Abstract
Neuronal activity controls the correct establishment and refinement of neuronal circuits by regulating key aspects such as dendritogenesis and spine development. Both transcriptional and post-transcriptional gene expression programs induced by neuronal activity have to be coordinated in a tight spatio-temporal manner in order for proper functioning of the neuron. In this context microRNAs (miRNAs), which are implicated in post-transcriptional gene regulation, are good candidates to control dendritic and spine development. In a recent study we have demonstrated that neuronal activity induces myocyte enhancing factor 2 (Mef2) dependent transcription of a large cluster of brain-specific miRNAs (miR379-410). Expression of at least three microRNAs (miR-329,-134 and -381) from this cluster is essential for activity-dependent dendritic outgrowth of hippocampal neurons. One of these three miRNAs, miR-134, promotes dendritic outgrowth by inhibiting translation of the mRNA encoding the translational regulator Pumilio2 (Pum2). In brief, our results suggest a novel role for Mef2 in promoting activity-dependent dendritogenesis by inducing the transcription of the miR379-410 cluster.
Key words: mef2, pumilio, miRNA, plasticity, dendrite, neuron
The proper development and functioning of neuronal circuits require elaborate gene expression programs that have to be coordinated in a tight spatio-temporal manner. While intrinsic gene expression programs are heavily involved in the early stages of neuronal development activity-dependent gene expression is necessary for the correct establishment and refinement of neuronal circuits by regulating key aspects such as dendritogenesis and spine development.1 Increasing evidence suggests that non-coding RNAs, in particular miRNAs, cooperate with canonical activity regulated transcriptional and post-transcriptional factors (CREB and CPEB, respectively),2,3 to regulate gene expression in response to neuronal activity.4,5 MiRNAs are involved in the posttranscriptional regulation of gene expression by targeting the RNA-induced silencing complex (RISC) to the 3′-UTR of an mRNA, leading to its translational repression or degradation.
In our recent study we discovered that the transcriptional expression of miRNAs from the miR379-410 cluster, located within the Gtl2/Dlk1 locus and composed of more than 50 miRNAs, is induced in response to neuronal activity (KCl and BDNF) in a Mef2—dependent manner.6 At least three miRNAs (miR-134, -381 and -329) from the cluster are necessary for an increase in dendritic complexity observed upon neuronal activity (Fig. 1A). Activity-dependent changes in dendritic complexity are well documented both in in vitro cell culture models and in in vivo animal models. For example, the dendritic tree of cortical neurons is extensively elaborated in response to the increased neuronal network activity in animals exposed to an enriched environment.7 Furthermore, abnormalities of the dendritic tree are a common hallmark of several cognitive diseases characterized by synaptic dysfunction such as mental retardation.8 Recently, defects in miRNA biogenesis were shown to contribute to dendritic abnormalities in a mouse model of schizophrenia9 and in Dicerdeficient mice.10 Therefore, it will be important to study the role of the activity-dependent expression of the miR379-410 cluster in dendrite development in vivo. Since only a few members of the cluster appear to be required for activity-dependent dendritogenesis, it is likely that multiple other aspects of neuronal development (neuronal survival, synapse development) may be coordinately controlled by the miR379-410 cluster. Combining gene targeting approaches with genome wide profiling will yield valuable insight into the signaling pathways regulated by the miR379-410 cluster.
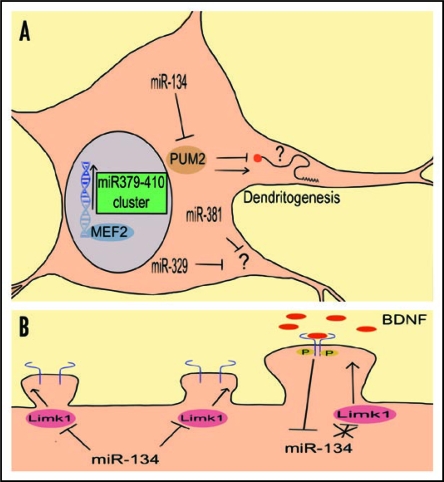
Figure 1.
The effect of neuronal activity on the function of the miR379-410 cluster member miR-134. (A) Schematic representation of miR379-410 cluster dependent dendritogenesis pathway. Neuronal activity induces Mef2-dependent transcription of microRNAs from the miR379-410 cluster. At least three of them (miR-134, -329 and -381) are necessary for activity-dependent dendritogenesis. The targets of miR-329/381 are unknown. One of the miR-134 targets important for dendritogenesis is Pum2. Enhanced expression of miR-134 blocks Pum2 translation. Reduced Pum2 expression, in turn, allows the synthesis of dendrite growth promoting factors (and/or interferes with the synthesis of inhibiting factors). (B) miR-134 regulates dendritic spine size.15 Bdnf (neuronal activity) application inhibits the repressive effect of miR-134 on translation of Limk1 mRNA which leads to increased spine size.
We identified the transcription factor Mef2 as necessary for activity-dependent regulation of the miR379-410 cluster. Mef2 was recently shown to act as a negative regulator of synapse number.11 We uncovered a novel role of Mef2, namely as a positive effector of dendritic outgrowth. Furthermore, it was recently shown that, during early postnatal development, dendritic arborization is accompanied by a concomitant reduction in unitary excitatory synaptic strength.12 This latter finding might provide a rational to reconcile the seemingly opposing functions of Mef2; upon neuronal activity Mef2 could in parallel induce excitatory synapse downscaling and dendritic outgrowth. A key role for Mef2 in reducing neuronal excitability is further suggested by the observation that under conditions of high neuronal activity, Mef2 induces expression of the neurotrophin BDNF and the transcription factor Npas4, two positive regulators of inhibitory synapse development.13,14
MiR-134 is one of the miR379-410 cluster miRNAs necessary for activity-dependent dendritogenesis. Previously we have shown that miR-134 restricts dendritic spine growth by reversibly inhibiting the local synthesis of the actin cytoskeleton regulator LimK1.15 In the recent sudy, the effect of miR-134 on dendritic outgrowth appears to be mediated by a different target, the RNA-binding protein Pumilio 2 (Pum2). Pumilio proteins can act either as translational activators or inhibitors and control several aspects of neuronal function, including neuronal morphology and excitability.16,17 Both the Pum2 protein and mRNA are localized in dendrites. Thus miR-134 might couple global nuclear programs of gene expression with the spatially restricted control of protein synthesis in dendrites. To test this hypothesis it will be necessary to determine the localization of the Pum2-miR-134 interaction and the subset of mRNAs whose translation is regulated by Pum2. These studies could elucidate how a neuron coordinates transcriptional and post-transcriptional programs of gene expression in response to activity.
Surprisingly, our study also revealed a dual effect of neuronal activity on miR-134 function. In young neurons, activity increases miR-134 levels to facilitate the translational inhibition of Pum2 and dendritic outgrowth. In more mature neuronal cultures, BDNF release locally suppresses the miR-134 mediated Limk1 translational inhibition. It is tempting to speculate that these different effects might not only reflect different functions of miR-134 at different stages of development, but also represent a mechanism of neuronal homeostasis in response to increased or decreased network activity. Homeostatic plasticity is defined as the capability of individual neurons within a circuit to adjust to different levels of presynaptic input by changing the strength of the postsynaptic responses. One example is synaptic downscaling, a mechanism that decreases the average magnitude of postsynaptic responses to keep the overall neuronal excitability within the physiological range.18 One could speculate that the increased global levels of miR-134 in response to activity might contribute to synaptic downscaling by restricting spine growth. Moreover, synaptic activity leads to BDNF secretion that can locally block miR-134 activity. These two forms of activity-dependent miR-134 regulation would permit the potentiation of individual active synapses without compromising neuronal homeostasis in the very same neuron (Fig. 1B). The coordination of global and local control of miRNAs by neuronal activity and their function in homeostatic and Hebbian forms of synaptic plasticity is an exciting topic for future investigation.
It is becoming evident that many neurological diseases arise from a failure in homeostatic mechanisms within the neuronal network.19 Understanding how miRNAs modulate experience-dependent neuronal responses should shed some light on the etiology of complex brain disorders and potentially provide new means for therapeutic intervention.
Acknowledgements
We thank R. Saba for critically reading the manuscript. This work was supported by grants from the DFG (SFB488), HFSP (CDA) and NIDA (4R21DAO25102-01).
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8834
References
- 1.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 3.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 5.Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779:471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, et al. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005;46:401–415. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- 9.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 10.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 12.Peng YR, He S, Marie H, Zeng SY, Ma J, Tan ZJ, et al. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron. 2009;61:71–84. doi: 10.1016/j.neuron.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 16.Kaye JA, Rose NC, Goldsworthy B, Goga A, L'Etoile ND. A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron. 2009;61:57–70. doi: 10.1016/j.neuron.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baines RA. Neuronal homeostasis through translational control. Mol Neurobiol. 2005;32:113–121. doi: 10.1385/MN:32:2:113. [DOI] [PubMed] [Google Scholar]
- 18.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]