Abstract
Successful cytokinesis is critical for cell proliferation and development. In animal cells, cytokinesis relies on temporally and spatially regulated membrane addition to the cleavage site. An important source for the new membrane is recycling endosomes. Yet how these endocytic vesicles are transported and regulated remains unclear. Several potential factors have been recently identified that regulate the trafficking of recycling endosomes during cytokinesis. Dynein and dynactin are required for the retrograde transport of recycling endosomes, while Kinesin-1 is responsible for endosome delivery to the furrow and midbody. Other regulators of recycling endosome trafficking have been identified, including RACK1, JIP3/4 and ECT2, which target recycling endosomes during the cell cycle. Here, we provide insights into the mechanisms controlling endosomal trafficking during cytokinesis.
Key words: recycling endosomes, cytokinesis, membrane trafficking, RACK1, RAB11, RAB11FIP3, JIP4, ARF6, kinesin, dynein, dynactin
In animal cells, cytokinesis is driven by constriction of the actomyosin contractile ring. Membrane addition is required for the increase in cell surface area and the drastic cell shape changes during cytokinesis.1–3 The requirement of membrane trafficking in cytokinesis had long been recognized as solely associated with plant cell division, as Golgi-derived vesicles form the phragmoplast structure in the center of the cell to build a new cell wall.4 However, numerous studies over the past decade have identified membrane trafficking components as key regulators of animal cytokinesis.5–9 As the cleavage furrow ingresses, lipids and trans- membrane proteins are targeted to the ingressing furrow.10 During the final phase of cytokinesis, abscission, elaborate membrane targeting and fusion events within the midbody allow for proper daughter cell separation.11 Membrane trafficking is also necessary for local enrichment of particular signaling molecules at the cleavage furrow.12
New membrane within the cleavage furrow is thought to be derived from the Golgi compartment, namely the trans-Golgi network.13 Blocking Golgi function by Brefeldin A prevents vesicle accumulation at the furrow and inhibits the completion of cytokinesis.14,15 A recent study showed that Golgi-derived vesicles are targeted to the furrow where they fuse with the plasma membrane.16 However, Golgi is not the only source for the membrane addition during cytokinesis. Accumulating evidence suggests that endocytic recycling is important for the delivery of membrane to sites of division in several animal models, not unlike plants.17 During cellularization in Drosophila, recycling endosomes (REs) provide material for the growth of the lateral membrane.18 The Drosophila pericentrosomal proteins RAB11 and NUF (Nuclear fallout) localize to REs and are required for the dramatic remodeling of the cortical cytoskeleton as well as membrane addition.19 In mammalian cells, REs accumulate near the cleavage furrow and are required for the successful completion of cytokinesis. The delivery, targeting and fusion of REs to the furrow are controlled by RAB11 and RAB11FIP3 (also known as FIP3), which shares homology with Drosophila NUF.20 Thus, cells might use REs for the delivery of membrane to locations that are subject to dynamic reorganization. This process is likely mediated through interactions with the exocyst that is thought to recruit vesicles to areas of membrane fusion and growth.21,22
Questions still remain on how membrane trafficking events during cytokinesis are regulated. What signals target the RE vesicles to the cleavage site? What are the motors for their trafficking? In this review, we will highlight several new studies that provide a glimpse into how endocytic vesicles are likely regulated and transported during cytokinesis.
Recycling Endosomes Cluster at MTOC
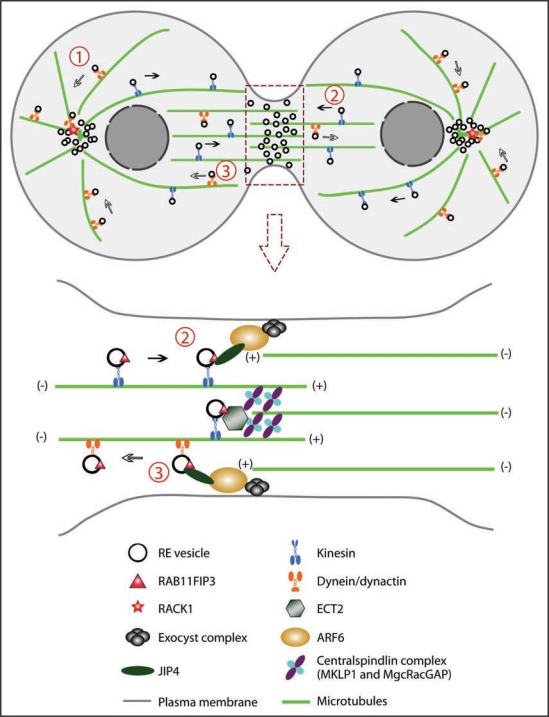
Prior to cytokinesis, various membrane organelles including recycling endosomes are clustered at the pericentrosomal region near the microtubule organizing center (MTOC), where they can target vesicles to the site of cleavage. Clustering of the endosomes is dependent on the activity of microtubule motors.23 Several data show that the minus-end-directed motor dynein associates with REs24,25 and is responsible for the retrograde trafficking of these vesicles, as overexpression of dynamitin, the p50 subunit of dynein activator dynactin, leads to scattered cytoplasmic distribution of recycling endosomes.26 Studies in C. elegans embryos also show that depletion of DNC-2, the homolog of p50/dynamitin, disrupts the pericentrosomal localization of REs and results in cytoplasmic clumps of RAB-11-labeled recycling endosomes.27 These findings support the model that dynactin mediates the minus-end-directed transport of REs by dynein.28 (Fig. 1)
Figure 1.
Model of recycling endosome (RE) trafficking during cytokinesis. (1) REs are clustered at the MTOC. The endosomes are transported along the microtubules via dynein/dynactin motors. RACK1 at the centrosomes anchors dynactin and targets REs to the centrosomes. (2) RE vesicles are directed to the cleavage site via kinesin motors. They are trafficked along both spindle microtubules and midzone microtubules. At the cleavage site these vesicles interact with ARF6 and the exocyst complex and insert membrane into the plasma membrane and the midbody. (3) RE vesicles may also be transported away from the cleavage site by dynein/dynactin. ARF6 and JIP3/4 likely serve as the switch controlling whether RE vesicles associate with kinesin or dynein.
So how does the cell know to where the RE vesicles should be trafficked? Recent work identified a known scaffolding protein, RACK-1 (Receptor for Activated C Kinase 1), as a potential factor that may serve as a localization cue for the REs. RACK-1 localizes to the centrosomes and is required for proper distribution of REs during metaphase and anaphase.27 Depletion of RACK-1 in C. elegans embryos results in a scattered localization of the REs in the cytoplasm and subsequent cytokinesis failures. Moreover, RACK-1 can directly bind to DNC-2 in vivo, providing a biochemical basis for its role in anchoring and targeting RE and its motor to the MTOC.27
RACK-1 does not seem to function alone. Being an adaptor protein, mammalian RACK1 has been shown to interact with a number of signaling molecules including PKC-betaII.29,30 Interestingly, long-term activation of PKC-βII results in sequestration of REs to the pericentrosomal region.31,32 RACK1 was identified as an anchor protein for PKC-βII that keeps it in the activated form.33 This leads to a possible model that RACK1 may anchor PKC-βII to the centrosome possibly mediating the recruitment of the REs by PKC signaling.
RE Vesicles are Trafficked to the Cleavage Site
Clusters of RE vesicles at the minus ends of microtubules (MTs) facilitate fast and robust transport of membrane to the cleavage sites. These vesicles are believed to be trafficked along microtubules, both astral MTs20,34 and midzone MTs.35,36 While dynein serves as the minus-end-directed motor for endosomes, RE vesicles are likely trafficked to the cell surface via plus-end-directed kinesins. Kinesin-1 was proposed to mediate the delivery of RE vesicles to the plasma membrane.37,38 A recent study in mammalian HeLa cells also provides evidence that Kinesin-1 is required for the trafficking of RE vesicles into the midbody region.39 In this work, they show that after knockdown of Kinesin light chain, KLC1, transferrin-labeled endosomes are not present at the intercellular bridge and are retained at the minus-ends of microtubules. The authors also demonstrate that the Kinesin-1-dependent trafficking is controlled by the interaction between the small GTPase ARF6 and the JNK-interacting proteins JIP3/JIP4.39
In order to add membrane at the right place and at the right time, the delivery of recycling endosomes to the furrow must be precisely controlled. Although the exact role of ARF6 at the cleavage furrow is not clear, it has been suggested that ARF6 may be required for the targeting of RE vesicles to the furrow.34 The exocyst complex has also been shown to target both Golgi-derived vesicles and REs to the cleavage site.22,34 Interestingly, SEC15 and SEC10, two components of the exocyst complex, bind to RAB11 and ARF6, respectively.34 More recently, the Centralspindlin complex was shown to mediate the accumulation of RE vesicles at the midbody. CYK-4/MgcRacGAP was identified to interact with RAB11FIP3. The RhoGEF ECT2 competes with RAB11FIP3 for the interaction with CYK-4/MgcRacGAP.40 It is likely that multiple mechanisms function together to ensure the proper targeting and delivery of REs to the cleavage site.
While more and more studies are trying to answer how recycling endosomes are trafficked and regulated, this field remains open with a number of questions. The dynein and dynactin complex may be responsible for the clearance of REs from the midbody region.39 Why and how are the RE vesicles recycled back from the cleavage site? Besides Kinesin-1, other kinesins have been shown to play key roles in cytokinesis.41 The Kinesin-II family member, KIF3, transports essential proteins, such as RhoA regulator p0071 (also known as plakophilin 4/PKP4), to the midbody. PKP4 has been proposed to mediate the activation of Rho locally in the midbody thus promoting successful cytokinesis.42 Thus, it is possible that other motors are responsible for the trafficking of REs during cytokinesis. Future work will need to pinpoint other motors and factors that modulate and regulate the elaborate endosomal trafficking events, which appear to be tremendously important for cytokinesis.
Acknowledgements
We are grateful to DS Poole for critically reading the manuscript. This work is supported by a National Science Foundation CAREER award (MCB-0546398) to A.R.S.
Abbreviations
- RE
recycling endosome
- MT
microtubule
- MTOC
microtubule organizing center
- C. elegans
Caenorhabditis elegans
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8931
References
- 1.Bluemink JG, de Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis I. Electron microscope observations. J Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prekeris R, Gould GW. Breaking up is hard to do—membrane traffic in cytokinesis. J Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strickland LI, Burgess DR. Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 2004;14:115–118. doi: 10.1016/j.tcb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Staehelin LA, Hepler PK. Cytokinesis in higher plants. Cell. 1996;84:821–824. doi: 10.1016/s0092-8674(00)81060-0. [DOI] [PubMed] [Google Scholar]
- 5.Echard A, Hickson GR, Foley E, O'Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggert US, Kiger AA, Richter C, Perlman ZE, Perrimon N, Mitchison TJ, et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2004;2:379. doi: 10.1371/journal.pbio.0020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian mid-body proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Schweitzer JK, D'Souza-Schorey C. Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp Cell Res. 2004;295:1–8. doi: 10.1016/j.yexcr.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.VerPlank L, Li R. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol Biol Cell. 2005;16:2529–2543. doi: 10.1091/mbc.E04-12-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeffer SR, Rothman JE. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- 14.Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baluska F, Menzel D, Barlow PW. Cytokinesis in plant and animal cells: endosomes ‘shut the door’. Dev Biol. 2006;294:1–10. doi: 10.1016/j.ydbio.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Riggs B, Rothwell W, Mische S, Hickson GR, Matheson J, Hays TS, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163:143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, et al. The FIP3Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipschutz JH, Mostov KE. Exocytosis: the many masters of the exocyst. Curr Biol. 2002;12:212–214. doi: 10.1016/s0960-9822(02)00753-4. [DOI] [PubMed] [Google Scholar]
- 22.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 24.Palmer KJ, Hughes H, Stephens DJ. Specificity of cytoplasmic dynein subunits in discrete membrane trafficking steps. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-12-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traer CJ, Rutherford AC, Palmer KJ, Wassmer T, Oakley J, Attar N, et al. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol. 2007;9:1370–1380. doi: 10.1038/ncb1656. [DOI] [PubMed] [Google Scholar]
- 26.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai E, Poole DS, Skop AR. RACK-1 directs dynactin-dependent RAB-11 endosomal recycling during mitosis in Caenorhabditis elegans. Mol Biol Cell. 2009;20:1629–1638. doi: 10.1091/mbc.E08-09-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan KT. Microtubule plus ends, motors and traffic of Golgi membranes. Biochim Biophys Acta. 2005;1744:316–324. doi: 10.1016/j.bbamcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 30.Sklan EH, Podoly E, Soreq H. RACK1 has the nerve to act: structure meets function in the nervous system. Prog Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Becker KP, Hannun YA. Isoenzyme-specific translocation of protein kinase C (PKC) betaII and not PKCbetaI to a juxtanuclear subset of recycling endosomes: involvement of phospholipase D. J Biol Chem. 2004;279:28251–28256. doi: 10.1074/jbc.M400770200. [DOI] [PubMed] [Google Scholar]
- 32.Idkowiak-Baldys J, Becker KP, Kitatani K, Hannun YA. Dynamic sequestration of the recycling compartment by classical protein kinase C. J Biol Chem. 2006;281:22321–22331. doi: 10.1074/jbc.M512540200. [DOI] [PubMed] [Google Scholar]
- 33.Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, et al. Rab11FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweitzer JK, Burke EE, Goodson HV, D'Souza-Schorey C. Endocytosis resumes during late mitosis and is required for cytokinesis. J Biol Chem. 2005;280:41628–41635. doi: 10.1074/jbc.M504497200. [DOI] [PubMed] [Google Scholar]
- 36.Albertson R, Cao J, Hsieh TS, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181:777–790. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SX, Gundersen GG, Maxfield FR. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol Biol Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans. 2008;36:391–394. doi: 10.1042/BST0360391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montagnac G, Sibarita JB, Loubery S, Daviet L, Romao M, Raposo G, et al. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19:184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 40.Simon GC, Schonteich E, Wu CC, Piekny A, Ekiert D, Yu X, et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–1803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu C, Zhao J, Bibikova M, Leverson JD, Bossy-Wetzel E, Fan JB, et al. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol Biol Cell. 2005;16:3187–3199. doi: 10.1091/mbc.E05-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keil R, Kiessling C, Hatzfeld M. Targeting of p0071 to the midbody depends on KIF3. J Cell Sci. 2009;122:1174–1183. doi: 10.1242/jcs.045377. [DOI] [PubMed] [Google Scholar]