Abstract
The biosynthetic pathway allows the transport of newly synthesized proteins to the TGN via the reticulum endoplasmic and Golgi apparatus. However, many large particles reach the TGN by unconventional means. For instance, Herpes simplex virus type 1(HSV-1) capsids assemble within the nucleus, bud into the perinuclear space, fuse with the outer nuclear membrane and finally travel unenveloped towards the TGN. Given the central role of protein kinase D in the transport of small cargo from the TGN to the cell surface, we probed its potential contribution in HSV-1 egress, as a model for studying large cargo exiting from the TGN. Using a synchronized infection, we show that inactivation of protein kinase D with pharmacological inhibitors, a kinase dead mutant or siRNA all causes the retention of HSV-1 at the TGN. This highlights the role of PKD in viral exit and a dependence of the virus on the classical host cell machinery to leave the TGN, unlike its previous transport steps. Conceptually, this supports a model in which the TGN is a meeting point where conventional and unconventional routes encounter.
Key words: trans golgi network, protein kinase D, HSV-1, intracellular transport, egress, herpesvirus, virus, sorting
Newly synthesized transmembrane and secreted proteins typically travel via the classical biosynthetic pathway from the ER to the Golgi apparatus and the TGN.1 They are then segregated at the TGN and sorted to multiple different targets (extracellular environment, intracellular organelles, cell surface).2,3 Proteins destined for the cell surface and extracellular milieu are often packaged in transport carriers regulated by the serine-threonine protein kinase D (PKD).4,5 At the TGN, PKD mediates the fission of carriers packed with small cargo. This activity requires diacyl glycerol (DAG), which recruits PKD from the cytosol to the TGN.6 The biosynthetic route is not applicable to all cargo, since small COPII vesicles that carry proteins from the ER towards the Golgi apparatus cannot accommodate large cargo such as chylomicrons, procollagen and viral capsids.7 Although, these outsized cargos use unconventional pathways to travel through the cell, they all reach the TGN. Given these unconventional routes to get to the TGN, it was of interest to study the way they escape this organelle to reach their final destination. Moreover, it was unclear whether they employed the PKD mediated cellular machinery or an alternative path to escape the TGN.
Herpes simplex virus type 1 (HSV-1) is a double stranded DNA enveloped virus. The assembly of new capsids takes place in the nucleus of the host cell. The large 125 nm capsids then pass through the double nuclear membranes by unique sequential budding and fusion processes,8 which release unenveloped capsids in the cytosol. These capsids then travel along the cytoskeleton and reach the TGN, where they acquire their final envelope.9,10 From the TGN, they journey to the plasma membrane via an unknown pathway. Given their unconventional route of transport to the TGN, we probed the role of the conventional PKD mediated transport machinery on viral egress downstream of that compartment. In the study described in this addendum,11 the egress of HSV-1 from the TGN to the plasma membrane was monitored using a synchronized infection protocol, in which HSV-1 capsids are accumulated at the TGN and then released to the cell exterior. Under these conditions, HSV-1 egress is strongly affected by three different DAG inhibitors, which all prevent the recruitment of PKD to the TGN and thus impede the fission of carriers travelling from the TGN to the plasma membrane.6,12 Moreover, immunofluorescence studies revealed the entrapment of the virus at the TGN, supporting the contribution of DAG and PKD in the release of HSV-1 from the TGN. To directly address the role of PKD in HSV-1 transport, we used two additional and more specific methods, namely an inactive mutant of the protein (PKD K618N)4,13 and siRNA directed against PKD. Both systems confirmed the implication of PKD by strongly reducing the egress of the virus. Once again, virions could be visualized in inactivated PKD induced tubules. Altogether the results demonstrated the dependence of HSV-1 virions on the PKD-regulated cellular pathway to transit from the TGN to the plasma membrane, i.e., the same path as for small cargo. This is in sharp contrast with anterior steps of intracellular transport of the virus.
The use of the classical PKD pathway by HSV-1 to exit the TGN, but its unusual route to arrive there underlines the TGN as a hub, where conventional and unconventional pathways carrying various cargos meet (Fig. 1). This applies not only to HSV-1 but likely extends to other large cargo such as procollagen and chylomicrons. Both molecules also bypass the COPII vesicles to reach the Golgi apparatus or TGN before reaching their final destination.14,15 Though unproven at this point, they probably depend on PKD for the last leg of their journey. For instance, procollagen has been shown to co-localize with the small VSV G cargo.16 Other large cargo such as several viruses that replicate and assemble in the cytoplasm also travel through the TGN before being released at the cell surface. These include Rota-, Corona-, Bunya-, Flavi- and Poxviruses.17,18 Several unconventional and unrelated pathways thus meet at the TGN and merge with the conventional transport apparatus. The additional passage in the TGN of some molecules endocytosed at the cell surface before being recycled to the plasma membrane19 further highlights the importance of the TGN as a meeting hub for several pathways.
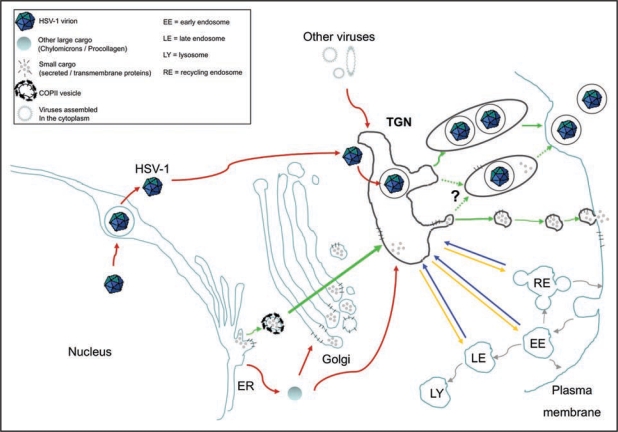
Figure 1.
The TGN is a meeting point where conventional and unconventional routes of intracellular transport merge. To illustrate this point, the classical biosynthetic pathway from the ER to the cell surface is depicted with green arrows. Alternative transport routes by which various large cargos such as HSV-1, chylomicrons, procollagen and some viruses that assemble in the cytoplasm reach the TGN are indicated by red arrows. In addition, the TGN is not only a merging point for molecules newly synthesized but also for proteins recycled from organelles along the endo-lysosomal system (shown with blue arrows). At the TGN, large and small cargos merge and are sorted towards the cell surface (green arrows) and various endosomal compartments (yellow arrows). Dotted green arrows illustrate the uncertainty as to whether large cargo, in particular viruses, share the PKD mediated pathway with smaller cargo or whether they monopolizes it.
The use of PKD by small, large and viral cargos raises interesting issues. For instance, it is not clear if these various cargos share the post-TGN transport machinery or compete for it (Fig. 1). This is particular relevant for viruses, as they often hijack host cell systems for their privileged use.20,21 These different cargos might also travel in parallel in distinct transport carriers. If so, what dictates their specific incorporation in the PKD generated carriers? What are the specific sorting signals? Particular coat adaptors, certain lipids, modulators and members of the Rab and ARF families of small GTPases might also be implicated.22–26 Would components be shared between these various cargos? Given the complexity of the intracellular machinery, cross-talk between the different pathways downstream of the TGN is indispensable.27,28 As new paths transiting through the TGN are elucidated for multiple types of cargo, this cross-talking is becoming more and more complex and will need additional investigations to describe it. As PKD impairment didn't completely shut down virus egress in our studies, this hints that other components, cellular or viral, are implicated in HSV-1 post-TGN transport. Finally, HSV-1 could encode novel modulators of the cellular transport mechanisms or alternatively mimic existing host molecules29,30 to emulate cellular pathways.
A final issue is the enduring debate regarding the site of final envelopment of HSV-1 capsids in neuronal cells. The pathogenesis of αherpesviruses is characterized by neuronal spread. Several studies suggest the envelopment of the virus at the cell body and transport of fully mature enveloped virions down the axon. Others studies rather propose the axonal transport of naked capsids and the acquisition of an envelope at the cone.31,32 Although our study showed a clear involvement of host cell PKD in TGN to plasma membrane transport in epithelial cells, it remains to be proven whether this is also the case in neurons. If so, it could argue in favour of a re-envelopment in the neuron body, where TGN and PKD are usually present, as opposed to a more distant envelopment in the axon end. However, it has been shown that PKD is only needed in dendritic transport for small cargo,33 not in axonal transport. This could mean an alternative and unique pathway of HSV-1 post-TGN transport in neuronal cells. It is also conceivable that the PKD multiple isoforms (three in humans4,13) assume different functions in different cell types.
Acknowledgements
This research was funded by the Canadian Institutes of Health Research and an equipment grant from the Canadian Foundation for Innovation.
Abbreviations
- TGN
trans golgi network
- PKD
protein kinase D
- HSV-1
herpes simplex virus type 1
- COPII
coat protein type II
- DAG
diacyl glycerol
- ER
endoplasmic reticulum
- ARF
ADP ribosylation factors
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/9217
References
- 1.Rodriguez-Boulan E, Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta. 2005;1744:455–464. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Muniz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–320. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 3.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 4.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanekar Y, Lowe M. Protein kinase D: activation for Golgi carrier formation. Trends Cell Biol. 2005;15:511–514. doi: 10.1016/j.tcb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 7.Fromme JC, Schekman R. COPII-coated vesicles: flexible enough for large cargo? Curr Opin Cell Biol. 2005;17:345–352. doi: 10.1016/j.ceb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: a tale of two membranes. Curr Opin Microbiol. 2006;9:423–429. doi: 10.1016/j.mib.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Turcotte S, Letellier J, Lippe R. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J Virol. 2005;79:8847–8860. doi: 10.1128/JVI.79.14.8847-8860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harley CA, Dasgupta A, Wilson DW. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: Role for organelle acidification in assembly of infectious particles. J Virol. 2001;75:1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rémillard-Labrosse G, Mihai C, Duron J, Guay G, Lippé R. Protein kinase D dependent trafficking of the large HSV-1 capsids from the TGN to plasma membrane. Traffic. 2009;10:1074–1083. doi: 10.1111/j.1600-0854.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 12.van Ooij C, Kalman L, van I, Nishijima M, Hanada K, Mostov K, Engel JN. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 13.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 14.Sabesin SM, Frase S. Electron microscopic studies of the assembly, intracellular transport and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 15.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 16.Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion and en bloc cleavage of large trans-golgi network tubular domains. Mol Biol Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambleton S, Gershon MD, Gershon AA. The role of the trans-Golgi network in varicella zoster virus biology. Cell Mol Life Sci. 2004;61:3047–3056. doi: 10.1007/s00018-004-4269-7. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths G, Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992;3:367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Meel E, Klumperman J. Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol. 2008;129:253–266. doi: 10.1007/s00418-008-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann-Che J, Saib A. Early stages of HIV replication: how to hijack cellular functions for a successful infection. AIDS Rev. 2004;6:199–207. [PubMed] [Google Scholar]
- 21.Ross EM. Cellular signalling. Viral hijack of receptors. Nature. 1990;344:707–708. doi: 10.1038/344707a0. [DOI] [PubMed] [Google Scholar]
- 22.Kreis TE, Pepperkok R. Coat proteins in intracellular membrane transport. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 23.Gu F, Crump CM, Thomas G. Trans-Golgi network sorting. Cell Mol Life Sci. 2001;58:1067–1084. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleeson PA, Lock JG, Luke MR, Stow JL. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5:315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 26.Chavrier P, Goud B. The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- 27.Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- 28.Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, et al. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- 29.Boomker JM, van Luyn MJ, The TH, de Leij LF, Harmsen MC. US28 actions in HCMV infection: lessons from a versatile hijacker. Rev Med Virol. 2005;15:269–282. doi: 10.1002/rmv.468. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi Y, Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev Med Virol. 2003;13:331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- 31.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 32.Tomishima MJ, Smith GA, Enquist LW. Sorting and transport of alpha herpesviruses in axons. Traffic. 2001;2:429–436. doi: 10.1034/j.1600-0854.2001.020701.x. [DOI] [PubMed] [Google Scholar]
- 33.Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, Quiroga S, et al. Protein kinase d regulates trafficking of dendritic membrane proteins in developing neurons. J Neurosci. 2008;28:9297–9308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]