Abstract
The formation of a multinucleated muscle fiber from individual myoblasts is a complex morphological event that requires dramatic cytoskeletal rearrangements. This multistep process includes myoblast fusion, myotube migration and elongation, myotube target recognition, and finally attachment to form a stable adhesion complex. Many of the studies directed towards understanding the developmental process of muscle morphogenesis at the cellular level have relied on forward genetic screens in model systems such as Drosophila melanogaster for mutations affecting individual stages in myogenesis. Through the analyses of these gene products, proteins that regulate the actin or microtubule cytoskeleton have emerged as important players in each of these steps. We recently demonstrated that RacGAP50C, an essential protein that functions as a cytoskeletal regulator during cell division, also plays an important role in organizing the polarized microtubule network in the elongating myotube. Here we review the current literature regarding Drosophila myogenesis and illustrate several steps of muscle development with respect to the diverse roles that the cytoskeleton plays during this process. Furthermore, we discuss the significance of cytoskeletal coordination during these multiple steps.
Key words: muscle, myotube guidance, actin, microtubule, cytoskeleton
Myoblast Fusion
The Drosophila embryonic somatic musculature forms a complex pattern underlying the epidermis of the developing embryo. There are thirty muscles per abdominal hemisegment that form a stereotypical repeated pattern. Muscles form from two myoblast populations, founder cells and fusion competent myoblasts.1 There is a founder cell for each individual muscle fiber and it contains the developmental programming required to direct the unique location, orientation, attachment sites, and size for each muscle.1–3 Each muscle undergoes a specific number of fusion events to give rise to syncitial multinucleated myotubes with 3–25 nuclei.4,5 Genetic screens in Drosophila have uncovered many genes that function in this pathway and subsequent analysis has uncovered the importance of remodeling the actin cytoskeleton during the fusion process.6–11 Specifically, confocal and ultrastructural analysis has revealed the presence of an F-actin focus (or fusion-restricted myogenic-adhesive structure) at the site of myoblast fusion (Fig. 1A).11,12 Further studies have also uncovered the important role of the actin-nucleator WASp at the point of contact between the fusion competent myoblast and the enlarging myotube.13 It is hypothesized that WASp-dependent actin-polymerization is required for the proper targeting of vesicles at the site of fusion to facilitate the fusion process.9,10 This phase of muscle development has been well studied and is the subject of several recent reviews.14–17
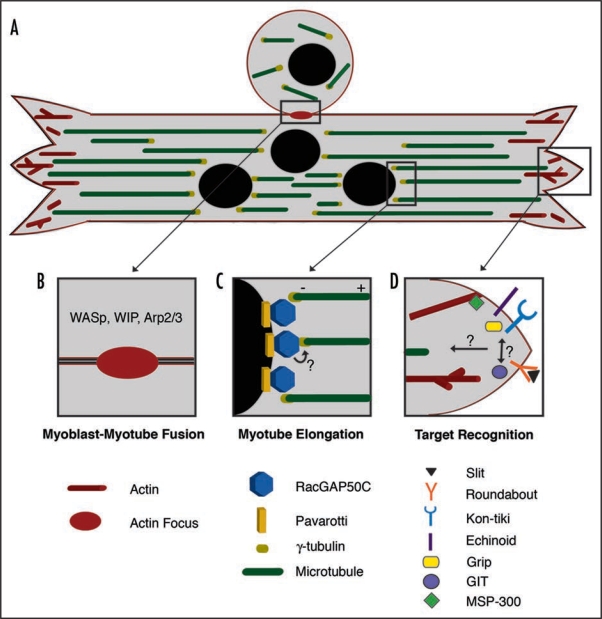
Figure 1.
Multiple roles of the cytoskeleton during Drosophila myogenesis. (A) Muscle assembly requires multiple steps, some of which occur simultaneously. Shown is a myoblast fusing to a multinucleated myotube as it elongates and searches for the proper attachment sites. The actin cytoskeleton (shown in red) is required for fusion at the central region of the myotube as well as at the myotube ends for target recognition during muscle attachment site selection. A polarized microtubule array (green) is found along the linear axis of the myotube with minus ends anchored near the nuclei, at the interior of the muscle fiber (black) and the plus ends directed outwards. (B) Myoblast-Myotube Fusion. Recent studies have shown that a dense actin focus (red oval) is critical for myoblast fusion to a multinucleated myotube. The formation of the actin focus is driven by actin regulators such as WASp, WIP and Arp2/3. (C) Myotube Elongation. Myotube elongation requires a uniform microtubule array based at the nuclear periphery. Through interaction with Pavarotti, RacGAP localizes to the nuclear periphery where it colocalizes with the microtubule nucleator protein γ-tubulin to establish a polarized microtubule array. It is not clear whether RacGAP and γ-tubulin directly interact in the myotube. (D) Target Recognition. Many molecules accumulate at myotube ends where they are required for the selection of appropriate attachment sites. These molecules include the well-known axon guidance molecules Slit and Roundabout. Kon-tiki and Echinoid are also involved in myotube target recognition, and both molecules are able to bind Grip, a downstream scaffolding protein. These molecules are required for target recognition within the same subset of myotubes, but it is unclear how they are coordinated. The Drosophila homologue of GIT was also recently implicated in target recognition. To date, common downstream signals that direct cytoskeletal rearrangements required for target recognition remain largely unknown. MSP-300 may be one molecule that mediates actin dynamics at the plasma membrane.
Myotube Attachment Site Selection: The Role of the Actin Cytoskeleton
Remodeling of the actin cytoskeleton is important not only for the morphological changes required for the cell-cell recognition and fusion between myoblasts, but also has been implicated in the precise matching between myotubes and their attachment sites. During Drosophila myogenesis, the multinucleated myotube elongates in two directions along a linear axis towards its specific sites of attachment in the epidermis, called tendon cells.1,18 At the ends of the myotube, extensive filopodia, similar to neuronal growth cones, search the environment for their attachment sites.1,19 Migrating myotubes have been shown to respond to signals from the tendon cells, which are specified by the Stripe transcription factor and induce attraction and adhesion of the approaching myotube.20–23
The identification of the first molecules known to guide muscles to their target sites came from studies in Drosophila, which revealed that the axon guidance molecules Derailed (Drl) and Roundabout (Robo) also played a role in guiding muscle fibers.24–26 These cell surface receptors were found to function cell autonomously in specific muscle subsets to sense guidance cues, much in the same way as axons do. Drl is expressed both in the lateral transverse (LT) muscles as well as the intrasegmental tendon cells to which they attach. In drl mutants, the LT myotubes extend past their normal tendon cell attachment sites25 suggesting that the Drl pathway is required in the myotube to interpret a stop signal from the tendon cells. In a similar manner, Robo is expressed at the ends of a subset of myotubes where it functions as the receptor for Slit, an attractive guidance cue secreted from segment border tendon cells.26 The downstream signaling pathways through which Robo and Drl function in the myotube have not been fully examined. However, based on the function of these proteins during axon guidance,27–30 it is highly likely that Robo and Drl function to remodel the actin network in order to facilitate directional motility and/or cell adhesion at the leading edge of the myotube. Both Drl and Robo appear to function primarily as repulsive guidance receptors during axon guidance,24,31 where as in myotubes, they appear to function as positive signals to promote myotube-tendon cell interactions.25,26 It is not yet clear whether these opposing functions are transduced to the actin cytoskeleton via similar or distinct signal transduction pathways.
More recent studies have identified additional molecules that function in attachment site selection including DGrip, and its interacting proteins Kon-tiki (Kon; also known as Perdido) and Echinoid (Ed).32–35 Kon is a large transmembrane protein that concentrates at muscle tips and is required for a specific subset of ventral myotubes to recognize their tendon cell targets.33 Kon was shown to interact with tendon-cell expressed PS1 integrin heterodimers.32 Together these studies suggest a mechanism for myotube-tendon cell recognition via a Kon-Integrin interaction. Mutants for the PDZ-domain protein Grip (for glutamate receptor interacting protein) have a similar phenotype to kon mutants,34 and subsequent studies showed that Kon was required to localize Grip to the myotube plasma membrane via its cytoplasmic domain.32,33 This interaction suggests that these proteins form a signaling complex during attachment site selection, although the intracellular mechanisms underlying this process remain unclear. One clue has come from studies that show that Grip also interacts with the cell adhesion molecule Echiniod,35 which has been shown to be a component of adherens junctions and also serves as a link to the actin cytoskeleton via binding to the PDZ protein Canoe, the Drosophila counterpart to mammalian AF-6 and Afadin.36,37 While there is no evidence to date showing that this interaction is important for regulating the actin cytoskeleton within myotubes, it may explain how Ed and Grip promote filopodia formation and muscle motility. Consistent with this idea, Kon and Grip cause ectopic projections and excessive filopodia formation when overexpressed in myotubes, supporting a role for these proteins in locally controlling membrane adhesiveness by modulation of the actin cytoskeleton at the myotube ends.33,35
Recently, dGIT, the Drosophila homologue for the GTPase activating protein GIT1, was identified as an additional protein required for muscle attachment site selection.38 Together with the proteins PIX and PAK, dGIT has been shown to play an important role in the remodeling of the actin cytoskeletal architecture during the regulation of cell motility, morphogenesis and cell-cell contacts.39–41 In Drosophila, dGIT was shown to function in complex with PAK to regulate muscle morphogenesis and guidance of a subset of ventral muscles.38 Mutations in dGit show a ventral bypass phenotype, suggesting that these embryos have defects in attachment site recognition, once the elongating myotubes reach their target sites.
Studies of Drl, Robo, Grip, Kon, Ed and dGIT have identified molecules required at the muscle ends for the accurate matching of specific subsets of myotubes to their target attachment sites. Extensive study of axonal guidance suggests that guidance information is interpreted by the growth cone via signaling pathways that control the actin cytoskeleton at the leading edge27,29,30 and it is highly likely that myotubes find their targets via a similar actinbased mechanism (Fig. 1D). The presence of actin-rich filopodia at the myotube ends further supports this hypothesis.19,42
How are external guidance signals transduced to the actin cytoskeleton in myotubes? The specific signaling pathways that transduce external guidance signals to the actin cytoskeleton in myotubes have not been well described. In the case of the axon guidance molecules Robo and Drl, similar signaling pathways may be utilized to mediate responses in both growth cones and the ends of myotubes. Alternatively, myotubes may utilize novel effector proteins to communicate with the cytoskeleton. One molecule that may participate in mediating the cytoskeletal response to guidance cues in the myotube is the Drosophila protein MSP-300. MSP-300 decorates actin filaments in cultured myotubes and is specifically enriched at sites where actin filaments are closely associated with the plasma membrane.43 Embryos mutant for MSP-300 show defects in myotube extension suggesting that MSP-300 is required to mediate the proper extension of the myotube towards its epidermal attachment site.44 MSP-300 is expressed in all myotubes during the stage of attachment site selection.43 Further study will help to determine whether MSP-300 functions downstream of any of the known myotube guidance molecules we describe here.
Microtubules and Myotube Guidance
As reviewed above, studies have revealed that many of the genes required for both myoblast fusion and attachment site selection have been shown to regulate the actin cytoskeleton, suggesting that both myoblast fusion and myotube-tendon cell target recognition are primarily actin-based processes. But what about the role of the microtubule (MT) network during myogenesis? Clues about this process have come primarily from cell culture studies, which have revealed an abundance of MTs in the long axis of young myotubes suggesting that these cytoskeletal elements may be required for elongation of the muscle fiber during development.45 Furthermore, it was shown that in cultured cells, the MT cytoskeleton is completely reorganized during myogenesis from a radial, centrosomal array in individual myoblasts to a linear, non-centrosomal array in multinucleated myotubes46,47 and that microtubule re-growth from sites in the cytoplasm and nuclear periphery in the myotube is closely associated with γ-tubulin, a known microtubule nucleator.47–49
In Drosophila embryos, this linear array of MTs was also observed using a minus-ended MT marker which showed that the MT network is polarized with minus ends towards the interior of the myotube and plus ends directed outwards.50 However, until very recently, the in vivo significance of the linear and polarized MT array for Drosophila myotube guidance had not been examined. In a recent study, we describe a novel role for RacGAP50C (RacGAP) (also know as Tumbleweed) in organizing the MT array in migrating myotubes42 (Fig. 1C). In embryos mutant for RacGAP, γ-tubulin is not properly localized, resulting in myotubes with non-uniform MT polarity. As a result, the myotubes are not able to properly extend towards their attachment sites and display abnormal morphology. RacGAP co-localizes with γ-tubulin in vivo, although a direct association between these two proteins has not yet been established. However, the vertebrate counterpart of RacGAP, MgcRacGAP, was shown to associate with γ-tubulin in vitro,51 which suggests that this binding activity may be conserved.
Our study provides the first in vivo evidence for the role of polarized MTs in promoting myotube elongation in the long axis of the myotube. The myotube is an unusually elongated cell in comparison to other cell types and so it is not surprising that it would rely on the MT cytoskeleton to maintain its unique shape during extension. The assembly of a polarized MT array in the long axis of the myotube may help restrict elongation to a single axis of the myotube. One question that remains to be answered is whether this axis is predetermined during myoblast fusion, prior to extension, or whether it is set up in the multinucleated myotube in response to extracelluar guidance cues. Close examination of the myotube guidance mutants Drl and dGit suggests that this axis is at least in part predetermined prior to myotube target recognition. For example, one hallmark of many of the muscle phenotypes in embryos mutant for Drl or dGit is the “by-pass phenotype,” where myotubes are capable of elongating but migrate past their normal sites of attachment.25,38 These findings reveal that in the context of muscle development, Drl and dGit function primarily in mediating the proper matching of specific subsets of myotubes with their attachment sites.
Coordination of the Actin and MT Cytoskeletons during Myogenesis
Breaking down muscle morphogenesis into discrete cellular processes has allowed for the identification of molecules that function specifically in myoblast fusion, myoblast elongation and target recognition. However muscle development is not a strictly sequential process. For example, myotubes begin to extend filopodia at their ends while fusion continues to occur at the center of the fiber to add to the size of the extending myotube4,19 (Fig. 1A). Further support for the separation between myoblast fusion and myotube migration comes from the observation that founder cells can extend and make attachments even when myoblast fusion is blocked.52 However, there must be some regulatory mechanism present to prevent fusion events from interfering with the migratory machinery. How is the actin cytoskeleton regulated to allow for two different activities at once? One possibility is that that the myotube simultaneously utilizes two distinct pathways for regulation of actin dynamics. For example, for myoblast fusion, F-actin polymerization at myoblast-myotube fusion sites was shown to be dependant on the WASp protein,9,10,13 which regulates actin nucleation via the Arp2/3 complex.53,54 Conversely, recent studies which have identified key components of the signaling pathways downstream of the guidance and cell adhesion molecules Robo and Ed shown a potential connection to the actin cytoskeleton via alternative pathways which include the Ena/VASP and Canoe/Af-6/Afadin family of proteins.27,36,55 Alternatively, simultaneous regulation of actin dynamics in the myotube could be a simple matter of spatial restriction. It is known that the fusion of additional myoblasts to the myotube occurs primarily at the interior region of the myotube, while actin-rich filopodia are limited to the growing ends. The polarity of the myotube, which is set up by the MT network may play an important role in ensuring this spatial restriction.
Finally, another matter that remains unclear is the amount of crosstalk that occurs between the actin and MT networks in the myotube. The myotube is a large, elongated and polarized cell with multiple nuclei that is simultaneously fusing with myoblasts to increase its size while elongating at its ends. Due to these unique features, it may be possible that the myotube has utilized different cytoskeletal elements to perform specific tasks. For example, polarized elongation of the myotube along a linear axis may be primarily MT based, while actin filopodia at the myotube ends may be utilized specifically seek attachment sites. However, although the respective roles of the actin and MTs in myogenesis appear to be well defined, their functions are also very likely to be overlapping.
What are some examples of the known molecules that mediate crosstalk between the actin and MT cytoskeleton? The vertebrate Shroom family of actin-binding proteins have been recently shown to regulate both actin and MTs for apicobasal cell elongation in epithelium.56 A Drosophila counterpart to Shroom has been identified,57 but its role in myogenesis has not yet been addressed. Other proteins known to interact with both the actin and microtubule cytoskeletons include Myosin II (Zipper in Drosophila) as well as the APC2 and Diaphanous/Formin proteins.58–61 Interestingly, RacGAP has also been shown to link the actin and MT cytoskeletons. RacGAP is best known for its role in cytokinesis, where it was shown to interact with Pavarotti (Pav), a plus-end MT motor to facilitate bundling of spindle MTs.62–65 RacGAP also binds to Anillin, an actin-binding protein, and serves to provide a link between the microtubule central spindle and the actomyosin contractile ring for cleavage furrow formation during cell division.66–68 In our study, mutations that removed the MT binding protein Pav also disrupted myotube guidance in a manner similar to RacGAP mutants, while a mutation in scraps, which encodes Drosophila Anillin, showed normal muscle patterning. Furthermore, RacGAP mutants were not defective in myoblast fusion, an actin based process, and myotubes were able to form filopodia at their ends.42 Together, these findings suggest that the actin machinery required for fusion and target recognition is not completely disrupted in RacGAP mutants and that RacGAP's function in myotubes may be limited to regulating the MT network for myotube elongation via γ-tubulin. Alternatively, in ΡαχΓΑΠ mutants, the link between the actin and MT cytoskeletons could be lost, which may prevent coordination of the actin and MT cytoskeletons during elongation. Continued investigation of the mechanisms by which molecules such as RacGAP function during myogenesis and the identification of new factors that regulate the cytoskeleton, will shed light on this important topic.
Acknowlegments
This work was supported by National Institutes of Health Grant ARO54482 to S.G.K.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/9158
References
- 1.Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- 2.Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Gomez M, Romani S, Hartmann C, Jackle H, Bate M. Specific muscle identities are regulated by Kruppel during Drosophila embryogenesis. Development. 1997;124:3407–3414. doi: 10.1242/dev.124.17.3407. [DOI] [PubMed] [Google Scholar]
- 4.Bate M, Rushton E. Myogenesis and muscle patterning in Drosophila. C R Acad Sci III. 1993;316:1047–1061. [PubMed] [Google Scholar]
- 5.Beckett K, Baylies MK. 3D analysis of founder cell and fusion competent myoblast arrangements outlines a new model of myoblast fusion. Dev Biol. 2007;309:113–125. doi: 10.1016/j.ydbio.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Gomez M, Coutts N, Price A, Taylor MV, Bate M. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell. 2000;102:189–198. doi: 10.1016/s0092-8674(00)00024-6. [DOI] [PubMed] [Google Scholar]
- 7.Bour BA, Chakravarti M, West JM, Abmayr SM. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 2000;14:1498–1511. [PMC free article] [PubMed] [Google Scholar]
- 8.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 9.Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesper DA, Stute C, Buttgereit D, Kreiskother N, Vishnu S, Fischbach KF, et al. Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS) Dev Dyn. 2007;236:404–415. doi: 10.1002/dvdy.21035. [DOI] [PubMed] [Google Scholar]
- 13.Schafer G, Weber S, Holz A, Bogdan S, Schumacher S, Muller A, et al. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev Biol. 2007;304:664–674. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Richardson B, Beckett K, Baylies M. Visualizing new dimensions in Drosophila myoblast fusion. Bioessays. 2008;30:423–431. doi: 10.1002/bies.20756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abmayr SM, Zhuang S, Geisbrecht ER. Myoblast fusion in Drosophila. Methods Mol Biol. 2008;475:75–97. doi: 10.1007/978-1-59745-250-2_5. [DOI] [PubMed] [Google Scholar]
- 16.Peckham M. Engineering a multi-nucleated myotube, the role of the actin cytoskeleton. J Microsc. 2008;231:486–493. doi: 10.1111/j.1365-2818.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 17.Onel SF, Renkawitz-Pohl R. FuRMAS: triggering myoblast fusion in Drosophila. Dev Dyn. 2009;238:1513–1525. doi: 10.1002/dvdy.21961. [DOI] [PubMed] [Google Scholar]
- 18.Volk T, VijayRaghavan K. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development. 1994;120:59–70. doi: 10.1242/dev.120.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Schnorrer F, Dickson BJ. Muscle building; mechanisms of myotube guidance and attachment site selection. Dev Cell. 2004;7:9–20. doi: 10.1016/j.devcel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Becker S, Pasca G, Strumpf D, Min L, Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–2622. doi: 10.1242/dev.124.13.2615. [DOI] [PubMed] [Google Scholar]
- 21.Vorbruggen G, Jackle H. Epidermal muscle attachment site-specific target gene expression and interference with myotube guidance in response to ectopic stripe expression in the developing Drosophila epidermis. Proc Natl Acad Sci USA. 1997;94:8606–8611. doi: 10.1073/pnas.94.16.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JC, VijayRaghavan K, Celniker SE, Tanouye MA. Identification of a Drosophila muscle development gene with structural homology to mammalian early growth response transcription factors. Proc Natl Acad Sci USA. 1995;92:10344–10348. doi: 10.1073/pnas.92.22.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frommer G, Vorbruggen G, Pasca G, Jackle H, Volk T. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 1996;15:1642–1649. [PMC free article] [PubMed] [Google Scholar]
- 24.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 25.Callahan CA, Bonkovsky JL, Scully AL, Thomas JB. derailed is required for muscle attachment site selection in Drosophila. Development. 1996;122:2761–2767. doi: 10.1242/dev.122.9.2761. [DOI] [PubMed] [Google Scholar]
- 26.Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- 27.Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 28.Callahan CA, Muralidhar MG, Lundgren SE, Scully AL, Thomas JB. Control of neuronal pathway selection by a Drosophila receptor protein-tyrosine kinase family member. Nature. 1995;376:171–174. doi: 10.1038/376171a0. [DOI] [PubMed] [Google Scholar]
- 29.Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–127. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- 30.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- 31.Bonkowsky JL, Yoshikawa S, O'Keefe DD, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402:540–544. doi: 10.1038/990122. [DOI] [PubMed] [Google Scholar]
- 32.Estrada B, Gisselbrecht SS, Michelson AM. The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–4478. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- 33.Schnorrer F, Kalchhauser I, Dickson BJ. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev Cell. 2007;12:751–766. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Swan LE, Wichmann C, Prange U, Schmid A, Schmidt M, Schwarz T, et al. A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev. 2004;18:223–237. doi: 10.1101/gad.287604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swan LE, Schmidt M, Schwarz T, Ponimaskin E, Prange U, Boeckers T, et al. Complex interaction of Drosophila GRIP PDZ domains and Echinoid during muscle morphogenesis. EMBO J. 2006;25:3640–3651. doi: 10.1038/sj.emboj.7601216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahri SM, Choy JM, Manser E, Lim L, Yang X. The Drosophila homologue of Arf-GAP GIT1, dGIT, is required for proper muscle morphogenesis and guidance during embryo- genesis. Dev Biol. 2009;325:15–23. doi: 10.1016/j.ydbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucanic M, Cheng HJ. A RAC/CDC-42-independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans. PLoS Genet. 2008;4:1000269. doi: 10.1371/journal.pgen.1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerin CM, Kramer SG. RacGAP50C directs perinuclear {gamma}-tubulin localization to organize the uniform microtubule array required for Drosophila myotube extension. Development. 2009;136:1411–1421. doi: 10.1242/dev.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volk T. A new member of the spectrin superfamily may participate in the formation of embryonic muscle attachments in Drosophila. Development. 1992;116:721–730. doi: 10.1242/dev.116.3.721. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg-Hasson Y, Renert-Pasca M, Volk T. A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech Dev. 1996;60:83–94. doi: 10.1016/s0925-4773(96)00602-8. [DOI] [PubMed] [Google Scholar]
- 45.Saitoh O, Arai T, Obinata T. Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 1988;252:263–273. doi: 10.1007/BF00214368. [DOI] [PubMed] [Google Scholar]
- 46.Tassin AM, Maro B, Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biol. 1985;100:35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bugnard E, Zaal KJ, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil Cytoskel. 2005;60:1–13. doi: 10.1002/cm.20042. [DOI] [PubMed] [Google Scholar]
- 48.Cottam DM, Tucker JB, Rogers-Bald MM, Mackie JB, Macintyre J, Scarborough JA, et al. Non-centrosomal microtubule-organising centres in cold-treated cultured Drosophila cells. Cell Motil Cytoskel. 2006;63:88–100. doi: 10.1002/cm.20103. [DOI] [PubMed] [Google Scholar]
- 49.Musa H, Orton C, Morrison EE, Peckham M. Microtubule assembly in cultured myoblasts and myotubes following nocodazole induced microtubule depolymerisation. J Muscle Res Cell Motil. 2003;24:301–308. [PMC free article] [PubMed] [Google Scholar]
- 50.Clark IE, Jan LY, Jan YN. Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development. 1997;124:461–470. doi: 10.1242/dev.124.2.461. [DOI] [PubMed] [Google Scholar]
- 51.Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- 52.Rushton E, Drysdale R, Abmayr SM, Michelson AM, Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- 53.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 54.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, et al. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5:1147–1154. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]
- 56.Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–1441. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- 57.Hagens O, Ballabio A, Kalscheuer V, Kraehenbuhl JP, Schiaffino MV, Smith P, et al. A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol. 2006;7:18. doi: 10.1186/1471-2121-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 59.McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pawson C, Eaton BA, Davis GW. Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J Neurosci. 2008;28:11111–11123. doi: 10.1523/JNEUROSCI.0833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 63.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 64.Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zavortink M, Contreras N, Addy T, Bejsovec A, Saint R. Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J Cell Sci. 2005;118:5381–5392. doi: 10.1242/jcs.02652. [DOI] [PubMed] [Google Scholar]
- 67.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 68.D'Avino PP, Takeda T, Capalbo L, Zhang W, Lilley KS, Laue ED, et al. Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J Cell Sci. 2008 doi: 10.1242/jcs.026716. [DOI] [PubMed] [Google Scholar]