Abstract
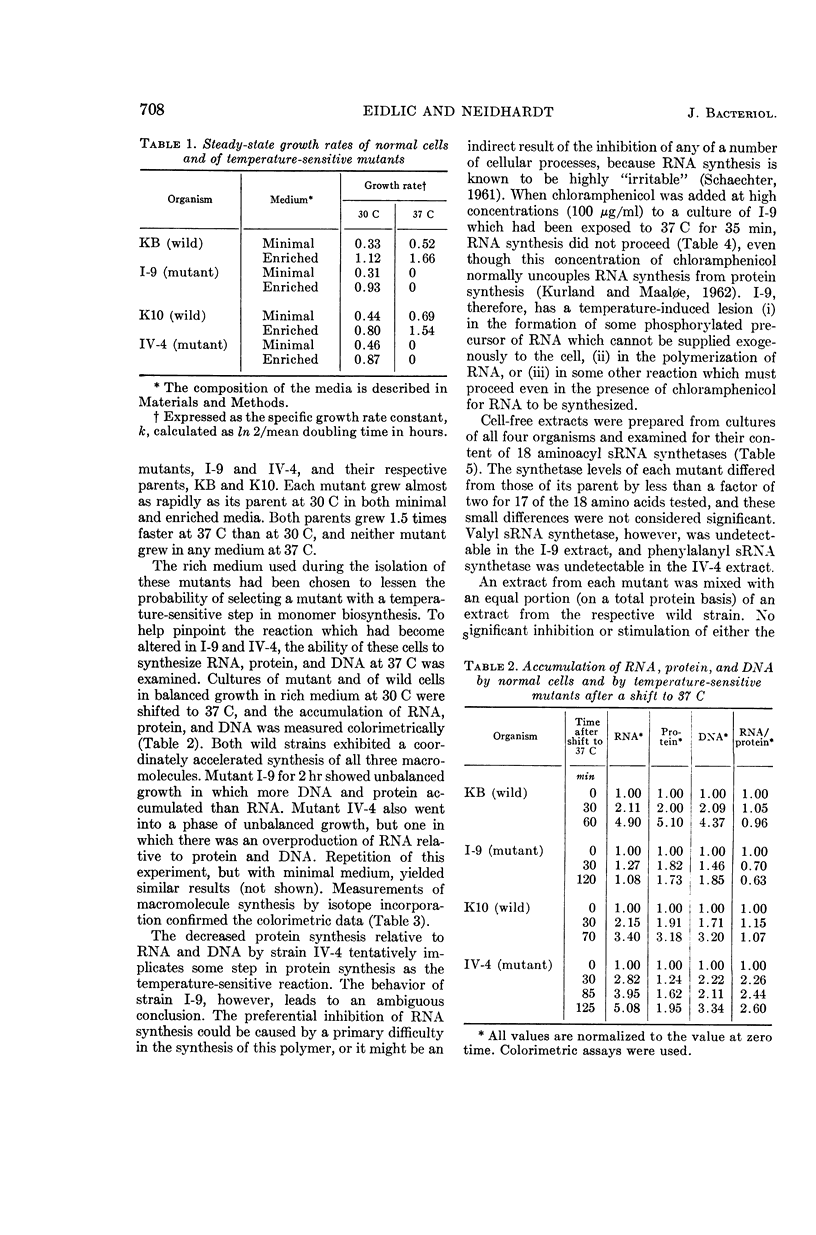
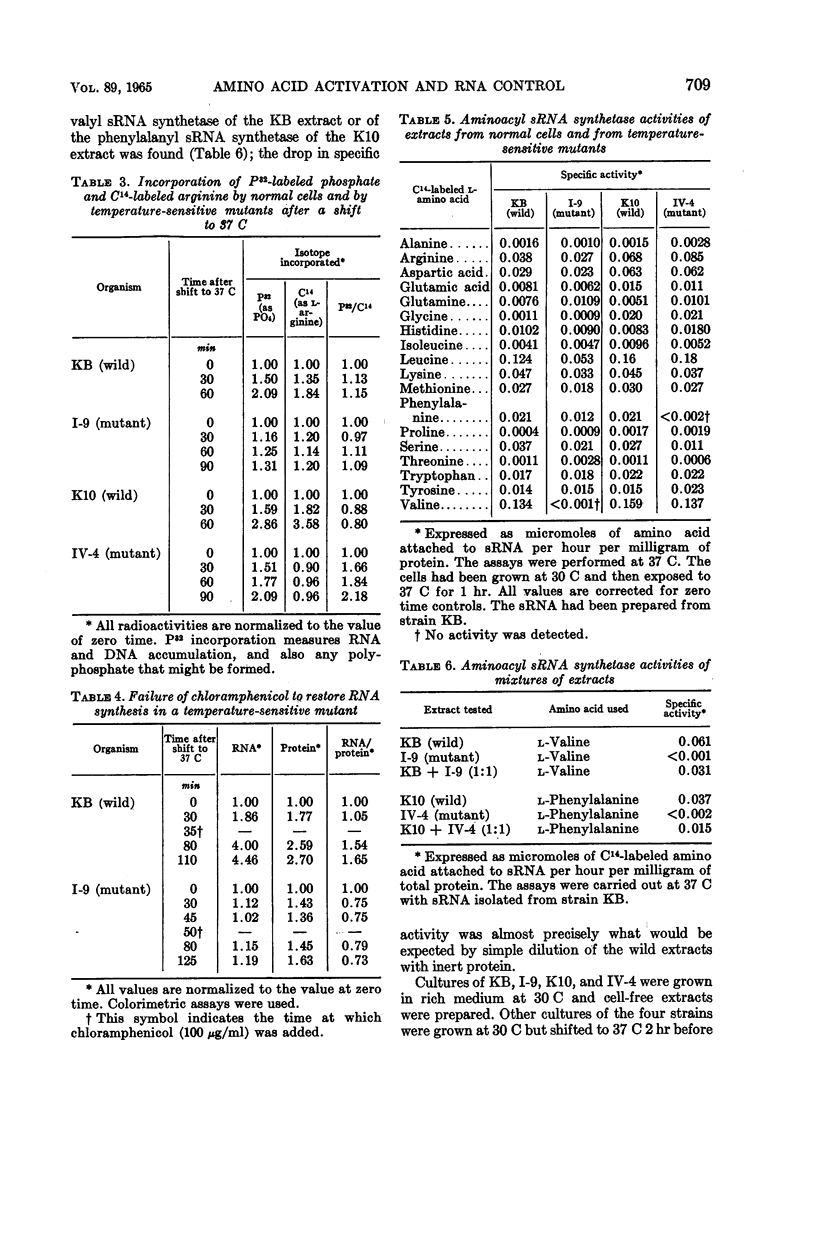
Eidlic, Lia (Purdue University, Lafayette, Ind.), and Frederick C. Neidhardt. Protein and nucleic acid synthesis in two mutants of Escherichia coli with temperature-sensitive aminoacyl ribonucleic acid synthetases. J. Bacteriol. 89:706–711. 1965.—Two temperature-sensitive mutants of Escherichia coli were isolated which grow almost normally at 30 C and fail to grow at 37 C. One (I-9) was derived from a strain with stringent amino acid control of ribonucleic acid (RNA) synthesis; the other (IV-4) was derived from a strain with relaxed amino acid control of RNA synthesis. When cultures of these mutants growing at 30 C were shifted to 37 C, IV-4 synthesized RNA preferentially to protein but I-9 did not. Cell-free extracts of both mutants and their parent strains were examined for their ability to catalyze adenosine triphosphate (ATP)-dependent attachment of amino acids to soluble RNA (sRNA). These measurements indicated that I-9 possesses a temperature-sensitive valyl sRNA synthetase, and that IV-4 possesses a temperature-sensitive phenylalanyl sRNA synthetase. The behavior of these mutants suggests that amino acids permit RNA synthesis in stringent strains only after activation or attachment to sRNA, that relaxed strains can overproduce RNA without a complete array of fully functioning aminoacyl sRNA synthetases, and that these enzymes are obligatory for the biosynthesis of proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H. Intracellular charging of soluble ribonucleic acid in Escherichia coli subjected to isoleucine starvation and chloramphenicol treatment. Biochem Biophys Res Commun. 1964;14:64–68. doi: 10.1016/0006-291x(63)90212-2. [DOI] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. PROTEIN AND RIBONUCLEIC ACID SYNTHESIS IN A MUTANT OF ESCHERICHIA COLI WITH AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE. J Biol Chem. 1964 Jun;239:1844–1847. [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg J, Cavalli L L, Lederberg E M. Sex Compatibility in Escherichia Coli. Genetics. 1952 Nov;37(6):720–730. doi: 10.1093/genetics/37.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C. The regulation RNA synthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1964;3:145–181. doi: 10.1016/s0079-6603(08)60741-2. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M. Patterns of cellular control during unbalanced growth. Cold Spring Harb Symp Quant Biol. 1961;26:53–62. doi: 10.1101/sqb.1961.026.01.011. [DOI] [PubMed] [Google Scholar]
- SIMPSON M. V. Protein biosynthesis. Annu Rev Biochem. 1962;31:333–368. doi: 10.1146/annurev.bi.31.070162.002001. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., BOURGEOIS S., GROS F. Inhibition of RNA polymerase by RNA. J Mol Biol. 1963 Jul;7:100–103. doi: 10.1016/s0022-2836(63)80024-8. [DOI] [PubMed] [Google Scholar]


