Abstract
In addition to its well established role in balance, coordination, and other motor skills, the cerebellum is increasingly recognized as a prominent contributor to a wide array of cognitive and emotional functions. Many of these capacities undergo dramatic changes during childhood and adolescence. However, accurate characterization of co-occurring anatomical changes has been hindered by lack of longitudinal data and methodologic challenges in quantifying subdivisions of the cerebellum. In this study we apply an innovative image analysis technique to quantify total cerebellar volume and 11 subdivisions (i.e. anterior, superior posterior, and inferior posterior lobes, corpus medullare, and three vermal regions) from anatomic brain MRI scans from 25 healthy females and 25 healthy males aged 5–24 years, each of whom was scanned at least three times at approximately two year intervals. Total cerebellum volume followed an inverted U shaped developmental trajectory peaking at age 11.8 years in females and 15.6 years in males. Cerebellar volume was 10% to 13% larger in males depending on the age of comparison and the sexual dimorphism remained significant after covarying for total brain volume. Subdivisions of the cerebellum had distinctive developmental trajectories with more phylogenetically recent regions maturing particularly late. The cerebellum's unique protracted developmental trajectories, sexual dimorphism, preferential vulnerability to environmental influences, and frequent implication in childhood onset disorders such as autism and ADHD make it a prime target for pediatric neuroimaging investigations.
INTRODUCTION
The cerebellum has traditionally been associated with balance, motor control, and the ability to learn complex motor sequences. However, a growing body of literature indicates that the cerebellum also plays a prominent role in higher cognitive functions. For instance, cognition but also emotion regulation are affected in patients with vascular and degenerative cerebellar disease (Schmahmann, 2004; Riva and Giorgi, 2000). Electrophysiologic studies indicate that the activity of neurons in selected regions of the cerebellum is related more to cognitive aspects of performance than to motor function (Middleton and Strick, 1997), and functional MRI studies show cerebellar activation in tasks involving language, visuo-spatial analyses, learning and working memory (Stoodley and Schmahmann, 2009, Desmond et al., 1998). Finally, there have been several histological studies demonstrating cerebellar connections to dorsolateral prefrontal cortex, the medial frontal cortex, the parietal and superior temporal areas (Ramnani, 2006, Middleton and Strick, 1998, 2001).
These cerebellar-subserved higher cognitive functions continue to improve during childhood and adolescence, suggesting that the cerebellum may be undergoing substantial development during this period. Little is known, however, regarding normal development of the cerebellum during childhood or adolescence (Diamond, 2000), including whether developmental trajectories are different between females and males as previous studies, to the best of our knowledge, did not include repeated measurements of the same individuals. Also, little is known regarding differences in the development of cerebellar compartments, despite their having distinct characteristics regarding function, anatomical connections with the cortex, embryological sources and phylogenetic histories, and an important role in neurodevelopmental disorders (Ramnani, 2006). The cerebellum has been implicated in several neurodevelopmental disorders such as Attention Deficit / Hyperactivity Disorder, Autism, and Schizophrenia (Bishop, 2002; Seidman et al., 2005) (Courchesne et al., 1994), and it may be particularly vulnerable to environmental insults (Lesnik et al., 1998.
The relative paucity of previous neuroimaging studies reporting quantitative morphology of the cerebellum and its subdivisions compared to cerebral structures reflects methodologic challenges specific to the cerebellum, including its thinner striations of gray and white matter, its foliated shape, and less obvious anatomic demarcations of functionally distinct subdivisions. We therefore collaborated with the University of Iowa to implement a novel expert-guided cerebellar quantification method on longitudinally-acquired MRI scans from a pediatric cohort. We hypothesized that cerebellar regions would show distinct growth curves, and that these trajectories would differ between males and females, similar to what we and others have observed in cerebral development (Lenroot et al., 2007), and that ontologically diverse cerebellar compartments would have different developmental trajectories.
METHODS
Participant Selection
Subjects are healthy participants from an ongoing longitudinal study at the Child Psychiatry Branch of the National Institute of Mental Health. Subjects are recruited from the local community. When multiple siblings were available only one child per family was included; the sibling with 3 or more scans were chosen to maximize the number of available longitudinal scans and otherwise at random. We included only right handed children in this sample. The healthy participants were screened via previously published criteria (Giedd et al., 1996) which included an initial telephone interview parent and teacher rating versions of the Child Behavior Checklist (Achenbach and Edelbrock, 1983), a physical and neurological assessment, and neuropsychological testing. Parental socioeconomic status (SES) was calculated from parental occupation and education (Hollingshead, 1975). IQ data was acquired for 49 of the 50 subjects, measured using age-appropriate subtests of the Wechsler intelligence scales (Wechsler, 1974). Exclusion criteria included suspected psychiatric diagnosis, first-degree relatives with psychiatric diagnoses, head injury, any condition known to potentially affect brain development, and adverse prenatal or perinatal events including gestational age of < 30 weeks; birth weight < 3 lbs 4 oz., any known exposure to psychotropic medications during pregnancy, or significant birth complications. Longitudinal samples are acquired at approximately 2-year intervals. There were a total of 50 subjects (25 males and 25 females) and 183 scans. Of these, 27 children contributed 3 scans, 14 each had 4 scans, 8 had 5 scans, and 1 person had 6 scans.
MRI Acquisition
All images were acquired using the same scanning sequence on the same scanner which was located at the NIH Clinical Center in Bethesda, Maryland. T1-weighted images with contiguous 1.5-mm slices in the axial plane and 2.0-mm slices in the coronal plane were obtained using 3-dimensional spoiled gradient recalled echo in the steady state on a 1.5-T General Electric Signa Scanner (Milwaukee, Wisconsin; echo time of 5 milliseconds, repetition time of 24 milliseconds, flip angle of 45°, acquisition matrix of 256 × 192, number of signals acquired, 1, and 24-cm field of view). The native MRIs were registered into standardized stereotaxic space using a linear transformation and intensity corrected for nonuniformity artefacts (Sled et al., 1998). An advanced neural net classifier was used to segment the registered and corrected volumes into white matter, gray matter, CSF, and background (Zijdenbos et al., 2002). Gray and white matter volumes of the total cerebrum were quantified using an automated technique developed at the Montreal Neurological Institute that combines voxel-intensity based tissue classification into gray matter, white matter, and CSF with a probabilistic atlas (Collins et al., 1994, Zijdenbos and Dawant, 1994). The cerebellum could not be classified with this method owing to the complexity of its gray-white border. The spatially normalized and intensity corrected images from the were then imported into the BRAINS2 image analysis package developed at the University of Iowa (Magnotta et al., 2002) for measurement of cerebellar regions.
Image Analysis
The cerebellar parcellation method developed by Ron Pierson and colleagues at the University of Iowa was used to generate outlines of each lobe of the cerebellar cortex and corpus medullare, as described previously (Pierson et al., 2002). Images were segmented into gray matter, white matter, and cerebrospinal fluid. However, a reliable automated grey and white matter classification of parts of the cerebellar lobes would have been feasible only with a very high resolution scanner or in prepared brain tissue (as has been done in animals, Bush and Allman, 2003).
Next, thirty-one landmarks were manually defined and used as the basis for the application of a neural net to generate surface masks of each of the cerebellar subregions. These were then reviewed and edited manually. Volumes of each subregion were quantified and also summed to provide the total cerebellar volume (see Figure 1).
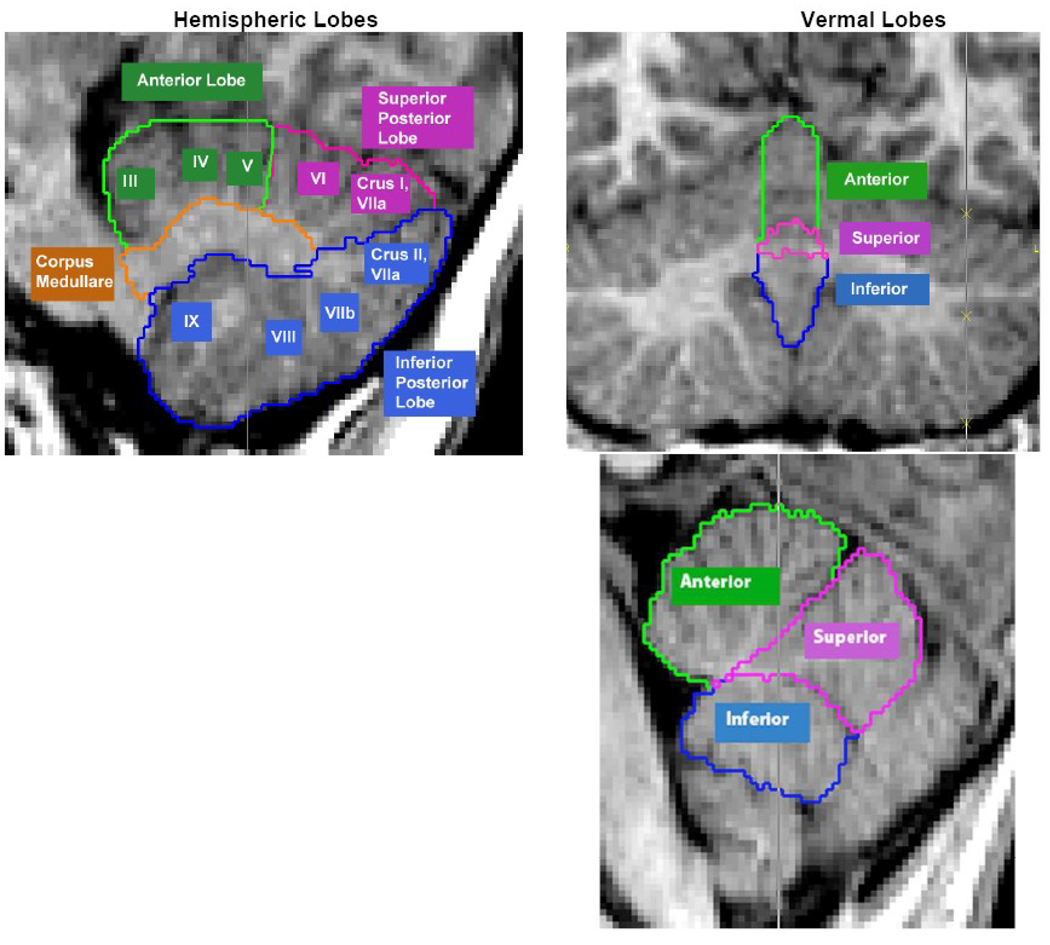
Figure 1.
Figure 1a: Cerebellar Subregions, Hemispheric lobes and vermal lobes
Example of an image as processed with BRAINS, acquired with a 1.5 Tesla; typical image, the quality reflects that machine and protocol are unchanged since 1990.
Masks of vermal subregions were manually traced. A midline guide trace in the sagittal plane marked the superior, inferior, anterior, and posterior limits of the vermis, while the axial plane was used to generate guide traces approximating the lateral borders. The vermis was defined in each coronal slice by manual tracing with reference to these guide traces. Regional subdivision was accomplished through placement of limiting boundaries in the sagittal plane, where the primary and prepyramidal fissures were readily apparent throughout the vermis. As there is not a clearly separated vermis within the anterior lobe, this term is applied to the midline and paramedian sectors of the anterior lobe as an extension of the structures visible in the posterior and inferior aspects of the cerebellum (Schmahmann, 2000).
The left and right cerebellar cortices were each divided into anterior, superior-posterior, and inferior-posterior lobes bordered by the primary and the horizontal fissure. This parcellation follows the work Larsell and Jansen and we use their nomenclature to describe the anatomical structures included in three lobes defined by our method (Larsell and Jansen, 1967). Although more detailed segmentation techniques are available (Makris et al., 2005, Diederichsen et al., 2009), parcellation of the cerebellum in units smaller than 10 mL make it extremely difficult to reliably detect small changes (<5%).
The anterior lobe was composed of the lobules I–V and delineated by the primary fissure. The superior-posterior lobe was defined to include lobules VI and VIIA-folium in the vermis region and lobule VI and crus I of VIIA in the hemispheres. It was separated from the inferior-posterior lobe by the horizontal fissure. The inferior-posterior lobe consisted of the lobules VIIa-tuber through X. The three hemispheric lobes consist predominantly of grey matter, although white matter branching off into the folia was included in the respective lobes. The left and right corpus medullare were also defined on each side, including the portion of the central region which appears as white matter. The MRI acquisition method used for this study did not have the resolution or contrast necessary to separate out the deep nuclei within the corpus medullare. The vermis was divided in to anterior, superior, and inferior regions. The primary fissure determined the posterior extent of the anterior lobe, and the pre-pyramidal fissure separated the superior from the inferior lobe. These regions are illustrated in Figures 1.
All cerebellar measurements were done by one of two raters. Ten images, randomly selected and introduced in a blinded fashion during the image analyses procedure were quantified twice by both raters to determine intra- and inter-rater reliability. Intraclass correlations (ICCs) for all hemispheric regions and the corpus medullare were > 0.80 for both intra-rater and inter-rater reliability. In the vermis, intra-rater reliability ICC’s were also > 0.80, and inter-rater ICCs were all > 0.68.
Statistical Analysis
Mixed model regression, which accounts for missing data, irregular intervals between measurements, and within-person correlation, was used to examine the developmental trajectories. For a given structure, the ith individual’s jth measurement was modeled as Volumeij = intercept + di + B1(Age - mean Age) + B2(Age- mean Age)2+ B3(Age -mean Age)3 +eij. In the equation above, di is a normally distributed random effect that models within-person dependence. The eij term represents the usual normally distributed residual error. The B1, B2, and B3 coefficients show how volume changes with age. The intercept and B terms were modeled as fixed effects. We allowed the intercept and B terms to vary by sex group, producing two growth curves with different height and shape characteristics. Age was centered at the sample average age to permit interpretation of intercept differences. The most parsimonious growth curve for each subregion was fitted. F tests (with numerator df=2) were used to determine whether male and female polynomial age terms combined significantly contributed to the explanatory power of the model. If neither cubic (tested first) nor quadratic age terms were significant contributors, we employed a constant growth model. F tests were used to determine if the diagnostic curves differed in shape, and t tests were used to determine if the groups’ curves differed in height at the average age. Fixed effects (slopes and intercepts) were used to generate fitted values used for graphing purposes. Total cerebral volume (TCV) was calculated as the sum of volumes of gray matter and white matter excluding the cerebellum. Values were compared both with and without adjustment for total brain volume.
We used mixed effect regression to compare trajectories between boys and girls and between cerebellar regions. If trajectories were gender specific, our models allowed regional relationships to vary over time differently for males and females by including three way interactions (a region*time*sex interaction). If these interactions with age were not significant, we quantified the relationship between the volumes of different regions with linear regression.
Statistical assumptions were checked using normal probability plots, plots of predicted values vs. residuals, and tests of normality (Shapiro-Wilks and Kolmogorov-Smirnov tests). We present predicted volumes for ages 7 to 22 in our figures as only 10% of scans were made outside this age range. Statistics were performed using SPSS 11.0.1. and SAS 9.1
RESULTS
Demographic characteristics
All participants were right handed, singletons and self-described as being of Caucasian origin. There was no significant difference between the male and female groups in terms of average age at time of scan (females 13.7 years, males 13.9 years). Females came from families with a higher socio-economic status than males (parental Hollingshead Index of Social Status Score was 30 in females versus 21 in males, Mann-Whitney U=202, df=48, p=0.02). There was a trend for group difference in IQ with a male mean of 122 and a female mean of 116 (t=1.87, df=48, p=0.07).
Neuroanatomic results
Cerebellar subregions were defined and measured in all 183 scans. Two scans had volumes smaller than three standard deviations from the mean as identified by visual inspection and descriptive statistics were excluded as outliers, which occurred due to processing errors. This left 181 scans for analysis.
Total Cerebellum
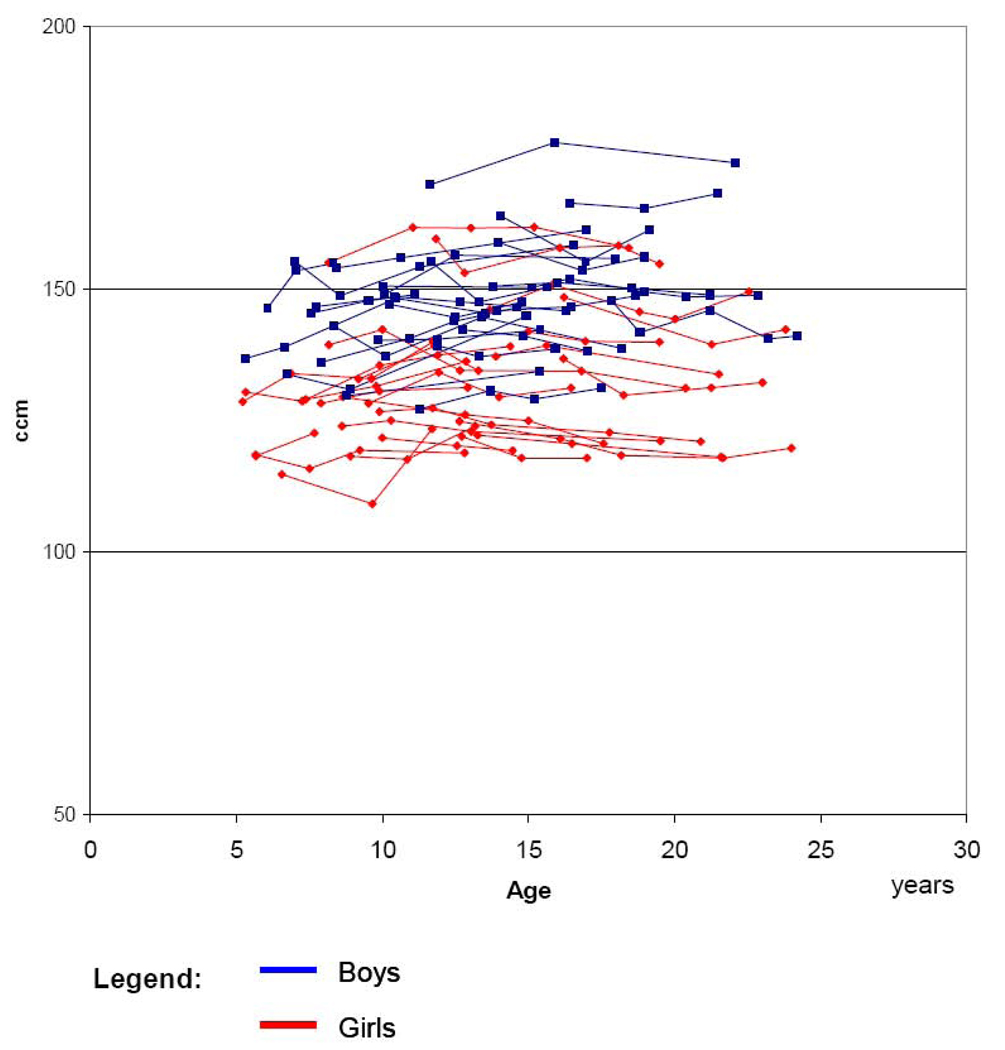
Figure 2 depicts the individual data points of the total cerebellar volume by age and gender; repeated measures of one individual are combined. This figure shows that the age range depicted in the subsequent figures (7–22 years) is covered well by the repeated assessments of the participants at ages (5–24 years). Furthermore, the figure indicates the individual variation of cerebellar volume. The variation is relatively small and suggests that there is relatively little random error in this measure.
Figure 2.
Individual data of total cerebellar volume by age and gender
 Boys
Boys
 Girls Observations from one individual are connected by lines
Girls Observations from one individual are connected by lines
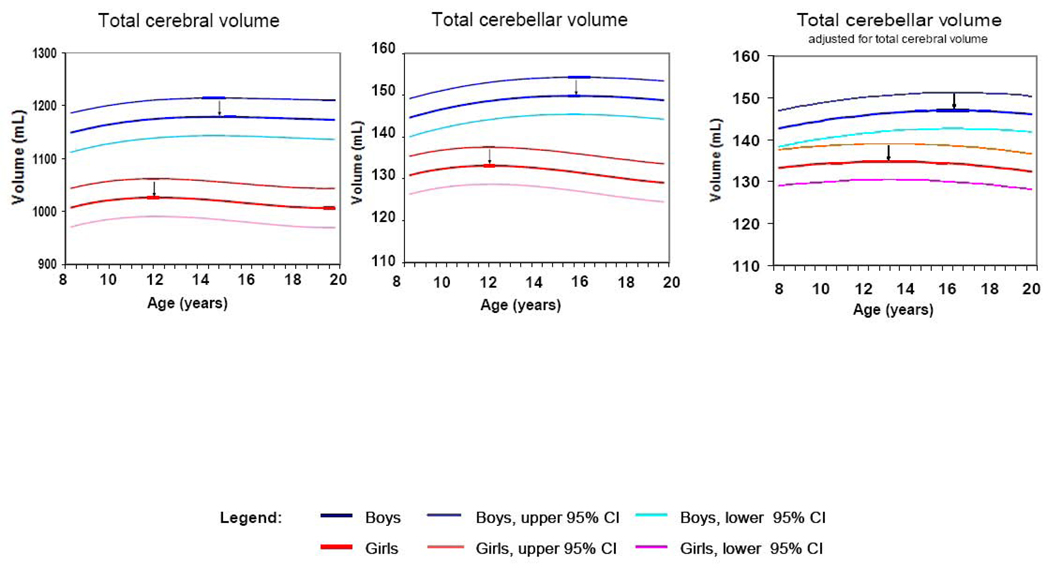
Maturation of the total cerebellum followed an inverted U shaped trajectory. While total cerebellar volume was larger in males than females at all ages the magnitude of the difference was age-dependent (see Table 1 and Figure 3): The difference in the fitted value increased from 13.8 mL larger cerebellar volume in males at age 8 (10%) to 19.8 mL at age 20 (13%; Figure 3). After covarying for TCV (larger in males), total cerebellar volume remained significantly larger in males at the average age and the sex difference in developmental trajectory remained significant as well.
Table 1.
Parameters for developmental trajectories of cerebellar regions
| Cerebellar structure | Best fitting model, p-value ¶ |
Mean age centered intercept* (SE) |
Age at peak volume, years |
Age coefficient β1 (SE) |
Age2 Coefficient β2 (SE) |
Age3 coefficient β3 (SE) |
|---|---|---|---|---|---|---|
| Total cerebellar volume | Cubic, 0.005 | |||||
| Female | 132.68 (2.24) | 11.8 | −0.417 (0.15) | −0.078 (0.01) | 0.008 (0.00) | |
| Male | 149.59 (2.24) | 15.5 | 0.289 (0.15) | −0.087 (0.02) | 0.003 (0.00) | |
| Inferior posterior lobe | Cubic, 0.006 | |||||
| Female | 53.83 (0.96) | 11.1 | −0.307 (0.08) | −0.307 (0.08) | 0.004 (0.00) | |
| Male | 61.13 (0.96) | 13.8 | 0.014 (0.08) | −0.030 (0.01) | 0.001 (0.00) | |
| Superior posterior lobe | Quadratic, 0.01 |
|||||
| Female | 42.76 (1.09) | 15.8 | 0.040 (0.05) | −0.010 (0.01) | n.a. | |
| Male | 9.08 (1.09) | 18.2 | 0.254 (0.05) | −0.031 (0.01) | n.a. | |
| Anterior lobe | Quadratic, 0.04 |
|||||
| Female | 12.29 (0.54) | 13.5 | −0.004 (0.02) | −0.006 (0.00) | n.a. | |
| Male | 12.84 (0.54) | 15.7 | 0.029 (0.03) | −0.007 (0.00) | n.a. | |
| Corps medullare | Quadratic, 0.04 |
|||||
| Female | 14.70 (0.37) | 17.2 | 0.083 (0.04) | −0.013 (0.01) | n.a. | |
| Male | 16.62 (0.37) | n.a.** | 0.092 (0.04) | −0.005 (0.01) | n.a. | |
| Total cerebellar volume/total cerebral volume |
Linear, 0.06‡ |
|||||
| Female | 0.13 (0.00) | n.a. | −0.002 (0.00)‡ | n.a. | n.a. | |
| Male |
0.13 (0.00) |
n.a. | 0.001 (0.00) | n.a. | n.a. |
See methods section in text for the multivariate model. n.a. = not applicable
p-value of term with highest power in polynomial function.
The mean age-centered intercept gives the predicted volume in mL at 13.8 years.
The trajectory does not reach its peak within the age range included in this study
This p-value refers to the best model.
Although the age-coefficient β1 of girls differed significantly from 0, overall the model shows that the ratio of the total cerebellar and total cerebral volume does not change with age.
Figure 3.
Developmental trajectories of total cerebral and total cerebellar volume
 Boys —— Boys, upper 95% CI
Boys —— Boys, upper 95% CI  Boys, lower 95% CI
Boys, lower 95% CI
 Girls
Girls  Girls, upper 95% CI
Girls, upper 95% CI  Girls, lower 95% CI
Girls, lower 95% CI
 Indicates age of peak volume
Indicates age of peak volume
Subregions
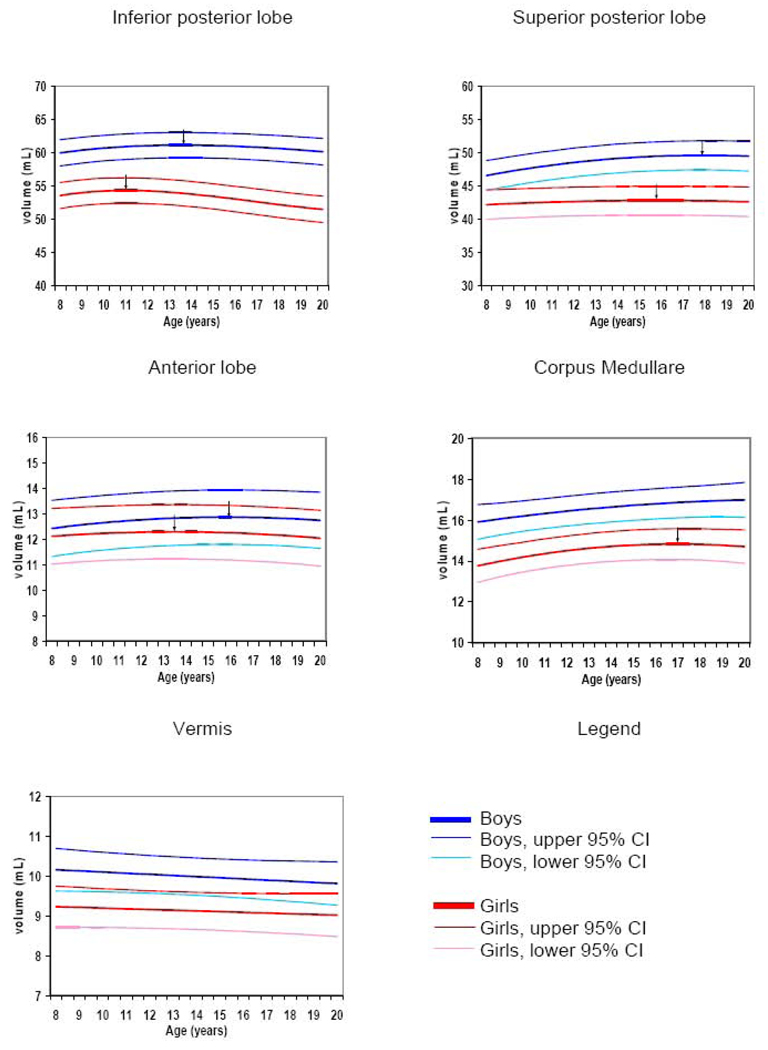
Analysis was first performed combining left and right sides for each cerebellar lobe. The growth of the inferior posterior cerebellum was best described using a cubic trajectory; the anterior, superior posterior, and corpus medullare regions were modelled by a quadratic trajectory, and the vermis and its subregions by a linear trajectory (Table 1, Figure 3 and figure 4). The volumes of the vermal lobuli did not show significant changes with age (see Table 2). The total volume of the vermis throughout the age range was approximately 9.5% larger in males than in females (difference at mean age is 0.86 mL, T=2.57, df=47, p=0.01). Volumes for all cerebellar lobes at the mean age were significantly larger in males than females with the exception of the anterior lobe, which was not significantly different (Table 3 and Figure 4). Inverted U-shaped trajectories for the anterior, superior posterior, and inferior posterior lobes peaked earlier in females and at different ages in different regions (see Table 1). A sex difference in developmental trajectory was evident for the superior posterior lobe, indicating that the magnitude of the difference in volume between sexes changes across development. After covarying for TCV, superior and inferior posterior lobes remained significantly larger in males, and the superior posterior developmental trajectory sex difference survived as well (Table 1).
Figure 4.
Developmental trajectories of cerebellar hemispheric lobes, central white matter and vermis
 Boys —— Boys, upper 95% CI
Boys —— Boys, upper 95% CI  Boys, lower 95% CI
Boys, lower 95% CI
 Girls
Girls  Girls, upper 95% CI
Girls, upper 95% CI  Girls, lower 95% CI
Girls, lower 95% CI
 Indicates age of peak volume
Indicates age of peak volume
Table 2.
Volumes of the vermal lobuli by age in boys and girls
| Vermal subregion | Mean age centered intercept* (SE) |
Age coefficient β1 (SE) |
P-values for β1, age change |
|---|---|---|---|
| Total Vermal volume | |||
| Female | 9.13 (0.24) | −0.018 (0.02) | 0.39 |
| Male | 9.99 (0.24) | −0.029 (0.02) | 0.21 |
| Inferior posterior vermal lobulus | |||
| Female | 2.96 (0.10) | −0.018 (0.01) | 0.04 |
| Male | 3.44 (0.10) | −0.011 (0.01) | 0.26 |
| Superior posterior vermal lobulus | |||
| Female | 2.53 (0.08) | −0.007 (0.01) | 0.35 |
| Male | 2.57 (0.08) | −0.016 (0.01) | 0.07 |
| Anterior vermal lobulus | |||
| Female | 3.63 (0.11) | 0.009 (0.01) | 0.39 |
| Male | 3.98 (0.11) | −0.002 (0.01) | 0.83 |
See methods section in text for the multivariate model.
The mean age-centered intercept gives the predicted volume at 13.8 years.
Table 3.
Sex differences in developmental trajectories
| Cerebellar structure | Male-female comparisons | Male-female comparisons adjusted for total brain volume |
||||||
|---|---|---|---|---|---|---|---|---|
| Height Differences | Shape Differences | Height Differences | Shape Differences | |||||
| F | p values | F | p values | F | p values | F | p values | |
| Total cerebellar volume | 28.56 | <0.01 | 4.41 | <0.01 | 13.46 | <0.01 | 3.58 | 0.03 |
| Right | 31.22 | <0.01 | 3.89 | 0.01 | 15.34 | <0.01 | 3.29 | 0.04 |
| Left | 28.77 | <0.01 | 3.84 | 0.01 | 12.31 | <0.01 | 3.30 | 0.04 |
| Inferior posterior lobe | 29.09 | <0.01 | 2.65 | 0.05 | 16.06 | <0.01 | 2.19 | 0.09 |
| Right | 31.02 | <0.01 | 2.30 | 0.08 | 15.66 | <0.01 | 1.66 | 0.19 |
| Left | 25.03 | <0.01 | 2.04 | 0.11 | 13.04 | <0.01 | 1.72 | 0.17 |
| Superior posterior lobe | 16.72 | <0.01 | 5.47 | 0.01 | 8.79 | <0.01 | 4.93 | 0.01 |
| Right | 17.15 | <0.01 | 4.50 | 0.01 | 9.66 | <0.01 | 4.09 | 0.02 |
| Left | 14.61 | <0.01 | 3.22 | 0.03 | 5.93 | 0.02 | 3.00 | 0.06 |
| Anterior lobe | 0.52 | 0.47 | 0.49 | 0.61 | 0.00 | 0.97 | 0.37 | 0.69 |
| Right | 0.02 | 0.89 | 0.46 | 0.63 | 0.16 | 0.69 | 0.47 | 0.63 |
| Left | 2.12 | 0.15 | 4.58 | 0.03 | 0.32 | 0.57 | 3.86 | 0.05 |
| Corpus medullare | 13.55 | <0.01 | 0.53 | 0.59 | 2.20 | 0.14 | 0.63 | 0.54 |
| Right | 14.72 | <0.01 | 0.31 | 0.58 | 1.43 | 0.23 | 1.15 | 0.32 |
| Left | 18.78 | <0.01 | 0.07 | 0.80 | 3.00 | 0.09 | 0.01 | 0.92 |
Sex differences were obtained with multilevel models; height differences are estimated mean differences across the age range studied.
When considering sides separately, males had significantly greater volumes for left and right corpus medullare, left and right inferior posterior and left and right superior posterior lobes (Table 3). Significant sex differences in developmental trajectory were evident for the left and right superior posterior lobes and the left anterior lobe. After covarying for TCV males continued to have significant volumetric sex differences at the average age in the left and right inferior posterior and left and right superior posterior lobes, and the significant interaction was still present between sex and age in the development of the right superior posterior lobe.
Relationships Between Subregions
We then tested whether gender differences in cerebellar structures varied by region. We found a significant three-way interaction between region, sex, and age for the anterior and inferior lobes (p < 0.0073). Specifically, the relationship between the anterior and inferior lobe becomes progressively weaker with age for females, while it is similar at all ages in males. Table 3 shows that the inferior but not the anterior lobe develops different between males and females; and the trajectories of the two regions differing significantly in females are depicted in figure 4. For the remaining comparisons, no three way (sex*region or age*region) interaction reached significance. Thus, while controlling for sex and age (and sex*age for regions with sex difference in developmental trajectory (above)), we found significant positive relationships between the superior and inferior posterior lobes (b = 0.04398, p = < 0.001), all three vermal regions (anterior versus superior vermis: b=.67, p = < 0.0001, the anterior versus inferior vermis: b=0.4844, p = < 0.0001), and between the vermis and hemispheres (b = 1.42, p < = 0.0003). The relationship between the anterior and superior posterior lobe was not significant (b = 0.04596, p = 0.183).
In addition, we found a significant relationship between total cerebellum and TCV, (b = 0.04398, p < 0.001) which did not significantly vary over time or between sexes. We also compared trajectories of the ratio of total cerebellar volume and TCV and found that the ratio followed a linear trajectory which did not differ in height or shape between males and females.
Discussion
This is the first longitudinal study of normal cerebellar development during childhood and adolescence. Total cerebellar volume was larger in males, both with and without adjustment for total brain volume, this difference gradually increased during adolescence. The cerebellum as a whole reached its peak volume later than the cerebrum, suggesting a prolonged developmental course. Different cerebellar compartments had distinct growth patterns with varying degrees of sexual dimorphism. Growth trajectories between regions arising from different regions of the neural plate showed weaker relationships than regions of more homogeneous origin.
The cerebellum is a brain region that increased in size fairly late in evolution and is one of the brain regions most markedly enlarged in humans compared to other mammals (MacLeod et al., 2003). This is largely due to a phenomenal increase of the cerebellar hemispheres relative to the vermis, which is phylogenetically older and the predominant component of the cerebellar cortex in many mammals. One of the most remarkable characteristics of the cerebellum is the cytoarchitectural uniformity of its cortex. Functional specificity of different regions is conferred in large part by their membership within particular cerebro-cerebellar loops (Ito, 1990; Herrup and Kuemerle, 1997; Voogd, 2003). The cerebellar neurons associated with specific loops are contained within parasagittal zones that have shown high degree of conservation across species. The most obvious medio-lateral division in the cerebellum is between the vermis and the hemispheres. The extensive connections of lateral portions of the cerebellar hemispheres to the prefrontal cortex (Middleton and Strick, 2001) have provided support for the role of the cerebellum in cognitive processes.
The visually most striking major anatomical subdivisions of the cerebellum are the horizontally arranged lobes and folia. The clearest demarcations are the primary fissure, which divides the anterior and inferior lobes, and the posterolateral fissure, which divides the inferior lobe and the flocculonodular lobe. A less prominent division lies between the superior and posterior lobules of the inferior lobe. The functional specificity of these antero-posterior divisions is less well understood, but there is significant evidence to suggest that the anterior, inferior, and flocculonodular lobes are biologically distinct compartments (Larouche and Hawkes 2006). Support for a division on an ontologic basis comes from studies in quail-chick chimera that have demonstrated the cerebellum is formed by cells coming from both the mesencephalon and metencephalon (Hallonet 1990, Hallnot & LeDouarin 1993]. The primary fissure appears to be similar to the division between cells derived from these two major vesicles. Our findings that the developmental trajectories of the superior and posterior inferior lobes show a much stronger relationship with each other than to the trajectory of the anterior lobe may be reflective of their different neurodevelopmental origins.
Sex differences have been much less well characterized in the cerebellum than in the cerebrum. Although several studies have reported larger cerebellar volumes in males in adult populations, consistent with overall larger brain size (Rhyu et al., 1999; Raz et al., 2001; Szabo et al., 2003), only one to our knowledge has reported on the relationship between age, cerebellar volumes, and gender differences in children and adolescents. Caviness et al. in a cross-sectional sample of fifteen males and fifteen females aged 7–11 found that the cerebellum was at adult volume in females but not males at this age range, suggesting the presence of late development and gender dimorphism (Caviness Jr. et al., 1996). These results are consistent with the results reported here of later peak cerebellar volume in males than females, similar to findings of later maturation of other cerebral structures in males (Sowell et al., 2003; Lenroot et al., 2007). We found that cerebellar volume in males was larger than in females even after correction for larger brain volume in males. However, this difference decreased as subjects became older, approaching the reported sex differences in cerebellar volume of adults that are proportionate to overall differences in brain volume.
In an earlier study of this cohort, Giedd et al. reported that changes in cortical gray matter were regionally specific, with developmental curves for the frontal and parietal lobe peaking around age 11 in girls and 12 in boys and for the temporal lobe at about age 16 years in both boys and girls (Giedd et al., 1999).
We now found that the developmental trajectory of the cerebellum peaks earlier in the inferior posterior lobe and later in the superior posterior and anterior lobe, although these differences were mostly not significant. Moreover, there are multiple connections between a large number of widely distributed brain areas of the cerebral cortex (particularly the prefrontal cortex) and the different cerebellar regions (Ramnani, 2006). Hence, we can only speculate cautiously that the cerebellar inferior posterior lobe and the frontal grey matter follow a similar developmental pattern. However, we can be more confident that the increase of the volume of the cortical grey matter does not continue longer than the increase of the volume of the cerebellar lobes, which comprise mostly grey matter.
Functionally, some researchers have concluded that the anterior lobe is primarily concerned with motor function, while the inferior lobes, which contain the zones connected to cerebral regions such as the parietal and prefrontal cortices, play a stronger role in cognitive processes (Middleton and Strick, 1998). The vermis is a region which appears relatively early in evolution, is associated with axial stability, and has been related to abnormalities in emotional function (Schmahmann, 2004). Vermal volumes did not show significant changes during the age range included within our study, in contrast to the protracted developmental course of the phylogenetically younger parts of the cerebellum. A similar pattern was seen in the cerebral cortex, where areas such as the prefrontal cortex thought to have appeared later in evolution also attained a mature state later in development (Fuster, 2002; Gogtay et al., 2004). Our data suggest that this principle may also be operative in the cerebellum. Alternatively, it is possible that the prolonged development of the cerebellar lobes seen here is related to the presence of both gray and white matter in the cerebellar lobar volumes. White matter in the cerebrum has been found to increase longer than gray matter, and if this pattern is also true in the cerebellum, the mixed tissue types could contribute to delaying age at peak volume for the lobes as a whole compared to what would be seen if it were possible to look at gray matter alone.
Volumetric differences occurring during development could arise from changes in neuropil, neuronal size, dendritic or axonal arborisation, or the vasculature. Synaptic pruning together with trophic glial changes has been shown associated with puberty both in primate and human cerebral development (Huttenlocher, 1979; Bourgeois and Rakic, 1993; Morrison and Hof, 1997; Chechik et al., 1999). Although the existing literature is sparse, there has been increasing evidence of sexual dimorphism in gene and protein expression in the developing cerebellum. Modulation of cerebellar function and development by neurosteroids via estrogen and progesterone receptors has been identified in the cerebellum in recent years (Perez et al., 2003; Ikeda and Nagai, 2006), and Purkinje cells have been identified as a major site for neurosteroid formation (Tsutsui, 2006). Differences in gene expression have also been reported by Vawter et al, who examined genes specific to sex chromosomes and found significantly different expression of six genes in the cerebellum as well as regions of the cerebrum (Vawter et al., 2004). Functional implications of these molecular findings or of the volumetric differences are largely unexplored. Although measures of pubertal status were not available for subjects in this study, the observation of later peak values in males, corresponding to the later age of puberty seen in males, suggests that the onset of volume decrease may be related to processes associated with pubertal maturation.
Cerebellar abnormalities are among the most consistently reported structural findings for both autism (Stanfield et al., 2007) and ADHD (Valera et al., 2007). The larger size of the cerebellum in males and differences between sexes in longitudinal development may reflect sex-specific factors related to the higher risk for these disorders in males. It has also been suggested that the protracted postnatal neurogenesis and development of the cerebellum may render it particularly vulnerable to effects of hypoxia, toxins, or other environmental factors (Ciesielski and Knight, 1994). It should be noted that this average IQ of this was sample was above population norms at 119. In addition, we included only typically developing Caucasian, right-handed singletons sample in order to maximize sample homogeneity and improve the ability to detect non-linear brain volume changes with age. Results should therefore be generalized to broader populations with care.
In summary, developmental trajectories of the cerebellum are sexually dimorphic. Phylogenetically and ontogenetically diverse subregions of the cerebellum also show differing developmental trajectories, with areas of the cerebellum linked to later developing regions of the cerebrum also showing prolonged maturation. Differences in brain size between males and females should not be interpreted as implying any sort of functional advantage or disadvantage. Relationships between size and function are complicated by the inverted U shape of developmental trajectories and by the many cytoarchitectural factors contributing to structure size. However, an understanding of sexual dimorphism of developmental trajectories and differences between subregions of the cerebellum may have important implications for the understanding of neurodevelopmental disorders, most of which have different ages of onset, prevalence, and symptomatology between males and females.
Acknowledgements
Dr. H. Tiemeier’s work on this project was supported by a research fellowship of the Sophia Stichting (2004-025/SWO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Edelbrock CS. Manual for child behavior checklist and revised behavior profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1983. [Google Scholar]
- Bishop DV. Cerebellar abnormalities in developmental dyslexia: cause, correlate or consequence? Cortex. 2002;38:491–498. doi: 10.1016/s0010-9452(08)70018-2. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. Journal of Neuroscience. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years, A volumetric analysis based on Magnetic Resonance Images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chechik G, Meilijson I, Ruppin E. Neuronal regulation: A mechanism for synaptic pruning during brain maturation. Neural Comput. 1999;11:2061–2080. doi: 10.1162/089976699300016089. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE. Cerebellar abnormality in autism: a nonspecific effect of early brain damage? Acta Neurobiol Exp (Wars) 1994;54:151–154. [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol. 1994;162:123–130. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH. Dissociation of Frontal and Cerebellar Activity in a Cognitive Task: Evidence for a Distinction between Selection and Search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, 3rd, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallonet ME, Le Douarin NM. Tracing neuroepithelial cells of the mesencephalic and metencephalic alar plates during cerebellar ontogeny in quail-chick chimaeras. Eur J Neurosci. 1993;5:1145–1155. doi: 10.1111/j.1460-9568.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven: Yale University Department of Sociology; 1975. In. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A. Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Res. 2006;1083:39–49. doi: 10.1016/j.brainres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Ito M. A new physiological concept on cerebellum. Rev Neurol (Paris) 1990;146:564–569. [PubMed] [Google Scholar]
- Larouche M, Hawkes R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum. 2006;5:77–88. doi: 10.1080/14734220600804668. [DOI] [PubMed] [Google Scholar]
- Larsell O, Jansen J. The comparative anatomy and histology of the cerebellum. Minneapolis: University of Minnesota Press; 1967. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik PG, Ciesielski KT, Hart BL, Benzel EC, Sanders JA. Evidence for cerebellar-frontal subsystem changes in children treated with intrathecal chemotherapy for leukemia: enhanced data analysis using an effect size model. Arch Neurol. 1998;55:1561–1568. doi: 10.1001/archneur.55.12.1561. [DOI] [PubMed] [Google Scholar]
- MacLeod CE, Zilles K, Schleicher A, Rilling JK, Gibson KR. Expansion of the neocerebellum in Hominoidea. J Hum Evol. 2003;44:401–429. doi: 10.1016/s0047-2484(03)00028-9. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, Rauch SL, Harris G, Biederman J, Caviness VS, Jr, Kennedy DN, Schmahmann JD. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage. 2005;25:1146–1160. doi: 10.1016/j.neuroimage.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output channels. Int Rev Neurobiol. 1997;41:61–82. doi: 10.1016/s0074-7742(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The cerebellum: an overview. Trends Neurosci. 1998;21:367–369. doi: 10.1016/s0166-2236(98)01330-7. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, Oleary D, Andreasen NC. Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999;270:149–152. doi: 10.1016/s0304-3940(99)00487-5. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. MRI atlas of the human cerebellum. San Diego: Academic Press; 2000. [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2007 doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmannm JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Lancaster JL, Xiong J, Cook C, Fox P. MR imaging volumetry of subcortical structures and cerebellar hemispheres in normal persons. AJNR Am J Neuroradiol. 2003;24:644–647. [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K. Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell. J Steroid Biochem Mol Biol. 2006;102:187–194. doi: 10.1016/j.jsbmb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, Li J, Lopez JF, Myers R, Cox D, Watson SJ, Akil H, Jones EG, Bunney WE. Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacology. 2004;29:373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J. The human cerebellum. J Chem Neuroanat. 2003;26:243–252. doi: 10.1016/j.jchemneu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children - revised. New York: The Psychological Corporation; 1974. [Google Scholar]
- Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]