are derived from arachidonic acid and other polyunsaturated fatty acids, respectively, and are considered for the treatment of inflammation-associated diseases including periodontitis (203, 232). A proof-of-principle study on the efficacy of lipoxin A4 in inhibiting P. gingivalis-induced periodontitis was performed in transgenic rabbits (204). These animals overexpress 15-lipooxygenase resulting in elevated production of lipoxin A4. Upon oral infection with P. gingivalis, 15-lipooxygenase transgenic rabbits displayed significantly reduced periodontal bone loss and gingival inflammation compared to non-transgenic control rabbits (204). Moreover, topical application of a stable analog of lipoxin A4 resulted in prevention of P. gingivalis-induced experimental periodontitis in normal (i.e., non-transgenic) rabbits. Similar protective effects in the same experimental model were observed after topical application of resolvin E1 (94). The protective effects of lipoxin A4 and resolvin E1 were attributed to their abilities to prevent excessive recruitment of neutrophils (and thereby inhibit neutrophil-mediated periodontal tissue destruction) and to promote resolution of inflammation (94, 204).
Pro-resolving agents may have advantages over non-steroidal anti-inflammatory drugs, which may in fact interfere with resolution of inflammation (71). Indeed, although cyclooxygenase-2 serves a pro-inflammatory role (e.g., via prostaglandin E2) during the early phase of neutrophil-dominated inflammation, it contributes to resolution of inflammation at a later, mononuclear cell-dominated phase through generation of pro-resolving prostaglandins (e.g., PGD2) (71). Therefore, non-steroidal anti-inflammatory drugs may abrogate the beneficial effects of pro-resolving prostaglandins. Traditional anti-inflammatory approaches may also interfere with protective responses against infection, especially when systemically administered (46), whereas pro-resolution agonists are thought to emulate the way inflammation resolves physiologically (202). A potential caveat with the exogenous application of pro-resolving agents in infection-driven inflammatory diseases is the possibility of prematurely resolving an inflammatory response before it has had the chance to control infection, therefore timing appears to be an important factor. Moreover, resolvins (at least of the D series) inhibit TLR4-dependent activation of macrophages (50), which might adversely affect protective innate immunity. There is a possibility, however, that these potential issues may, at least in part, be offset through the induction of antimicrobial effects by certain pro-resolution molecules (32, 41).
Bisphosphonates constitute a class of drugs that inhibit osteoclast function and bone resorption, and have thus been used for the prevention and treatment of osteoporosis (56). To determine their potential therapeutic use in periodontitis, several studies were performed in animal models of periodontitis. These studies showed that bisphosphonates have a protective effect against alveolar bone loss without, however, a concomitant improvement of gingival inflammation (reviewed in refs. 122, 197). Similar protective effects (reduced alveolar bone loss and improved mineral density) were observed in periodontitis patients administered bisphosphonates (alendronate or risedronate) as an adjunct to SRP. On the other hand, inconsistent results were obtained regarding their effects on other clinical parameters, such as clinical attachment level, probing pocket depth, and gingival index (122, 171, 197). It may therefore be concluded that bisphosphonates can protect at least against bone loss. However, more long-term studies may be necessary to establish safety, given the recent realization that bisphosphonates may be associated with osteonecrosis of the jaws (20, 193).
The inhibitory action of bisphosphonates on bone resorption could be explained, at least in part, through their effects on critical molecules involved in the regulation of osteoclast function (47, 146, 218). Specifically, a triad of proteins within the TNF/TNF-receptor family consisting of the receptor activator of nuclear factor-κB ligand (RANKL), its functional receptor RANK, and its decoy receptor osteoprotegerin (OPG) play a key role in osteoclast recruitment, differentiation, and activation (28). OPG serves a regulatory function by inhibiting the binding of RANKL (expressed by osteoblasts, fibroblasts, T cells, and B cells) to RANK on osteoclast precursors (28). Bisphosphonates appear to reduce the RANKL/OPG ratio, mostly by increasing OPG levels (47, 146, 218). The RANKL/RANK/OPG axis has moreover been targeted directly for the treatment of osteolytic pathologies (14). This may be an important therapeutic approach to suppressing inflammatory bone loss in periodontitis (91), although RANKL-mediated osteoclastogenesis may not be exclusively responsible for induction of bone loss in periodontal (228) or other experimental systems (124). Indeed, in proof-of-concept investigations, systemic or local treatment of mice or rats with OPG (as a fusion protein with the immunoglobulin Fc region) suppressed experimental induction of periodontal bone loss (90, 110, 142). Importantly, a recent clinical study showed that the RANKL/OPG ratio is significantly elevated in periodontitis and is positively correlated to probing pocket depth and clinical attachment level (27). Consistent with this, another study found that gingival expression of RANKL, but not OPG, is significantly correlated with increased numbers of P. gingivalis (237). These clinical observations provide a further rationale for the use of OPG as a RANKL antagonist.
However, a potential issue with the use of OPG-Fc as a therapeutic agent is the recent realization that OPG-Fc treatment in clinical trials results in elicitation of auto-antibodies which impair its biological activity (58). The development of a humanized anti-RANKL monoclonal antibody, known as denosumab or AMG 162, appears to overcome these shortcomings and is effective in the treatment of osteoporosis (17, 149). In fact, RANKL inhibitors may prove more effective than bisphosphonates, the only bone resorption inhibitors currently available (191). However, blockade of the RANK-RANKL interaction may not only affect bone metabolism but may also interfere with the normal function of the immune system. In this regard, RANKL enhances the survival and activity of dendritic cells and macrophages and promotes their antigen-presenting function (38, 172), thus necessitating additional studies investigating the safety of RANKL inhibitors (58). Moreover, a limitation of RANKL antagonistic approaches in rheumatoid arthritis is that they do not treat synovitis (189). By analogy, RANKL inhibition in periodontitis may inhibit bone resorption but will probably not treat inflammation or control the infection. Therefore, anti-RANKL therapy in human periodontitis may need to be combined with additional therapeutic modalities.
TLRs: Properties and roles in periodontitis
TLRs may offer novel targets for host-modulation therapy in periodontitis since manipulation of TLR signalling may contribute to control of infection or regulation of inflammation and, moreover, synthetic or natural TLR agonists could serve as novel periodontal vaccine adjuvants. For a better discussion of these new concepts, a brief background on TLR biology is deemed necessary. TLRs are primarily expressed by first-line professional defense cells (e.g., neutrophils, macrophages, and dendritic cells) as well as epithelial cells. They are thus strategically located at the host-pathogen interface, where they recognize microbe-associated molecular patterns for inducing innate immune and inflammatory responses and directing the development of adaptive immunity (96, 152, 175).
Structurally, the TLRs are type I transmembrane glycoproteins comprising an N-terminal leucine-rich repeat domain, a transmembrane region, and a C-terminal cytoplasmic signalling domain, known as the Toll/IL-1R domain (2). To date, 10 human TLRs have been identified which respond to different types of microbe-associated molecular patterns, endowing the innate response with a degree of specificity. For example, TLR2 responds to lipoteichoic acid, TLR3 to double-stranded viral RNA, TLR4 to lipopolysaccharide (LPS), TLR5 to flagellin, and TLR9 to bacterial CpG DNA (2, 21). These receptors are differentially expressed in terms of cellular localization; while TLRs mainly responsible for detecting extracellular microbial structures (TLRs 1, 2 , 4 , 5, and 6) are expressed on the surface, those TLRs which sense viral or bacterial nucleic acids (TLRs 3, 7, 8 and 9) are located intracellularly on endocytic vesicles or organelles. Another factor that enhances the capacity of TLRs to cope with infection is their property to intimately cooperate with other pattern recognition receptors (e.g., CD14, CD36, CR3) in multireceptor complexes in membrane lipid rafts (21, 86, 224). It is conceivable that the formation of TLR-containing receptor clusters may generate a combinatorial repertoire for discriminating among diverse microbial molecules and thereby to tailor the host response.
Following ligand binding, TLR signalling is triggered upon recruitment of adaptor proteins to the cytoplasmic domains of activated TLRs via Toll/IL-1R-Toll/IL-1R interactions (2, 165). MyD88 is a Toll/IL-1R-containing adaptor which is utilized by almost all TLRs except TLR3. However, only TLR2 and TLR4 utilize the MyD88-adaptor like (Mal) adaptor, which serves to recruit MyD88 to the TLR2 or TLR4 cytoplasmic domains (112). TLR4 is unique in that it initiates two divergent signalling cascades depending on distinct sets of adaptor molecules: The Mal/MyD88 pathway which activates mitogen-activated protein kinases and nuclear translocation of nuclear factor-κB, and the TRAM/TRIF (i.e., TRIF-related adapter molecule/Toll/IL-1R-domain-containing adapter inducing interferon-β) pathway, which additionally triggers activation of the interferon regulatory factor-3. These signalling cascades eventually result in the production of proinflammatorypro-inflammatory cytokines and type-I interferons. TRIF is additionally utilized for TLR3 downstream signalling (165). By contrast, a fifth known adaptor, designated sterile alpha and HEAT/Armadillo motif protein-1, functions as a negative regulator of TLR signalling (35). The existence of diverse intracellular pathways that can be activated downstream of distinct TLRs with different ligand specificities is indicative of the host’s potential to elicit a response that is appropriate for handling the invading pathogen. However, when excessively activated or inadequately controlled, TLRs become major players in chronic inflammation and autoimmunity through induction of high levels of pro-inflammatory cytokines. This and the documented upregulation of TLRs in diseased tissues have spurred an interest in the development of TLR antagonists as potential therapeutics for inflammatory diseases (5, 62, 150).
Using immunohistochemical methods, several groups have shown that the inflamed periodontium is infiltrated by TLR2- and TLR4-expressing cells (mostly monocytes/macrophages), whereas significantly lower levels of TLR2 and TLR4 expression are seen in relatively healthy gingivae (158, 160, 186). Using quantitative real-time PCR, another study has confirmed the upregulation of TLR2 and TLR4 and additionally demonstrated enhanced expression of TLR7 and TLR9 (but not of TLR5) in periodontitis lesions (114). TLR2 expression is readily detectable in gingival epithelia, being denser in the spinous epithelial layer than in the basal layer (126), and is upregulated in diseased (pocket) epithelia (186). However, TLR2-expressing cells are mostly found in the connective tissue subjacent to the pocket epithelium (158). Besides professional inflammatory cells, TLRs are expressed also by gingival fibroblasts, i.e., nonmyeloid cells responsible for the synthesis of extracellular matrix proteins. Gingival fibrobalsts constitutively express TLR2 and TLR4 (215), and their expression levels are elevated in periodontitis (236). No studies have been published yet examining TLR expression by human bone cells in periodontitis relative to health. Nonetheless, the documented expression of TLRs by osteoblasts and osteoclasts in the mouse model, and the findings that TLR activation influences osteoclastogenesis (4, 13, 119), suggests that TLRs may form a link between inflammation and bone metabolism (13, 117). However, the observed effects are quite complex ranging from TLR2- or TLR4-mediated induction of RANKL expression and osteoclast differentiation (119) to TLR9-mediated inhibition of RANKL-induced osteoclast differentiation (4). The latter effect depends on TLR9-induced expression of interleukin (IL)-12 which is known to inhibit osteoclastogenesis (103). Moreover, activation of several TLRs (e.g., TLR2, 4, and 9) in early osteoclast precursors inhibits their differentiation, whereas in cells already under osteoclastic differentiation, TLRs act in a stimulatory mode and enhance the survival rates of mature osteoclasts (13, 216). It has been proposed that TLR-mediated inhibition of osteoclastogenesis in early precursor cells may serve to reduce excessive bone loss caused by pathogen infection (13). Another initially unanticipated role of TLRs is their ability to detect tissue damage and initiate tissue regeneration (109, 130, 250). Whether TLRs also induce a tissue-repair gene expression program in the damaged periodontium has not been addressed yet.
Interestingly, the numbers of TLR2-expressing cells, but not of TLR4-expressing cells, tend to increase linearly with gingival inflammation (160). This may have to do with the fact that most suspected periodontal pathogens preferentially activate TLR2 rather than TLR4. Indeed, P. gingivalis, T. forsythia, T. denticola, P. intermedia, Prevotella nigrescens, Capnocytophaga ochracea, A. actinomycetemcomitans, Fusobacterium nucleatum and Veillonella parvula can all activate TLR2, but only the latter three can efficiently activate TLR4 (116, 192, 247). The regulated expression of TLRs in the periodontium and their activation by periodontal bacteria suggest that TLRs are potentially major players in periodontitis. Whether TLRs are involved in protective immunity or destructive inflammation (or both) has yet to be elucidated. Their potentially ambivalent role may be reflected in the studies aiming to correlate single nucleotide polymorphisms of TLR genes with susceptibility to periodontitis, which have been inconclusive when taken together (19, 57, 59, 107, 200). However, functional TLR polymorphisms, which impair the ability of affected individuals to respond properly to TLR ligands, have been correlated with susceptibility to certain infections (e.g., legionnaires’ disease) but with protection against chronic inflammatory diseases (e.g., atherosclerosis) (26, 225). Periodontal disease may be unique in that it is neither a classical bacterial infectious disease nor a conventional inflammatory disease. Consequently, the role of TLRs may be accordingly quite complex; their function should be necessary to keep pathogens at bay, although unregulated activation may contribute to the disease. Therefore, it becomes evident that to inhibit adverse host reactions and promote beneficial responses in periodontitis would necessitate highly selective and precisely targeted intervention. This would in turn require precise characterization of TLR signalling pathways and response outcomes in the periodontium. In this regard, research in animal models of periodontitis may be essential for complementing human studies, which are correlative at best as they are often limited by the difficulty to address mechanistic hypotheses (75). The intervention strategies discussed below are currently at a very early experimental stage. Nevertheless, the central importance of TLRs suggests that such approaches hold great promise for successful application in the treatment of periodontitis.
TLRs and control of inflammation
Despite the potential of TLR signalling cascades to activate expression of immunoregulatory molecules to fight infection, the same pathways may also induce serious immunopathological reactions if over stimulated or insufficiently controlled due to poor function of negative regulators of TLR signalling (134). Negative TLR regulation can be exerted either extracellularly (inhibition of receptor function) or intracellularly (inhibition of downstream signalling). Several strategies are being considered for the control of TLR-mediated inflammation (Table 2) and will be discussed below.
Table 2.
Strategies for inhibition of TLR function and signalling
| Approach | Mechanism | Refs. |
|---|---|---|
| 1) Monoclonal antibodies | blockade of ligand binding inhibition of TLR dimerization inhibition of TLR/coreceptor interactions |
(154, 222) |
| 2) Natural or synthetic antagonists | blockade of ligand binding | (36, 140, 141) |
| 3) Soluble decoy TLRs | blockade of ligand binding inhibition of TLR/coreceptor interactions |
(129, 156) |
| 4) BB loop decoy peptides | inhibition of Toll/IL-1R-dependent TLR/adaptor interactions |
(138, 139, 221) |
| 5) Dominant-negative versions of signalling adaptors |
abrogation of TLR-mediated signalling | (55, 195) |
| 6) RNA interference and microRNA-based approaches |
inhibition of expression of receptors or downstream signalling intermediates |
(6, 18, 53, 209) |
| 7) Kinase inhibitors | negative regulation of TLR downstream signalling |
(48, 145, 188) |
| 8) Anti-cytokine therapy (neutralizing antibodies, soluble cytokine receptors, receptor antagonists) |
neutralization of TLR-induced pro- inflammatory cytokines |
(74, 163) |
Monoclonal antibody blockade may be effective for inhibition of TLR signalling, at least for those TLRs that are expressed on the cell surface (TLR-1, -2, -4, -5, -6 and -10). Mechanistically, this could be achieved either by blocking ligand binding or inhibiting TLR dimerization or their interaction with essential co-receptors. Proof-of-concept prevention of lethal septic shock in mice has been demonstrated after TLR2 (154) or TLR4 (43) blockade. However, a possible caveat to this approach is the possible engagement of Fc receptors (by the antibodies) with potentially undesirable consequences. This may be overcome through the use of natural or synthetic non-antibody antagonists. For example, an LPS-like molecule extracted from Oscillatoria planktothrix FP1 inhibits LPS-activated MyD88- and TRIF-mediated signalling pathways and protects mice from endotoxic shock (141). This is based on the previously demonstrated concept of TLR antagonism, according to which atypical LPS molecules (tetra-acylated LPS from P. gingivalis or penta-acylated msbB LPS from E.coli) block the ability of classical enterobacterial LPS to bind MD-2 and activate the TLR4/MD-2 complex (36). Moreover, eritoran (E5564, alpha-D-glucopyranose; Eisai Inc.) is a promising synthetic analogue of bacterial lipid A which inhibits TLR4/MD2-dependent cell activation by LPS (140). Crystallographic analysis has confirmed that eritoran interacts with the hydrophobic internal pocket in MD-2 and prevents LPS from activating the TLR4/MD2 complex (118). Importantly, eritoran was shown to block the human endotoxin response in a model of clinical sepsis (140). This compound may thus potentially be used for the treatment or prevention of sepsis and perhaps other inflammatory conditions. At least in principle, soluble versions of TLR receptors could be used to inhibit the interaction of TLRs with ligands or with critical co-receptors, such as CD14 or MD-2. Experimental evidence for the feasibility of this antagonistic approach has been demonstrated using a natural soluble form of TLR2 (129) or recombinantly expressed soluble TLR4 (156).
Low-molecular weight compounds that interfere with the function of key signalling molecules in the TLR cascades are additional candidates for the treatment of inflammatory conditions. One such approach is based on the shared Toll/IL-1R domain by the TLRs and their adaptors. A short amino-acid sequence within the Toll/IL-1R domain, the so-called BB loop, is critical for the interaction between certain TLRs or between TLRs and their adaptors (165). Cell permeable versions of specific Toll/IL-1R BB loop amino-acid sequences (BB peptides) were constructed and evaluated (221). TLR2- and TLR4-specific BB peptides were shown to inhibit nuclear factor-κB activation by LPS or lipopeptides, respectively, suggesting their potential as therapeutic drugs (221). In an analogous manner, a cell permeable peptide targeting the BB loop of the MyD88 Toll/IL-1R domain specifically inhibits MyD88-mediated signalling by interfering with the homodimerization of the MyD88 Toll/IL-1R domains (139). Oral administration of this compound (named ST2825) inhibits CpG-induced activation of TLR9 or IL-1β-induced production of IL-6 in mice (138). Therefore, ST2825 can inhibit MyD88-dependent inflammatory signalling cascades, such as those induced by TLR or IL-1 receptor activation, and may find application in the treatment of chronic inflammatory diseases. Another approach to blocking the function of TLR signalling adaptors involves the use of dominant-negative versions of MyD88 or TIRAP (Mal), which have also been shown to suppress inflammatory responses (55, 195).
Inhibition of gene expression by means of RNA interference has successfully been used to study TLR signalling by targeting receptors or signalling intermediates (6). Although this approach can in principle be used to treat human TLR-mediated inflammatory diseases, it appears that small interfering RNAs themselves may be recognized by intracellular TLRs, like TLR7 and TLR8, in a sequence-specific mode (102, 209). Therefore, to develop RNA interference as a therapeutic tool, it would be necessary to screen and select appropriate sequences or chemically modified small interfering RNAs in ways that do not trigger TLR signalling (102, 209). More recently, microRNA-based approaches have been developed which may effectively manipulate TLR expression in vivo. The microRNAs constitute an evolutionarily conserved class of noncoding RNA molecules that negatively regulate gene expression at the level of translation, although microRNAs which are perfectly complimentary to their target mRNAs can act at the transcriptional level by causing direct mRNA cleavage (53, 144). Approaches based on microRNA technology could thus be used to regulate TLR expression for therapeutic purposes. For example, TLR2 was recently identified as a target of miR-105. Transfection of mature miR-105 mimic (same sequence as mature miR-105) into gingival epithelial cells repressed their ability to express TLR2 and mediate TLR2-induced signalling in response to P. gingivalis and other agonists (18). It is therefore conceivable that in vivo delivery of microRNAs targeting TLRs could be used for therapeutic intervention in periodontitis, although proof-of-concept animal studies have not yet been performed.
Intracellular signalling kinases also offer drug targets for negative regulation of TLR signalling. An effective way for downregulating TLR-mediated excessive inflammatory responses could be achieved through manipulation of the serine/threonine glycogen synthase kinase-3 (145). Specifically, although stimulation of human monocytes or peripheral blood mononuclear cells with diverse TLR agonists induces high levels of pro-inflammatory cytokines, these effects are significantly reversed after inhibition of glycogen synthase kinase-3. Moreover, inhibition of glycogen synthase kinase-3 activity upregulates the production of the anti-inflammatory cytokine IL-10 (145). Mechanistically, glycogen synthase kinase-3 inhibition allows the cAMP response element-binding protein to effectively compete with the p65 subunit of nuclear factor-κB for limiting amounts of a common transcriptional co-activator, the cAMP response element-binding protein—binding protein (145, 173). This explains both the inhibition of nuclear factor-κB-dependent pro-inflammatory cytokines and the upregulation of the cAMP response element-binding protein—dependent IL-10 production. Importantly, intravenous administration of a glycogen synthase kinase-3 inhibitor suppressed LPS-induced inflammation in mice and protected 70% of the animals against endotoxin shock and death (145). It remains to be established whether inhibition of glycogen synthase kinase-3 can also protect against periodontal inflammation in animal models. For possible future use in humans, it would be necessary to investigate potential side effects arising from general inhibition of glycogen synthase kinase-3, since this kinase plays diverse roles in immunity and other physiologic functions (242). However, it should be noted that lithium, one of many available glycogen synthase kinase-3 inhibitors, is relatively well tolerated as a mood stabilizer in human patients (251).
The p38 mitogen-activated protein kinase is another kinase activated downstream of TLRs that is considered as a target for the treatment of inflammatory diseases, such as psoriasis and rheumatoid arthritis, and certain p38 inhibitors have actually advanced to clinical trials (48, 167). Activation of p38 mitogen-activated protein kinase signalling results in production of pro-inflammatory cytokines and prostaglandin E2 and promotes osteoclast differentiation, though not osteoclast function (131, 234). An orally administered p38 antagonist (SD282) was used to determine the efficacy of p38 inhibition in a rat model of periodontitis, where bone loss was induced by gingival injections of A. actinomycetemcomitans LPS (188). The results showed that treatment with the p38 inhibitor suppressed bone resorption, concomitantly with reduced IL-1β and TNF-α expression and osteoclast formation, suggesting the therapeutic potential of this approach (188).
Other studies have focused on directly inhibiting the effects of pro-inflammatory cytokines with anti-cytokine therapy (74, 163). Although pro-inflammatory cytokines are important components of the innate response against infection, host over reaction to the inciting stimuli results in excessive production of IL-1, TNF, and other cytokines, which may cause collateral damage to host tissues in chronic inflammatory states (150). To prevent or minimize inflammatory tissue destruction, cytokine antagonists such as recombinant IL-1 receptor antagonist, soluble TNF receptors (as Fc fusion proteins), or anti-TNF specific antibodies are currently being considered for the treatment of rheumatoid arthritis and other autoimmune inflammatory conditions (150). Evidence from both clinical and experimental animal studies has strongly implicated IL-1 and TNF in periodontal tissue degradation, manifested as loss of attachment and bone resorption (reviewed in ref. 74). Proof-of-principle therapeutic neutralization of IL-1 and TNF in experimental periodontitis has been accomplished in the Macaca fascicularis primate model using soluble receptors to both cytokines (7). Specifically, administration by local injection of function-blocking soluble receptors to IL-1 and TNF caused 80% inhibition in inflammatory cell recruitment to the periodontium, 70% inhibition in osteoclast formation, and 60% reduction in P. gingivalis-induced alveolar bone loss (7). In another study, IL-11 was tested for its ability to inhibit experimental periodontitis (147), on the rationale that it displays anti-inflammatory properties including inhibition of IL-1 and TNF production (223). The investigation showed that subcutaneous administration of recombinant human IL-11 in the beagle dog model of ligature-induced periodontitis inhibits attachment loss and alveolar bone resorption compared to placebo treatment (147).
TLR adjuvants, periodontal vaccines, and immunomodulation
General considerations
Implicit in the definition of periodontitis given in the introduction is the concept that periodontal disease activity is determined by a complex interplay between the host immune system and periodontal pathogens. Accordingly, direct tissue damage by virulence factors of periodontal pathogens is not a major pathogenetic mechanism, nor is periodontal disease simply an aberrant or idiopathic inflammatory response. Rather, subgingival bacteria initiate and sustain a non-resolving inflammation which is ineffective at controlling the infection (60). Inasmuch as TLR agonists can function as adjuvants, linking innate to adaptive immunity and modulating both (105), it is conceivable that an appropriately adjuvanted periodontal vaccine could both reduce the bacterial challenge and favorably modulate the host response to infection. At least in principle, vaccination against periodontitis could be used preventively or therapeutically as an adjunct to SRP. Periodontal vaccines may confer specific protection via antibody-mediated blockade of bacterial colonization, neutralization of virulence factors, or through opsonophagocytosis and killing of periodontal pathogens. Moreover, a vaccine which induces specific cell-mediated immunity may potentially protect against periodontal intracellular pathogens which find refuge in permissive cells (reviewed in ref. 83). Induction of specific cell-mediated immunity with production of Type I interferons may be the most appropriate means to control the intracellular replication of herpesviruses, which are suspected to contribute to the pathogenesis of periodontitis (104, 210). For a successful periodontal vaccine, the importance of selecting appropriate adjuvant and protective immunogen(s) cannot be overstated. For a detailed discussion of candidate immunogens as promising periodontal vaccine targets, the reader is referred to more specialized reviews published recently (83, 206).
Adjuvanted vaccination against periodontal bacteria
The discovery of TLRs has not only resulted in a resurgent interest in innate immunity, but additionally suggested novel approaches to adjuvant development, some of which may be suitable for consideration in periodontal vaccine formulations. TLRs can function as adjuvant receptors by recognizing and responding to certain microbial molecules, thus effectively stimulating antigen-presenting cells and alerting the immune system (105). Prior to the discovery of TLRs, a great part of adjuvant research was focused on detoxified versions of several ganglioside-binding enterotoxins (reviewed in ref. 78). Currently, besides the enterotoxin-based approaches, TLR agonists and synthetic analogues are key targets of the pharmaceutical industry for developing vaccine adjuvants to prevent infectious diseases or destroy tumours (62). Certain adjuvants, such as the monophosphoryl lipid A or the peptidoglycan-derived muramyl dipeptide, which were developed before the discovery of TLRs, happen to work through activation of TLRs and other pattern-recognition receptors. For example, monophosphoryl lipid A is a chemically detoxified form of the lipid A from Salmonella minnesota LPS and stimulates the immune response through activation of TLR4 (148). Besides monophosphoryl lipid A, newly developed TLR9-immunostimulatory CpG oligonucleotides and synthetic TLR7/8 ligands are promising adjuvants under clinical development, being tested in vaccine formulations against various infectious diseases (115). Several other adjuvants are currently at an experimental level of development, including the B subunit of Type IIb E. coli enterotoxin, which is unique in that it interacts with both TLR and ganglioside receptors and can be effectively administered via mucosal routes (132, 133). Most of these novel adjuvants, with the exception of monophosphoryl lipid A, have not been tested yet in periodontal vaccines. Although mostly “old-generation” adjuvants have been used so far, significant advancements have been made in periodontal vaccine development (summarized below), and is likely that the future use of novel TLR adjuvants will lead to further progress.
The concept that vaccination against periodontal pathogens can confer protection against periodontitis was first demonstrated in proof-of-principle rodent studies where whole bacterial cells or sonicates thereof (e.g., P. gingivalis or E. corrodens) were used as immunogens (16, 123). However, due to potential problems relating to adverse reactogenicity, subsequent vaccine development efforts in animal models focused on defined microbial molecules or engineered subunits of such structures, in order to minimize detrimental side-effects. Subunit vaccine approaches have so far concentrated mainly on P. gingivalis virulence proteins, particularly its cysteine proteinases, as well as the fimbriae of both P. gingivalis and A. actinomycetemcomitans (Table 3). Vaccination with defined subunit immunogens requires the use of appropriate adjuvants even more so than in the case of immunization with whole bacterial cells which intrinsically contain adjuvant substances (e.g., LPS). Some of the studies have used Freund’s complete or incomplete adjuvant or cholera toxin, while other studies have used adjuvants that are more appropriate for human use, such as monophosphoryl lipid A, either alone or supplemented with trehalose dicorynomycolate (Ribi adjuvant system) (Table 3).
Table 3.
Adjuvanted immunization studies against periodontal pathogens
| Immunogen | Adjuvant | Animal model |
Immunization route |
Results | Refs. |
|---|---|---|---|---|---|
|
P. gingivalis hemoglobin- binding domain of gingipain (HA2) |
Freund’s complete adjuvant |
rats | subcutaneous | High levels of serum IgG antibody responses Protection against bone loss * |
(44) |
| Purified ariginine gingipains (RgpA or RgpB) from P. gingivalis |
Freund’s complete adjuvant |
mice | subcutaneous | Elevated IgG antibody responses Protection against bone loss in animals immunized with RgpA but not with RgpB |
(67) |
| Proteinase adhesin complex ( RgpA- Kgp) from P. gingivalis |
Freund’s incomplete adjuvant |
rats | subcutaneous | High-titer serum IgG2a antibody responses Protection against bone loss Inhibition of P. gingivalis colonization |
(184) |
|
P. gingivalis cysteine proteinase (porphypain-2) |
Freund’s incomplete adjuvant |
monkeys | intramuscular & subcutaneous |
Elevated IgG antibody responses Reduction in total number of gram-negative anaerobes but not of P. gingivalis |
(159) |
|
P. gingivalis recombinant hemagglutinin B |
monophosphoryl lipid A | mice | intranasal | High levels of serum IgG and salivary IgA antibody responses |
(245) |
| Fimbrial peptide from A. actinomycetemcomi tans |
Ribi adjuvant system R-700 ** |
mice | intramuscular | High-titer serum IgG antibody responses In vitro inhibition of A. actinomycetem- comitans adherence by anti-peptide serum IgG antibodies |
(101) |
| Fimbrial peptide from A. actinomycetemcomi tans |
cholera toxin and IL-4- expressing plasmid |
mice | intranasal | High levels of salivary IgA antibody responses |
(101) |
| Formalin-killed P. gingivalis |
Syntex Adjuvant Formulation-M *** |
monkeys | intramuscular & subcutaneous |
Elevated serum IgG antibody responses Protection against bone loss Modest inhibitory effect against P. gingivalis colonization |
(178) |
| Formalin-killed P. gingivalis |
Syntex Adjuvant Formulation-M *** |
monkeys | intramuscular & subcutaneous |
Elevated IgG antibody responses in gingival crevicular fluid Suppressed gingival crevicular fluid levels of prostaglandin E2 Inhibition of bone loss |
(187) |
The adjuvant was associated with enhanced IgG responses (vs. immunogen alone) but not with bone loss protection
Emulsion of monophosphoryl lipid A and synthetic trehalose dicorynomycolate
Oil-in-water emulsion stabilized by Tween 80 and pluronic polyoxyethlene/polyoxypropylene block copolymer L121(Syntex Laboratories, Palo Alto, CA)
Important recent periodontal vaccination studies are summarized in Table 3 and are briefly discussed here. Subcutaneous immunization of rats with the hemoglobin-binding domain of gingipain (HA2) from P. gingivalis resulted in specific IgG antibodies and modest protection against bone loss (44). The same immunogen was also administered with Freund’s complete adjuvant, which significantly potentiated the antibody responses but, strikingly, prevented the protective effect on bone loss (44). This appears paradoxical given that the magnitude of the antibody response was positively correlated with protection in the non-adjuvanted group. However, the adjuvant might have stimulated innate mechanisms of inflammatory bone resorption. The findings from this study (44) underscore the necessity for selecting appropriate adjuvants that can enhance specific protective immunity with minimal or no stimulation of inappropriate responses that contribute to periodontal pathogenesis. In another study, rats immunized with a combination of ariginine- and lysine-specific gingipains (RgpA/Kgp), in Freund’s incomplete adjuvant, elicited high-titer serum IgG2a antibody responses to the gingipains, resulting in protection against P. gingivalis-induced periodontal bone loss and suppression of colonization by the pathogen (184). The protective effect of immunization with RgpA is also supported by an independent study performed in mice, which additionally found that, unlike RgpA, RgpB failed to induce protection against bone loss despite inducing specific IgG antibodies (67).
Mucosal immunization (e.g., by the oral or intranasal route) represents a more convenient and safer approach to systemic immunization, and is the only method that can effectively generate secretory IgA antibodies for protection against mucosal pathogens at the portals of their entry (80, 81). In this regard, intranasal immunization with recombinant hemagglutinin B from P. gingivalis, in the presence of monophosphoryl lipid A, resulted in considerably higher serum IgG and salivary IgA antibody responses compared to immunization with immunogen alone, although protection against disease was not examined (245). In another study, systemic or intranasal immunization of mice with an adjuvanted synthetic peptide derived from the sequence of A. actinomycetemcomitans fimbriae resulted in preferential induction of specific serum IgG or salivary IgA antibodies, respectively (101) (Table 3). Protection against disease was not specifically addressed, although serum IgG antibodies to the same peptide inhibited the adherence of fimbriated A. actinomycetemcomitans strains to experimental salivary pellicles (101). Mucosal immunization of rats with Streptococcus gordonii vectors expressing segments of P. gingivalis FimA fimbriae induced specific salivary IgA and serum IgG antibody responses, which conferred protection against subsequent P. gingivalis-induced periodontal bone loss (205). Although this immunization was not adjuvanted, it is possible that its efficacy could be further improved by the coadministration of one of the novel TLR-based adjuvants.
Vaccination studies in monkeys are particularly important as this model is closer to the human condition than the more convenient and relatively inexpensive rodent models. Immunization of monkeys with purified P. gingivalis cysteine proteinase (porphypain-2) in Freund’s incomplete adjuvant elicited specific antibody responses but failed to suppress the emergence of this pathogen in the oral flora, although the total number of gram-negative anaerobes was suppressed (159). Furthermore, there were no significant differences in periodontal bone loss between immunized and control animals, despite a trend for relative protection in the immunized group (159) (Table 3). Therefore, specific immunization against a defined virulence protein displayed only limited potential for protective immunity in monkeys. However, vaccination of monkeys with formalin-killed P. gingivalis elicited specific IgG responses and conferred significant protection against bone loss, although the inhibitory effect against P. gingivalis colonization was less pronounced (178). This study utilized Syntex Adjuvant Formulation-M, which is an oil-in-water emulsion stabilized by Tween 80 and pluronic polyoxyethlene/polyoxypropylene block copolymer L121) (178). In a similar study by the same group, monkey vaccination resulted in dramatically suppressed levels of prostaglandin E2 in the gingival crevicular fluid and, importantly, this observation was correlated with decreased bone loss compared to non-immunized controls (187). This study suggests that suppression of inflammatory mediators by immunization, perhaps owing to reduced pathogenic challenge as a result of antibody-dependent inhibition of colonization, is a potentially protective mechanism against periodontitis. Nevertheless, more studies with defined subunit vaccines are warranted to limit the possibility of adverse reactions. Although it is uncertain whether oil-in-water emulsions will be sufficient to stimulate protective immunity when purified proteins or segments thereof are used for immunization, this can likely be achieved with powerful, yet safe, TLR-based adjuvants.
Antiviral immunity and periodontitis
Herpesviruses have emerged as putative periodontal pathogens (104, 210) with the potential to synergize with pathogenic bacteria in periodontal tissue destruction (211). For example, herpesviruses found in periodontal inflammatory cells (37) may compromise the ability of these immune cells to fight bacterial pathogens in the periodontium. In this regard, it was shown that human cytomegalovirus primarily infects monocytes and macrophages, herpes simplex virus infects T cells, and Epstein-Barr virus type 1 infects B cells, whereas neutrophils harbored no herpesviruses (37). The recent demonstration for elevated expression of a number of intereferon-stimulated genes in peripheral blood neutrophils from chronic periodontitis patients (243) is consistent with viral-induced production of Type I interferons. However, these responses can also be contributed via bacterial lipopolysaccharide-activated TLR4/TRIF signalling or bacterial DNA-activated TLR9 signalling (226).
Induction of cell-mediated immunity with production of Type I interferons may be the most appropriate means to control the intracellular replication of herpesviruses in periodontal tissues. As alluded to earlier in this review, a subset of TLRs (specifically, TLR-3, -7, -8, and -9) are expressed in endosomal compartments and can sense viral nucleic acids in the intraluminal space (226). TLR3 recognizes double-stranded viral RNA, TLR7 and TLR8 detect viral single-stranded viral RNA, and TLR9 detects viral DNA. Activation of signaling cascades downstream of these TLRs leads to induction of Type I interferons, such as interferon-α and interferon-β, and thus specific synthetic agonists are being considered for antiviral immunity (15, 226). Although TLR4 is expressed at the cell surface where it can activate the Mal/MyD88 pathway, it can also activate TRAM/TRIF signaling in early endosomes upon relocation from the cell surface, leading to induction of interferon-β responses (113). Thus, even though the prototypical TLR4 agonist (lipopolysaccharide) is not a viral component, lipopolysaccharide or detoxified derivatives (e.g., monophosphoryl lipid A) may in principle be exploited for inducing antiviral immunity.
Studies involving a limited number of human patients with deficiencies in antiviral signaling pathways, suggest that TLR7-, TLR8-, and TLR9-mediated antiviral responses play important but redundant roles in protective immunity to most viruses (248). On the other hand, TLR3-deficient patients appear to be specifically susceptible to herpes simplex virus type 1 encephalitis (249). However, human TLR3 appears to be redundant in host defense to most other viral infections (248). These findings suggest that agonists activating a specific endosomal TLR may be appropriate for inducing antiviral immunity to a number of different viral infections, including those associated with periodontitis.
Synthetic agonists capable of effectively activating TLR-mediated antiviral responses have been tested in mice and humans, either for enhancing antiviral innate immunity or as vaccine adjuvants, aiming to elicit protective adaptive immunity (15, 70, 155). For example, the TLR7 agonist imiquimod was found effective in clinical studies for topical treatment of genital warts caused by human papillomavirus (HPV), although mixed results were obtained for herpes simplex virus type 2 infections (155). Resiquimod, is an imiquimod analogue with dual TLR7/8 agonistic activity, which was tested in clinical topical applications for the treatment of genital herpes lesions (herpes simplex virus type 2) and met with mixed success (54, 143). In mice, the use of CpG oligonucleotides, as TLR9-activating adjuvants for intranasal or intravaginal vaccination with recombinant glycoprotein B of herpes simplex virus type 2, confers complete protection against intravaginal infection with this virus (15, 61, 127). Moreover, vaginal delivery of CpG or the TLR3 agonist Poly I:C in mice protects against genital herpes simplex virus type 2 infection, in a way that depends on interferon-α/β signaling (70, 214). Moreover, vaccines against other herpersviruses, such as cytomegalovirus, Epstein-Barr virus, and herpes simplex virus type 1, are currently in development (241, 244). It will be interesting to see if these vaccines, when available for clinical use, can positively influence periodontal disease.
TLR3- and TLR9-dependent cross-priming of CD8+ T cells by dendritic cells (the mechanism is discussed in the next subsection) is another important mechanism for the control of viruses or intracellular bacterial pathogens (161). Cross-priming of CD8+ T cells may thus be of relevance to periodontal immunotherapy, not only because herpesviruses are suspected as contributory factors in periodontal tissue destruction (210) but also since certain pathogenic bacteria (e.g., P. gingivalis) appear to replicate intracellularly in epithelial cells (128). In this context, it should be noted that MHC class I-responsive CD8+ T cells, unlike MHC class II-responsive CD4+ T cells, have not been implicated in host-mediated periodontal tissue destruction (10). Therefore, cross-priming of CD8+ T cells may result in protective immunity free of potentially adverse reactions.
Mechanisms of action of TLR adjuvants
The knowledge of the mode of action of TLR adjuvants is important for the selection of those that can potentially induce appropriate type of responses against a given infectious disease. Various mechanisms have been proposed whereby TLR-interacting adjuvants stimulate protective immunity (Table 4). Upon encounter with pathogens, dendritic cells undergo a maturation process characterized by enhanced surface expression of MHC class II and co-stimulatory molecules (e.g., CD40, CD80 and CD86) and migrate from peripheral tissues to the T cell areas of secondary lymphoid organs (12). Once in the lymph nodes, the activated dendritic cells produce immunostimulatory cytokines (e.g., IL-1β, IL-6, IL-12, and TNF-α) and prime naïve T lymphocytes through antigen presentation and induction of co-stimulatory signalling (12, 105). Pathogen-induced dendritic cell maturation can be replicated by purified TLR agonists, which moreover up-regulate CCR7, a lymph node-homing chemokine receptor (108). In this regard, a dendritic cell vaccine genetically engineered to co-express antigen and CCR7 elicits augmented antigen-specific immune responses compared to a similar dendritic cell vaccine transduced with antigen alone (168). Moreover, the efficacy of a DNA vaccine against herpes simplex virus is enhanced if co-delivered with a plasmid DNA expressing CCR7 ligands (52).
Table 4.
TLR adjuvant mechanisms
| Mechanisms | Refs. |
|---|---|
| 1) Stimulation of dendritic cell maturation and migration | (12, 105, 108, 168) |
| 2) Induction of phagosome maturation and presentation of their peptide cargo by MHC class II |
(25) |
| 3) Enhancement of antigen uptake | (106, 199) |
| 4) Direct action on T cell function a) Maintenance of T cell memory b) Expansion of effector T cells and attenuation of T regulatory cell suppressive activity |
(125, 137) |
| 5) Reversal of established T regulatory cell-induced tolerance |
(174, 177, 246) |
| 6) Costimulation of naïve B cells (“third signal”) | (194) |
| 7) Cross-priming of CD8+ T cells | (42, 98, 201) |
In addition to inducing antigen-presenting cell maturation, an effective vaccine should stimulate peptide loading of MHC class II molecules for inducing CD4+ T cell responses. In this context, TLRs dictate the fate of internalized antigens (25). Specifically, the presence of TLR ligands within the internalized antigen cargo of a phagosome (e.g., when a TLR adjuvant is used for vaccination) “marks” such phagosomes (but not those containing self-antigens which do not engage TLR signalling) for an inducible mode of maturation and presentation of their peptide cargo by MHC Class II (25). In addition, an independent study showed that ligand binding to TLR2 on human dendritic cells triggers receptor-mediated endocytosis of the ligand resulting in efficient presentation of ligand epitopes by MHC class II molecules (199). This principle was applied for vaccine development. Specifically, totally synthetic vaccines consisting of (i) a T helper cell epitope, (ii) a target antigen epitope and (iii) a TLR2-targeting lipid moiety, were capable of inducing protective immunity in different models (e.g., infection with influenza virus or Listeria monocytogenes) (106). In contrast, unlipidated versions of the same vaccines failed to induce protection against viral or bacterial infection, which suggests that including a TLR2-targeting lipid moiety confers self-adjuvanticity in these synthetic vaccines (106).
Although naïve human T cells do not typically express TLRs, TLR2 appears to be expressed on the surface of activated and memory T cells (125). In terms of function, TLR2 was shown to act as a co-stimulatory receptor molecule for T cell activation and to contribute to the maintenance of T cell memory (125). This suggests that TLR2 adjuvants may contribute directly to the establishment of long-term T cell memory, in the absence of specific pathogen or vaccine immunogen. In a more recent study, the same group showed that TLR2 signalling regulates the adaptive immune response by enhancing the proliferation of TCR-activated regulatory (CD4+CD25+) as well as effector (CD4+CD25-) T cells (137). However, TLR2 signalling transiently suppresses the induction of Foxp3 in T regs which are temporarily switched off, thereby allowing strong activation of effector T cells (137). The transient nature of TLR-mediated immunomodulation of T regulatory cells allows them to regain their suppressive function and thus to prevent potential autoimmunity.
Even when T regulatory cell-mediated peripheral tolerance has been established, this can be reversed by TLR activation. Indeed, the suppressive function of T regulatory cells is counteracted by TLR stimulation of dendritic cells in a way that depends on induction of IL-6 release (174). Moreover, viral vaccine-induced TLR signals were shown to be essential for reversing T regulatory cell-mediated suppression of CD8+ T cells in vivo (246). Furthermore, synthetic and natural TLR8 agonists can also reverse T regulatory cell-induced tolerance, although this mechanism is independent of dendritic cell function and requires direct TLR8 activation of T regulatory cells (177).
With regard to humoral immunity, it is thought that the activation of naïve B cells depends upon two signals. The first signal is mediated by specific antigen recognition resulting in cross-linking of the B cell receptor. This is followed by a second signal mediated by cognate interaction with T helper cells leading to effective B cell stimulation rather than anergy (105). However, this paradigm was recently challenged in that these two signals, i.e., B cell receptor activation and T cell help, were found insufficient in themselves to promote survival and differentiation of human naïve B cells, despite initial induction of cell division (194). Therefore, a third signal is apparently required for B cell differentiation and production of antibodies. The requisite third signal is provided by microbial products via stimulation of B cell TLRs that are upregulated in naïve B cells upon B cell receptor triggering (e.g., TLR2/1, TLR2/6, or TLR9, but not TLR3 or TLR4). The third signal may also be provided indirectly, albeit less efficiently, by cytokines released from TLR-activated dendritic cells (194). This novel information can be exploited for vaccine development. For instance, if the immunization strategy against a given infectious disease calls for induction of humoral immunity, then the adjuvant included should be capable of stimulating upregulatable B cell TLRs (i.e., a TLR2 agonist may be more effective than a TLR4-interacting adjuvant).
Activation of CD8+ T cells by cross-priming is an important mechanism for vaccine responses against intracellular pathogens. The cross-priming mechanism depends on the exogenous pathway for MHC class I antigen, which allows foreign antigen to be taken up by antigen-presenting cells, processed and presented on MHC class I molecules for cross-priming of CD8+ T cells (98). Therefore, this pathway is important for the development of cytotoxic lymphocyte responses against infectious pathogens that do not directly infect the cross-presenting antigen-presenting cells. Cross-presentation can be induced by a subset of TLR ligands, specifically those that can activate TLR3 or TLR9 (42, 201). Cross-priming of CD8+ T cells may be of relevance to periodontal immunotherapy as it can be exploited for the control of both intracellular periodontal bacteria and herpesviruses.
TLR adjuvants and skewing of the T cell responses
Inasmuch as TLRs link innate to adaptive immunity and antigen-presenting cells receive cues from bacterial TLR agonists (73, 105), it can be reasonably expected that TLR-interacting adjuvants can influence the nature of T cell responses in periodontitis. For example, it is thought that TLR2 or TLR4 activation preferentially induces Th2- or Th1-biased responses, respectively (1, 73, 185). The existence of an array of natural, modified, or synthetic TLR agonists with both overlapping and unique immunostimulatory signalling (reviewed in refs. 62, 115) suggests the feasibility of selecting adjuvants that are appropriate for vaccine formulations against a given disease. With regard to periodontal disease, however, it is not yet quite clear what type(s) of T cell responses provide protection or, conversely, mediate destructive effects. In this context, periodontitis is not accurately described in straightforward Th1 vs. Th2 dichotomous terms and, in fact, may involve additional modes of T cell function such as the recently described Th17 (reviewed in ref. 60).
There is supportive, though not conclusive, evidence that Th1 cells and their cytokines characterize early/stable periodontal lesions (reviewed in ref. 64). If the Th1 response cannot be sustained, however, this may lead to Th2-driven disease progression characterized by increased infiltration by plasma cells secreting low-affinity, non-protective antibodies (64). By contrast, certain other studies have ascribed destructive effects to Th1 cells (217, 227). Nevertheless, the implication of Th1 cells in destructive inflammation in various diseases requires re-interpretation. This is because the presence or involvement of IL-12, a signature Th1 cytokine, was until recently concluded by assessment of the IL-12/23 common p40 subunit, and thus involvement of the Th17 subset (rather than the Th1) cannot be ruled out (240). In fact, recent evidence implicates Th17 as a dedicated osteoclastogenic subset (198). Under the extended Th1/Th2/Th17 paradigm, it may be possible to clarify what constitutes protective vs. destructive host responses in periodontitis. This in turn would facilitate the selection of vaccine/adjuvant intervention modalities to maximize the protective and minimize the destructive aspects of the periodontal T cell responses. In other words, the choice of adjuvant is a critical parameter, not only for enhancing the magnitude of specific immune responses, but also for modulating the T cell response to the desired outcome. Lack of such knowledge in periodontal vaccine development may result in vaccine-induced host responses which could potentially do more harm than good.
Counteracting microbial subversion of TLR-mediated immunity
The complexity of the interplay between the immune system and periodontal pathogens is best described by a circular rather than an arrow model (60). An arrow would be an accurate representation only if the role of the bacteria was to simply initiate inflammation, thus “permitting” the host inflammatory response to subsequently act independently in causing tissue damage. The circular model predicts that periodontal disease progression is determined by a continuous host-pathogen cross-talk (60). Accordingly, the skewing, magnitude, and quality of the immune response is constantly shaped by periodontal pathogens. These may subvert the host response and trigger a non-resolving inflammation that is ineffective to control the infection. This non-productive inflammation in turn provides the bacteria with serum-derived nutrients and new niches for colonization through the generation of deep periodontal pockets (60). Therefore, it becomes essential to develop strategies for counteracting microbial immune subversion in order to redirect the immune response to benefit the host. In other words, if a specific signalling pathway is exploited by a pathogen for enhancing its adaptive fitness, interventions that inhibit this pathway (by blocking the receptor involved or by targeting a downstream signalling intermediate) may deprive the pathogen of an immune evasion mechanism.
Although P. gingivalis and other periodontal organisms are successful pathogens, the mechanisms whereby they manipulate innate immunity and resist TLR-mediated elimination are only now starting to be investigated and understood (Table 5). As discussed above, most suspected periodontal pathogens preferentially activate TLR2 rather than TLR4 (116, 192, 247), although more studies examining additional species would be required to conclusively assert this notion. Since there are several examples of pathogens capable of exploiting TLR2 signalling for inducing immunosuppression and escaping immunosurveillance (170, 176, 208, 235, 239), it is tempting to speculate that periodontal pathogens may activate TLR2 for this reason. Alternatively, TLR2 may not necessarily be exploited by periodontal pathogens, but TLR2 innate responses may be less harmful for the bacteria than TLR4 signalling which activates both MyD88-dependent and MyD88-independent pathways (166). Interestingly, a limited subset of TLRs that includes TLR4, but not TLR2 (as either a TLR2/1 or TLR2/6 heterodimer), activate autophagy in a way that eliminates intracellular pathogens in macrophages (45). The molecular basis, however, for the failure of TLR2 to stimulate this protective response is not clear since TLR4 stimulates autophagy through the MyD88 signalling pathway which is also activated by TLR2. The importance of TLR4 in controlling infection is also suggested by findings that certain pathogens have opted to modify their surface structures so as to escape TLR4 recognition (8, 157, 207). In a similar context from a periodontal viewpoint, P. gingivalis expresses a diverse mixture of atypical LPS molecules, including species that trigger TLR2 signalling, weakly stimulate TLR4, but potently antagonize TLR4 activation by other stronger agonists (40). Importantly, the differential expression of distinct lipid A moities in P. gingivalis represents an actively regulated process in response to microenvironmental concentrations of hemin (3). It could be speculated, therefore, that by altering the proportions of its different lipid A moieties, P. gingivalis may increase its virulence through manipulation of the innate response (40). Approaches interfering with the ability of P. gingivalis to sense hemin concentrations in its microenvironment could, at least in principle, block its capacity to regulate its TLR2 vs. TLR4 interactive profile. Moreover, P. gingivalis LPS is significantly more potent than classical enterobacterial LPS in upregulating the IL-1R-associated kinase (IRAK)-M, a negative regulator of the TLR signalling pathway (49). This mechanism inhibits the innate response of macrophages and in principle could serve as an immune evasion strategy. Targeting IRAK-M for blocking its activity might be a way to neutralize this putative escape strategy, although it could compromise the ability of the cells to regulate their pro-inflammatory activities. The same challenge would apply to any case of periodontal pathogen capable of up-regulating negative regulators of protective TLR responses. Although in principle such a microbial strategy could be negated by suppressing the expression of the identified negative regulator(s) (e.g., by means of RNA interference; Table 2), the intervention would require sufficient, if not exquisite, specificity. This, however, is a formidable undertaking since many signalling molecules are common to multiple TLRs or even other receptors and, therefore, regulating the activity of a certain target may have mixed (both protective and destructive) results.
Table 5.
Manipulation of TLR-mediated immunity by P. gingivalis and possible counterstrategies
| Mechanisms | Intervention | Refs. |
|---|---|---|
| 1) Manipulation of TLR2 vs. TLR4 responses by altering its lipid A structure |
Blockade of hemin- binding proteins? * |
(3, 40) |
| 2) Upregulation of negative regulators of TLR signalling(IRAK-M) |
Inhibition of IRAK-M activity? ** |
(49) |
| 3) Induction of TLR2 inside-out signalling in macrophages for CR3-dependent inhibition of IL-12 |
CR3 antagonists (e.g., XVA143) |
(84, 235) |
| 4) Degradation of essential TLR coreceptors (CD14) or immunostimulatory cytokines |
Gingipain inhibitors (e.g., KYT-1, KYT-36) or anti-gingipain vaccines |
(11, 111, 213) |
| 5) Instigation of CXCR4/TLR2 cross-talk for suppressing macrophage-mediated clearance |
CXCR4 antagonists (e.g. AMD3100) |
(89) |
The rationale is based on the finding that alteration of lipid A structure is regulated by microenvironmental hemin concentrations (3).
This could be achieved through specific RNA interference or microRNA-based approaches, although upregulation of undesirable inflammatory responses may be a serious side-effect.
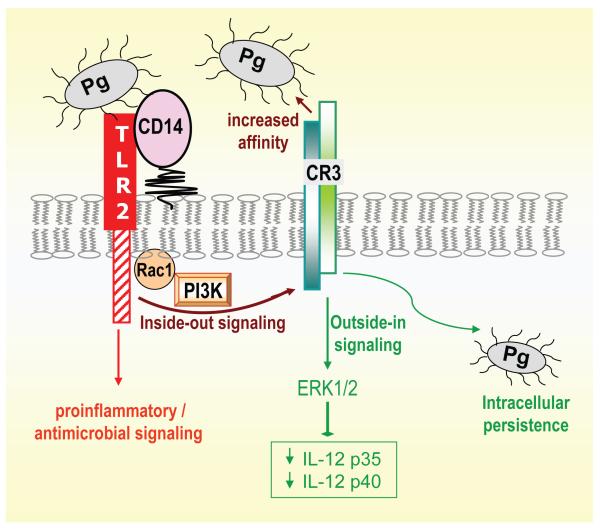
There is intriguing in vivo evidence that P. gingivalis-induced TLR2 signalling is detrimental for the host in the context of periodontitis (30, 69), although not in acute infections (87). Specifically, in a periodontal context, TLR2-deficient mice are more resistant to P. gingivalis-induced bone loss than wild-type controls (30, 69), which could be attributed to TLR2-dependent osteoclastogenesis via TNF-α induction (228) and/or to decreased phagocytosis and killing of P. gingivalis (30). Although the latter appears paradoxical, given the ability of TLR2 to induce antimicrobial responses for pathogen control (23, 87, 229, 230), the authors speculated that a TLR2-induced secreted immunosuppressive factor may reduce neutrophil-dependent bacterial killing (30). The notion that P. gingivalis may be exploiting at least some aspects of TLR2 signalling is also supported by another study. Specifically, recent evidence suggests that P. gingivalis fimbriae activate TLR2 inside-out signalling for activating the ligand-binding capacity of macrophage complement receptor-3 (CR3), a multitask β2 integrin (CD11b/CD18) (79, 82) (Fig. 1). In its activated/high-affinity state, CR3 recognizes structurally unrelated molecules from either the host (e.g., complement C3 fragment and intercellular adhesion molecule-1) or pathogens (e.g., Bordetella pertussis filamentous hemagglutinin) (51, 79). CR3 activation is a potentially protective host mechanism since this molecule plays a role in the migration of phagocytes to sites of extravascular inflammation (153). However, CR3 activation by P. gingivalis via TLR2 inside-out signalling (92, 93) leads to CR3-mediated uptake of this oral pathogen by macrophages (88). Intriguingly, CR3 uptake does not promote the killing of P. gingivalis. Indeed, CR3 blocking studies and experiments in wild-type or CR3-deficient mice indicate that the interaction of CR3 with P. gingivalis causes IL-12 suppression in vitro and in vivo, resulting in increased intracellular survival of the pathogen in macrophages (Fig. 1) and enhanced tissue persistence and periodontal bone loss in mice (84, 235). CR3 blockade was attempted as an intervention counter-strategy to block the ability of P. gingivalis for manipulating the TLR2/CR3 inside-out pathway. Indeed, treatment of mice with gingival injections of XVA143, a small-molecule CR3 antagonist, promoted induction of IL-12 and inhibited P. gingivalis-induced periodontitis (84). The ability of CR3 antagonism to negate immune evasion by P. gingivalis holds promise for a potential therapeutic immunomodulation in human periodontitis.
Fig. 1. P. gingivalis exploits TLR2 inside-out signalling to subvert innate immunity.
P. gingivalis activates the high-affinity conformation of CR3 through TLR2 inside-out signalling involving Rac1 and phosphatidylinositol 3-kinase (PI3K) (92, 93). CD14 participates in the pathway as a coreceptor for efficient P. gingivalis-induced TLR2 stimulation, which in parallel activates a distinct nuclear factor-κB-dependent pro-inflammatory/antimicrobial pathway (82, 86). Intriguingly, P. gingivalis can interact with activated CR3 leading to its internalization and outside-in signalling, which via extracellular signal-related kinase 1/2 (ERK1/2) downregulates IL-12 p35 and p40 mRNA expression. This in turn inhibits production of bioactive IL-12 and undermines IL-12-mediated immune clearance of the pathogen (84, 235). Blocking TLR2 would counteract this immune evasion strategy but may also suppress protective nuclear factor-κB-dependent antimicrobial responses. On the other hand, inhibitors of signalling intermediates of the inside-out pathway or antagonists of CR3 may selectively target this immune evasion mechanism. The latter approach (CR3 antagonism) was successfully tested in the mouse periodontitis model (84).
P. gingivalis employs additional immune evasion tactics that depend on expression of other virulence factors, such as the Arg- and Lys-specific cysteine proteinases (gingipains). P. gingivalis gingipains are encoded by three genes, namely rgpA, rgpB and kgp (39), and play critical virulence roles in animal models (164). Gingipains can degrade host proteins, including extracellular matrix proteins as well as several that are involved in host defense (180). It is thus thought that gingipains may dysregulate normal defense function. For example, the gingipains inhibit human serum-dependent bactericidal activity by degrading IgG and IgM as well as complement factor C3 (77). CD14, which is an essential co-receptor for TLR-mediated innate defense, is also degraded by gingipains (213) and so are cytokines such as TNF-α (31) or IL-6 (11). There have been no reports yet as to whether gingipains can also degrade and inactivate TLRs. In an effort to neutralize immune evasion or other virulence activities of the gingipains, synthetic inhibitors specific for Rgp and Kgp have been developed and are being considered for therapeutic applications (111). In addition, neutralization of gingipain activity may be conferred by specific vaccines (83).
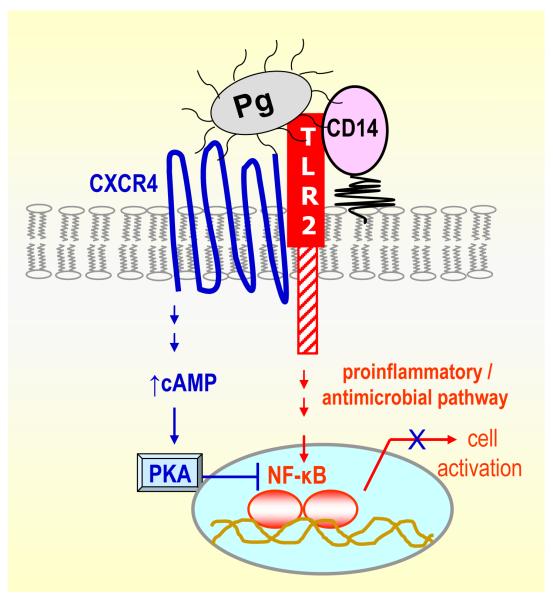
A novel mechanism of P. gingivalis evasion of TLR-mediated immunity was recently reported that is dependent upon exploitation of the CXC-chemokine receptor 4 (CXCR4) (89). Specifically, P. gingivalis instigates co-association of TLR2 and CXCR4 in macrophage lipid rafts and interacts with both receptors. Binding to CXCR4 induces cAMP-dependent protein kinase A (PKA) signalling, which in turn inhibits TLR2-mediated pro-inflammatory (nuclear factor-κB activation and TNF-α production) and antimicrobial (nitric oxide production) responses (Fig. 2). This molecular cross-talk is exploited by P. gingivalis for promoting its adaptive fitness as evidenced by its ability to resist clearance in vitro and in vivo in a CXCR4-dependent way. The use of the bicyclam drug AMD3100, a specific CXCR4 antagonist, abrogates this immune evasive tactic of P. gingivalis (89). Thus, CXCR4 antagonists may offer a promising strategy for the control of P. gingivalis infections. In general, if activation of a specific pathway facilitates pathogen persistence in the host, then strategies that block this pathway (by targeting the receptor or downstream signalling molecules; see Table 2) may attenuate microbial virulence.
Fig. 2. Inhibition of TLR2-mediated innate immune responses through P. gingivalis exploitation of CXCR4.
P. gingivalis instigates a molecular cross-talk between CXCR4 and TLR2 in macrophage lipid rafts. Unlike CD14 which facilitates TLR2 activation by P. gingivalis (86), CXCR4 suppresses TLR2 signalling (89). Specifically, the pathogen binds CXCR4 and induces cAMP-dependent PKA signalling, which in turn inhibits TLR2-mediated nuclear factor-κB activation. This promotes the in vivo survival of the pathogen, unless CXCR4 is blocked by specific antagonists (89).
Additional in vivo evidence is required for the support of the above discussed putative immune evasion strategies. However, how important could counteracting those strategies possibly be? Even though P. gingivalis is a major periodontal pathogen, periodontitis is caused by a pathogenic biofilm rather than by a specific pathogen. If, however, P. gingivalis adds a panoply of critical virulence properties to the biofilm, then approaches to neutralize P. gingivalis may impact upon the whole biofilm. For example, the interaction of P. gingivalis with CR3 at the macrophage cell surface suppresses IL-12 production also in response to other bacterial stimuli, such as A. actinomycetemcomitans (84). Therefore, this immune evasion mechanism may promote the survival of both P. gingivalis and other pathogens that are present in the mixed species biofilm in the subgingival pocket. Moreover, attempts to neutralize the proteolytic activity of P. gingivalis would probably impact on other species too; this is because the proteolytic degradation of antimicrobial molecules or the generation of peptide nutrients from uninhibited gingipain function would be beneficial to both P. gingivalis and other bacteria sharing the same niche.
Conclusions
Although TLR-based therapeutic modalities for treating periodontitis are in their infancy, they have the potential to uniquely target all conceivable pathways contributing to the disease. Specifically, TLRs or their induced pathways can be manipulated in ways that may enhance antimicrobial responses, negate microbial immune evasion strategies, suppress destructive inflammation, initiate tissue repair, and enhance the effectiveness of vaccination through effective adjuvant action. Other host-modulation approaches may not be that versatile. For example, the use of bisphosphonates or anti-RANKL monoclonal antibodies could readily suppress bone loss but would not control periodontal infection or soft tissue inflammation. Traditional anti-inflammatory approaches do not generally have antimicrobial effects and may adversely affect protective innate immunity. As we learn more about the intricacies of TLR signalling, it becomes evident that the control of adverse host responses in infection-driven inflammatory diseases would require highly selective and precisely targeted intervention. This in turn necessitates studies to precisely characterize TLR signalling pathways in response to important periodontal pathogens. The challenge becomes even greater if TLRs are to be manipulated also for enhancing their protective aspects, e.g., to elicit antimicrobial responses or to stimulate appropriate T cell responses through vaccine adjuvants. The continuous discovery and characterization of TLR-mediated signalling cascades with both overlapping and unique elements, and our ability to uncover their roles in the elicitation of protective or destructive responses, may open the Toll gates for novel host-modulation therapy in periodontitis.
Acknowledgements
Studies performed in the author’s lab and cited in this review were supported by U.S. Public Health Service Grants DE015254, DE017138, and DE018292 from the NIDCR, National Institutes of Health.
References
- 1.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 2006;74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amcheslavsky A, Bar-Shavit Z. Interleukin (IL)-12 mediates the anti-osteoclastogenic activity of CpG-oligodeoxynucleotides. J Cell Physiol. 2006;207:244–250. doi: 10.1002/jcp.20563. [DOI] [PubMed] [Google Scholar]
- 5.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 6.Aoki M, Ishii T, Kanaoka M, Kimura T. RNA interference in immune cells by use of osmotic delivery of siRNA. Biochem Biophys Res Commun. 2006;341:326–333. doi: 10.1016/j.bbrc.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 7.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 8.Backhed F, Normark S, Schweda EK, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057–1063. doi: 10.1016/s1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- 9.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 10.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banbula A, Bugno M, Kuster A, Heinrich PC, Travis J, Potempa J. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1999;261:598–602. doi: 10.1006/bbrc.1999.1075. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Shavit Z. Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity. 2008;41:195–203. doi: 10.1080/08916930701694469. [DOI] [PubMed] [Google Scholar]
- 14.Baud’huin M, Lamoureux F, Duplomb L, Redini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–2350. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer S, Pigisch S, Hangel D, Kaufmann A, Hamm S. Recognition of nucleic acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9. Immunobiology. 2008;213:315–328. doi: 10.1016/j.imbio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Behling UH, Sallay C, Sanavi F, Pham PH, Nowotny A. Humoral immunity and reduced periodontal bone loss in Eikenella corrodens-monoassociated rats. Infect Immun. 1981;33:801–805. doi: 10.1128/iai.33.3.801-805.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 18.Benakanakere MR, Li Q, Eskan MA, Singh AV, Zhao J, Galicia JC, Stathopoulou P, Kinane DF. MicroRNA-105 modulates TLR-2 responses in oral keratinocytes. J Biol Chem. 2009 submitted. [Google Scholar]
- 19.Berdeli A, Emingil G, Saygan B Han, Gurkan A, Atilla G, Kose T, Baylas H. TLR2 Arg753Gly, TLR4 Asp299Gly and Thr399Ile gene polymorphisms are not associated with chronic periodontitis in a Turkish population. J Clin Periodontol. 2007;34:551–557. doi: 10.1111/j.1600-051X.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertoldo F, Santini D, Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol. 2007;4:711–721. doi: 10.1038/ncponc1000. [DOI] [PubMed] [Google Scholar]
- 21.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 22.Bezerra MM, de Lima V, Alencar VB, Vieira IB, Brito GA, Ribeiro RA, Rocha FA. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2000;71:1009–1014. doi: 10.1902/jop.2000.71.6.1009. [DOI] [PubMed] [Google Scholar]
- 23.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–3137. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 25.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 26.Bochud PY, Bochud M, Telenti A, Calandra T. Innate immunogenetics: a tool for exploring new frontiers of host defence. Lancet Infect Dis. 2007;7:531–542. doi: 10.1016/S1473-3099(07)70185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H, Atilla G, Hughes FJ, Belibasakis GN. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 28.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 29.Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233:90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 31.Calkins CC, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-α by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J Biol Chem. 1998;273:6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- 32.Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci U S A. 2002;99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cario E, Podolsky DK. Toll-like receptor signaling and its relevance to intestinal inflammation. Ann N Y Acad Sci. 2006;1072:332–338. doi: 10.1196/annals.1326.006. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter S, O’Neill LA. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 35.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 36.Coats SR, Pham TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol. 2005;175:4490–4498. doi: 10.4049/jimmunol.175.7.4490. [DOI] [PubMed] [Google Scholar]
- 37.Contreras A, Zadeh HH, Nowzari H, Slots J. Herpesvirus infection of inflammatory cells in human periodontitis. Oral Microbiol Immunol. 1999;14:206–212. doi: 10.1034/j.1399-302x.1999.140402.x. [DOI] [PubMed] [Google Scholar]
- 38.Cremer I, Dieu-Nosjean MC, Marechal S, Dezutter-Dambuyant C, Goddard S, Adams D, Winter N, Menetrier-Caux C, Sautes-Fridman C, Fridman WH, Mueller CG. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood. 2002;100:3646–3655. doi: 10.1182/blood-2002-01-0312. [DOI] [PubMed] [Google Scholar]
- 39.Curtis MA, Kuramitsu HK, Lantz M, Macrina FL, Nakayama K, Potempa J, Reynolds EC, Aduse-Opoku J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodont Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 40.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das UN. Do unsaturated fatty acids function as endogenous antibacterial and antiviral molecules? Am J Clin Nutr. 2006;83:390–391. doi: 10.1093/ajcn/83.2.390. [DOI] [PubMed] [Google Scholar]
- 42.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 43.Daubeuf B, Mathison J, Spiller S, Hugues S, Herren S, Ferlin W, Kosco-Vilbois M, Wagner H, Kirschning CJ, Ulevitch R, Elson G. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol. 2007;179:6107–6114. doi: 10.4049/jimmunol.179.9.6107. [DOI] [PubMed] [Google Scholar]
- 44.DeCarlo AA, Huang Y, Collyer CA, Langley DB, Katz J. Feasibility of an HA2 domain-based periodontitis vaccine. Infect Immun. 2003;71:562–566. doi: 10.1128/IAI.71.1.562-566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. Embo J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimakou K, Papaioannides D, Latsi P, Katsimboula S, Korantzopoulos P, Orphanidou D. Disseminated tuberculosis complicating anti-TNF-alpha treatment. Int J Clin Pract. 2004;58:1052–1055. doi: 10.1111/j.1742-1241.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- 47.Dobnig H, Hofbauer LC, Viereck V, Obermayer-Pietsch B, Fahrleitner-Pammer A. Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos Int. 2006;17:693–703. doi: 10.1007/s00198-005-0035-4. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel. 2005;8:421–430. [PubMed] [Google Scholar]
- 49.Domon H, Honda T, Oda T, Yoshie H, Yamazaki K. Early and preferential induction of IL-1 receptor-associated kinase-M in THP-1 cells by LPS derived from Porphyromonas gingivalis. J Leukoc Biol. 2008;83:672–679. doi: 10.1189/jlb.0607432. [DOI] [PubMed] [Google Scholar]
- 50.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 51.Ehlers MRW. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 52.Eo SK, Lee S, Kumaraguru U, Rouse BT. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine. 2001;19:4685–4693. doi: 10.1016/s0264-410x(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 53.Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv Drug Deliv Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Fife KH, Meng TC, Ferris DG, Liu P. Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions. Antimicrob Agents Chemother. 2008;52:477–482. doi: 10.1128/AAC.01173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 56.Fleisch H. The role of bisphosphonates in breast cancer: Development of bisphosphonates. Breast Cancer Res. 2002;4:30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folwaczny M, Glas J, Torok HP, Limbersky O, Folwaczny C. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol. 2004;135:330–335. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fouque-Aubert A, Chapurlat R. Influence of RANKL inhibition on immune system in the treatment of bone diseases. Joint Bone Spine. 2008;75:5–10. doi: 10.1016/j.jbspin.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Fukusaki T, Ohara N, Hara Y, Yoshimura A, Yoshiura K. Evidence for association between a Toll-like receptor 4 gene polymorphism and moderate/severe periodontitis in the Japanese population. J Periodontal Res. 2007;42:541–545. doi: 10.1111/j.1600-0765.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 60.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL 17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol. 2001;166:3451–3457. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 62.Gearing AJH. Targeting toll-like receptors for drug development: a summary of commercial approaches. Immunol Cell Biol. 2007;85:490–494. doi: 10.1038/sj.icb.7100102. [DOI] [PubMed] [Google Scholar]
- 63.Geisinger ML, Mealey BL, Schoolfield J, Mellonig JT. The Effectiveness of Subgingival Scaling and Root Planing: An Evaluation of Therapy With and Without the Use of the Periodontal Endoscope. J Periodontol. 2007;78:22–28. doi: 10.1902/jop.2007.060186. [DOI] [PubMed] [Google Scholar]
- 64.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontology 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 65.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh M, Wang H, Ai Y, Romeo E, Luyendyk JP, Peters JM, Mackman N, Dey SK, Hla T. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARδ activation. J Exp Med. 2007;204:2053–2061. doi: 10.1084/jem.20070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibson FC, 3rd, Genco CA. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect Immun. 2001;69:7959–7963. doi: 10.1128/IAI.69.12.7959-7963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibson FC, 3rd, Genco CA. Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs. Curr Pharm Des. 2007;13:3665–3675. doi: 10.2174/138161207783018554. [DOI] [PubMed] [Google Scholar]
- 69.Gibson FC, 3rd, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci. 2008;13:2041–2059. doi: 10.2741/2822. [DOI] [PubMed] [Google Scholar]
- 70.Gill N, Davies EJ, Ashkar AA. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am J Reprod Immunol. 2008;59:35–43. doi: 10.1111/j.1600-0897.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 71.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 72.Golub LM, Goodson JM, Lee HM, Vidal AM, McNamara TF, Ramamurthy NS. Tetracyclines inhibit tissue collagenases. Effects of ingested low-dose and local delivery systems. J Periodontol. 1985;56:93–97. doi: 10.1902/jop.1985.56.11s.93. [DOI] [PubMed] [Google Scholar]
- 73.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 74.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 75.Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenwell H. Position paper: Guidelines for periodontal therapy. J Periodontol. 2001;72:1624–1628. doi: 10.1902/jop.2001.72.11.1624. [DOI] [PubMed] [Google Scholar]
- 77.Grenier D. Inactivation of human serum bactericidal activity by a trypsinlike protease isolated from Porphyromonas gingivalis. Infect Immun. 1992;60:1854–1857. doi: 10.1128/iai.60.5.1854-1857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005;84:1104–1116. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 79.Hajishengallis G, Harokopakis E. Porphyromonas gingivalis interactions with complement receptor 3 (CR3): innate immunity or immune evasion? Front Biosci. 2007;12:4547–4557. doi: 10.2741/2409. [DOI] [PubMed] [Google Scholar]
- 80.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 81.Hajishengallis G, Nikolova E, Russell MW. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A antibodies to the cell surface protein antigen I/II: Reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 83.Hajishengallis G, Russell MW. Molecular approaches to vaccination against oral infections. In: Rogers A, editor. Molecular Oral Microbiology. Caister Academic Press; Norfolk, UK: 2008. pp. 257–285. [Google Scholar]
- 84.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade Promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 85.Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. 2002;7:72–78. doi: 10.1902/annals.2002.7.1.72. [DOI] [PubMed] [Google Scholar]
- 86.Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S-I, Ratti P, Schifferle RE, Lyle EA, Triantafilou M, Triantafilou K, Yoshimura F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 87.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol. 2008;181:4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect Immun. 2006;74:5658–5666. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 91.Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 92.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 93.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 94.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 95.Hawkey CJ. Gastroduodenal problems associated with non-steroidal, anti-inflammatory drugs (NSAIDs) Scand J Gastroenterol Suppl. 1993;200:94–95. doi: 10.3109/00365529309101583. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 97.Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1 beta, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor alpha in experimental gingivitis in humans. J Periodontal Res. 1993;28:241–247. doi: 10.1111/j.1600-0765.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 98.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 99.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 100.Holzhausen M, Spolidorio DM, Muscara MN, Hebling J, Spolidorio LC. Protective effects of etoricoxib, a selective inhibitor of cyclooxygenase-2, in experimental periodontitis in rats. J Periodontal Res. 2005;40:208–211. doi: 10.1111/j.1600-0765.2005.00787.x. [DOI] [PubMed] [Google Scholar]
- 101.Honma K, Kato T, Okuda K. Salivary immunoglobulin A production against a synthetic oligopeptide antigen of Actinobacillus actinomycetemcomitans fimbriae. Oral Microbiol Immunol. 1999;14:288–292. doi: 10.1034/j.1399-302x.1999.140504.x. [DOI] [PubMed] [Google Scholar]
- 102.Hornung V, Barchet W, Schlee M, Hartmann G. RNA recognition via TLR7 and TLR8. Handb Exp Pharmacol. 2008:71–86. doi: 10.1007/978-3-540-72167-3_4. [DOI] [PubMed] [Google Scholar]
- 103.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001;166:4915–4921. doi: 10.4049/jimmunol.166.8.4915. [DOI] [PubMed] [Google Scholar]
- 104.Imbronito AV, Okuda OS, de Freitas N Maria, Lotufo RF Moreira, Nunes FD. Detection of Herpesviruses and Periodontal Pathogens in Subgingival Plaque of Patients With Chronic Periodontitis, Generalized Aggressive Periodontitis, or Gingivitis. J Periodontol. 2008;79:2313–2321. doi: 10.1902/jop.2008.070388. [DOI] [PubMed] [Google Scholar]
- 105.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 106.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, Brown LE. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.James JA, Poulton KV, Haworth SE, Payne D, McKay IJ, Clarke FM, Hughes FJ, Linden GJ. Polymorphisms of TLR4 but not CD14 are associated with a decreased risk of aggressive periodontitis. J Clin Periodontol. 2007;34:111–117. doi: 10.1111/j.1600-051X.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 108.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 109.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 110.Jin Q, Cirelli JA, Park CH, Sugai JV, Taba M, Jr., Kostenuik PJ, Giannobile WV. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol. 2007;78:1300–1308. doi: 10.1902/jop.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kadowaki T, Baba A, Abe N, Takii R, Hashimoto M, Tsukuba T, Okazaki S, Suda Y, Asao T, Yamamoto K. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol Pharmacol. 2004;66:1599–1606. doi: 10.1124/mol.104.004366. [DOI] [PubMed] [Google Scholar]
- 112.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 113.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kajita K, Honda T, Amanuma R, Domon H, Okui T, Ito H, Yoshie H, Tabeta K, Nakajima T, Yamazaki K. Quantitative messenger RNA expression of Toll-like receptors and interferon-alpha1 in gingivitis and periodontitis. Oral Microbiol Immunol. 2007;22:398–402. doi: 10.1111/j.1399-302X.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 115.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 116.Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ. Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiology and Immunology. 2007;22:145–151. doi: 10.1111/j.1399-302X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 117.Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T, Yoshikai Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol. 2001;166:3574–3579. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- 118.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 119.Kim KW, Cho ML, Lee SH, Oh HJ, Kang CM, Ju JH, Min SY, Cho YG, Park SH, Kim HY. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110:54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Kinane DF, Demuth DR, Gorr SU, Hajishengallis GN, Martin MH. Human variability in innate immunity. Periodontol 2000. 2007;45:14–34. doi: 10.1111/j.1600-0757.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 121.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 122.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klausen B, Evans RT, Ramamurthy NS, Golub LM, Sfintescu C, Lee JY, Bedi G, Zambon JJ, Genco RJ. Periodontal bone level and gingival proteinase activity in gnotobiotic rats immunized with Bacteroides gingivalis. Oral Microbiol Immunol. 1991;6:193–201. doi: 10.1111/j.1399-302x.1991.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kusumoto Y, Hirano H, Saitoh K, Yamada S, Takedachi M, Nozaki T, Ozawa Y, Nakahira Y, Saho T, Ogo H, Shimabukuro Y, Okada H, Murakami S. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J Periodontol. 2004;75:370–379. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- 127.Kwant A, Rosenthal KL. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV 2. Vaccine. 2004;22:3098–3104. doi: 10.1016/j.vaccine.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 128.Lamont RJ, Yilmaz O. In or out: the invasiveness of oral bacteria. Periodontol 2000. 2002;30:61–69. doi: 10.1034/j.1600-0757.2002.03006.x. [DOI] [PubMed] [Google Scholar]
- 129.LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, Labeta MO. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol. 2003;171:6680–6689. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 130.Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 131.Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143:3105–3113. doi: 10.1210/endo.143.8.8954. [DOI] [PubMed] [Google Scholar]
- 132.Liang S, Wang M, Tapping RI, Stepensky V, Nawar HF, Triantafilou M, Triantafilou K, Connell TD, Hajishengallis G. Ganglioside GD1a is an essential coreceptor for toll-like receptor 2 signaling in response to the B subunit of Type IIB enterotoxin. J Biol Chem. 2007;282:7532–7542. doi: 10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- 133.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of Type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 134.Liew FY, Xu D, Brint EK, O’Neill LAJ. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 135.Lim EJ, Lee SH, Lee JG, Kim JR, Yun SS, Baek SH, Lee C. Toll-like receptor 9 dependent activation of MAPK and NF-κB is required for the CpG ODN-induced matrix metalloproteinase-9 expression. Exp Mol Med. 2007;39:239–245. doi: 10.1038/emm.2007.27. [DOI] [PubMed] [Google Scholar]
- 136.Lindsley CB, Warady BA. Nonsteroidal antiinflammatory drugs. Renal toxicity. Review of pediatric issues. Clin Pediatr (Phila) 1990;29:10–13. doi: 10.1177/000992289002900101. [DOI] [PubMed] [Google Scholar]
- 137.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loiarro M, Capolunghi F, Fanto N, Gallo G, Campo S, Arseni B, Carsetti R, Carminati P, De Santis R, Ruggiero V, Sette C. Pivotal Advance: Inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol. 2007;82:801–810. doi: 10.1189/jlb.1206746. [DOI] [PubMed] [Google Scholar]
- 139.Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, Carminati P, Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J Biol Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- 140.Lynn M, Wong YN, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, Vargas R, D’Angelo T, Gotzkowsky S, McMahon FG, Wasan KM, Rossignol DP. Extended in vivo pharmacodynamic activity of E5564 in normal volunteers with experimental endotoxemia [corrected] J Pharmacol Exp Ther. 2004;308:175–181. doi: 10.1124/jpet.103.056531. [DOI] [PubMed] [Google Scholar]
- 141.Macagno A, Molteni M, Rinaldi A, Bertoni F, Lanzavecchia A, Rossetti C, Sallusto F. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J Exp Med. 2006;203:1481–1492. doi: 10.1084/jem.20060136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahamed DA, Marleau A, Alnaeeli M, Singh B, Zhang X, Penninger JM, Teng YT. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005;54:1477–1486. doi: 10.2337/diabetes.54.5.1477. [DOI] [PubMed] [Google Scholar]
- 143.Mark KE, Corey L, Meng TC, Magaret AS, Huang ML, Selke S, Slade HB, Tyring SK, Warren T, Sacks SL, Leone P, Bergland VA, Wald A. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis. 2007;195:1324–1331. doi: 10.1086/513276. [DOI] [PubMed] [Google Scholar]
- 144.Marquez RT, McCaffrey AP. Advances in microRNAs: implications for gene therapists. Hum Gene Ther. 2008;19:27–38. doi: 10.1089/hum.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martini G, Gozzetti A, Gennari L, Avanzati A, Nuti R, Lauria F. The effect of zoledronic acid on serum osteoprotegerin in early stage multiple myeloma. Haematologica. 2006;91:1720–1721. [PubMed] [Google Scholar]
- 147.Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000;71:573–578. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- 148.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 149.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 150.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 151.McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125:1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- 152.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 153.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 154.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004;113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21:69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 156.Mitsuzawa H, Nishitani C, Hyakushima N, Shimizu T, Sano H, Matsushima N, Fukase K, Kuroki Y. Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J Immunol. 2006;177:8133–8139. doi: 10.4049/jimmunol.177.11.8133. [DOI] [PubMed] [Google Scholar]
- 157.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 158.Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol Immunol. 2003;18:54–58. doi: 10.1034/j.1399-302x.2003.180109.x. [DOI] [PubMed] [Google Scholar]
- 159.Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–697. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- 160.Muthukuru M, Jotwani R, Cutler CW. Oral mucosal endotoxin tolerance induction in chronic periodontitis. Infect Immun. 2005;73:687–694. doi: 10.1128/IAI.73.2.687-694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neuenhahn M, Busch DH. Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens. Immunol Lett. 2007;114:66–72. doi: 10.1016/j.imlet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 162.Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 163.Numerof RP, Asadullah K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs. 2006;20:93–103. doi: 10.2165/00063030-200620020-00004. [DOI] [PubMed] [Google Scholar]
- 164.O’Brien-Simpson NM, Paolini RA, Hoffmann B, Slakeski N, Dashper SG, Reynolds EC. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect Immun. 2001;69:7527–7534. doi: 10.1128/IAI.69.12.7527-7534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 166.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 167.O’Neill LAJ. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 168.Okada N, Mori N, Koretomo R, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther. 2005;12:129–139. doi: 10.1038/sj.gt.3302358. [DOI] [PubMed] [Google Scholar]
- 169.Oringer RJ. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73:460–470. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- 170.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171:175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 171.Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother. 2007;8:309–315. doi: 10.1517/14656566.8.3.309. [DOI] [PubMed] [Google Scholar]
- 172.Park HJ, Park OJ, Shin J. Receptor activator of NF-kappaB ligand enhances the activity of macrophages as antigen presenting cells. Exp Mol Med. 2005;37:524–532. doi: 10.1038/emm.2005.65. [DOI] [PubMed] [Google Scholar]
- 173.Parry G, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 174.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 175.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 176.Pecora ND, Gehring AJ, Canaday DH, Boom WH, Harding CV. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol. 2006;177:422–429. doi: 10.4049/jimmunol.177.1.422. [DOI] [PubMed] [Google Scholar]
- 177.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 178.Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page RC. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–1031. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 180.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 181.Preshaw PM, Hefti AF, Jepsen S, Etienne D, Walker C, Bradshaw MH. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J Clin Periodontol. 2004;31:697–707. doi: 10.1111/j.1600-051X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 182.Preshaw PM, Novak MJ, Mellonig J, Magnusson I, Polson A, Giannobile WV, Rowland RW, Thomas J, Walker C, Dawson DR, Sharkey D, Bradshaw MH. Modified-Release Subantimicrobial Dose Doxycycline Enhances Scaling and Root Planing in Subjects With Periodontal Disease. J Periodontol. 2008;79:440–452. doi: 10.1902/jop.2008.070375. [DOI] [PubMed] [Google Scholar]
- 183.Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- 184.Rajapakse PS, O’Brien-Simpson NM, Slakeski N, Hoffmann B, Reynolds EC. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infect Immun. 2002;70:2480–2486. doi: 10.1128/IAI.70.5.2480-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 186.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–1959. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]
- 187.Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect Immun. 2004;72:1166–1168. doi: 10.1128/IAI.72.2.1166-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA, Higgins LS, Medicherla S, Kirkwood KL. A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol. 2007;78:1992–1998. doi: 10.1902/jop.2007.070101. [DOI] [PubMed] [Google Scholar]
- 189.Romas E, Gillespie MT. Inflammation-induced bone loss: can it be prevented? Rheum Dis Clin North Am. 2006;32:759–773. doi: 10.1016/j.rdc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 190.Rossa C, Jr., Liu M, Bronson P, Kirkwood KL. Transcriptional activation of MMP-13 by periodontal pathogenic LPS requires p38 MAP kinase. J Endotoxin Res. 2007;13:85–93. doi: 10.1177/0968051907079118. [DOI] [PubMed] [Google Scholar]
- 191.Roux S. RANKL inhibitors: a bright future? Joint Bone Spine. 2006;73:129–131. doi: 10.1016/j.jbspin.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 192.Ruby J, Rehani K, Martin M. Treponema denticola activates mitogen-activated protein kinase signal pathways through Toll-like receptor 2. Infect Immun. 2007;75:5763–5768. doi: 10.1128/IAI.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 194.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 195.Sacre SM, Andreakos E, Kiriakidis S, Amjadi P, Lundberg A, Giddins G, Feldmann M, Brennan F, Foxwell BM. The Toll-Like Receptor Adaptor Proteins MyD88 and Mal/TIRAP Contribute to the Inflammatory and Destructive Processes in a Human Model of Rheumatoid Arthritis. Am J Pathol. 2007;170:518–525. doi: 10.2353/ajpath.2007.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Salvi GE, Lang NP. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr Pharm Des. 2005;11:1757–1769. doi: 10.2174/1381612053764878. [DOI] [PubMed] [Google Scholar]
- 197.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):108–129. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 198.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schjetne KW, Thompson KM, Nilsen N, Flo TH, Fleckenstein B, Iversen JG, Espevik T, Bogen B. Cutting edge: link between innate and adaptive immunity: Toll-like receptor 2 internalizes antigen for presentation to CD4+ T cells and could be an efficient vaccine target. J Immunol. 2003;171:32–36. doi: 10.4049/jimmunol.171.1.32. [DOI] [PubMed] [Google Scholar]
- 200.Schroder NW, Meister D, Wolff V, Christan C, Kaner D, Haban V, Purucker P, Hermann C, Moter A, Gobel UB, Schumann RR. Chronic periodontal disease is associated with single-nucleotide polymorphisms of the human TLR-4 gene. Genes Immun. 2005;6:448–451. doi: 10.1038/sj.gene.6364221. [DOI] [PubMed] [Google Scholar]
- 201.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, e Sousa C Reis. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 202.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. Faseb J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 205.Sharma A, Honma K, Evans RT, Hruby DE, Genco RJ. Oral immunization with recombinant Streptococcus gordonii expressing Porphyromonas gingivalis FimA domains. Infect Immun. 2001;69:2928–2934. doi: 10.1128/IAI.69.5.2928-2934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sharma DC, Prasad SB, Karthikeyan BV. Vaccination against periodontitis: the saga continues. Expert Rev Vaccines. 2007;6:579–590. doi: 10.1586/14760584.6.4.579. [DOI] [PubMed] [Google Scholar]
- 207.Shin H, Mally M, Kuhn M, Paroz C, Cornelis GR. Escape from immune surveillance by Capnocytophaga canimorsus. J Infect Dis. 2007;195:375–386. doi: 10.1086/510243. [DOI] [PubMed] [Google Scholar]
- 208.Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci U S A. 2005;102:16049–16054. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sioud M. RNA interference and innate immunity. Adv Drug Deliv Rev. 2007;59:153–163. doi: 10.1016/j.addr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 210.Slots J. Herpesviruses in periodontal diseases. Periodontol 2000. 2005;38:33–62. doi: 10.1111/j.1600-0757.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- 211.Slots J. Herpesviral-bacterial synergy in the pathogenesis of human periodontitis. Curr Opin Infect Dis. 2007;20:278–283. doi: 10.1097/QCO.0b013e3280964da0. [DOI] [PubMed] [Google Scholar]
- 212.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 213.Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol. 2000;165:411–418. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]
- 214.Svensson A, Bellner L, Magnusson M, Eriksson K. Role of IFN-alpha/beta signaling in the prevention of genital herpes virus type 2 infection. J Reprod Immunol. 2007;74:114–123. doi: 10.1016/j.jri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 215.Tabeta K, Yamazaki K, Akashi S, Miyake K, Kumada H, Umemoto T, Yoshie H. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect Immun. 2000;68:3731–3735. doi: 10.1128/iai.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169:1516–1523. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 217.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 218.Tanishima S, Kishimoto Y, Fukata S, Mizumura H, Hagino H, Teshima R. Minodronic acid influences receptor activator of nuclear factor-κB ligand expression and suppresses bone resorption by osteoclasts in rats with collagen-induced arthritis. Mod Rheumatol. 2007;17:198–205. doi: 10.1007/s10165-007-0566-y. [DOI] [PubMed] [Google Scholar]
- 219.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12:125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 220.Teng YT. Protective and destructive immunity in the periodontium: Part 1-Innate and humoral immunity and the periodontium. J Dent Res. 2006;85:198–208. doi: 10.1177/154405910608500301. [DOI] [PubMed] [Google Scholar]
- 221.Toshchakov VY, Fenton MJ, Vogel SN. Cutting Edge: Differential inhibition of TLR signaling pathways by cell-permeable peptides representing BB loops of TLRs. J Immunol. 2007;178:2655–2660. doi: 10.4049/jimmunol.178.5.2655. [DOI] [PubMed] [Google Scholar]
- 222.Trent JO, Wang Z-x, Murray JL, Shao W, Tamamura H, Fujii N, Peiper SC. Lipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonists. J Biol Chem. 2003;278:47136–47144. doi: 10.1074/jbc.M307850200. [DOI] [PubMed] [Google Scholar]
- 223.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
- 224.Triantafilou M, Brandenburg K, Gutsmann T, Seydel U, Triantafilou K. Innate recognition of bacteria: engagement of multiple receptors. Crit Rev Immunol. 2002;22:251–268. [PubMed] [Google Scholar]
- 225.Uematsu S, Akira S. The role of Toll-like receptors in immune disorders. Expert Opin Biol Ther. 2006;6:203–214. doi: 10.1517/14712598.6.3.203. [DOI] [PubMed] [Google Scholar]
- 226.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 227.Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001;46:901–908. doi: 10.1016/s0003-9969(01)00057-7. [DOI] [PubMed] [Google Scholar]
- 228.Ukai T, Yumoto H, Gibson FC, 3rd, Genco CA. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-α production. Infect Immun. 2008;76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 230.Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T, Yoshimura T. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004–1012. doi: 10.1152/ajpgi.00096.2007. [DOI] [PubMed] [Google Scholar]
- 231.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 232.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 233.Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, Abreu MT. β-Defensin-2 Expression Is Regulated by TLR Signaling in Intestinal Epithelial Cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 234.Wada Y, Shimada K, Kimura T, Ushiyama S. Novel p38 MAP kinase inhibitor R-130823 suppresses IL-6, IL-8 and MMP-13 production in spheroid culture of human synovial sarcoma cell line SW 982. Immunol Lett. 2005;101:50–59. doi: 10.1016/j.imlet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 235.Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 236.Wang PL, Ohura K, Fujii T, Oido-Mori M, Kowashi Y, Kikuchi M, Suetsugu Y, Tanaka J. DNA microarray analysis of human gingival fibroblasts from healthy and inflammatory gingival tissues. Biochem Biophys Res Commun. 2003;305:970–973. doi: 10.1016/s0006-291x(03)00821-0. [DOI] [PubMed] [Google Scholar]
- 237.Wara-aswapati N, Surarit R, Chayasadom A, Boch JA, Pitiphat W. RANKL upregulation associated with periodontitis and Porphyromonas gingivalis. J Periodontol. 2007;78:1062–1069. doi: 10.1902/jop.2007.060398. [DOI] [PubMed] [Google Scholar]
- 238.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 239.Watanabe I, Ichiki M, Shiratsuchi A, Nakanishi Y. TLR2-mediated survival of Staphylococcus aureus in macrophages: A novel bacterial strategy against host innate immunity. J Immunol. 2007;178:4917–4925. doi: 10.4049/jimmunol.178.8.4917. [DOI] [PubMed] [Google Scholar]
- 240.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 241.Wills MR, Carmichael AJ, Sissons JG. Vaccines against persistent DNA virus infections. Br Med Bull. 2002;62:125–138. doi: 10.1093/bmb/62.1.125. [DOI] [PubMed] [Google Scholar]
- 242.Woodgett JR, Ohashi PS. GSK3: an in-Toll-erant protein kinase? Nat Immunol. 2005;6:751–752. doi: 10.1038/ni0805-751. [DOI] [PubMed] [Google Scholar]
- 243.Wright HJ, Matthews JB, Chapple IL, Ling-Mountford N, Cooper PR. Periodontitis associates with a type 1 IFN signature in peripheral blood neutrophils. J Immunol. 2008;181:5775–5784. doi: 10.4049/jimmunol.181.8.5775. [DOI] [PubMed] [Google Scholar]
- 244.Wu JJ, Pang KR, Huang DB, Tyring SK. Advances in antiviral therapy. Dermatol Clin. 2005;23:313–322. doi: 10.1016/j.det.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 245.Yang QB, Martin M, Michalek SM, Katz J. Mechanisms of monophosphoryl lipid A augmentation of host responses to recombinant HagB from Porphyromonas gingivalis. Infect Immun. 2002;70:3557–3565. doi: 10.1128/IAI.70.7.3557-3565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 247.Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor 4. Infect Immun. 2002;70:218–225. doi: 10.1128/IAI.70.1.218-225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Z, Schluesener HJ. Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci. 2006;63:2901–2907. doi: 10.1007/s00018-006-6189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhong J, Lee W-H. Lithium: a novel treatment for Alzheimer’s disease? Expert Opinion on Drug Safety. 2007;6:375–383. doi: 10.1517/14740338.6.4.375. [DOI] [PubMed] [Google Scholar]