Abstract
Helicobacter pylori-initiated chronic gastritis is characterized by the cag pathogenicity island-dependent upregulation of proinflammatory cytokines, which is largely mediated by the transcription factor nuclear factor (NF)-κB. However, the cag pathogenicity island-encoded proteins and cellular signalling molecules that are involved in H. pylori-induced NF-κB activation and inflammatory response remain unclear. Here, we show that H. pylori virulence factor CagA and host protein transforming growth factor-β-activated kinase 1 (TAK1) are essential for H. pylori-induced activation of NF-κB. CagA physically associates with TAK1 and enhances its activity and TAK1-induced NF-κB activation through the tumour necrosis factor receptor-associated factor 6-mediated, Lys 63-linked ubiquitination of TAK1. These findings show that polyubiquitination of TAK1 regulates the activation of NF-κB, which in turn is used by H. pylori CagA for the H. pylori-induced inflammatory response.
Keywords: CagA, NF-κB, TAK1, TRAF6/ubiquitination
Introduction
Helicobacter pylori infection causes chronic gastritis and peptic ulceration, and is the strongest risk factor for the development of gastric cancer (Peek & Blaser, 2002; Hatakeyama, 2008). The inflammatory response associated with gastritis is characterized by the expression of various proinflammatory cytokines, and transcription factor nuclear factor (NF)-κB has an important role in the regulation of their expression (Ghosh & Karin, 2002). H. pylori infection activates NF-κB and the expression of interleukin-8 (IL-8) in epithelial cells, which is believed to be critical for H. pylori-initiated chronic inflammation (Sharma et al, 1998; Maeda et al, 2001; Naumann, 2001; Hirata et al, 2006).
The H. pylori cag pathogenicity island is required for activation of NF-κB and expression of NF-κB target genes (Glocker et al, 1998; Foryst-Ludwig & Naumann, 2000), and its encoded virulence factor, CagA, has an important role in the pathogenicity of H. pylori, including H. pylori-induced inflammation (Brandt et al, 2005; Kim et al, 2006). Infection with H. pylori cagA-positive strains is associated with higher grades of gastric inflammation and an increased risk of gastric cancer than infection with cagA-negative strains (Blaser et al, 1995; Parsonnet et al, 1997), thereby highlighting the role of CagA in the H. pylori-mediated inflammatory response. However, the exact function of CagA in the activation of NF-κB and the NF-κB-dependent inflammatory response is yet to be addressed.
CagA is injected into H. pylori-infected host epithelial cells—by using a type-IV secretion system—where it is often activated by tyrosine phosphorylation through the host src kinase and targets host proteins to modify cellular responses (Peek, 2005; Hatakeyama, 2008). For example, phosphorylated and non-phosphorylated CagA interact, respectively, with Src homology 2 domain-containing protein tyrosine phosphatase (SHP2) and with host proteins such as growth-factor-receptor-bound protein 2 (GRB2) and zona occludens 1 (ZO-1) to induce abnormal proliferation and movement of gastric epithelial cells (Mimuro et al, 2002; Amieva et al, 2003; Hatakeyama, 2004). Non-phosphorylated CagA also destabilizes the E-cadherin/β-catenin complex to induce the activation of the β-catenin signal that underlies intestinal metaplasia (Franco et al, 2005; Murata-Kamiya et al, 2007). However, the cellular proteins CagA targets that are involved in inducing the inflammatory response remain elusive.
NF-κB has an important role in the regulation of inflammatory responses in mammals. The prototypical NF-κB complex, which is a heterodimer of p50 and RelA, is sequestered in the cytoplasm by its inhibitor IκBα. On stimulation, the IκB kinase (IKK) complex is activated, leading to phosphorylation and degradation of IκBα, nuclear translocation of NF-κB and activation of its target genes (Ghosh & Karin, 2002). Transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) is a key regulator of signal transduction cascades that lead to stimulus-coupled phosphorylation and activation of IKK (Adhikari et al, 2007). Emerging evidence indicates that activation of TAK1 and IKK is regulated by tumour necrosis factor receptor-associated factor 6 (TRAF6)-mediated, Lys 63-linked ubiquitination of TRAF6, interleukin 1 receptor-associated kinase 1 and NF-κB essential modulator (Adhikari et al, 2007). Recent studies also show that TAK1 is polyubiquitinated by TRAF6 in response to TGF-β, and that Lys 63-linked ubiquitination is required for TGF-β-induced activation of p38/Jun N-terminal kinase and AP-1 (Sorrentino et al, 2008), indicating that TAK1 ubiquitination might also be crucial for the activation of IKK and NF-κB.
Here, we show that CagA is essential for H. pylori-induced activation of NF-κB, and identify TAK1 as a new cellular target of CagA.
Results And Discussion
H. pylori-mediated activation of NF-κB requires CagA
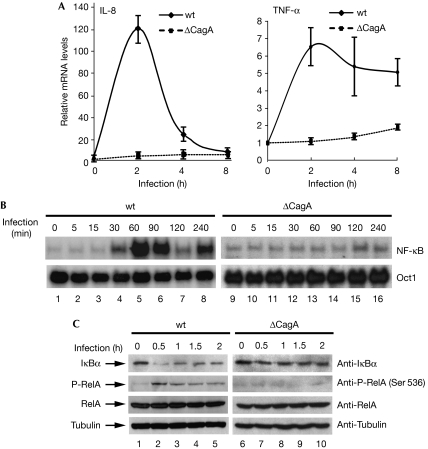
To investigate the role of CagA in the H. pylori-induced inflammatory response, we compared wild-type (wt) and cagA-deficient H. pylori for their ability to induce the expression of inflammatory genes in AGS gastric epithelial cells. Infection of AGS cells with wt H. pylori strain G27, but not its cagA-deficient isogenic mutant, induced the expression of IL-8 and tumour necrosis factor-α (TNF-α) messenger RNA (mRNA; Fig 1A). Similar results were obtained with two other H. pylori strains, NCTC11637 and 7.13 (supplementary Fig S1 online). These data indicate that induction of proinflammatory genes can be generalized for most cagA-positive H. pylori strains and that the defect from the cagA-deficient mutants is unlikely to be due to a polar effect.
Figure 1.
CagA is required for Helicobacter pylori-induced activation of NF-κB. (A) AGS cells were infected with G27 wt or ΔCagA H. pylori and quantitative RT–PCR was performed to analyse the expression of NF-κB target genes IL-8 and TNF-α. (B) EMSA was performed using whole-cell extracts from AGS cells infected with wt or ΔCagA H. pylori by using radiolabelled NF-κB and Oct1 probes. (C) Levels of IκBα, phosphorylated Ser 536 RelA, RelA, CagA and tubulin were detected in lysates from AGS cells infected with G27 wt or ΔCagA H. pylori by immunoblotting with the indicated antibodies. EMSA, electrophoretic mobility-shift assay; IL-8, interleukin-8; NF-κB, nuclear factor-κB; RT–PCR, real-time PCR; TNF-α, tumour necrosis factor-α; wt, wild type.
As NF-κB regulates the expression of many inflammatory cytokines, including IL-8 and TNF-α, we next investigated whether H. pylori infection activated NF-κB and whether this activation was CagA-dependent. wt H. pylori, but not the cagA-deficient mutant, activated all three κB luciferase reporters, including two that contain natural promoters from NF-κB target genes IL-8 and E-selectin (supplementary Fig S2 online). Furthermore, only wt H. pylori, but not the cagA-deficient mutant, stimulated the binding of NF-κB, heterodimers of RelA and p50, to DNA (Fig 1B; supplementary Fig S3 online).
To define the signalling pathway leading to the activation of NF-κB, we examined the degradation and resynthesis of IκBα and phosphorylation of RelA in AGS cells infected with wt and cagA-deficient H. pylori. IκBα was degraded and resynthesized in cells infected with wt but not in those infected with cagA-deficient H. pylori (Fig 1C). In addition, wt H. pylori, but not the cagA-deficient mutant, stimulated the phosphorylation of RelA at Ser 536, which is also mediated by activated IKK (Fig 1C). Altogether, these data indicate that H. pylori infection stimulates CagA-dependent activation of NF-κB through the activation of IKK. Other studies also indicate that CagA induces cytokine release through activation of NF-κB (Brandt et al, 2005; Shibata et al, 2006); although some reports indicate that CagA might not be required for activation of NF-κB (Viala et al, 2004), this discrepancy is due to the different cell lines used in the studies. In 293 cells and macrophages, CagA does not seem to be essential for activation of NF-κB (Maeda et al, 2001; Viala et al, 2004). However, in gastric epithelial cells—the most physiologically relevant cells—CagA is essential for activation of NF-κB (Fig 1; Brandt et al, 2005).
TAK1 is required for NF-κB activation by H. pylori
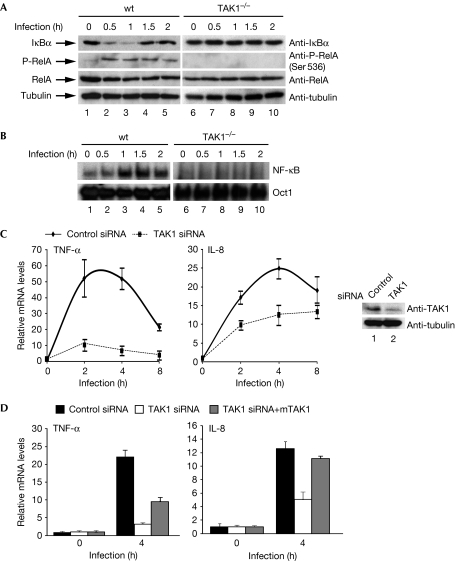
As TAK1 is the upstream kinase for IKK (Wang et al, 2001)—and is also used by effectors from other pathogens, such as human T-lymphotrophic virus-1 protein TAX (Wu & Sun, 2007)—we next evaluated the role of TAK1 in the H. pylori-induced activation of IKK and NF-κB. When wt or TAK1-deficient (TAK1−/−) mouse embryonic fibroblasts (MEFs) were infected with H. pylori, IκBα was degraded and resynthesized, and phosphorylation of Ser 536 of RelA was induced in wt MEFs but not in TAK1−/− MEFs (Fig 2A). Furthermore, H. pylori stimulated the DNA-binding activity of NF-κB in wt but not in TAK1−/− MEFs (Fig 2B). These data indicate that TAK1 is essential for the activation of IKK and NF-κB after H. pylori infection. Consistently, infection of wt MEFs, but not TAK1−/− MEFs, with H. pylori stimulated mRNA expression of TNF-α, IL-6 and E-selectin—three NF-κB target genes (supplementary Fig S4 online).
Figure 2.
TAK1 is required for Helicobacter pylori-induced NF-κB activation. (A) Levels of IκBα, phosphorylated Ser 536 RelA, RelA, TAK1 and tubulin were detected in the lysates from wt or TAK1−/− MEFs infected with H. pylori. (B) EMSA was performed using whole-cell extracts from wt or TAK1−/− MEFs infected with H. pylori, as in Fig 1B. (C) AGS cells transfected with control or TAK1 siRNA were infected with H. pylori and RT–PCR was performed to analyse NF-κB target gene expression. Levels of TAK1 and tubulin are shown in the right panels. (D) AGS cells transfected with control siRNA or TAK1 siRNA were reconstituted with mouse TAK1 (mTAK1), followed by infection for 4 h with H. pylori. Gene expression was analysed as shown in (C). EMSA, electrophoretic mobility-shift assay; IL-8, interleukin-8; MEF, mouse embryonic fibroblast; NF-κB, nuclear factor-κB; RT–PCR, real-time PCR; siRNA, short interfering RNA; TAK1, transforming growth factor-β-activated kinase 1; TNF-α, tumour necrosis factor-α; wt, wild type.
To demonstrate further the role of TAK1 in H. pylori-induced NF-κB activation, we knocked down the expression of TAK1 in AGS cells. Depletion of TAK1 by short interfering RNA (siRNA) impaired the phosphorylation of RelA (supplementary Fig S5 online) and H. pylori-induced mRNA expression of TNF-α and IL-8 (Fig 2C). The partial decrease in expression of TNF-α and IL-8 mRNA in TAK1-knockdown cells (compared with TAK1−/− MEFs) probably reflects the incomplete depletion of TAK1 by siRNA in AGS cells (with ∼80% knockdown efficiency; Fig 2C). The impaired expression of TNF-α and IL-8 could be restored when the siRNA-resistant mouse TAK1 was re-introduced into the knockdown cells (Fig 2D). These data confirm that TAK1 is essential for H. pylori-induced activation of NF-κB.
H. pylori stimulates ubiquitination of TAK1
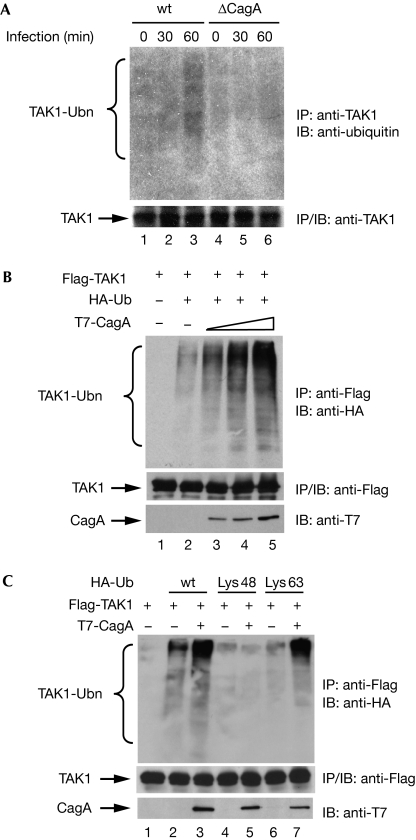
As Lys 63-linked polyubiquitination is important for the activation of TAK1 and IKK (Wang et al, 2001; Sorrentino et al, 2008), and TAK1 is essential for H. pylori-induced activation of NF-κB (Fig 2), we next examined whether H. pylori stimulated polyubiquitination of TAK1. Infection of AGS cells with wt, but not cagA-deficient, H. pylori induced polyubiquitination of endogenous TAK1 (Fig 3A), indicating that CagA is crucial for H. pylori-induced polyubiquitination of TAK1.
Figure 3.
Helicobacter pylori infection induces CagA-dependent TAK1 ubiquitination. (A) Endogenous TAK1 was immunoprecipitated (IP) from AGS cells infected with wt or ΔCagA H. pylori and ubiquitination of TAK1 was detected by immunoblotting (IB) with ubiquitin antibodies. (B) Flag-TAK1 from HEK293T cells transfected as indicated was immunoprecipitated and immunoblotted for ubiquitination using HA antibodies. (C) HEK293T cells were transfected with Flag-TAK1 with or without T7-CagA and with wt, Lys 48-only or Lys 63-only HA–ubiquitin as indicated. Ubiquitination of TAK1 was detected by immunoblotting the TAK1 immunoprecipitates with HA antibodies. HA, haemagglutinin; HEK, human embryonic kidney; TAK1, transforming growth factor-β-activated kinase 1; Ub, ubiquitin; Ubn, ubiquitination; wt, wild type.
Next, we determined the potential effect of CagA on the ubiquitination of TAK1. TAK1 is modestly ubiquitinated in the presence of ubiquitin, and this ubiquitination is markedly enhanced by co-transfection of CagA, independently of the tyrosine phosphorylation of CagA (Fig 3B; supplementary Figs S6 and S7 online). The enhanced ubiquitination of TAK1 is Lys 63-linked rather than Lys 48-linked, as enhanced ubiquitination of TAK1 could only be detected in the presence of wt or Lys 63-only ubiquitin, but not in the presence of Lys 48-only ubiquitin (Fig 3C), indicating that ubiquitination of TAK1 enhanced by CagA probably promotes the activity of TAK1 rather than its proteasomal degradation.
CagA physically associates with TAK1 in vitro and in vivo
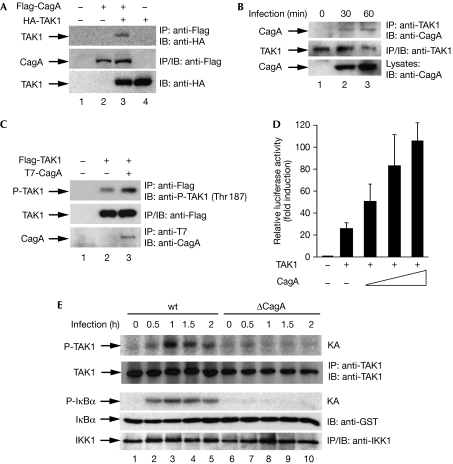
As CagA often elicits its cellular functions by interacting with cellular proteins (Hatakeyama, 2008), we next assessed the physical interaction between CagA and TAK1. Immunoprecipitation of CagA from transfected human embryonic kidney (HEK) 293T cells co-immunoprecipitated TAK1 (Fig 4A). Furthermore, CagA colocalized with TAK1 on the plasma membrane in transfected AGS cells (supplementary Fig S8 online). An in vitro glutathione-S-transferase (GST) pull-down assay showed that CagA associated directly with TAK1 through the amino (N)-terminal and carboxy (C)-terminal regions of CagA and the N-terminal region of TAK1 (supplementary Figs S9–S11 online). More importantly, endogenous TAK1 associated with CagA after H. pylori infection (Fig 4B). Collectively, these data show that CagA associates with TAK1 in vitro and in vivo in response to H. pylori infection.
Figure 4.
Helicobacter pylori CagA interacts with TAK1 and enhances its activity. (A) Ectopically expressed CagA and TAK1 co-immunoprecipitate in HEK293T cells. (B) Endogenous TAK1 was immunoprecipitated (IP) from H. pylori-infected AGS cells and immunoblotted (IB) for associated CagA with CagA antibodies. (C) TAK1 immunoprecipitates from HEK293T cells transfected as indicated were immunoblotted with phospho-TAK1 (Thr 187) antibodies. Levels of immunoprecipitated TAK1 and CagA are shown in the lower panels. (D) TAK1 and CagA were co-transfected with an IL-8–luciferase reporter plasmid into HEK293T cells, as indicated. At 30 h after transfection, luciferase activity was measured. (E) AGS cells were infected with wt or ΔCagA H. pylori. Immunoprecipitates of endogenous TAK1 or IKK were subjected to in vitro kinase assays. Kinase activity of TAK1 or IKK was assessed by the auto-phosphorylation of TAK1 immunoprecipitates or phosphorylation of GST–IκBα by IKK1 immunoprecipitates, respectively. GST, glutathione-S-transferase; HEK, human embryonic kidney; IKK, IκB kinase; KA, kinase assay; IL-8, interleukin-8; TAK1, transforming growth factor-β-activated kinase 1; wt, wild type.
CagA enhances the activity of TAK1
As CagA interacts with, and enhances the ubiquitination of, TAK1 (Figs 3 and 4), we next examined whether CagA could activate TAK1. Co-expression of CagA enhanced the kinase activity of TAK1, which is demonstrated by the auto-phosphorylation of TAK1 at Thr 187 (Fig 4C). TAK1-mediated activation of NF-κB was consistently enhanced by CagA in a dose-dependent manner in a κB luciferase reporter assay (Fig 4D). H. pylori infection in AGS cells also activated TAK1 and IKK with similar kinetics in a CagA-dependent manner (Fig 4E; supplementary Figs S12 and S13 online). Together, these data indicate that the association of CagA with TAK1 enhances the ability of TAK1 to activate IKK and NF-κB, and that CagA is essential for H. pylori-induced TAK1 activation.
CagA is localized to the plasma membrane and undergoes oligomerization—two events that are essential for its signal transduction activity (Hatakeyama, 2008). As CagA colocalizes with TAK1 on the plasma membrane (supplementary Fig S8 online), and the oligomerization-defective mutant of CagA fails to induce the ubiquitination of TAK1 or the activation of NF-κB (supplementary Figs S14 and S15 online), the membrane-binding and oligomerization properties of CagA might be important for the activation of TAK1 and NF-κB.
Ubiquitination and activation of TAK1 require TRAF6
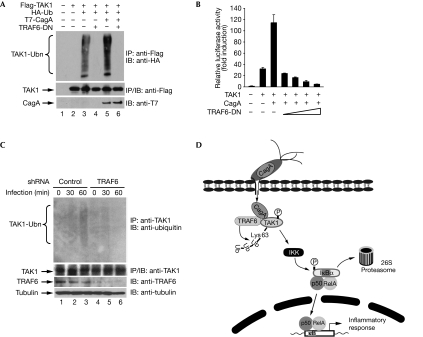
As TRAF6-mediated Lys 63-linked ubiquitination is crucial for activation of TAK1 (Wang et al, 2001; Sorrentino et al, 2008), we next determined the role of TRAF6 in CagA-induced ubiquitination of TAK1. We first assessed the effect of a dominant-negative mutant of TRAF6 (TRAF6-DN; Jono et al, 2004) on the polyubiquitination of TAK1. TRAF6-DN not only blocked the ubiquitination of TAK1 but also inhibited the enhanced ubiquitination of TAK1 facilitated by CagA (Fig 5A). The ability of CagA to enhance TAK1-mediated NF-κB activation was also inhibited by TRAF6-DN (Fig 5B), indicating that ubiquitination of TAK1 is crucial for its activation. Although Lys 34 of TAK1 is ubiquitinated by TRAF6 in response to TGF-β, mutation of Lys 34 to arginine did not affect TAK1 ubiquitination or its ability to activate NF-κB (supplementary Figs S16 and S17 online), indicating that other unidentified lysine residues might also undergo Lys 63-linked ubiquitination, leading to activation of IKK and NF-κB.
Figure 5.
TRAF6 mediates the ubiquitination and activation of TAK1 by CagA. (A) HEK293T cells were transfected as indicated and TAK1 ubiquitination was detected by immunoblotting (IB) Flag immunoprecipitates (IP) with HA antibodies. (B) HEK293T cells were transfected with IL-8–luciferase reporter and other plasmids as indicated. Luciferase activity was measured 30 h after transfection. (C) AGS cells stably expressing control or TRAF6 shRNA were infected with Helicobacter pylori and ubiquitination of TAK1 was detected by immunoblotting TAK1 immunoprecipitates with ubiquitin antibodies. (D) A schematic model illustrating the role of CagA in H. pylori infection-induced activation of NF-κB. After injection into cells, CagA associates with TAK1 and TRAF6, and initiates the TRAF6-mediated, Lys 63-linked ubiquitination of TAK1, leading to activation of IKK, NF-κB and the NF-κB-dependent inflammatory response. HA, haemagglutinin; HEK, human embryonic kidney; IL-8, interleukin-8; NF-κB, nuclear factor-κB; shRNA, short hairpin RNA; TAK1, transforming growth factor-β-activated kinase 1; TRAF6-DN, tumour necrosis factor receptor-associated factor 6 dominant-negative; Ub, ubiquitin; Ubn, ubiquitination.
To analyse further the role of TRAF6 in H. pylori-induced ubiquitination of TAK1 and NF-κB activation, we generated an AGS cell line stably expressing short hairpin RNA (shRNA) against TRAF6 and examined the ubiquitination of TAK1 in these cells. H. pylori-induced ubiquitination of TAK1 and mRNA expression of IL-8 and TNF-α were markedly attenuated in the TRAF6-depleted cells compared with AGS cells stably expressing control shRNA (Fig 5C; supplementary Fig S18 online). These data demonstrate that ubiquitination of TAK1 is crucial for CagA-enhanced NF-κB activation and that TRAF6 is probably the E3 ubiquitin ligase for H. pylori-induced TAK1 ubiquitination.
Although CagA enhances TRAF6-mediated Lys 63-linked ubiquitination of TAK1 (Figs 3,5), it is not clear as to how this occurs. CagA fails to enhance the interaction between TRAF6 and TAK1 (supplementary Fig S19 online), excluding the possibility that CagA might function as a bridging factor to facilitate the TAK1–TRAF6 interaction. It is possible that CagA could target TRAF6 and enhance its E3 ligase activity or prevent TAK1 from interacting with deubiquitination enzymes such as CYLD, which inhibit ubiquitination and auto-activation of TAK1 (Reiley et al, 2007). Supporting this argument, we found that CagA also interacts with TRAF6 in vitro and enhances its auto-ubiquitination (supplementary Figs S20,S21 online). However, binding of TRAF6 to CagA does not seem to affect the interaction between CagA and TAK1 (supplementary Fig S22 online).
In addition to NF-κB, other transcription factors, including AP-1, are activated by H. pylori and are involved in the induction of proinflammatory cytokines, including IL-8 (Mitsuno et al, 2001; O'Hara et al, 2006). Interestingly, AP-1 is also a downstream target of activated TAK1 (Adhikari et al, 2007). Further studies are needed to determine whether CagA-enhanced ubiquitination of TAK1 also contributes to H. pylori-induced AP-1 activation. It is probable that H. pylori uses CagA for activation of several signalling pathways that, in combination, contribute to the ultimate inflammatory response.
In conclusion, our current studies explore the mechanism by which H. pylori stimulates NF-κB activation and expression of inflammatory cytokines through the CagA-dependent, TRAF6-mediated Lys 63-ubiquitination and activation of TAK1 (Fig 5D). These studies also contribute to a more comprehensive understanding of how H. pylori infection stimulates inflammation and how pathogens, in general, might use cellular signalling molecules to induce cellular responses.
Methods
Cell lines, H. pylori culture and infection. Human AGS gastric adenocarcinoma, HEK293T and MEF cells were cultured in DMEM supplemented with 10% fetal bovine serum. H. pylori G27, NCTC11637 and 7.13 strains and their cagA-deficient mutants were cultured in bisulphite-free Brucella broth on agar media containing Ham's F-12 medium supplemented with 10% fetal bovine serum and 5 μg/ml vancomycin at 37°C in the presence of 10% CO2. H. pylori was added to AGS or MEF cells for infection at a multiplicity of infection of 50–100. All wt H. pylori and the corresponding cagA-deficient strains have similar abilities to adhere to the cells.
Immunoprecipitation, immunoblotting analysis and EMSA. Immunoprecipitation and immunoblotting analysis were performed as described previously (Chen et al, 2002). For electrophoretic mobility-shift assay (EMSA), whole-cell extracts were collected from infected cells by freeze–thaw lysis (Chen & Greene, 2005) and used for DNA binding assay as described previously (Chen et al, 2001).
Quantitative real-time PCR analysis. AGS or MEF cell lines were infected with H. pylori for various times and total RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). Complementary DNA was synthesized using the Omniscript RT kit (Qiagen). Quantitative real-time PCR was performed using the Qiagen SYBR green PCR kit, with the aid of the 7300 Real-time PCR system (ABI, Foster City, CA, USA). PCR primers for human β-actin, IL-8 and TNF-α, as well as mouse β-actin, E-selectin, IL-6 and TNF-α, were purchased from Qiagen.
Transient transfection and luciferase reporter assay. Transient transfection and luciferase reporter assays were performed as described previously (Chen et al, 2002). In each luciferase experiment, cells were also co-transfected with the EF1–Renilla luciferase reporter plasmid, which was used as the internal control. Results represent the average of three independent experiments±s.d.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Figs S1–S22
Acknowledgments
We thank K. Guillemin (University of Oregon, Eugene) for providing the H. pylori G27 strain and its cagA-deficient isogenic mutant, and B.G. Darnay, X. Lin, Z.J. Chen and J. Ninomiya-Tsuji for the gift of reagents. This work was supported in part by the Indirect Cost Recovery provided by University of Illinois at Urbana-Champaign. A.L. is a recipient of the Cell and Molecular Biology Training Grant. Y.-H.N.T. is an A*STAR-Illinois Partnership fellow. R.M.P is supported by National Institutes of Health Grants DK-58587, CA-77955 and CA-116087. S.R.B. is supported by National Institutes of Health Grant AI45928 and the Robert A. Welch Foundation Grant E-1311.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adhikari A, Xu M, Chen ZJ (2007) Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26: 3214–3226 [DOI] [PubMed] [Google Scholar]
- Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S (2003) Disruption of the epithelial apical–junctional complex by Helicobacter pylori CagA. Science 300: 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A (1995) Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55: 2111–2115 [PubMed] [Google Scholar]
- Brandt S, Kwok T, Hartig R, Konig W, Backert S (2005) NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA 102: 9300–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293: 1653–1657 [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J 21: 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC (2005) Assessing acetylation of NF-κB. Methods 36: 368–375 [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Naumann M (2000) p21-activated kinase 1 activates the nuclear factor kappa B (NF-κB)-inducing kinase-Ikappa B kinases NF-κB pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem 275: 39779–39785 [DOI] [PubMed] [Google Scholar]
- Franco AT et al. (2005) Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA 102: 10646–10651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl HL (1998) Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect Immun 66: 2346–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M (2004) Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 4: 688–694 [DOI] [PubMed] [Google Scholar]
- Hatakeyama M (2008) SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol 11: 30–37 [DOI] [PubMed] [Google Scholar]
- Hirata Y, Ohmae T, Shibata W, Maeda S, Ogura K, Yoshida H, Kawabe T, Omata M (2006) MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori-infected human epithelial cells. J Immunol 176: 3796–3803 [DOI] [PubMed] [Google Scholar]
- Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, Mosialos G, Li JD (2004) NF-κB is essential for induction of CYLD, the negative regulator of NF-κB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem 279: 36171–36174 [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee YC, Kim HK, Blaser MJ (2006) Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol 8: 97–106 [DOI] [PubMed] [Google Scholar]
- Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M (2001) Distinct mechanism of Helicobacter pylori-mediated NF-κB activation between gastric cancer cells and monocytic cells. J Biol Chem 276: 44856–44864 [DOI] [PubMed] [Google Scholar]
- Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C (2002) Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol Cell 10: 745–755 [DOI] [PubMed] [Google Scholar]
- Mitsuno Y, Yoshida H, Maeda S, Ogura K, Hirata Y, Kawabe T, Shiratori Y, Omata M (2001) Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 49: 18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Kamiya N et al. (2007) Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26: 4617–4626 [DOI] [PubMed] [Google Scholar]
- Naumann M (2001) Host cell signaling in Helicobacter pylori infection. Int J Med Microbiol 291: 299–305 [DOI] [PubMed] [Google Scholar]
- O'Hara AM et al. (2006) Interleukin-8 induction by Helicobacter pylori in gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J Immunol 177: 7990–7999 [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Orentreich N, Vogelman H (1997) Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM Jr (2005) Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA. Sci STKE 2005: pe14. [DOI] [PubMed] [Google Scholar]
- Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37 [DOI] [PubMed] [Google Scholar]
- Reiley WW et al. (2007) Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med 204: 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SA, Tummuru MK, Blaser MJ, Kerr LD (1998) Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol 160: 2401–2407 [PubMed] [Google Scholar]
- Shibata W et al. (2006) CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol 210: 306–314 [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M (2008) The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 10: 1199–1207 [DOI] [PubMed] [Google Scholar]
- Viala J et al. (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5: 1166–1174 [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351 [DOI] [PubMed] [Google Scholar]
- Wu X, Sun SC (2007) Retroviral oncoprotein Tax deregulates NF-κB by activating TAK1 and mediating the physical association of Tak1–IKK. EMBO Rep 8: 510–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figs S1–S22