Abstract
Haematopoietic stem cell (HSC) homeostasis is tightly controlled by growth factors, signalling molecules and transcription factors. Definitive HSCs derived during embryogenesis in the aorta-gonad-mesonephros region subsequently colonize fetal and adult haematopoietic organs1,2. To identify new modulators of HSC formation and homeostasis, a panel of biologically active compounds was screened for effects on stem cell induction in the zebrafish aorta-gonad-mesonephros region. Here, we show that chemicals that enhance prostaglandin (PG) E2 synthesis increased HSC numbers, and those that block prostaglandin synthesis decreased stem cell numbers. The cyclooxygenases responsible for PGE2 synthesis were required for HSC formation. A stable derivative of PGE2 improved kidney marrow recovery following irradiation injury in the adult zebrafish. In murine embryonic stem cell differentiation assays, PGE2 caused amplification of multipotent progenitors. Furthermore, ex vivo exposure to stabilized PGE2 enhanced spleen colony forming units at day 12 post transplant and increased the frequency of long-term repopulating HSCs present in murine bone marrow after limiting dilution competitive transplantation. The conserved role for PGE2 in the regulation of vertebrate HSC homeostasis indicates that modulation of the prostaglandin pathway may facilitate expansion of HSC number for therapeutic purposes.
A chemical genetic screen was conducted to identify new pathways modulating definitive HSC formation during zebrafish embryogenesis. runx1 and cmyb, required for mammalian HSC development, are expressed in the ventral wall of the dorsal aorta in a region analogous to the mammalian aorta-gonad-mesonephros (AGM) at 36 h post fertilization (h.p.f.)3-5. Wild-type embryos, incubated with individual chemicals, were examined for alterations in runx1+/cmyb+ HSCs by in situ hybridization expression at 36 h.p.f. A high percentage of compounds (91.7%, 2,275 of 2,357) failed to alter HSC expression, whereas 35 (1.4%) and 47 (1.9%) led to increased or decreased numbers of HSCs, respectively. Among these substances, 10 affected the prostaglandin pathway (Supplementary Table 1). runx1+/cmyb+ HSCs comprise a line of flattened endothelial cells (arrow) and haematopoietic clusters (arrowhead) in the aorta (Fig. 1a-c); linoleic acid increased HSC numbers (22 altered out of 30 scored), whereas celecoxib, a cyclooxygenase (Cox)2 inhibitor, decreased HSCs (26/31). PGE2 is the main effector prostanoid produced in the zebrafish6 and is regulated by both Cox1 (also known as Ptgs1) and Cox2 (also known as Ptgs2a). Treatment of zebrafish embryos with PGE2 increased expression of runx1/cmyb (25/49), whereas Cox inhibition with SC560 (Cox1) and NS398 (Cox2) (Supplementary Fig. 1a-e) decreased HSC numbers in 30/36 and 35/44 cases, respectively. These findings argue persuasively for a specific role of PGE2 in the formation of AGM HSCs.
Figure 1. Prostaglandin agonists and antagonists alter runx1/cmyb expression without affecting vascular development.
In situ hybridization for runx1/cmyb or flk1 at 36 h.p.f. Photomicrographs were taken with Nomarski optics at 10× (a-c, left panels) and 40× (a-c, right panels, and e-m) magnification. a-c, Representative examples of chemicals in the prostaglandin pathway discovered in the screen are shown; 10 μM linoleic acid increases, and 20 μM celecoxib reduces HSC numbers. runx1+/myb+ HSCs are indicated: endothelial cells (arrow); haematopoietic clusters (arrowhead). d, Quantitative PCR profile of endothelial and HSC-specific gene expression following exposure to long-acting dmPGE2 (10 μM, blue) or the nonspecific Cox inhibitor indomethacin (10 μM, green) versus control (red). Both treatments resulted in statistically significant differences compared with controls for each gene examined (ANOVA, *P < 0.05; mean, s.d. and n are listed in Supplementary Table 2). e-m, dmPGE2 and indomethacin exert opposing effects on runx1/cmyb expression by in situ hybridization (e, h, k); flk1 is used to assess the effects on vascular development (f, i, l). Confocal microscopy images of cmyb-gfp; lmo2-dsRed bigenic fish exposed to dmPGE2 and indomethacin showing an increase and decrease in HSC (yellow) number along the ventral wall (yellow arrowhead) of the aorta, respectively (g, j, m). Quantitative analysis of 10 embryos in each treatment group revealed significant differences in HSC numbers (Supplementary Fig. 1i).
Cox1 is required for the development of the aorta-vein endothelial boundary during zebrafish development7; thus, alteration in Cox1 activity could have an impact on endothelial-derived HSCs. By in situ hybridization, cox2 was diffusely expressed in the tail region at 36 h.p.f. (Supplementary Fig. 1f, g). In FACS-isolated blood and endothelial cell populations, both cox1 and cox2 were found to be highly expressed during the onset of definitive haematopoiesis. cox1 was detected in both Lmo2+ endothelial cells and in Cd41+ HSCs, whereas cox2 was only found in HSCs (Supplementary Fig. 1h). This suggests that Cox1 and Cox2 participate in HSC induction through regulation of the stem cell niche and the HSC itself.
A long-acting derivative of PGE2, 16,16-dimethyl-PGE2 (dmPGE2) caused an increase in runx1+/cmyb+ AGM HSCs in 78% of embryos (97/124) (Fig. 1e, h), whereas HSCs were inhibited by indomethacin (10 μM) treatment in 90% of embryos (92/102) (Fig. 1k and Supplementary Fig. 1j-r). Mass spectrometry of 36 h.p.f. embryos demonstrated that indomethacin treatment depressed PGE2 formation below detectable levels (from 18±6 pg per 50 embryos to <2 pg per 50 embryos; n = 3)6. dmPGE2 had minimal effects on the vasculature, as shown by flk1 staining (Fig. 1f, i) whereas indomethacin slightly altered the intersomitic vessels in 30% (15/49) of embryos (Fig. 1l). At 36 h.p.f., live bigenic cmyb-gfp; lmo2-dsRed (green-fluorescent-protein-labelled HSCs and progenitors; red-labelled HSCs and endothelium) embryos imaged by confocal microscopy exhibited significantly decreased numbers of HSCs (yellow) following indomethacin treatment, and significantly increased HSCs after dmPGE2 exposure (Fig. 1g, j, m and Supplementary Fig. 1i). Quantitative PCR confirmed an enhancement in runx1 and cmyb expression by dmPGE2, whereas indomethacin significantly reduced the expression of each gene (Fig. 1d).
To confirm the requirement of PGE2 activity, we used morpholino oligonucleotides to knock down expression of Cox1 and Cox2; a low dose (40 μM) inhibition of Cox1 minimizes toxicity, while mimicking Cox-dependent developmental defects6. Morpholino oligonucleotide knockdown of Cox1/Cox2 decreased the levels of prostaglandins and inhibited AGM HSCs (Cox1, 54/74; Cox2, 60/71) (Supplementary Fig. 1s-u). The morpholino-mediated effects on HSCs were reversed by dmPGE2 (Cox1 + dmPGE2, 29/52 rescued; Cox2 + dmPGE2, 43/60) (Supplementary Fig. 1y, z, a’). dmPGE2 rescued (25/45) morpholino-mediated knockdown of PGE2 synthase (35/50) indicating that PGE2 signalling was sufficient to modulate HSC formation (Supplementary Fig. 1u, b’). PGE2 signals through receptors Ptger1l-Ptger4l (ref. 8). Morpholino-mediated knockdown of Ptger2l and Ptger4l diminished runx1/cmyb expression (Ptger2l, 39/63; Ptger4l, 44/67) and was not reversed by dmPGE2 (Supplementary Fig. 1s, t, c’, d’). Quantitative PCR analysis showed ptger2l/ptger4l are present in HSCs (Supplementary Fig. 1e’). These experiments confirm that PGE2-mediated signalling regulates the formation of HSCs in the AGM region.
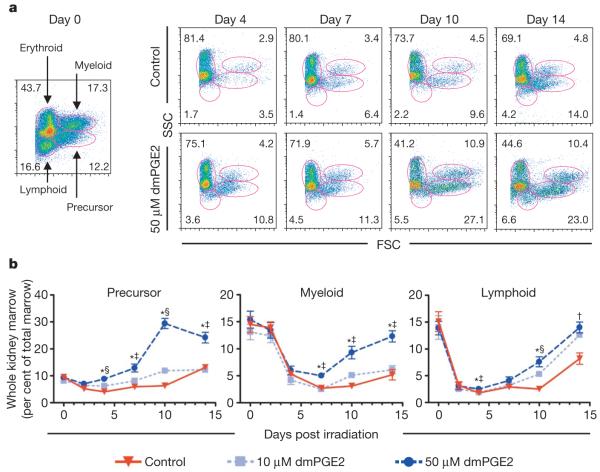
To examine the role of PGE2 in HSC homeostasis in adult zebrafish, we performed a kidney marrow irradiation-recovery assay in sublethally irradiated wild-type fish9 (Fig. 2a). The rate of kidney marrow repopulation was significantly enhanced after exposure to 50 μM dmPGE2 (Fig. 2a, b), with progenitor recovery preceding reconstitution of the myeloid and lymphoid populations. Significant upregulation of stem, progenitor and endothelial cell markers was found after dmPGE2 treatment (Supplementary Fig. 2a). Inhibition of Cox activity significantly decreased kidney marrow recovery and affected overall survival (Supplementary Fig. 2b). Our results indicate that PGE2 has an important role in kidney marrow homeostasis.
Figure 2. Treatment with dmPGE2 enhances haematopoietic recovery in sublethally irradiated adult zebrafish.
Zebrafish whole kidney marrow irradiation recovery experiments were performed. a, Representative FSC/SSC FACS profiles of haematopoietic cell lineages in the kidney marrow on days 0, 4, 7, 10 and 14 of irradiation recovery in DMSO and dmPGE2-treated (50 μM) zebrafish. b, Kinetics of kidney marrow reconstitution of precursor, lymphoid and myeloid cells in control and dmPGE2-treated fish. Statistically significant differences: †, 50 μM versus control; ‡, 50 μM versus 10 μM, and 50 μM versus control; and §, all variables significant (ANOVA, *P < 0.05; mean, s.d. and n listed in Supplementary Table 3).
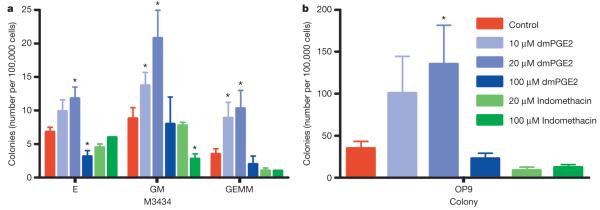
We then evaluated the effects of PGE2 on murine HSC and progenitor populations. Addition of dmPGE2 to embryonic stem cells during embryoid body expansion increased haematopoietic colonies on an OP9 stromal cell layer and in methylcellulose assays10 (Fig. 3a, b). OP9, definitive erythroid and granulocyte/monocyte colonies increased in a dose-dependent manner after exposure to 10 μM (granulocyte/monocyte, P = 0.005) and 20 μM (OP9, P = 0.047; definitive erythroid, P = 0.04; granulocyte/monocyte, P = 0.007) dmPGE2. The number of multipotent granulocyte/erythrocyte/monocyte/macrophage colonies was enhanced 2.9-fold following dmPGE2 treatment (10 μM, P = 0.017; 20 μM, P = 0.016). Cox1 (also known as Ptgs1), Cox2 (also known as Ptgs2), PGE2 synthase (Ptges) and Ptger1-Ptger4 were present in embryonic stem cells at all stages examined (Supplementary Fig. 3a). Indomethacin inhibited colony growth at 20 μM (OP9, P = 0.069) and 100 μM (granulocyte/monocyte, P = 0.024) (Fig. 3a, b) and could be rescued by dmPGE2 (Supplementary Fig. 3b, c). These data suggest the role of PGE2 in regulating haematopoiesis is conserved between zebrafish and mammals.
Figure 3. dmPGE2 modulates colony number and haematopoietic differentiation in mouse embryonic stem cells.
M3434 and OP9 embryonic stem cell colony-forming assays were performed; counts are per 100,000 cells plated. An asterisk (*) indicates a statistically significant difference (two-tailed t-test; mean, s.d. and n listed in Supplementary Table 4). a, Effect of increasing doses of dmPGE2 and inhibition of cyclooxygenase activity by indomethacin on haematopoietic differentiation in methylcellulose; numbers of definitive erythroid (E), mixed granulocyte/monocyte (GM), and multi-potent (GEMM) progenitor colonies are shown (10 μM dmPGE2: GM, P = 0.005; GEMM, P = 0.017; 20 μM dmPGE2: E, P = 0.04; GM, P = 0.007; GEMM, P = 0.016; 100 μM indomethacin: GM, P = 0.024). b, Effect of dmPGE2 and indomethacin on OP9 haematopoietic colony number (20 μM dmPGE2, P = 0.047).
To explore effects in an intact mammalian model, murine whole bone marrow (WBM) was exposed ex vivo to dmPGE2 (1 μM per 106 cells) and irradiated recipients were transplanted with 6 × 104 treated WBM cells. The number of spleen colony-forming units at day 12 post transplant (CFU-S12) was increased threefold (P < 0.0001) in recipients of dmPGE2-treated WBM (Fig. 4b, Supplementary Fig. 4b, Supplementary Table 6); numbers of more mature CFU-S8 colonies were also enhanced (Fig. 4a, Supplementary Fig. 3a, Supplementary Table 5). To assess the endogenous PGE2 requirement, WBM cells were incubated with indomethacin (1 μM 10-6 cells) or specific COX1 and COX2 inhibitors. After transplantation of 1 × 105 cells, a significant decrease (P = 0.0001) in the number of CFU-S12 was observed (Fig. 4c; Supplementary Fig. 4c, k, l; Supplementary Table 6). These results suggest that PGE2 enhances haematopoietic progenitor formation, and is required for CFU-S activity.
Figure 4. Exposure of murine bone marrow to dmPGE2 increases the number of CFU-S and repopulating HSCs.
An asterisk (*) indicates a statistically significant difference. a, b, Effect of ex vivo treatment of WBM (2 h on ice) with ethanol control (red) or dmPGE2 (1 μM per 106 cells) on CFU-S8 and CFU-S12 (60,000 cells per recipient; CFU-S12: two-tailed t-test, control (mean/s.d./n) = 5.78/2.73/9, dmPGE2 = 15.22/2.39/9, P < 0.0001). c, Effect on CFU-S12 following ex vivo treatment with indomethacin (1 μM per 106 cells) (100,000 cells/recipient; two-tailed t-test, control (mean/s.d./n) = 8.8/2.10/10, indomethacin = 2.5/1.43/10, P = 0.0001). d, CFU-S12 evaluation after treatment of cKit+Sca1+Lineage- stem cells with dmPGE2 or ethanol control (two-tailed t-test, 100 cells: control (mean/s.d./n) = 3/1.63/4, dmPGE2 = 6.2/1.3/5, P = 0.013; 300 cells: control (mean/s.d./n) = 5/1.22/5, dmPGE2 = 11/1.87/5, P = 0.0003). e, f, Limiting dilution competitive repopulation assay. The number of negative recipients as determined by FACS analysis (e) in relation to the total number of cells transplanted for control or dmPGE2-treated cell samples is shown at 12 weeks. P0 = 67,884 (control) and 16,970 (dmPGE2 treated). The frequency of engraftment (f) at 6, 12, and 24 weeks post transplantation in recipients of ethanol- versus dmPGE2-treated WBM calculated by Poisson statistics (ANOVA, n = 10 per variable; 6 wks, P = 0.005; 12 wks, P = 0.002; 24 wks, P = 0.05); the number of recipients surviving to analysis at each time point is shown in Supplementary Tables 7-9.
The prostaglandin pathway components are present in both stromal cell and HSC populations in mice and humans11,12. Cox1, Cox2, PGE2-synthase, Ptger2 and Ptger4 are present in fetal liver HSCs and in bone marrow HSCs after 5-fluorouracil (5-FU) injury, suggesting PGE2 signalling functions in murine HSCs13. To determine if the increase in CFU-S number is due to a direct effect of PGE2 on the stem/progenitor cell population, FACS-isolated cKit+Sca1+ Lineage- (KSL) bone marrow cells were exposed to dmPGE2 and transplanted into irradiated recipients. Both splenic weight (Supplementary Fig. 4d) and CFU-S12 were significantly increased, indicating that dmPGE2 can lead to cell-autonomous activation of HSCs and immature progenitors (Fig. 4d, Supplementary Table 6).
A limiting dilution competitive repopulation analysis was conducted to determine the effects of dmPGE2 on HSC reconstitution14. WBM (CD45.1) exposed to dmPGE2 ex vivo was mixed independently at varying doses with a fixed number of untreated competitor cells (CD45.1/CD45.2) and injected into congenic recipient mice (CD45.2). Peripheral blood obtained at 6, 12 and 24 weeks post transplantation was examined by FACS to determine percentage test-cell contribution to haematopoietic repopulation (Supplementary Fig. 4e-j); positive reconstitution was defined as test-cell multilineage chimaerism >5% (Supplementary Fig. 4f, h, i). A significant increase in the number of repopulating cells as determined by Poisson statistics was seen in dmPGE2-treated bone marrow (Fig. 4e, Supplementary Fig. 4g, j). At 6 weeks, the calculated frequency of engrafting cells per 106 WBM cells was enhanced 3.3-fold (P = 0.005) in dmPGE2-treated WBM recipients, and the frequency of short-term repopulating HSCs was 4.0-fold (P = 0.002) higher at 12-weeks post-transplantation (Fig. 4e, f, Supplementary Fig. 4g). At 24 weeks, the frequency of long-term repopulating HSCs was 2.3-fold enhanced (P = 0.05) in recipients of dmPGE2-treated cells (Fig. 4f; Supplementary Fig. 4j). dmPGE2 treatment increased the frequency of repopulating HSCs in the mouse, but did not impair the differentiative capacity as seen by multilineage analysis. To determine whether dmPGE2 treatment enhanced homing to the bone marrow niche, WBM was labelled with a vital dye, CDFA, before transplantation; no significant difference in homing could be detected (P = 0.83) (Supplementary Fig. 5).
Here we have demonstrated that PGE2 enhances the number of HSCs and multipotent progenitors in two vertebrate species, zebrafish and mice. Prior studies have documented that unmodified PGE2 impairs blood-cell maturation in the mouse15,16 and cell cycle stimulation in CFU-S8 progenitors17; however, the effects of prostaglandin-mediated cell signalling on HSCs have not been examined previously. Cox1 and Cox2 seem to have distinct functions in AGM HSC formation: Cox1 is important in the formation of the haematopoietic niche, whereas Cox2 is probably involved in self-renewal and proliferation of HSCs themselves. Conversely, homozygous Cox1 or Cox2 knockout mice are viable, without apparent defects in HSC formation18, due to maternal and sibling PGE2 contribution7,19. Analyses of Cox2-/- mice demonstrated alterations in haematocrit levels and an impaired recovery from 5-FU-induced bone marrow injury20; these findings imply HSC defects in adult Cox2-/- mice that are compatible with our proposed role for prostaglandin in HSC homeostasis. To clarify the roles of COX1 and COX2 in regulating HSC homeostasis, we performed CFU-S12 (Fig. 4k, l) and 5-FU bone marrow recovery assays using selective inhibitors of COX1 (SC560) or COX2 (NS398). Inhibition of either enzyme significantly diminished CFU-S activity, as well as the recovery of peripheral blood and bone marrow WBC numbers (Supplementary Fig. 4m, n). Administration of dmPGE2 following 5-FU treatment significantly enhanced bone marrow recovery. These data suggest that both COX1 and COX2 have a role in regulating HSC homeostasis in adult mice, as in the zebrafish, and that PGE2 is the mediator of this HSC regulation. The precise mechanism of PGE2 modulation of vertebrate HSC homeostasis remains to be elucidated.
Patients undergoing bone marrow transplantation show increased endogenous PGE2 levels21. Our studies raise the possibility that administration of COX inhibitors following human bone marrow transplantation might impair HSC engraftment and result in delayed recovery of the WBC counts, in addition to adversely affecting platelet function. PGE2 and its analogues are safely administered to patients22,23. The concentration of dmPGE2 used to expand the number of murine HSCs falls within the physiological range of PGE2 in human serum24, thus dmPGE2 or its derivatives may be useful for ex vivo or in vivo expansion of HSCs. Our studies illustrate that PGE2 functions as a potent regulator of HSCs in vertebrates, and may prove useful in treating patients with bone marrow failure or following transplantation.
METHODS SUMMARY
Wild-type age-matched embryos were exposed to individual test compounds from 3-somites until 36 h.p.f. and effects on HSCs were evaluated by in situ hybridization for runx1 and cmyb. Treatment with PGE2, dmPGE2 and Cox inhibitors (indomethacin, SC560, NS398) at 10 μM was used to confirm and quantify the effects of prostaglandin signalling on HSCs by in situ hybridization, quantitative PCR9 and confocal microscopy25. Expression of prostaglandin pathway components in HSCs was characterized by microarray analysis26, quantitative PCR and in situ hybridization. Morpholino knockdown of prostaglandin pathway components6,8,27, and subsequent rescue by dmPGE2, was used to confirm the specificity of the results of the chemical treatments. Functional inhibition of prostaglandin synthesis was measured by mass spectroscopy for chemical and morpholino experiments. A flow-cytometry-based irradiation recovery assay was used to assess the impact of PGE2-mediated signalling on adult kidney marrow28. The effect of dmPGE2 and indomethacin on haematopoietic colony forming potential of embryonic stem cells was analysed by standard OP9 and methylcellulose colony forming assays29,30. CFU-S assays and limiting dilution competitive transplantation assays were used to test the effects of ex vivo dmPGE2 treatment or COX inhibition on haematopoietic stem and progenitor populations. Bone marrow ablation13 by 5-FU was used to test the in vivo effect of dmPGE2 or COX inhibitor treatment on haematopoietic recovery in mammals.
Supplementary Material
Acknowledgements
We thank the Institute of Chemical and Cellular Biology at Harvard Medical School for access to the chemical libraries used in the screen. We thank: A. Flint, E. Mayhall and C.E. Burns for technical assistance and advice on the kidney marrow analysis; C. Thisse and B. Thisse for information and plasmids for PTGER2; and A. Meyers and J. Ojeda for technical help with the zebrafish chemical screen. This work was supported by grants from the National Institutes of Health (T.E.N., W.G., G.Q.D., S.H.O., G.A.F. and L.I.Z), the American Cancer Socitey (T.E.N.), the American Gastroenterological Association (W.G.), the Leukemia and Lymphoma Society (C.R.W.), the American Heart Association (T.G.) and the Dr. Mildred Scheel Foundation for Cancer Research (C.L.). S.H.O. and L.I.Z. are Howard Hughes Medical Institute investigators.
Appendix
METHODS
Chemical screen design and confirmatory testing
Wild-type age-matched embryos were arrayed into 48-well plates (~5 embryos per well) of individual test compounds and exposed from 3-somites until 36 h.p.f. Three compound libraries were used: NINDS Custom Collection (1,040 compounds), SpecPlus Collection (960) and BIOMOL ICCB Known Bioactives (480). Five per cent (123/2480) of the compounds were toxic, resulting in death or severe morphological abnormalities. In situ hybridization for runx1 and cmyb was performed to assess HSC numbers. Compounds were retested at 10, 20 and 50 μM. Stem cell specificity was assessed using flk1 at 36 h.p.f. PGE2, PGI2, dmPGE2 and all Cox inhibitors (Sigma) were used at 10 to 20 μM.
Qualitative scoring (number of embryos with altered HSCs per number scored) of runx1/cmyb was conducted using the following criteria: Normal/unchanged, continuous line of runx1+/cmyb+ endothelial cells and occasional haematopoietic clusters; decreased/absent, reduction in runx1+/cmyb+ cells, including the presence of large gaps in the line of HSCs, isolated positive cells, or absence of expression; increased/excess, enhancement in runx1+/cmyb+ cells, including many HSC clusters, a thickened line of HSCs, or ectopic expression.
Confocal imaging
Live 36 h.p.f. treated bigenic zebrafish embryos were embedded in 1% low-melting point agarose containing 0.04 mg ml-1 Tricaine-S for confocal imaging. lmo2-dsRed fish were created as described25. cmyb-gfp transgenic reporter lines were created by homologous recombination of a 3.7 kb EGFP construct downstream of the 5′ untranslated region and precisely before the start site of a PAC clone containing cmyb (J. Galloway, H. Zhu, S. Lin and L.I.Z., unpublished data). For HSC quantification, cMyb+/Lmo2+ cells were counted in projections of z-stack images (n = 10 per treatment).
Morpholino knockdown
Morpholino oligonucleotides (GeneTools) directed against zebrafish cox1 and cox2, PGE2 synthase, and ptger2l and ptger4l (refs 6, 8, 27) were injected (40 μM) into zebrafish embryos at the one-cell stage. For rescue experiments, 3-somite-stage morpholino-injected embryos were exposed to 10 μM dmPGE2.
Microarray gene expression profiling
Gata1-Gfp+ (12 somites), Lmo2-Gfp+ (12 somites and 35 h.p.f.) and Cd41-Gfp+ (35 h.p.f.) cells were FACS-sorted; total RNA was purified and analysed using Affymetrix zebrafish gene chips as described previously26.
Quantitative PCR
Quantitative PCR (qPCR) was performed using previously described primer sets9. Embryos (n = 50) were treated as described. qPCR (60 °C annealing) was performed using SYBR Green Supermix on the iQ5 Multicolour RTPCR Detection System (BioRad) (n = 10 replicates) and relative expression levels were determined. Primer pairs for Ptger2 and Ptger4 are shown (Supplementary Table 10). qPCR of whole kidney marrow RNA (n = 15 per variable) was performed on day 3 post irradiation as described. qPCR on S cell RNA (harvested in Stat-60, Tel-Test) was performed using the Stratagene Sybrgreen kit on the Stratagene qPCR machine. Prostaglandin pathway component primer sequences are shown (Supplementary Table 10).
Mass spectroscopy
PGE2 and the stable PGI2 metabolite, 6-keto-PGF1α, were measured using high-performance liquid chromatography-tandem mass spectrometry. Ethylacetate extracts from homogenized embryos were spiked with the corresponding stable-isotope-labelled internal standards (d4-PGE2 and d4-6-keto PGF1α) and allowed to react with methoxylamine. The following mass transitions were monitored: m/z 384→272 (PGE), m/z 398→368 (6-keto PGF1α and TxB2).
Irradiation recovery assay
Adult zebrafish were exposed to 23 Gy of γ-irradiation. On day 2 post irradiation, fish were exposed overnight to DMSO control, dmPGE2 (10 or 50 μM), indomethacin (10 μM), SC560 (10 μM) or NS398 (10 μM) in fish water. Whole kidney marrow isolated on days 0, 2, 4, 7, 10, 14 was subjected to forward scatter/side scatter (FSC/SSC) FACS analysis to identify haematopoietic lineages (n = 5 per treatment, 3 replicates)28.
Embryonic stem cell differentiation assays
Embryonic stem cell haematopoietic differentiation assays were performed as previously described29,30. dmPGE2 (10, 20 or 100 μM) or indomethacin (20, 100 μM) were added at day 4 and 5 during embryoid body expansion. M3434 methylcellulose colony forming and OP9 colony assays were conducted on day 6 and analysed at days 8 and 5, respectively. Colony type was identified by morphological analysis; duplicate chemical exposures were averaged to determine the reported colony number (n = 3 replicates minimum).
Murine colony-forming units-spleen (CFU-S)
WBM cells from the femurs of 8-week-old C57Bl/6 mice were incubated ex vivo with (1 μM per 106 cells) dmPGE2, indomethacin, SC560, NS398 or ethanol control on ice for 2 h. Two independent bone marrow samples were treated (n = 5 per treatment, 2 replicates) for each variable. Recipient mice were lethally irradiated with a split dose of 10 Gy. Sixty thousand unfractionated dmPGE2 or control-treated bone marrow cells were injected retro-orbitally into irradiated recipient mice. Spleens were dissected on day 8 or 12, weighed and fixed with Bouin’s solution; haematopoietic colonies per spleen were counted. Cells (1 × 105 per recipient) were transplanted after treatment with the COX inhibitors. FACS-sorted cKit+Sca1+Lineage- bone marrow cells were treated as above and transplanted at a dose of 100 or 300 cells per recipient.
5-fluorouracil bone marrow injury
Mice were treated with 5-FU (150 mg kg-1) as described13. SC560, NS398, dmPGE2 (1 mg kg-1) or ethanol control were administered by intraperitoneal injection on days 1, 5, 9, 13 and 17 post injection. Peripheral blood was obtained on day 7 and 14, quantified and subjected to multilineage FACS analysis using antibodies (eBioscience) to B220/IgM (B-lymphoid), CD4/8 (T-lymphoid), Mac1/Gr1 (myeloid), Ter119/CD71 (erythroid) and cKit/Sca1 (stem/progenitor). Mice were killed on day 16, and bone marrow was isolated, quantified and analysed by FACS.
Limiting dilution competitive transplantation
WBM from CD45.1 C57Bl/6 mice was incubated with dmPGE2 or ethanol control ex vivo, as described. Treated test cells were independently transplanted into irradiated CD45.2 recipients (n = 5 per variable, 2 replicates) with untreated CD45.1/CD45.2 competitor at the following ratios: 15,000:200,000 (0.075:1), 50,000:200,000 (0.25:1), 200,000:200,000 (1:1) or 2,000,000:200,000 (10:1). Peripheral blood was obtained at 6, 12 and 24 weeks post transplantation, and white blood cells were FACS-analysed to determine test reconstitution for each series of treatment populations. Frequency of peripheral blood chimaerism >5% was used to calculate the number of repopulating cells using the L-Calc program (Stem Cell Technologies). For 12- and 24-week peripheral blood samples, multilineage reconstitution was measured by FACS analysis as above.
Footnotes
The authors declare no competing financial interests.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
References
- 1.Dzierzak E. The emergence of definitive hematopoietic stem cells in the mammal. Curr. Opin. Hematol. 2005;12:197–202. doi: 10.1097/01.moh.0000160736.44726.0e. [DOI] [PubMed] [Google Scholar]
- 2.Galloway JL, Zon LI. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr. Top. Dev. Biol. 2003;53:139–158. doi: 10.1016/s0070-2153(03)53004-6. [DOI] [PubMed] [Google Scholar]
- 3.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 4.Mukouyama Y, et al. Hematopoietic cells in cultures of the murine embryonic aorta-gonad-mesonephros region are induced by c-Myb. Curr. Biol. 1999;9:833–836. doi: 10.1016/s0960-9822(99)80368-6. [DOI] [PubMed] [Google Scholar]
- 5.Kalev-Zylinska ML, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 6.Grosser T, Yusuff S, Cheskis E, Pack MA, FitzGerald GA. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl Acad. Sci. USA. 2002;99:8418–8423. doi: 10.1073/pnas.112217799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha YI, Kim SH, Solnica-Krezel L, Dubois RN. Cyclooxygenase-1 signaling is required for vascular tube formation during development. Dev. Biol. 2005;282:274–283. doi: 10.1016/j.ydbio.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Cha YI, et al. Cyclooxygenase-1-derived PGE2 promotes cell motility via the G-protein-coupled EP4 receptor during vertebrate gastrulation. Genes Dev. 2006;20:77–86. doi: 10.1101/gad.1374506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Kodama H, Honjo T. In vitro development of primitive and definitive erythrocytes from different precursors. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- 11.Ivanova NB, et al. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 12.Akashi K, et al. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–389. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- 13.Venezia TA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 15.Boer AK, Drayer AL, Rui H, Vellenga E. Prostaglandin-E2 enhances EPO-mediated STAT5 transcriptional activity by serine phosphorylation of CREB. Blood. 2002;100:467–473. doi: 10.1182/blood.v100.2.467. [DOI] [PubMed] [Google Scholar]
- 16.Rocca B, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc. Natl Acad. Sci. USA. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feher I, Gidali J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature. 1974;247:550–551. doi: 10.1038/247550a0. [DOI] [PubMed] [Google Scholar]
- 18.Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem. Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 19.Langenbach R, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz M, et al. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp. Hematol. 1999;27:1494–1502. doi: 10.1016/s0301-472x(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 21.Cayeux SJ, Beverley PC, Schulz R, Dorken B. Elevated plasma prostaglandin E2 levels found in 14 patients undergoing autologous bone marrow or stem cell transplantation. Bone Marrow Transplant. 1993;12:603–608. [PubMed] [Google Scholar]
- 22.Talosi G, et al. Prostaglandin E1 treatment in patent ductus arteriosus dependent congenital heart defects. J. Perinat. Med. 2004;32:368–374. doi: 10.1515/JPM.2004.069. [DOI] [PubMed] [Google Scholar]
- 23.Thanopoulos BD, Andreou A, Frimas C. Prostaglandin E2 administration in infants with ductus-dependent cyanotic congenital heart disease. Eur. J. Pediatr. 1987;146:279–282. doi: 10.1007/BF00716473. [DOI] [PubMed] [Google Scholar]
- 24.Hertelendy F, Woods R, Jaffe BM. Prostaglandin E levels in peripheral blood during labor. Prostaglandins. 1973;3:223–227. doi: 10.1016/0090-6980(73)90090-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, et al. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev. Biol. 2005;281:256–269. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Weber GJ, et al. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pini B, et al. Prostaglandin E synthases in zebrafish. Arterioscler. Thromb. Vasc. Biol. 2005;25:315–320. doi: 10.1161/01.ATV.0000152355.97808.10. [DOI] [PubMed] [Google Scholar]
- 28.Traver D, et al. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- 29.Kyba M, et al. Enhanced hematopoietic differentiation of embryonic stem cells conditionally expressing Stat5. Proc. Natl Acad. Sci. USA. 2003;100(Suppl 1):11904–11910. doi: 10.1073/pnas.1734140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc. Natl Acad. Sci. USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.