Abstract
N-myristoyltransferase (NMT) attaches a 14 carbon fatty acid, myristic acid, to the N-terminal glycine residue of proteins. NMT exists in two isoforms NMT1 and NMT2. Myristoylated proteins play critical roles in protein–protein interactions, cell signaling and oncogenesis. Although elevated expression of NMT1 has been described in colorectal carcinoma, its expression and roles in normal and inflamed lungs of the cattle are unknown. Therefore, we investigated the expression and activity of NMT in a bovine model of lung inflammation induced with Mannheimia hemolytica and in vitro in neutrophils and macrophages. Western blots revealed increased expression of NMT1 in lungs from infected animals compared to control animals. Total NMT activity was reduced in inflamed lungs compared to control animals (p < 0.05) along with increased expression of enolase, a putative inhibitor of NMT. NMT1 staining was observed in the septum, vascular endothelium and the epithelium in the lungs from control as well as infected calves. NMT1 expression was intense in neutrophils in the necrotic areas in the inflamed lungs. Immuno-electron microscopy localized NMT1 in cytoplasm and nuclei of endothelium, pulmonary intravascular macrophages and airway epithelium. Total NMT activity and NMT1 expression were increased in neutrophils and macrophages exposed to Escherichia coli LPS in vitro. NMT knockdown increased apoptosis in activated neutrophils. This is the first report demonstrating expression of NMT in normal and inflamed lungs and a novel role for NMT in regulation of neutrophil lifespan.
Keywords: NMT, neutrophil, macrophage, apoptosis, RNA knockdown
1. INTRODUCTION
N-myristoylation is a lipidic modification referring to the covalent attachment of myristate, a 14 carbon saturated fatty acid, to the N-terminal glycine residue of a number of mammalian, viral and fungal proteins [3, 6, 19, 25, 28, 32]. Myristoylation of proteins is catalyzed by the ubiquitously distributed eukaryotic enzyme N-myristoyltransferase (NMT) which is a member of GCN5-related N-acetyltransferase (GNAT) superfamily of proteins. NMT has been purified and characterized from different sources [3, 6, 19, 25, 28]. The cDNA encoding NMT from human, yeast, bovine spleen, malaria parasite and higher plant have been characterized. NMT in lower eukaryotes is encoded by a single copy gene whereas higher eukaryotes such as bovine, human and Arabidopsis NMT are encoded by two gene copies. The second genetically distinct mammalian NMT cDNA (NMT2) has been cloned from a human liver library and the respective mouse homologues for the two human NMT have also been cloned [7].
We demonstrated for the first time in a rat model higher activity of NMT in colonic epithelial neoplasm than in the normal appearing colonic tissue, and an increase in NMT activity at an early stage in colonic carcinogenesis [14]. Furthermore, we have reported a potential role for NMT in cardiac muscle in the experimentally induced ischemia-reperfusion, in Parkinson syndrome in rat model and also in the streptozotocin induced diabetic rat [30]. Earlier we demonstrated an essential role of NMT1 in early development of mice. Although homozygous Nmt −/− mice did not survive, we were able to isolate homozygous Nmt−/− embryonic stem cells [37] suggesting NMT1 knock out is not lethal to cell survival. Our recent data also showed that NMT1 is important for the proper differentiation of monocytes [33]. Taken together, NMT is emerging as an important regulator of cell signaling in normal, tumor and immune cells.
The expression, activity and role of NMT have not been demonstrated in acute lung inflammation (ALI). ALI underlies most of the lung diseases and results in significant mortality and morbidity in humans and domestic animals and imposes enormous economic burden on the health care system and animal industry [27, 36]. For example, therapeutic and preventive measures needed to tackle pneumonia in cattle cost nearly $1 billion every year in the USA alone [26]. ALI is characterized by excessive activation and recruitment of neutrophils and monocytes/macrophages. Activated neutrophils live longer and cause tissue damage, which is believed to contribute to morbidity and mortality [13, 16, 17]. There is an ongoing search to explore the role of new molecules to better understand the pathogenesis of ALI with an aim to develop more effective therapeutics.
Considering role of NMT in regulating differentiation of monocytes [33] and current lack of any data on the role of NMT1 in bacterial acute lung inflammation, we investigated the expression and activity of NMT1 in a calf model of lung inflammation induced following intratracheal instillation of Mannheimia hemolytica [34]. The data from these experiments show that both NMT activity and NMT1 expression are altered in inflamed lungs. The in vitro data show changes in NMT activity in neutrophils upon exposure to lipopolysaccharide (LPS) and the effect of NMT1 expression on the lifespan of neutrophils.
2. MATERIALS AND METHODS
2.1. Materials
LPS isolated from Escherichia coli 0127.B8 was purchased from Sigma-Aldrich, St. Louis, USA (L3129). 9,10−3H-Myristic acid (39.3 Ci/mM) was purchased from Perkin Elmer (Waltham, USA). Pseudomonas acyl CoA synthetase was purchased from ICN Biochemicals (Aurora, USA). PVDF membrane was purchased from BioRad Laboratories (Mississauga, Canada). NMT1 mAb and HRP-conjugated goat anti mouse antibody was obtained from BD-Biosciences (Mississauga, Canada). Gold conjugated anti rabbit secondary antibody was procured from British Biocell International. Polyclonal (Cardiff, UK) antibody was raised against purified NIP71 in New Zealand white rabbits and its specificity has been described previously [20]. Cell culture reagents, RPMI 1640, and fetal bovine serum were purchased from Invitrogen/GIBCO (Burlington, Canada). Disposable plastic tissue culture plates and other supplies were from VWR Canlab (Mississauga, Canada). Chemiluminescence reagent plus was obtained from NEN Life Science products (Boston, USA). Peptide substrate based on the N-terminal ends of pp60c-Src (GSSKSKPKR) was synthesized by the Alberta Peptide Institute, Edmonton, Canada. PMA and general laboratory reagents of analytical grade were obtained from Sigma Chemical Co. (Oakville, Canada).
2.2. Tissue samples
Lung tissue samples were obtained as described earlier [34]. Briefly, 4 to 6 week old male Holstein-Friesian calves were infected intratracheally with M. hemolytica A1 (20 × 109 CFU in 10 mL volume) or with endotoxin-free saline. This strain of M. hemolytica was isolated from a calf lung in 1982 at the Vaccine and Infectious Disease Organization (University of Saskatchewan, Saskatoon, Canada). Lung samples were collected according to a pre-determined plan which included dividing the each lung lobe into 10 arbitrary slices and collecting a piece from the 5th slice of each lobe. Lung samples were frozen for protein analyses and embedded in paraffin for light microscopy or for immuno-gold electron microscopy. While immunohistology was performed on sections prepared from lung tissues obtained from all the animals, the Western blots and other analyses were done on lung samples tissues from only three animals each of the groups.
2.3. Homogenization
Tissue samples from infected (n = 3) and control animals (n = 3), that were previously stored at −80 °C were used for the experiment. Using a polytron homogenizer, 1 g of each tissue sample collected from an animal was homogenized together at 4 °C in 2 mL of phosphate buffered saline (PBS) containing 0.1 mM EGTA and 10 mM 2-mercaptoethanol. Samples were homogenized twice for 30 s each with a cooling interval of 1 min and centrifuged at 400 g for 15 min. The supernatant was removed and used for biochemical studies.
2.4. Cell culture
Promonocytic U937 cell line was obtained from ATCC and grown in RPMI 1640 supplemented with 10% fetal calf serum, 1% antibiotic solution in a humidified air and 5% CO2 at 37 °C. Cells were induced to differentiate in to macrophage by the treatment of PMA (10 ng/mL) for 96 h and treated LPS for indicated dose and time period. Cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 10 mM DTT, 1 mM PMSF and 1% protease inhibitor cocktail.
2.5. Neutrophil isolation and treatment
Polymorphonuclear leukocytes (PMN) were isolated from the blood collected from healthy cattle by density gradient centrifugation with lymphocyte separation media (LSM, MP Biomedicals, Solon, USA) after using ammonium chloride for erythrocyte lysis. Isolated cells were suspended in RPMI 1640 medium modified with 10% fetal bovine serum and L-glutamine. The viability of isolated PMN was found to be greater than 97% with trypan blue (Sigma, St. Louis, USA) exclusion. Neutrophils purity determined by staining cytospin slides with Grunwald Giemsa stain was more than 90%.
2.6. N-myristoyltransferase assay
N-myristoyltransferase activity was assayed as described earlier [11, 24]. Briefly, 3H-myristoyl-CoA was synthesized according to the literature [24]. The reaction was carried out for 30 min at 30 °C in a mixture that contained 40 mM Tris-HCl, pH 7.4, 0.1 mM EGTA, 10 mM MgCl2, 5 mM ATP, 1 mM LiCoA, 1 μM 3H-myristic acid (7.5 μCi) and 0.3 unit/mL Pseudomonas acyl-CoA synthetase in a total volume of 200 μL. The conversion to 3H-myristoyl-CoA was generally greater than 95%. The assay mixture contained 40 mM Tris-HCl, pH7.4, 0.5 mM EGTA, 0.45 mM 2-mercaptoethanol, 1% Triton X-100, peptide substrate (500 μM) and NMT source in a total volume of 25 μL. The transferase reaction was initiated by adding freshly generated 3H-myristoyl-CoA, allowed to proceed at 30 °C for 30 min, terminated by spotting 15 μL aliquots of incubation mixture onto P81 phosphocellulose paper discs and drying under a stream of warm air. The P81 phosphocellulose paper discs were washed in two changes of 40 mM Tris-HCl, pH 7.3 for 60 min. The radioactivity was quantified in 7.5 mL of Beckman Ready Safe Liquid Scintillation mixture in a Beckman Liquid Scintillation Counter. One unit of NMT activity was expressed as 1 pmol of myristoyl peptide formed per min.
2.7. Western blot
Western blot analysis was performed as described previously [35]. Equal amount of tissue homogenates (25 μg) were electrophoresed on a SDS-PAGE and transferred to a PVDF membrane. Transblotted PVDF membrane was incubated with blocking buffer (PBS-Tween 20 plus 5% powdered milk) for 1 h at room temperature to block non-specific binding. After washing, the blots were probed with antibodies listed in Table I. HRP-conjugated secondary antibodies were used and the bands were detected using the chemiluminescence reagent plus and exposed to X-ray films.
Table I.
Antibodies used in the present study.
| Antibody | Clone designate | Specificity | Source |
|---|---|---|---|
| NMT1 | Monoclonal | Human, rat, mouse and bovine | BD Biosciences Canada |
| NIP71 | Polyclonal | Human, rat, mouse, chicken and bovine | [20] |
| Enolase | Monoclonal | Human, rat, mouse and bovine | BD Biosciences Canada |
2.8. Immunohistochemistry
The method has been described previously [31]. Briefly, the sections were deparaffinzed, rehydrated followed by quenching of endogenous tissue peroxidase. The sections were incubated with pepsin (2 mg/mL 0.01 N HCl) for 45 min followed by BSA for 30 min. Lung sections were stained with polyclonal anti-NMT1 antibody (1:100) for 1 h, HRP conjugated anti-rabbit secondary antibody for 30 min followed by color development. Controls included omission of primary antibody.
2.9. Immuno-gold electron microscopy
Thin sections were labeled for immuno-gold electron microscopy as reported previously [32]. To summarize, 1 μm thick sections stained with toluidine blue were examined to select desirable areas for ultramicrotomy. Thin sections (90 nm) were collected on 200 mesh nickel grids, blocked with albumin, and exposed to a polyclonal NMT antibody (1:50) for 60 min. The grids were washed with tris-buffered saline and then incubated with gold conjugated anti-rabbit secondary antibody (1:100) for 60 min. Lung sections stained with only secondary antibody conjugated to gold particles were used as the controls. The sections were stained with 2% aqueous uranyl acetate with triton-X and lead citrate followed by examination in a Philips 410LS electron microscope at 60 kV.
2.10. RNA interference
Small interfering RNA (siRNA) duplexes designed against human NMT1 and NMT2 were obtained from Dharmacon, Inc. (Lafayette, CO, USA). Scrambled siRNA from Dharmacon was used as the control siRNA. siRNA were transfected into cells by DharmaFect4. Neutrophils (104/well) were plated in 96 well plate, treated with 1 μg/mL E. coli LPS and lipid mediated transfections were performed. We mixed 105 neutrophils, 50 μL (2 mM siRNA), 4 mL DharmaFECT 1 in 146 mL of OptiMEM media according to procedures provided by Dharmacon. Thereafter, they were allowed to incubate in a humidified air and 5% CO2 at 37 °C for 18 h. The transfected cells were placed in a 96 well plate and MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) assay was performed to determine cell death after siRNA treatment.
2.11. Other methods
Proteins were estimated by the Bradford method. The group comparisons were done with Student t-test with SigmaPlot software and p < 0.05 was accepted as a significant value.
3. RESULTS
3.1. N-myristoyltransferase activity in normal and inflamed lungs
NMT activity was determined in homogenates of the lung samples collected from the control or the infected calves as described in the Materials and Methods section. Interestingly, NMT activity was significantly reduced in the lungs of the infected animals compared to the controls (Fig. 1A). Control animals had a mean activity of 0.0181 pmol/mg/mL, while the mean activity of NMT within infected animal tissue was found to be 0.00403 pmol/mg/mL.
Figure 1.
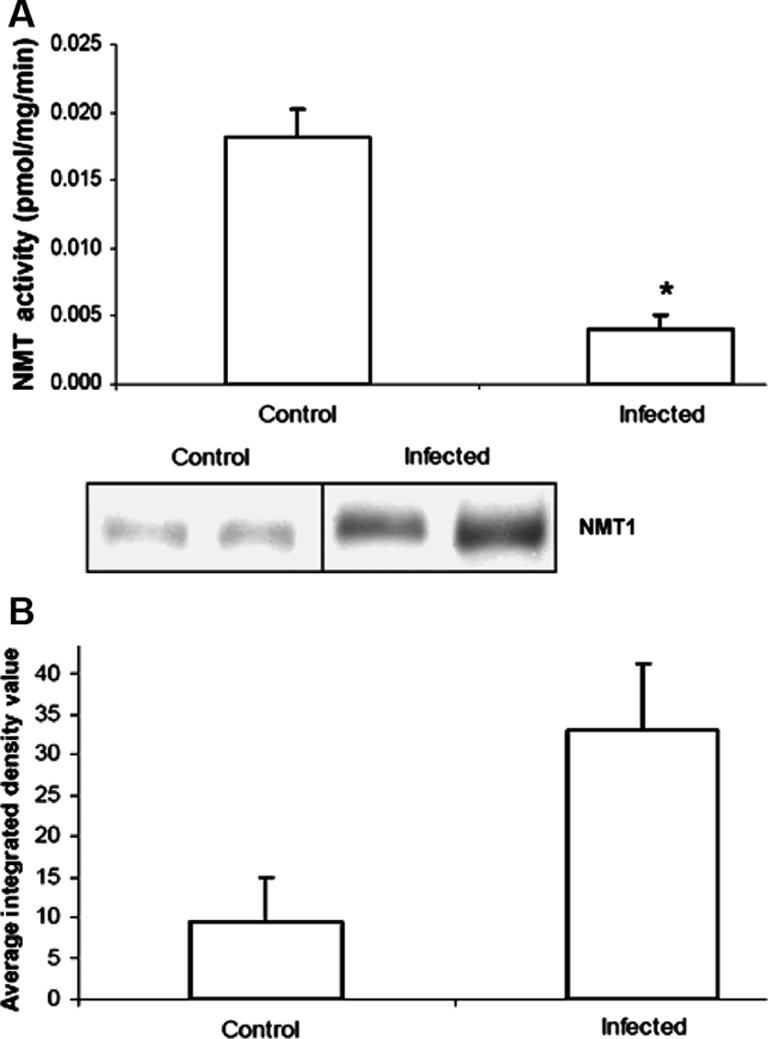
NMT in bovine lung homogenates: (A) NMT activity was determined in control and infected bovine lung homogenates described in Materials and Methods section using peptide substrate based on the N-terminal ends of pp60c-Src (GSSKSKPKR). Values are mean of at least three independent experiments ± standard deviation (p = 0.00146). (B) NMT1 expression in bovine lung homogenates. Western blot analysis was carried out as described in Materials and Methods section. Band density was measured using NIH Image program.
3.2. N-myristoyltransferase protein expression in lungs
Reduced NMT activity in the infected lungs could be due to the overall reduction in the NMT expression as a result of infection. Therefore, we examined the levels of protein expression in control and infected lung homogenates. Western blot analysis (Fig. 1B) revealed that infection of lung with M. hemolytica altered the expression level of NMT. Contrary to lower activity of NMT in infected bovine lungs, the protein expression was high in infected lung homogenates compared to that of control ones. This suggests that reduced NMT activity may be due to the presence of NMT inhibitor(s). Earlier, we discovered an N-myristoyltransferase inhibitor protein with a molecular mass of 71 kDa (NIP71) from bovine brain and later on we reported additional inhibitors of NMT. Therefore, we examined the presence of NMT inhibitor(s) by Western blot analysis in control and infected lung homogenates. We observed a polypeptide band on Western blot corresponding to 48 kDa and band densitometry showed that this peptide had higher expression in infected lung homogenates compared to control lung homogenate (Fig. 2). Earlier, we have discovered that boiled sample of enolase with 48 kDa molecular mass is a potent inhibitor NMT. Enolase cross reacts with NIP71 polyclonal antibody, therefore, we further confirmed that 48 kDa polypeptide band is in fact enolase by probing with monoclonal anti-enolase antibody.
Figure 2.
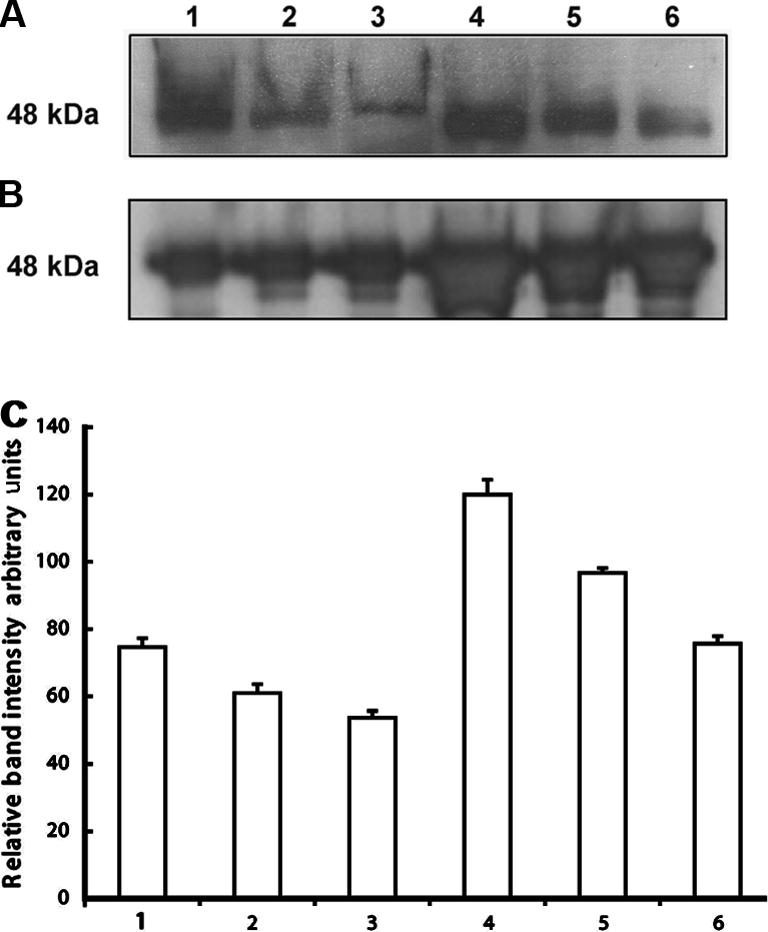
NIP expression in bovine lung homogenates: proteins (25 μg) from control or infected bovine lung homogenates were subjected to 10% SDS-PAGE, transblotted onto PVDF membrane and was probed with (A) polyclonal anti-NIP antibody (1:1 000 dilutions) or (B) monoclonal anti-enolase (1:1 000 dilutions). 1, 2 and 3 are control whereas, 4, 5 and 6 are infected lungs. (C) Band intensity analysis (p = 0.01816). Band density was measured using NIH Image program.
3.3. Immunocytochemical expression of N-myristoyltransferase-1
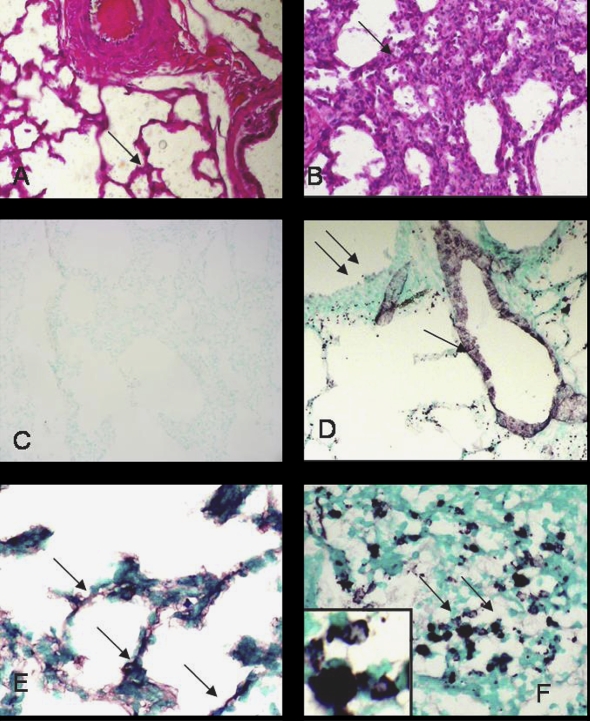
Lung sections from uninfected calves showed normal histology while those from the infected animals showed inflammation including accumulation of inflammatory cells in alveoli and septa (Figs. 3A and 3B). Some of the lung sections contained large necrotic areas that were densely packed with leukocytes. Tissues stained without primary antibody lacked any staining (Fig. 3C) while those with von Willebrand antibody delineated lung vasculature (Fig. 3D). Lung sections from the control as well as infected animals showed mild NMT staining in the septum (Fig. 3E), vascular endothelium and airway epithelium (data not shown). Some of the leukocytes in the necrotic areas in the inflamed lungs showed amplified expression of NMT1 (Fig. 3F).
Figure 3.
Histopathology and NMT1 expression: lung sections from control calves (A) showed normal tissue architecture (arrow) while those from the infected calves (B) showed extensive inflammation (arrow). Lung sections stained with only secondary antibody (C) did not show any staining while von Willebrand Factor antibody (D) reacted with vascular endothelium (single arrow) but not bronchiolar epithelium (double arrows). Lung sections from control calves (E) showed light staining for NMT in alveolar septa but the staining was intense in neutrophils (F) in inflamed lung. Note NMT staining nuclei and cytoplasm of neutrophils (inset). Original magnification × 100. (A color version of this figure is available at www.vetres.org.)
3.4. Immunogold electron microscopy
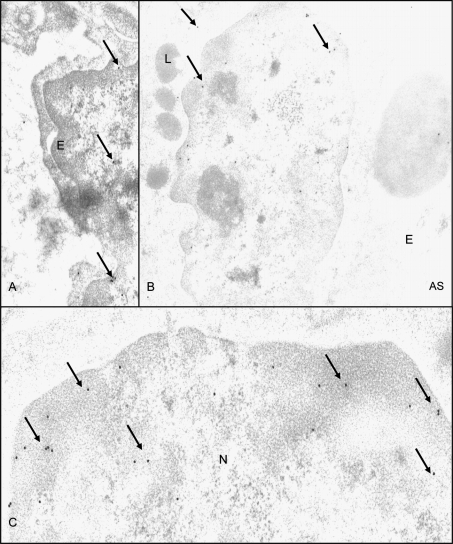
NMT1 antibody labeled capillary endothelium (Fig. 4A), pulmonary intravascular macrophages (Fig. 4B) and airway epithelium (data not shown) of control and inflamed lungs. Nuclear labeling with NMT1 in lung cells (Fig. 4B) appeared to be increased in pulmonary intravascular macrophages in tissues from infected calves (Fig. 4C). Lung sections stained with only secondary antibody attached to gold particles did show only occasional gold particle (data not shown).
Figure 4.
Immuno-electron microscopy for NMT: NMT was localized in lung capillary endothelium (A) and pulmonary intravascular macrophages (B). Figure C shows higher magnification view to demonstrate extensive nuclear staining for NMT. E: endothelium; AS: alveolar space; N: nucleus; L: lysosome; original magnification × 10 000.
3.5. N-myristoyltransferase activity in activated macrophages and neutrophils
Peripheral blood neutrophils were treated with E. coli LPS in vitro and NMT activity assessed at various time points. Following an initial increase, NMT activity decreased after 60 min (Fig. 5A). We differentiated U937 promonocytic cells into monocytes/macrophages by PMA assessed, NMT activity following treatment with E. coli LPS at indicated concentrations and time periods (Fig. 5B). Although NMT activity increased with the increasing concentrations over the short-term treatment with LPS, prolonged treatment with LPS did not have a significant effect on NMT activity (Fig. 5B). These results are similar to those observed in neutrophils. Immuno-histochemistry showed increased expression of NMT1 in LPS-treated neutrophils (Fig. 6). Furthermore, we determined the expression profile of NMT1 in PMA induced U937 cells treated with LPS at various concentrations and time points by Western blot analysis. As shown in Figure 7, short-term (1 h), but not long-term, treatment of the cells with increasing concentrations of LPS enhanced expression of NMT1. Expression of NIP71, which is an inhibitor of NMT activity, was not altered upon short and long term LPS treatment suggesting that LPS activated NMT in a time dependent manner.
Figure 5.
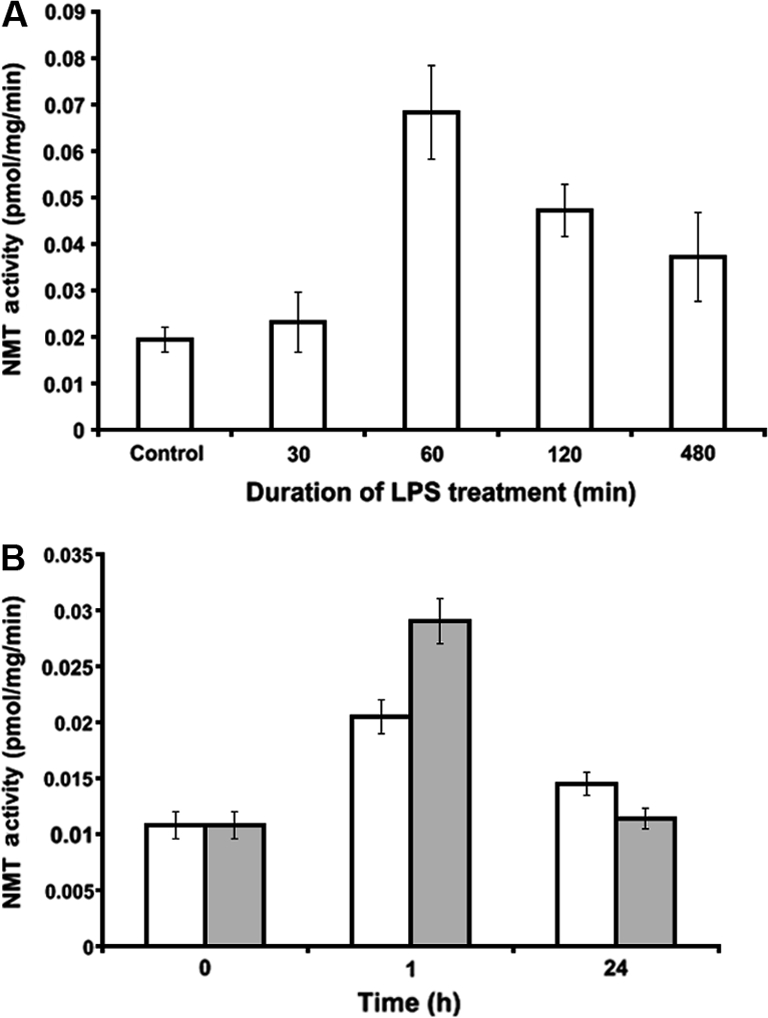
NMT activity in phagocytic cells: NMT activity was determined using peptide substrate based on the N-terminal ends of pp60c-Src (GSSKSKPKR) in (A) neutrophils isolated from peripheral blood of control animals as described in Materials and methods section and were treated for indicated time points with LPS (10 μg/mL), and (B) PMA induced differentiated U937 cells treated with LPS (open bar, 1 μg/mL and closed bar, 10 μg/mL) for indicated time points.
Figure 6.
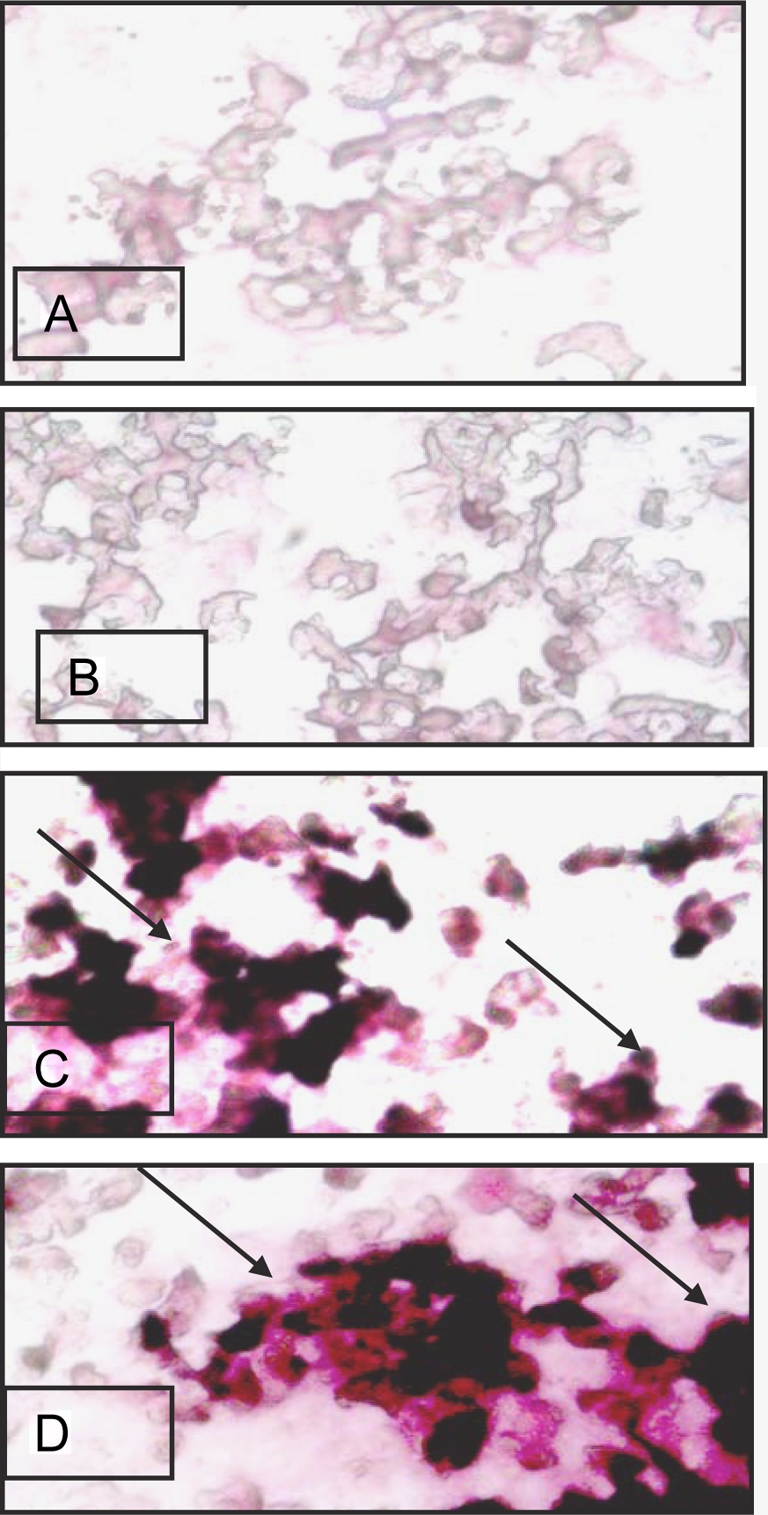
NMT1 staining in neutrophils: no staining was observed in neutrophils stained with only secondary antibody (A). Control neutrophils (B) showed light NMT1 staining. Intense NMT1 staining was noticed in neutrophils incubated with LPS for 30 min (C) and in neutrophils stained with NMT1 30 min after 5 min LPS exposure. (A color version of this figure is available at www.vetres.org.)
Figure 7.
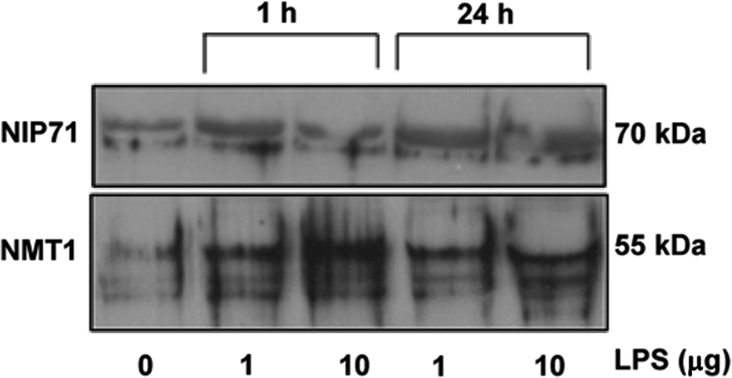
NMT1 expression in phagocytic cells: NMT1 expression was determined by Western analysis in PMA induced differentiated U937 cells treated with LPS with indicated doses and time points (antibody dilution, 1:1 000).
3.6. N-myristoyltransferase activity and neutrophil apoptosis
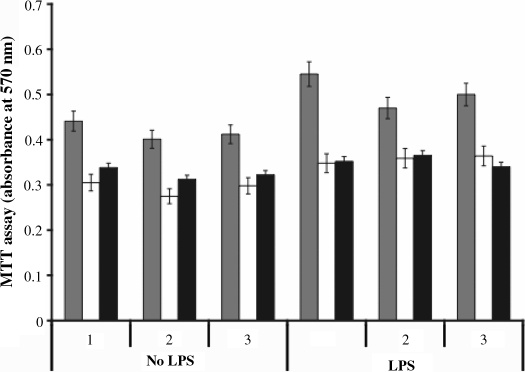
Activated neutrophils live longer through suppression of their constitutive apoptosis. Because of previously reported roles of NMT1 and NMT2 in the cell survival and embryogenesis, we investigated the role of NMT1 in neutrophil lifespan by knocking down NMT1. As depicted in Figure 8 NMT1 or NMT2 knock-down in neutrophils in the presence or absence of LPS induced ca 30% cell death compared to percent control (scramble siRNA).
Figure 8.
MTT Assay: neutrophil survival assay was carried out as described in Material and Methods section. Neutrophils were incubated with or without LPS (10 μg/mL) and transfected with siRNA against NMT1 (white) or NMT2 (black). Controls were transfected with scrambled siRNA (grey). Data presented are from three sets of experiments. The percent cell death was calculated by compared the siRNA transfected cells with those transfected with scrambled siRNA.
4. DISCUSSION
We report here for the first time the expression and activity of NMT in bacterial lung inflammation, phagocytic cells and the putative roles of NMT1 in regulation of neutrophil lifespan. We found that NMT activity was reduced but protein expression of NMT1 was apparently increased in inflamed lungs compared to the controls. NMT1 activity was transiently increased in LPS-treated neutrophils and macrophages. Furthermore, a knock-down of NMT1 resulted in increased cell death in normal and LPS-treated neutrophils.
Total NMT activity in control lung homogenates was four fold higher than that in the infected lung homogenates. Interestingly, immunohistology and Western blots showed an apparent increase in expression of NMT in normal and inflamed lungs. The increased expression of NMT1 observed is due to the infection and not due to the bacteria because bacteria lack NMT gene. Therefore, it is possible that reduced activity of NMT may be due to endogenous tissue inhibitor(s) and not due to the dilution of the NMT protein because expression of many other proteins. We detected a 48 kDa protein in lung homogenates which corresponds to a recently described NMT inhibitor in boiled supernatant of enolase [8, 12, 31]. Enolase is a glycolytic enzyme with a molecular weight of 48 kDa which catalyzes the reversible removal of a water molecule from 2-phohosphoglycerate to yield phosphoenolpyruvate. Enolase is a potent inhibitor of N-myristoylation in vitro. A γ–γ isoform of enolase called neuron-specific enolase is a putative marker of small cell lung carcinoma [18]. Our study does not address the mechanisms or effects of increased expression of NMT inhibitor on lung inflammation. The data, however, indicate alteration of NMT activity in bacterial lung inflammation. Based on our siRNA mediated knockdown experiments and previous studies [33], we speculate that increased activity of NMT activity may impact longevity of inflammatory cells such as neutrophils in inflamed lungs.
Immunohistology revealed light staining for NMT1 in the lung parenchyma of both experimental groups. We made another interesting observation with immuno-electron microscopy that NMT1 is predominantly localized in nuclei of leukocytes in inflamed lungs. Although NMT shuttles between cytoplasm and the nucleus in cardiac cells following ischemia and reperfusion, our recent data show its predominant nuclear localization in the bone marrow cells of colon cancer patients compared to cytoplasmic localization in the cells from healthy subjects [22, 33]. Previously, we have reported nuclear localization of inflammatory proteins such as Toll-like receptor 4 and integrin αvβ3 in leukocytes and endothelial cells in inflamed lungs [9, 10]. The consequences of nuclear migration of NMT are not known. Because NMT impacts the fundamental cell processes such as cell cycle and apoptosis, it is possible that NMT shuttles between cytoplasm and nucleus depending upon the demand for the myristoylation of cytoplasmic or nuclear proteins involved in such processes [2, 15].
We also observed strong expression of NMT in cytoplasm and nuclei of leucocytes trapped in necrotic areas in the inflamed lungs as well as LPS-treated neutrophils in vitro. It is known that most of recruited leukocytes in inflamed lungs are activated and that activated leukocytes live longer [13]. Although activated neutrophils are necessary to combat bacterial infection, they also contribute to tissue damage, morbidity and mortality [17]. Recently, we have demonstrated that NMT1 and regulated total NMT activity is essential for the development of myeloid lineage [33] and it is implicated in regulation of cell cycle [5]. Therefore, increased expression of NMT in recruited leukocytes in inflamed lungs and the LPS-activated neutrophils may inhibit their apoptosis and prolong their lifespan.
We moved to in vitro setting to examine expression and activity of NMT in normal and LPS-treated neutrophils and macrophages. The data showed that LPS treatment increased NMT activity in neutrophils and U937 macrophage cells. While the short-term treatment showed a dose-dependent increase in NMT activity, the long-term treatment did not have a similar effect. In contrast to the in vivo data, we did not find any change in the expression of NMT inhibitor called NIP. Nevertheless, the increased activity of NMT in neutrophils and macrophages parallels the data of increased NMT expression in inflamed lungs. This suggests that increased expression of NMT in the homogenates is potentially due to its over expression in recruited neutrophils, monocytes and macrophages and the reduction in total NMT activity is due to the expression of inhibitor protein in other cell types. We assessed the role of NMT1 in neutrophil apoptosis because of the previously reported increased expression of NMT in colorectral carcinoma and its potential role as a regulator of cell cycle [21, 23, 29, 37]. We found that the NMT knockdown resulted in 30% reduction in the lifespan of normal and activated neutrophils. The regulation of neutrophils lifespan is complex and is modulated upon activation with bacteria and their products such as LPS [1, 4]. Our data showing increased activity of NMT and expression of NMT1 in activated neutrophils and their reduced lifespan following NMT1 knockdown provide novel insights in the role of NMT1 in regulation of neutrophil life.
In conclusion, the data reported in this manuscript show expression of NMT1 in endothelium, epithelium and macrophages in both normal and inflamed lungs in a bovine model of pneumonia. However, NMT activity was significantly reduced due to the increased expression of NMT inhibitor protein in the inflamed lungs. In phagocytic cells NMT is activated upon LPS treatment. This is the first report wherein NMT knock down in neutrophils resulted in cell death suggesting involvement of NMT in neutrophils activation. Detail studies are in progress to further elucidate the NMT mediated activation of neutrophils.
Acknowledgments
This work was supported through a Discovery Grant from Natural Sciences and Engineering Research Council of Canada to Baljit Singh and a grant from Canadian Institutes of Health Research (CIHR) to Rajendra K. Sharma. Anuraag Shrivastav thanks CIHR and Saskatchewan Health Research Foundation for the postdoctoral fellowship. Mr. Ryan Mohr was supported by a scholarship from Merck-Merial Veterinary Scholars Program and Interprovincial Undergraduate Summer Research Program.
References
- 1.Abraham E., Neutrophils and acute lung injury, Crit. Care Med. (2003) 31:S195–S199 [DOI] [PubMed] [Google Scholar]
- 2.Benetka W., Mehlmer N., Maurer-Stroh S., Sammer M., Koranda M., Neumuller R., et al. , Experimental testing of predicted myristoylation targets involved in asymmetric cell division and calcium-dependent signalling, Cell Cycle (2008) 7:3709–3719 [DOI] [PubMed] [Google Scholar]
- 3.Boutin J.A., Myristoylation, Cell. Signal. (1997) 9:15–35 [DOI] [PubMed] [Google Scholar]
- 4.Dockrell D.H., Whyte M.K., Regulation of phagocyte lifespan in the lung during bacterial infection, J. Leukoc. Biol. (2006) 79:904–908 [DOI] [PubMed] [Google Scholar]
- 5.Ducker C.E., Upson J.J., French K.J., Smith C.D., Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis, Mol. Cancer Res. (2005) 3:463–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farazi T.A., Waksman G., Gordon J.I., The biology and enzymology of protein N-myristoylation, J. Biol. Chem. (2001) 276:39501–39504 [DOI] [PubMed] [Google Scholar]
- 7.Giang D.K., Cravatt B.F., A second mammalian N-myristoyltransferase, J. Biol. Chem. (1998) 273:6595–6598 [DOI] [PubMed] [Google Scholar]
- 8.Gowda S., Shrivastav A., Selvakumar P., Dimmock J.R., Sharma R.K., A novel inhibitor protein of N-myristoyltransferase from Escherichia coli, Biochem. Biophys. Res. Commun. (2004) 314:984–987 [DOI] [PubMed] [Google Scholar]
- 9.Janardhan K.S., Appleyard G.D., Singh B., Expression of integrin subunits alphav and beta3 in acute lung inflammation, Histochem. Cell Biol. (2004) 121:383–390 [DOI] [PubMed] [Google Scholar]
- 10.Janardhan K.S., McIsaac M., Fowlie J., Shrivastav A., Caldwell S., Sharma R.K., Singh B., Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation, Histol. Histopathol. (2006) 21:687–696 [DOI] [PubMed] [Google Scholar]
- 11.King M.J., Sharma R.K., N-myristoyl transferase assay using phosphocellulose paper binding, Anal. Biochem. (1991) 199:149–153 [DOI] [PubMed] [Google Scholar]
- 12.King M.J., Sharma R.K., Identification, purification and characterization of a membrane-associated N-myristoyltransferase inhibitor protein from bovine brain, Biochem. J. (1993) 291:635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W.L., Downey G.P., Neutrophil activation and acute lung injury, Curr. Opin. Crit. Care (2001) 7:1–7 [DOI] [PubMed] [Google Scholar]
- 14.Magnuson B.A., Raju R.V., Moyana T.N., Sharma R.K., Increased N-myristoyltransferase activity observed in rat and human colonic tumors, J. Natl. Cancer Inst. (1995) 87:1630–1635 [DOI] [PubMed] [Google Scholar]
- 15.Maurer-Stroh S., Eisenhaber F., Myristoylation of viral and bacterial proteins, Trends Microbiol. (2004) 12:178–185 [DOI] [PubMed] [Google Scholar]
- 16.Moraes T.J., Chow C.W., Downey G.P., Proteases and lung injury, Crit. Care Med. (2003) 31:S189–S194 [DOI] [PubMed] [Google Scholar]
- 17.Moraes T.J., Zurawska J.H., Downey G.P., Neutrophil granule contents in the pathogenesis of lung injury, Curr. Opin. Hematol. (2006) 13:21–27 [DOI] [PubMed] [Google Scholar]
- 18.Pujol J.L., Quantin X., Jacot W., Boher J.M., Grenier J., Lamy P.J., Neuroendocrine and cytokeratin serum markers as prognostic determinants of small cell lung cancer, Lung Cancer (2003) 39:131–138 [DOI] [PubMed] [Google Scholar]
- 19.Rajala R.V., Datla R.S., Moyana T.N., Kakkar R., Carlsen S.A., Sharma R.K., N-myristoyltransferase, Mol. Cell. Biochem. (2000) 204:135–155 [DOI] [PubMed] [Google Scholar]
- 20.Rajala R.V., Dehm S., Bi X., Bonham K., Sharma R.K., Expression of N-myristoyltransferase inhibitor protein and its relationship to c-Src levels in human colon cancer cell lines, Biochem. Biophys. Res. Commun. (2000) 273:1116–1120 [DOI] [PubMed] [Google Scholar]
- 21.Rajala R.V., Radhi J.M., Kakkar R., Datla R.S., Sharma R.K., Increased expression of N-myristoyltransferase in gallbladder carcinomas, Cancer (2000) 88:1992–1999 [PubMed] [Google Scholar]
- 22.Rajala R.V., Kakkar R., Kanthan R., Radhi J.M., Wang X., Wang R., et al. , Altered expression and localization of N-myristoyltransferase in experimentally induced rat model of ischemia-reperfusion, J. Cell. Biochem. (2002) 86:509–519 [DOI] [PubMed] [Google Scholar]
- 23.Raju R.V., Moyana T.N., Sharma R.K., N-Myristoyltransferase overexpression in human colorectal adenocarcinomas, Exp. Cell Res. (1997) 235:145–154 [DOI] [PubMed] [Google Scholar]
- 24.Raju R.V.S., Sharma R.K., Preparation and assay of myristoyl-CoA: protein N-myristoyltransferase, Methods Mol. Biol. (1999) 116:193–211 [DOI] [PubMed] [Google Scholar]
- 25.Resh M.D., Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins, Biochim. Biophys. Acta (1999) 1451:1–16 [DOI] [PubMed] [Google Scholar]
- 26.Ribble C.S., Meek A.H., Janzen E.D., Jim G.K., Effect of time of year, weather, and the pattern of auction market sales on fatal fibrinous pneumonia (shipping fever) in calves in a larger feedlot in Alberta (1985–1988), Can. J. Vet. Res. (1995) 59:167–172 [PMC free article] [PubMed] [Google Scholar]
- 27.Ribble C.S., Meek A.H., Jim G.K., Guichon P.T., The pattern of fatal fibrinous pneumonia (shipping fever) affecting calves in a large feedlot in Alberta (1985–1988), Can. Vet. J. (1995) 36:753–757 [PMC free article] [PubMed] [Google Scholar]
- 28.Selvakumar P., Lakshmikuttyamma A., Shrivastav A., Das S.B., Dimmock J.R., Sharma R.K., Potential role of N-myristoyltransferase in cancer, Prog. Lipid Res. (2007) 46:1–36 [DOI] [PubMed] [Google Scholar]
- 29.Selvakumar P., Sharma R.K., Role of calpain and caspase system in the regulation of N-myristoyltransferase in human colon cancer (Review), Int. J. Mol. Med. (2007) 19:823–827 [PubMed] [Google Scholar]
- 30.Sharma R.K., Potential role of N-myristoyltransferase in pathogenic conditions, Can. J. Physiol. Pharmacol. (2004) 82:849–859 [DOI] [PubMed] [Google Scholar]
- 31.Shrivastav A., Pasha M.K., Selvakumar P., Gowda S., Olson D.J., Ross A.R., et al. , Potent inhibitor of N-myristoylation: a novel molecular target for cancer, Cancer Res. (2003) 63:7975–7978 [PubMed] [Google Scholar]
- 32.Shrivastav A., Selvakumar P., Bajaj G., Lu Y., Dimmock J.R., Sharma R.K., Regulation of N-myristoyltransferase by novel inhibitor proteins, Cell Biochem. Biophys. (2005) 43:189–202 [DOI] [PubMed] [Google Scholar]
- 33.Shrivastav A., Varma S., Lawman Z., Yang S.H., Ritchie S.A., Bonham K., et al. , Requirement of N-myristoyltransferase 1 in the development of monocytic lineage, J. Immunol. (2008) 180:1019–1028 [DOI] [PubMed] [Google Scholar]
- 34.Singh B., Pearce J.W., Gamage L.N., Janardhan K., Caldwell S., Depletion of pulmonary intravascular macrophages inhibits acute lung inflammation, Am. J. Physiol. Lung Cell. Mol. Physiol. (2004) 286:L363–L372 [DOI] [PubMed] [Google Scholar]
- 35.Towbin H., Staehelin T., Gordon J., Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications, Proc. Natl. Acad. Sci. USA (1979) 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware L.B., Matthay M.A., The acute respiratory distress syndrome, N. Eng. J. Med. (2000) 342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 37.Yang S.H., Shrivastav A., Kosinski C., Sharma R.K., Chen M.H., Berthiaume L.G., et al. , N-myristoyltransferase 1 is essential in early mouse development, J. Biol. Chem. (2005) 280:18990–18995 [DOI] [PubMed] [Google Scholar]