Abstract
The difficulty to simultaneously record neural activity and behavior presents a considerable limitation for studying mechanisms of insect learning and memory. The challenge is finding a model suitable for the use of behavioral paradigms under the restrained conditions necessary for neural recording. In honeybees, Pavlovian conditioning relying on the proboscis extension reflex (PER) has been used with great success to study different aspects of insect cognition. However, it is desirable to combine the advantages of the PER with a more robust model that allows simultaneous electrical or optical recording of neural activity. Here, we briefly discuss the potential use of bumblebees as models for the study of learning and memory under restrained conditions. We base our arguments on the well-known cognitive abilities of bumblebees, their social organization and phylogenetic proximity to honeybees, our recent success using Pavlovian conditioning to study learning in two bumblebee species, and on the recently demonstrated robustness of bumblebees under conditions suitable for electrophysiological recording.
Key words: bombus, insect cognition, apis, animal model, bees
Learning and memory, defined as the acquisition and storage of neuronal representations of information through experience,1 are two of the most intensively explored processes of animal cognition. Learning represents one of the contributions of the nervous system to an animal's overall phenotypic plasticity that facilitate the accommodation of the phenotype to diverse environments.2,3 However, despite the great interest in learning and memory research, a longstanding challenge for the study at the proximate level has been the direct quantification of the acquisition, storage and retrieval of information associated with a neuronal representation.1,4 Thus, learning and memory are typically quantified through ‘performance’ (i.e., through a behavioral change associated with learning), which represents what the animal does with what it has learnt, but which also depends on other factors (e.g., motivation, attention) modulating the final behavioral output. Realistically, performance reflects the role of learning and memory in a more integrative ecological context, especially under unrestrained conditions. Therefore, using performance as a measure limits the analysis of proximate phenomena underlying learning and memory. When addressing these mechanisms at the level of brains and neurons, an additional problem arises from the restrained conditions required during recording of neural activity. Therefore, a growing interest exists in developing models and methods that may enable the ‘visualization’ of the process of memory formation and retrieval underlying the performance during training and testing.
Recently, we examined the use of Pavlovian conditioning to study associative learning in bumblebees under restrained conditions.5 There are several reasons for the selection of both, the methodology and the animal model. Pavlovian conditioning has been widely established as a paradigm for the study of cognitive abilities in model systems such as the round worm Caenorhabditis elegans, the marine snail Aplysia californica, the fruit fly Drosophila melanogaster and the honeybee Apis mellifera. As in the original paradigm,6 in honeybees, an unconditioned stimulus (typically sucrose) administered to the antenna or the tarsi of the leg evokes a reflexive tongue extension (the proboscis extension reflex; PER). Simultaneous presentation of sucrose and other (conditioned) stimuli (e.g., odors, touch or light) results in an association of the conditioned stimulus and the reward, and eventually the conditioned stimulus alone evokes the PER. The experimental paradigm allows for changes of the quality and quantity of the conditioned stimulus (e.g., odor concentration) as well as of the reward (e.g., sucrose concentration and/or amount).7 Thus, the entire paradigm emulates, under restrained conditions, the natural sequence of behaviors during foraging. Bees approaching a flower receive visual and/or olfactory information, which may vary between or within species of flowers (e.g., differences in shape, color, odor). Once on the flower, antennal exploration and the contact with nectar elicit the extension of the proboscis, followed by the ingestion of the reward, which may also vary in its quality (e.g., carbohydrate concentration) and/or quantity (i.e., nectar amount).
Despite the convenience and success of the PER paradigm in honeybees,8,9 our understanding of the neuronal processes underlying this learning behavior is still at an early stage. Intracellularly recording electrical activity is the technique of choice for analyzing learning-dependent changes of information processing in individual neurons. This technique has been used with some success,10,11 leading to the discovery of specific components of the neural pathway involved in the conditioned response,10,12 yet it faces the problem of fragility of the bees under extended experimental manipulations. The more recent calcium imaging technique for measuring brain activity has also met with some success, particularly regarding neuronal activity of the olfactory centers of bees, the antennal lobes.13 However, calcium imaging has a much lower temporal resolution than electrophysiological recordings and only enables visualization of pattern activity on the superficial layers of the neuropil. Another technique that has recently been introduced into brain research of larger insects, multi-unit recording using multi-channel silicon probes,14 has not been successfully applied in honeybees because of their size and fragility,15 although the use of thin (<15 um) electrodes has been suggested as an alternative.15
Therefore, given the convenience of the PER and the large amount of knowledge obtained from honeybees, it would be advantageous to establish a model system that combines the advanced behavioral and learning capabilities of honeybees with the experimental robustness and ease of larger insects such as locusts or hawk moths. Our success of establishing a robust PER learning paradigm in bumblebees5 (genus Bombus) together with the recently shown suitability of bumblebees for long-term intracellular brain recording16,17 suggest the use of bumblebees as such a model system.
Bumblebees as Models
Bumblebees are closely related to honeybees, which favors comparative approaches, in particular given their contrasting systems of social organization. In honeybees, age plays a predominant role in organizing the division of labor. Young bees typically remain inside the hive performing the tasks of nursing, cleaning and storage of resources brought in by foragers. Older workers engage in foraging activities, collecting mainly nectar, pollen and water. Along with these behavioral transitions, workers change their performance in learning and memory tasks, presumably preparing for higher cognitive challenges associated with navigation, predation risk and resource evaluation outside of the protected hive.18 In contrast to honeybees, bumblebee societies are strongly influenced by individuals' body size and only weakly by their age. Larger workers in the colony mostly form the foraging force, whereas smaller workers mostly participate in in-hive tasks.19 A second difference is the contrasting colony size, which reach up to 60,000 workers in honeybees, and typically between 200–400 workers in bumblebees. Besides potential behavioral differences associated with colony size (see below), smaller colonies of bumblebees certainly facilitate their maintenance under laboratory conditions. A third difference is the solitary system of foraging in bumblebees, which, in contrast to honeybees, do not provide nest mates with direct information about location and quality of potential resources (reviewed in refs. 20–22). A fourth difference is the independent foundation of colonies by individual bumblebee queens,19 whereas in honeybees the queen always swarms with a large number of her workers. A fifth difference is the single mating system in most bumblebee species,19 which enables an easier control of the behavioral variation associated with different genotypes.
From these contrasting characteristics, several predictions regarding learning and memory capacities in bumblebees can be drawn. Small colonies and a system of division of labor affected by body size may lead to earlier cognitive maturation of bumblebees because worker foragers may start foraging within two days after eclosion. Furthermore, losses of workers during foraging might have a stronger effect on a smaller colony, which, giving limitations for surplus storage of food, needs to rapidly replace foragers with the fewer workers remaining in the colony. In contrast, removal of foragers in honeybee colonies leads to an accelerated, yet not immediate, neural and physiological maturation of in-hive workers. Regarding the role of body size in bumblebee colonies, larger workers may be expected to show better learning performance and longer memory retention, as they are exposed to the cognitive challenges of foraging.
As for the mode of colony foundation, it is interesting to consider potential transitions in the queen learning and memory capacities: young, founding bumblebee queens have to perform all the cognitively demanding tasks of a forager to raise their first brood, while later they concentrate on egg-laying and their workers care for the brood. In this context, potential trade-offs between brain development, learning and reproduction may be identified in bumblebee queens as have been found in other species.23 Although very scarce, previous evidence indeed shows high performance of foraging queens on learning and on categorization tasks.24 This possibility might contrasts with the behavior observed in honeybee queens, which never need to forage.
Besides their use in a comparative approach, bumblebees have proved to be useful models per se in cognitive research25 as demonstrated by the growing body of literature on diverse aspects of learning and memory such as acquisition, retention, transfer and interference in individual bumblebees (reviewed in refs. 21, 26–32) and in social contexts (reviewed in refs. 20–22). Other areas have been also explored, such as decision-making (reviewed in refs. 25, 33–35) and navigation (reviewed in ref. 36). Importantly, most of these phenomena can be directly linked to ecological contexts of bumblebee life histories, such as risk-sensitive foraging,37 traplining foraging38 (and references therein), resource categorization,24 and predation avoidance.34,39 This context is of major significance because it considers the ‘cognitive architecture’ (reviewed in ref. 25) as part of the phenotype affecting individual performance and species evolution32 and also speaks for the relevance of bumblebees as models.
Bumblebees and the Proboscis Extension Reflex
In our research, we attempt to combine the benefits of the PER paradigm established in honeybees and the well-developed cognitive capacities of bumblebees, envisioning their use to incorporate neuronal recordings during training and testing. Our attempts are further encouraged by the robustness of bumblebees under restrained conditions (Fig. 1) and during electrophysiology recordings,16,17 and also by their large brains (reviewed in ref. 40; Riveros AJ and Gronenberg W, unpublished), both features favoring intra and extracellular recordings. Although an attempt to use the PER paradigm in bumblebees has been previously reported,29 the level of performance was much lower than that observed under free-flying conditions. Improving those early attempts, we have worked toward establishing a PER paradigm in two species of bumblebees, Bombus occidentalis and B. impatiens.
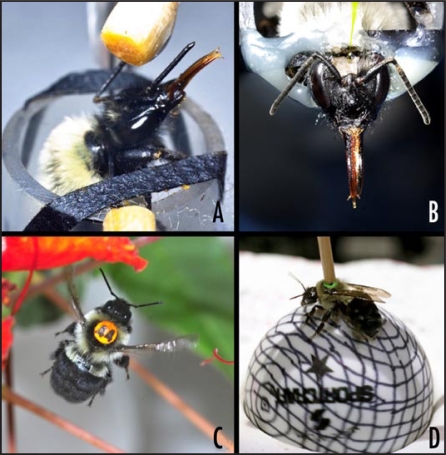
Figure 1.
Examples for different degrees of physical restriction in bumblebee behavioral paradigms. (A) Harnessed bee undergoing PER conditioning; (B) harnessed bee during electrophysiological recording; (C) tagged bee flying in foraging cage; (D) walking on a treadmill.
Bumblebee colonies can be kept under laboratory conditions, where colonies are connected to a foraging cage with feeders filled with a solution of sucrose. In our studies pollen and water are provided ad libitum inside the hive, assuring the use of bees inclined to forage for sugars. Individual workers can be tracked using numbers glued on the back of newly emerged bees. Foraging activity is recorded using a camera directed at the nest entrance. Thus, for each individual it is possible to record its age at first foraging trip, time spent during each trip and number of trips before training.
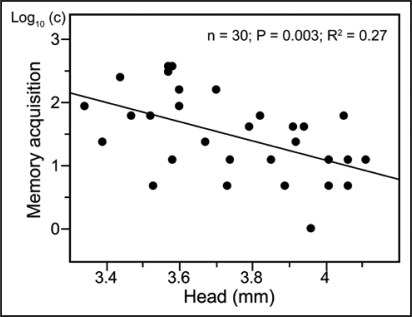
Our results using Bombus occidentalis5 and B. impatiens point to three major aspects of olfactory learning in bumblebees. First, bumblebees do not require several days of maturation before reaching the level of performance of older adults. Indeed, we found two hour old bees to learn as well as their older sisters. Second, once workers engage in foraging activities, their overall performance seems to increase along with foraging experience. Third, the association between body size and learning seems to vary across species. In B. occidentalis5 and B. terrestris,32 size is not associated with increasing learning performance (although it is associated with memory retention in B. occidentalis). In contrast, our current research using B. impatiens shows large workers performing better than the smaller ones in an olfactory learning paradigm (Fig. 2; cf references 21).
Figure 2.
Correlation between acquisition performance and body size in the bumblebee B. impatiens. c = number of training trials necessary for the first conditioned response to appear.
These results are relevant considering both, the ecological context and the potential use of electrophysiology during bumblebee learning. Together with information on honeybees, our findings suggest that learning ability of the worker caste is associated with division of labor and probably reflects the cognitive challenges of different tasks. Also associated with the social organization, size, but not age, significantly affects learning and memory in bumblebees. These two results point toward young bees as a good option for future studies in electrophysiological systems, because young bees lack the effects of experience on learning, which we found to be significant. We may also be able to electrophysiologically address the effect of size: are there certain tasks that a larger nerve cell (in a large bee) can do better than its smaller counterpart in a small bee? Do larger eyes or antenna or other sense organs endow their bearers with advanced sensory abilities?
Honeybee and bumblebee brains are organized very similar, and our neuroanatomical knowledge of the substrates presumably underlying learning and memory will benefit future attempts combining electrophysiology and the PER learning paradigm in bumblebees. Olfactory information is first processed by the olfactory (antennal) lobes and then transferred to the mushroom bodies and other central brain components, where the associations are presumably formed. Simultaneously recording from the antennal lobe and the mushroom body while the bumblebee is learning to associate an odor will probably greatly enhance our understanding of the neuronal mechanisms resulting in learning and memory.
Recent studies on bumblebees including our own findings open new possibilities for research and provide a protocol and an animal model that will likely contribute to our understanding of learning and memory beyond individual performance.
Acknowledgements
Our research is currently funded by an NSF grant to WG (IOB-0519483) and by research awards of the Graduate Interdisciplinary Program in Insect Science (University of Arizona) to A.J.R.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/9240
References
- 1.Dukas R. Evolutionary biology of insect learning. Annu Rev Entomol. 2008;53:145–160. doi: 10.1146/annurev.ento.53.103106.093343. [DOI] [PubMed] [Google Scholar]
- 2.West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; 2003. [Google Scholar]
- 3.Papaj DR. Learning, adaptation and the lessons of O. In: Papaj DR, Lewis AC, editors. Insect Learning: Ecological and Evolutionary Perspectives. Chapman and Hall; 1993. pp. 374–386. [Google Scholar]
- 4.Shettleworth SJ. Cognition, Evolution and Behavior. New York: Oxford University Press; 1998. [Google Scholar]
- 5.Riveros AJ, Gronenberg W. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften. 2009;96:851–856. doi: 10.1007/s00114-009-0532-y. [DOI] [PubMed] [Google Scholar]
- 6.Pavlov IP. In: Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Anrep GV, translator. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzel R. Memory dynamics in the honeybee. J Comp Physiol. 1999;185:323–340. [Google Scholar]
- 8.Giurfa M. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A. 2007;193:801–824. doi: 10.1007/s00359-007-0235-9. [DOI] [PubMed] [Google Scholar]
- 9.Menzel R, Mueller U. Learning and memory in honeybees: From behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- 10.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 11.Mauelshagen J. Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J Neurophysiol. 1993;69:609–625. doi: 10.1152/jn.1993.69.2.609. [DOI] [PubMed] [Google Scholar]
- 12.Menzel R, Manz G. Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. J Exp Biol. 2005;4:4317–4332. doi: 10.1242/jeb.01908. [DOI] [PubMed] [Google Scholar]
- 13.Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- 14.Lei H, Christensen TA, Hildebrand JG. Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci. 2004;24:11108–11119. doi: 10.1523/JNEUROSCI.3677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzel R. Electrophysiology and optophysiology of complex brain functions in insects. In: North G, Greenpan RJ, editors. Invertebrate Neurobiology. New York: Cold Spring Harbor Laboratory Press; 2007. pp. 53–78. [Google Scholar]
- 16.Paulk AC, Phillips-Portillo J, Dacks AM, Fellous J-M, Gronenberg W. The processing of color, motion and stimulus timing are anatomically segregated in the bumblebee brain. J Neurosci. 2008;28:6319–6332. doi: 10.1523/JNEUROSCI.1196-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulk A, Dacks A, Gronenberg W. Color processing in the medulla of the bumblebee (Apidae: Bombus impatiens) J Comp Neurol. 2009;513:441–456. doi: 10.1002/cne.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa N, Sasaki M. Importance of social stimuli for the development of learning capability in honeybees. Appl Entomol Zool. 2003;38:203–209. [Google Scholar]
- 19.Goulson D. Bumblebees: Their behaviour and ecology. Oxford: Oxford University Press; 2003. [Google Scholar]
- 20.Leadbeater E, Chittka L. A new mode of information transfer in foraging bumblebees? Curr Biol. 2005;15:447–448. doi: 10.1016/j.cub.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Worden BD, Skemp AK, Papaj DR. Learning in two contexts: the effects of interference and body size in bumblebees. J Exp Biol. 2005;208:2045–2053. doi: 10.1242/jeb.01582. [DOI] [PubMed] [Google Scholar]
- 22.Leadbeater E, Chittka L. The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris) Behav Ecol Sociobiol. 2007;61:1789–1796. [Google Scholar]
- 23.Snell-Rood EC, Papaj DR, Gronenberg W. Brain size: a global or induced cost of learning? Brain Behav Evolut. 2009;73:111–128. doi: 10.1159/000213647. [DOI] [PubMed] [Google Scholar]
- 24.Dukas R, Waser NM. Categorization of food types enhances foraging performance of bumblebees. Anim Behav. 1994;48:1001–1006. [Google Scholar]
- 25.Real L. Information processing and evolutionary ecology of cognitive architecture. In: Real L, editor. Behavioral mechanisms in evolutionary ecology. Chicago: The University of Chicago Press; 1994. pp. 99–132. [Google Scholar]
- 26.Laverty TM. Bumble bee learning and flower morphology. Anim Behav. 1994;47:531–545. [Google Scholar]
- 27.Dukas R. Transfer and interference in bumblebee learning. Anim Behav. 1995;49:1481–1490. [Google Scholar]
- 28.Keasar T, Motro U, Shur Y, Shmida A. Overnight memory retention of foraging skills by bumblebees is imperfect. Anim Behav. 1996;52:95–104. [Google Scholar]
- 29.Laloi D, Sandoz JC, Picard-Nizou AL, Marchesi A, Pouvreau A, Taséi JN, et al. Olfactory conditioning of the proboscis extension in bumble bees. Entomol Exp Appl. 1999;90:123–129. [Google Scholar]
- 30.Cnaani J, Thomson JD, Papaj DR. Flower choice and learning in foraging bumblebees: effects of variation in nectar volume and concentration. Ethology. 2006;112:278–285. [Google Scholar]
- 31.Raine NE, Chittka L. Pollen foraging: learning a complex motor skill by bumblebees (Bombus terrestris) Naturwissenschaften. 2007;94:459–464. doi: 10.1007/s00114-006-0184-0. [DOI] [PubMed] [Google Scholar]
- 32.Raine NE, Chittka L. The correlation of learning speed and natural foraging success in bumblebees. Proc R Soc B. 2008;275:803–808. doi: 10.1098/rspb.2007.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chittka L, Dyer AG, Block F, Dornhaus A. Bees trade off foraging speed for accuracy. Nature. 2003;424:388. doi: 10.1038/424388a. [DOI] [PubMed] [Google Scholar]
- 34.Ings TC, Chittka L. Speed-Accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr Biol. 2008;18:1520–1524. doi: 10.1016/j.cub.2008.07.074. [DOI] [PubMed] [Google Scholar]
- 35.Kulahci IG, Dornhaus A, Papaj DR. Multimodal signals enhance decision making in foraging bumble-bees. Proc R Soc B. 2008;275:797–802. doi: 10.1098/rspb.2007.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Church DL, Plowright CMS. Spatial encoding by bumblebees (Bombus impatiens) of a reward within an artificial flower array. Anim Cogn. 2006;9:131–140. doi: 10.1007/s10071-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 37.Bateson M, Kacelnik A. Risk-Sensitive Foraging: Decision Making in Variable Environments. In: Dukas R, editor. Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision making. 1998. pp. 297–341. [Google Scholar]
- 38.Saleh N, Chittka L. Traplining in bumblebees (Bombus impatiens): a foraging strategy's ontogeny and the importance of spatial reference memory in short-range foraging. Oecologia. 2007;151:719–730. doi: 10.1007/s00442-006-0607-9. [DOI] [PubMed] [Google Scholar]
- 39.Dukas R, Morse DH. Crab spiders affect flower visitation by bees. Oikos. 2003;101:157–163. [Google Scholar]
- 40.Mares S, Ash A, Gronenberg W. Brain allometry in bumblebee and honeybee workers. Brain Behav Evolut. 2005;66:50–61. doi: 10.1159/000085047. [DOI] [PubMed] [Google Scholar]