Abstract
The α6 nicotinic acetylcholine receptor (nAChR) subunit is involved in nicotine-stimulated dopamine release in the striatum. It is expressed in brain regions and coexpressed with nAChR subtypes implicated in nicotine dependence behaviors; hence, this subunit may play a role in nicotine dependence. Using the α6-selective antagonist α-conotoxin H9A;L15A (MII[H9A;L15A]), we determined the role of α6* nAChRs in the pharmacological and behavioral effects of nicotine. We measured effects of pretreatment with MII[H9A;L15A] on analgesia, locomotion, and body temperature after a single injection of nicotine. Effects of MII[H9A;L15A] on nicotine reward were measured using the conditioned place preference (CPP) paradigm. We further measured physical (somatic signs and hyperalgesia) and affective [anxiety-related behavior and conditioned place aversion (CPA)] nicotine withdrawal behaviors after extended nicotine exposure. Results showed that MII[H9A;L15A] did not block acute nicotine effects on the behaviors measured. Conversely, MII[H9A:l15A] blocked the expression of nicotine CPP, as well as withdrawal-associated CPA and anxiety-related behavior in the elevated plus maze, but not withdrawal-induced somatic signs or hyperalgesia. These results suggest a role for the α6 nAChR subunit in nicotine reward and affective nicotine withdrawal but not acute nicotine-induced or physical withdrawal behaviors.
The neurotransmitter dopamine plays a vital role in the rewarding and reinforcing effects of nicotine (Dani and Heinemann, 1996). Nicotine induced increases in ventral tegmental area (VTA) dopaminergic neuron firing rate (Grenhoff et al., 1986), and subsequent dopamine release in the nucleus accumbens (NAc) is a process thought to underlie the rewarding and reinforcing properties of nicotine (Pontieri et al., 1996). Alternatively, studies report decreased dopamine neuronal activity in the VTA (Liu and Jin, 2004) and decreased dopamine output in the NAc after nicotine withdrawal (Hildebrand et al., 1998; Rada et al., 2001).
The α4α6β2* nicotinic acetylcholine receptor (nAChR) subtype (where * denotes possible assembly with other nicotinic receptor subunits) is involved in nicotine-stimulated dopamine release in the striatum (Champtiaux et al., 2003; Salminen et al., 2004; Lai et al., 2005). Expression of α6-containing nAChRs in the brain is largely confined to catecholaminergic nuclei, such as the VTA, substantia nigra, and locus coeruleus (LC) (le Novère et al., 1996; Klink et al., 2001), brain areas that have also been implicated as having a role in mediating behaviors associated with drugs of abuse, including nicotine and morphine (Laviolette and van der Kooy, 2003; Bruijnzeel and Markou, 2004; Dizgah et al., 2005). The α6 nAChR subunit was shown to play a role in the locomotor-stimulating effects of nicotine in rats, an effect suggested to be due to enhanced mesolimbic dopamine transmission (Benwell and Balfour, 1992; le Novère et al., 1999; Drenan et al., 2008). Recent evidence suggests that recovery of the α6 nAChR subunit in the VTA of α6 nAChR knockout mice is sufficient for tail-vein self-administration (Pons et al., 2008). Our studies expand on this research to examine α6* nAChR contributions to the pharmacological and behavioral effects of nicotine. Using the α6-selective nAChR antagonist α-conotoxin H9A;L15A (MII[H9A;L15A]) (McIntosh et al., 2004), the role of α6 subunit-containing nAChRs was examined in the initial acute effects of nicotine, nicotine reward using the conditioned place preference (CPP) paradigm, and physical (somatic signs and hyperalgesia) and affective [anxiety-related behavior and conditioned place aversion (CPA)] nicotine withdrawal measures.
Materials and Methods
Animals
Male C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME) were used for the studies. Animals were 8 to 10 weeks of age at the start of the studies and were group-housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility, with ad libitum access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (Richmond, VA).
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl)pyrrolidine (+)-bitartrate salt] and mecamylamine hydrochloride [2-(methylamino) isocamphane hydrochloride] were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously at a volume of 10 ml/kg body weight. Nicotine doses are expressed as the free base of the drug. The α6*-selective antagonist MII[H9A;L15A] was synthesized as described in McIntosh et al. (2004). The compound was dissolved in physiological saline (0.9% sodium chloride) and administered to each animal by intracerebroventricular injection. The highest dose for intracerebroventricular injections in mice (6 pmol of MII[H9A;L15A]) was calculated based on the functional IC50 values of MII[H9A;L15A] at nAChRs (McIntosh et al., 2004).
Intracerebroventricular Surgery
On the evening before testing, mice were anesthetized with sodium pentobarbital (45 mg/kg i.p.), and a scalp incision was made to expose bregma and prepare an injection sight. A 26-gauge needle with a sleeve of polyurethane tubing to control depth of the needle at 2 mm ventral to the skull was used to make a hole in the skull at a site 2 mm rostral and 2 mm lateral to the bregma. Animals were loosely sutured, leaving the injection site accessible with gentle displacement of the scalp. Ten minutes before testing, 1% procaine topical anesthetic was applied to the scalp of mice, and animals were gently restrained for approximately 30 s during intracerebroventricular drug delivery. At the end of the study, a subset of animals were injected intracerebroventricularly with 5 μl of cresyl violet dye 10 min before a lethal overdose of 45 mg/kg sodium pentobarbital and perfused to confirm drug diffusion from the injection site to the lateral ventricle. Brain slices were collected, and mice were observed to have blue cresyl violet dye in both lateral ventricles.
Acute Nicotine Assessment
Naive male mice were injected subcutaneously with nicotine and tested for antinociception using the tail-flick and hot-plate tests, spontaneous activity, and body temperature. Groups of 8 to 10 mice were used for each dose and test. Dose-response curves were generated previously (data not shown), and doses for each test were chosen based on an 85% maximal response.
Behavioral Tests
Tail-Flick Test.
Mice were tested 5 min after a subcutaneous injection of nicotine at 2.5 mg/kg. Mice were lightly restrained by hand while a radiant heat light source was shone onto the upper portion of the tail. Latency to remove the tail from the heat source was recorded for each animal. A control response (2–4-s latency) was determined for each mouse before treatment, and test latency was determined after drug administration. To minimize tissue damage, a maximal latency of 10 s was imposed. Antinociceptive response was calculated as percentage of maximal possible effect (%MPE) = [(test value − baseline)/(cut-off time (10 s) − control value)] × 100, where baseline represents the value before nicotine.
Hot-Plate Test.
Mice were tested 2 h before and 5 min after an injection of nicotine (2 mg/kg s.c.). The animals were placed on a 55°C platform (Harvard Apparatus Inc., Holliston, MA) and were observed until they started to show pain avoidance behavior such as jumping or licking of the paws. Animals that did not respond to the noxious heat stimulus after 40 s were removed from the plate. Latency to pain avoidance measured in seconds was used to calculate %MPE, with the following equation: [(test value − baseline)/(cut-off time (40 s) − baseline)] × 100. Baseline latency that lasted 8 to 12 s was assessed with a saline injection. Antagonism studies for antinociception tests were carried out by pretreating the mice with either vehicle or MII[H9A;L15A] (6 pmol i.c.v.) 10 min before nicotine.
Locomotor Activity.
Mice were placed into individual Omnitech photocell activity cages (28 × 16.5 cm; Omnitech Electronics, Columbus, OH) 5 min after subcutaneous administration of either saline or nicotine (1.5 mg/kg). Antagonism studies were carried out by pretreating the mice with either vehicle or MII[H9A;L15A] (6 pmol i.c.v.) 10 min before nicotine. Interruptions of the photocell beams (two banks of eight cells each) were then recorded for the next 10 min. Data are expressed as number of photocell interruptions.
Body Temperature.
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH). Readings were taken just before and at 30 min after the subcutaneous injection of either saline or nicotine (1.5 mg/kg). Antagonism studies were carried out by pretreating the mice with either vehicle or MII[H9A;L15A] (6 pmol i.c.v.) 10 min before nicotine. The difference in rectal temperature (Δ°C) before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from day to day (21–24°C).
Nicotine CPP Assessment
An unbiased CPP paradigm was used in this study as described in Kota et al. (2007). In brief, place conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period, and then they were allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2 to 4 were the conditioning days during which the saline group received saline in both compartments and drug groups received nicotine (0.5 mg/kg s.c.) in one compartment and saline in the opposite compartment. The dose of nicotine was chosen based on previous CPP studies from Walters et al. (2006), which showed optimal preference at this dose in C57BL/6 mice. Drug-paired compartments were randomized among all groups.
MII[H9A;L15A] Assessment
Approximately 2 h after the final conditioning session on day 4, mice underwent intracerebroventricular surgery. On day 5, mice were injected intracerebroventricular with either vehicle or MII[H9A;L15A]) (1.5, 4.5, or 6 pmol) 10 min before being placed in the center habituation compartment. During testing, as on day 1, mice were allowed to move freely between all three compartments for 15 min. Activity counts and time spent on each side were recorded via photosensors using MED Associates (St. Albans, VT) interface and software. Data were expressed as time spent on drug-paired side minus time spent on saline-paired side. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
Chronic Nicotine Administration Protocol
Mice were anesthetized with sodium pentobarbital (45 mg/kg i.p.) and implanted with Alzet osmotic mini pumps [model 2002 (14 days, for withdrawal studies) or model 2004 (28 days, for CPA studies); Durect Corporation, Cupertino, CA] filled with (−)-nicotine or saline solution as described in Jackson et al. (2008). The concentration of nicotine was adjusted according to animal weight and the mini pump flow rate, resulting in 36 mg/kg/day for 14 or 28 days.
Nicotine Withdrawal Assessment
Withdrawal studies were conducted as described previously (Jackson et al., 2008). In brief, mini pumps were removed on day 14, and testing was initiated on day 15, approximately 18 to 24 h after mini pump removal. Mice were injected intracerebroventricularly with MII[H9A;L15A], and withdrawal signs were measured 10 min after injection. The mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior, followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Hyperalgesia was evaluated immediately after the somatic sign observation period. The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results.
For precipitated withdrawal experiments, mice were infused with nicotine or saline for 14 days. On the evening of day 14, intracerebroventricular injection sites were prepared and mice were allowed to recover overnight. Mini pumps were not removed. On the morning of day 15, mice were injected intracerebroventricularly with MII[H9A;L15A], and testing was initiated as described with spontaneous withdrawal studies, 10 min after injection.
Nicotine CPA-MII[H9A;L15A] Assessment
The CPA protocol was conducted over the course of 4 days in a biased manner as described in Jackson et al. (2008). In brief, mice were implanted with 28-day mini pumps 14 days before initiation of CPA testing to induce tolerance. Infusion continued throughout the duration of testing. The conditioning chambers were the same as those used for CPP studies. On day 1 of the CPA procedure, after a 5-min habituation period in the center compartment, mice were allowed to roam freely between compartments for 15 min to determine baseline responses. The prepreference score was used to pair each mouse with mecamylamine (3.5. mg/kg s.c.) to its initially preferred compartment. On days 2 and 3 of CPA training, all mice received injections of saline in the morning and mecamylamine in the afternoon. After the mecamylamine conditioning session on day 3, intracerebroventricular injection sites were prepared. On day 4, mice received intracerebroventricular injections of vehicle or MII[H9A;L15A] (4.5 or 6 pmol) 10 min before being placed in the test chambers. Mice moved freely between compartments as on day 1, and the time spent in each compartment was recorded for each mouse. A reduction in time spent in their initially preferred compartment was interpreted as CPA.
Statistical Analysis
For all data, statistical analyses were performed using StatView (SAS Institute, Cary, NC). Data were analyzed by one-way analysis of variance with treatment as the between-subject factor. Significant results were further analyzed using the Newman-Keuls post hoc test. p values less than 0.05 are considered significant.
Results
α6* nAChRs Are Necessary for Nicotine CPP.
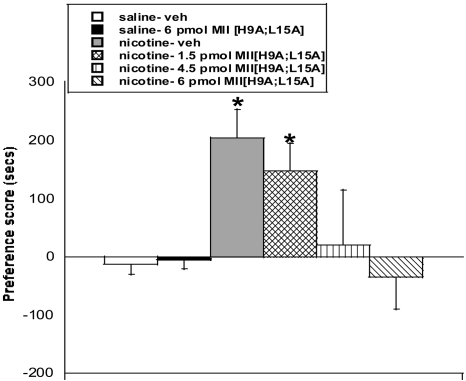
The CPP model was used to measure the role of the α6 nAChR subunit in the rewarding effects of nicotine. As shown in Fig. 1, mice conditioned with nicotine and treated with vehicle intracerebroventricularly on test day found nicotine (0.5 mg/kg s.c.) rewarding [F(5,43) = 3.3; p < 0.05)]. Conversely, intracerebroventricular pretreatment with MII[H9A;L15A] dose-dependently blocked the expression of nicotine CPP in mice. Pretreatment with 4.5 and 6 pmol of MII[H9A;L15A], but not 1.5 pmol MII[H9A;L15A], significantly blocked the expression of nicotine CPP [F(5,43) = 3.3; p < 0.05]. Activity counts were measured for each animal during the CPP test. There was no significant difference in activity count on postconditioning day between any test group, indicating that the results were not attributed to antagonist-induced differences in locomotor activity in the chambers (Table 1). As expected, mice conditioned with saline and treated with vehicle intracerebroventricularly on test day showed no preference for either side. The highest dose of MII[H9A;L15A] (6 pmol) by itself, did not produce significant results in saline-treated mice in the CPP model (Fig. 1).
Fig. 1.
The α6-selective antagonist MII[H9A;L15A] dose-dependently blocks expression of nicotine reward. Doses of 6 and 4.5 pmol of MII[H9A;L15A], but not 1.5 pmol of MII[H9A;L15A], block the expression of nicotine CPP in mice. The 6-pmol dose of MII[H9A;L15A] had no effect in saline-treated mice. Each point represents the mean ± S.E.M. of eight to 10 mice per group. *, p < 0.05 versus saline groups. veh, vehicle.
TABLE 1.
Average activity counts in the CPP model after central MII[H9A;L15A] administration
Mice were treated with vehicle or MII[H9A;L15A] (1.5–6 pmol i.c.v.) for the pharmacological assessment on test day. Activity counts were taken for each compartment in the chamber. Numbers represent the total activity counts in the drug-paired compartment on test day for each group and are presented as the average activity count on test day (postconditioning day) ± S.E.M. for 12 mice.
| Treatment Group | Avg. Activity Counts in Drug-Paired Compartment |
|---|---|
| Saline MP-vehicle | 509.8 ± 31.7 |
| Saline MP-MII[H9A;L15A] (6 pmol) | 524.8 ± 140.1 |
| Nicotine MP-vehicle | 471.1 ± 59.4 |
| Nicotine MP-MII[H9A;L15A] (1.5 pmol) | 455.8 ± 185.1 |
| Nicotine MP-MII[H9A;L15A] (4.5 pmol) | 492.9 ± 92.9 |
| Nicotine MP-MII[H9A;L15A] (6 pmol) | 486.1 ± 75.1 |
MP, mini pump.
α6* nAChRs Are Involved in Affective but Not Physical Nicotine Withdrawal.
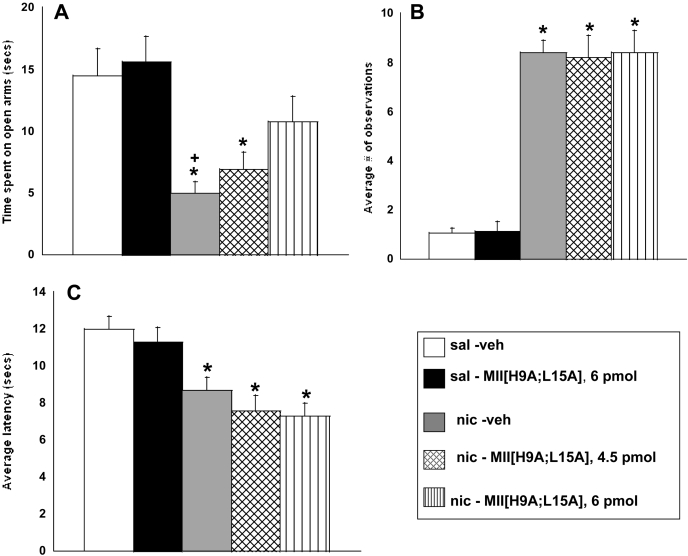
The role of α6* nAChRs in physical and affective nicotine withdrawal signs was evaluated using a spontaneous withdrawal model. As expected, nicotine-withdrawn mice, compared with saline-infused mice, spent significantly less time on the open arms of the plus maze, indicating withdrawal-associated anxiety-like behavior; showed decreased latency on the hot-plate, indicating a withdrawal-induced hyperalgesia response; and showed indications of somatic withdrawal (Fig. 2).
Fig. 2.
MII[H9A;L15A] dose-dependently blocks expression of the nicotine withdrawal-induced anxiety-related response in mice. Nicotine withdrawn mice treated with 6 pmol of MII[H9A;L15A] display a loss of anxiety-related response (A) but no change in average number of somatic signs (B) or hyperalgesia response (C). Each point represents the mean ± S.E.M. of 12 mice per group. *, p < 0.05 versus saline groups; +, p < 0.05 versus 6 pmol of MII[H9A;L15A]. sal, saline; nic, nicotine.
Pretreatment with the highest (6-pmol) dose of MII[H9A;L15A] blocked the expression of an anxiety-related response in nicotine-withdrawn mice [F(4,59) = 6.1; p < 0.001] (Fig. 2A). The average number of arm crosses was tallied as a measure of locomotor activity on the plus maze. There was no significant difference between any treatment group, suggesting that the observed effects on the plus maze were not the result of a difference in activity (Table 2). Somatic withdrawal assessment revealed that nicotine-withdrawn mice treated with vehicle, 4.5 pmol of MII[H9A;L15A], or 6 pmol of MII[H9A;L15A] intracerebroventricularly exhibited significantly more total somatic signs than saline-infused mice [F(4,54) = 63.4; p < 0.0001], indicating that MII[H9A;L15A] had no effect on somatic withdrawal at any dose tested (Fig. 2B). The hot-plate assessment also revealed a significant hyperalgesia response in all nicotine-withdrawn mice compared with saline exposed mice regardless of intracerebroventricular injection [F(4,57) = 8.1; p < 0.0001], indicating that MII[H9A;L15A] had no effects on the nicotine withdrawal-induced hyperalgesia response at the doses tested (Fig. 2C). Saline-exposed mice treated with 6 pmol of MII[H9A;L15A] intracerebroventricularly did not differ from saline-exposed mice treated with vehicle intracerebroventricularly in any behavioral test, indicating that the antagonist at the highest dose used was not behaviorally active by itself (Fig. 2).
TABLE 2.
Average number of arm crosses in the MII[H9A;L15A] plus maze assessment
Nicotine-withdrawn mice were treated with vehicle or MII[H9A;L15A] (4.5 and 6 pmol i.c.v.), and the total number of crosses between the open and closed arms of the plus maze was counted. Numbers are presented as the total average number of arm crosses ± S.E.M. for 8 to 10 mice per group.
| Treatment Group | Avg. No. of Arm Crosses |
|---|---|
| Saline MP-vehicle | 2.4 ± 0.3 |
| Saline MP-MII[H9A;L15A] (6 pmol) | 2.6 ± 0.5 |
| Nicotine MP-vehicle | 2.5 ± 0.4 |
| Nicotine MP-MII[H9A;L15A] (4.5 pmol) | 2.3 ± 0.4 |
| Nicotine MP-MII[H9A;L15A] (6 pmol) | 2.6 ± 0.7 |
MP, mini pump.
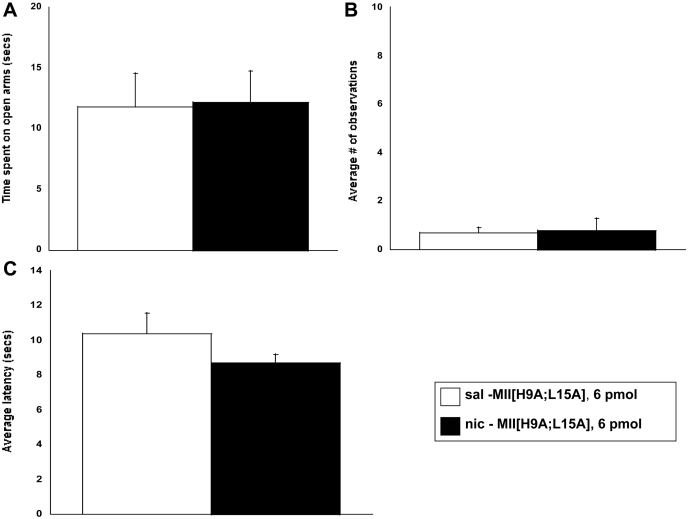
To further examine the observed withdrawal response, the effect of MII[H9A;L15A] was tested in a precipitated nicotine withdrawal model. Mini pumps were not removed on day 14, and withdrawal signs were measured the morning of day 15 after central administration of MII[H9A;L15A]. Pretreatment with MII[H9A;L15A] (6 pmol i.c.v.) failed to precipitate affective or physical nicotine withdrawal signs in chronic nicotine infused mice. There was no significant difference in time spent in the open arms of the plus maze (Fig. 3A), somatic signs (Fig. 3B), or hyperalgesia response (Fig. 3C) in nicotine-infused mice compared with saline-infused mice after antagonist treatment.
Fig. 3.
MII[H9A;L15A] does not precipitate physical or affective nicotine withdrawal signs in mice. Mice chronically infused with nicotine for 14 days were pretreated with MII[H9A;L15A] (6 pmol i.c.v.) on day 15, 10 min before testing initiated. The α6-selective antagonist did not precipitate an anxiety-related response (A), somatic signs (B), or withdrawal-induced hyperalgesia (C). Each point represents the mean ± S.E.M. of six mice per group. *, p < 0.05 versus saline group. sal, saline; nic, nicotine.
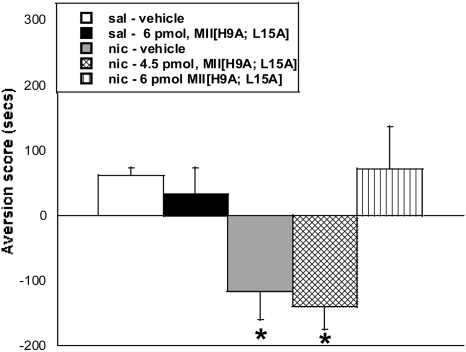
A CPA model was used to measure aversion associated with nicotine withdrawal (Fig. 4). Mecamylamine treatment (3.5 mg/kg s.c.) resulted in a significant CPA in chronic nicotine-exposed mice pretreated with vehicle intracerebroventricularly [F(4,49) = 5.2; p < 0.001]. In contrast, pretreatment with 6 pmol of MII[H9A;L15A], but not 4.5 pmol, on test day blocked the expression of mecamylamine-precipitated CPA in nicotine-dependent mice [F(4,49) = 5.2; p < 0.001].
Fig. 4.
The α6-selective antagonist MII[H9A;L15A] dose-dependently blocks expression of CPA during nicotine withdrawal. Mecamylamine (3.5 mg/kg s.c.) precipitated aversion in chronic nicotine-exposed mice was tested using conditioned place aversion. The 6-pmol dose of MII[H9A;L15A] had no effect in saline treated mice, but it blocked CPA in nicotine-exposed mice. Each point represents the mean ± S.E.M. of 12 mice per group. *, p < 0.05 versus saline groups. sal, saline; nic, nicotine.
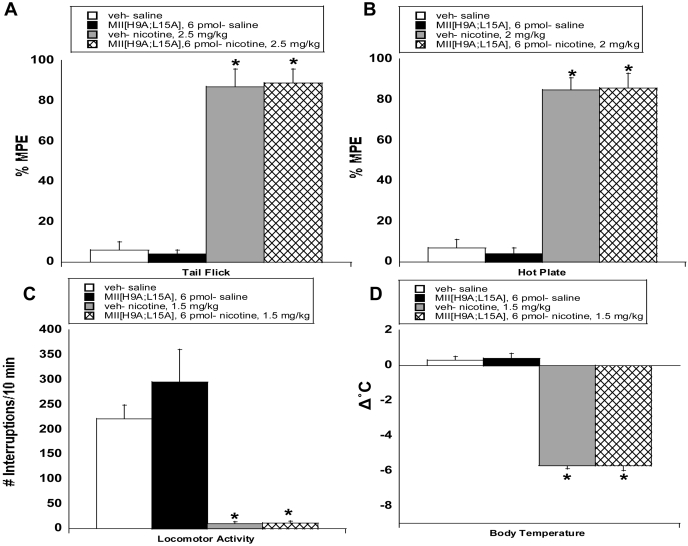
α6* nAChRs Do Not Regulate the Initial Effects of Nicotine on Analgesia, Temperature, or Locomotion.
The highest dose of the α6-selective antagonist MII[H9A;L15A], 6 pmol i.c.v., was found to be effective in blocking nicotine reward and affective withdrawal signs; thus, this dose was used to evaluate the role of α6 subunit-containing nicotinic receptors in the initial acute effects of nicotine. Results are shown in Fig. 5. Overall, pretreatment with MII[H9A;L15A] had no significant result on the effects of a single injection of nicotine on analgesia, body temperature, or locomotion. Nicotine significantly increased the latency in the tail-flick [2.5 mg/kg nicotine s.c.; F(3,19) = 52.5; p < 0.0001] and hot-plate [2 mg/kg nicotine, s.c; F(3,19) = 22.8; p < 0.0001] tests (Fig. 5, A and B). Pretreatment with MII[H9A;L15A] had no significant effect on acute nicotine-induced antinociception in either test. Acute nicotine (1.5 mg/kg s.c.) significantly reduced locomotor activity [F(3,23) = 16.9; p < 0.0001], and this effect was not altered by pretreatment with MII[H9A;L15A] (Fig. 5C). In addition, hypothermia was present 30 min after acute nicotine [1.5 mg/kg s.c.; F(3,19) = 68.1; p < 0.0001], and this effect remained after pretreatment with MII[H9A;L15A] (Fig. 5D). The dose of antagonist used did not produce significant effects in saline mice in any behavioral measure.
Fig. 5.
The α6 nicotinic acetylcholine receptor is not involved in the initial acute effects of nicotine on analgesia, locomotor activity, or body temperature. Mice were pretreated with either vehicle or MII[H9A;L15A] (6 pmol i.c.v.), 10 min before subcutaneous nicotine injection. Antinociception was measured using the tail-flick (A) and hot-plate (B) tests, and spontaneous activity (C) and difference in body temperature (D) were measured. Testing began 5 min after nicotine injection for antinociception and locomotor activity measures and after 30 min for the body temperature assessment. Vehicle (veh)-MII[H9A;L15A] mice did not differ from veh-saline mice in any test. Each point represents eight to 10 mice per group. *, p < 0.05 versus veh-saline.
Discussion
Our behavioral studies suggest that α6* nAChRs play a critical role in nicotine's rewarding effects and affective nicotine withdrawal behaviors but that they are not involved in the initial effects of nicotine induced after a single exposure. Central administration of a highly selective α6* nAChR antagonist blocked the expression of nicotine CPP, suggesting an important role for α6-containing nAChRs in nicotine's rewarding effects. In addition, expression of anxiety-related behavior and CPA were blocked by the α6 antagonist, whereas there was no effect on the expression of somatic signs or the hyperalgesia response, identifying a role for α6* nAChRs in affective, but not physical withdrawal. Treatment with the α6 antagonist had no effect on any acute nicotine measure, suggesting that α6* nAChRs are not involved in initial effects of nicotine on analgesia, body temperature, and locomotion.
Although several α-conotoxins have affinity for both α3 and α6 nAChR subunits, MII[H9A;L15A] is a much more potent at α6* than at α3* nAChRs (McIntosh et al., 2004). In the current study, we injected 1.5 to 6 pmol of peptide. Based on diffusion studies by Matta et al. (1995), the highest dose, 6 pmol, would correspond to a tissue concentration of ∼1.2 μM. At 1 μM, MII[H9A;L15A] has no effect on α2β2, α2β4, α4β2, α4β4, or α7 nAChRs. The peptide is also >2000-fold selective for α6/α3β2β3 nAChRs compared with α3β2 and α3β4 nAChRs. The IC50 value at α3β2 and α3β4 nAChRs is 4.8 and 7.8 μM, respectively. Thus, at concentrations used, there would be significantly less blockade of these receptor subtypes. The IC50 value at α6/α3β2β3 and α6β4 nAChRs is 2.4 and 270 nM, respectively (McIntosh et al., 2004). Thus, at the concentration used, selective block of α6* nAChRs is expected.
Our study suggests a critical role for α6* nAChRs in rewarding effects of nicotine using the CPP paradigm. Results revealed that doses of 4.5 and 6 pmol of MII[H9A;L15A] blocked the expression of nicotine CPP. These studies are consistent with findings from Pons et al. (2008) showing that the α6 nAChR subunit is critical for nicotine tail-vein self-administration. Immunoprecipitation and ligand-binding studies have shown that the α6 nAChR subunit is expressed with the α4 and β2 subunits on midbrain dopaminergic neurons and terminals (le Novère et al., 1996; Klink et al., 2001; Champtiaux et al., 2002; Zoli et al., 2002). The use of α4 knock-in mice, which have a point mutation rendering the α4 subunit hypersensitive to nicotine, revealed that these mice express nicotine CPP at doses that had no effect in wild-type mice (Tapper et al., 2004), suggesting that activation of the α4 nAChR subunit is important for nicotine reward. Studies in nAChR knockout mice indicate that β2* nAChRs are necessary for nicotine CPP and self-administration (Picciotto et al., 1998; Walters et al., 2006). Furthermore, rescue of the β2, α4, or α6 nAChR subunit in the VTA on a null mutant background is sufficient for rescue of intra-VTA nicotine self-administration (Maskos et al., 2005; Pons et al., 2008), and antagonism of β2* nAChRs in the VTA, but not the NAc, blocks nicotine self-administration (Corrigall et al., 1994). It is also shown that nicotine produces CPP in rats at doses that increase dopamine accumbal output (Janhunen and Ahtee, 2004; Janhunen et al., 2005). Indeed, studies using a single point mutation of the α6 nAChR subunit rendering it hypersensitive to nicotine reveal that the activity of VTA α6* nAChRs is primarily to stimulate dopamine and not GABA neurons (Drenan et al., 2008). Previous studies have also implicated the α6* nAChRs in nicotine-associated changes in locomotor activity (le Novère et al., 1999; Drenan et al., 2008). The concentrations of MII[H9A;L15A] used in these studies, however, failed to reveal an effect of acute nicotine exposure on nicotine locomotor activity. Although increases in dopamine transmission are associated with increased locomotor activity (Benwell and Balfour, 1992), the observed effects of MII[H9A;L15A] do not seem to be due to effects of the antagonist on locomotor activity, because there was no significant difference in activity counts between vehicle- and MII[H9A;L15A]-treated mice at a dose that blocked nicotine CPP. Together with the present data, these studies suggest that α4α6β2* nAChRs in the VTA may play an important role in nicotine reward.
Central administration of MII[H9A;L15A] had no effect on physical withdrawal but dose-dependently blocked anxiety-like behavior in the plus maze, as well as expression of aversion in the CPA model. Furthermore, MII[H9A;L15A] failed to precipitate an anxiety-related response, somatic signs, or a hyperalgesia response in chronic nicotine-infused mice. These results indicate a role for α6 nAChRs in affective but not physical nicotine withdrawal behaviors. In addition to regulating nicotine reinforcement (Corrigall et al., 1992), β2* nAChRs in the VTA also regulate affective withdrawal (Bruijnzeel and Markou, 2004), and some of these β2* nAChRs assemble with α6. Intra-VTA injections of a selective β2 antagonist dihydro-β-erythroidine precipitate elevations in brain reward thresholds during nicotine withdrawal in chronically exposed rats (Bruijnzeel and Markou, 2004). This is consistent with studies showing that β2* nAChRs are critical for affective but not somatic withdrawal (Damaj et al., 2003; Besson et al., 2006; Jackson et al., 2008).
Taken together, the results of this study suggest that the α6 nAChR subunit is involved in both nicotine reward and affective nicotine withdrawal. Although this effect may seem somewhat paradoxical, it is possible that these results reflect differences in nAChR subunit composition (i.e., α4α6β2* versus α6β2* versus α4α6α5β2), different anatomical distribution of brain nAChR receptors, and/or different intracellular mechanisms in nicotine reward and withdrawal. Although the VTA has been implicated in nicotine reinforcement (Corrigall et al., 1994; Maskos et al., 2005, Pons et al., 2008) and withdrawal (Bruijnzeel and Markou, 2004), the LC has also been implicated in withdrawal effects from drugs of abuse, such as morphine (Dizgah et al., 2005). Indeed, α6β2* nAChRs are expressed in the LC where they support noradrenaline release (Léna et al., 1999). Thus, it is possible that the contributions of α6* nAChRs to nicotine reward are mediated via the VTA, whereas α6* nAChRs in the LC regulate nicotine withdrawal. Future studies involving intrategmental and intra-LC injections of MII[H9A;L15A] would aid in elucidation of this possibility.
Our results also show that higher antagonist doses are required to block nicotine CPA (6 pmol) compared with nicotine CPP (4.5 pmol). The effect seems to be more dose-responsive in CPP compared with CPA. Although the in vivo pharmacokinetics of MII[H9A;L15A] is currently unknown, the observed variation between the dose necessary to block CPP versus CPA further suggests that there are different mechanisms that contribute to reward and aversive withdrawal effects associated with nicotine. In addition, it is possible that different intracellular mechanisms, resulting in different protein interactions during nicotine reward and withdrawal, can alter the function of the receptor. Immunoprecipitation studies would help elucidate the proteins that interact with the α6 nAChR subunit during nicotine reward versus nicotine withdrawal.
Together with previous data, the present results indicate that the α6 nAChR subunit supports behavior that promotes nicotine dependence. We further examined the role of α6* nAChRs in the initial effects of nicotine. Our results suggest that α6 subunit-containing receptors do not mediate the acute effects of nicotine on analgesia, locomotor activity, or body temperature. Although acute nicotine induced antinociception and decreases in locomotor activity and body temperature in mice, pretreatment with the α6-selective antagonist MII[H9A;L15A] had no effect on any of these measures. Previous studies support an important role for the α4 and β2 nAChR subunits in the acute pharmacological effects of nicotine (Marubio et al., 1999; Tritto et al., 2004). Our data suggest that the α4α6β2* nAChR subtype is unlikely to mediate these effects. Another possible candidate could be the α5 subunit, which was also found to coassemble with the α4β2* subtype (Wada et al., 1990; Klink et al., 2001).
Overall, the results of this study, together with previous studies, suggest that α6β2* and/or α4α6β2* nAChR subtypes are critical for nicotine reward and affective withdrawal. These behavioral findings provide better insight into potential targets for more effective smoking cessation therapies.
Acknowledgments
We thank Cindy Evans and Dr. Dena Kota for assistance with MII[H9A;L15A] conditioned place preference studies.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grants DA12610, DA05274, DA023114] (to M.I.D. and D.H.B., respectively); the National Institutes of Health National Institute of Mental Health [Grant MH53631] (to J.M.M.); and the National Institutes of Health National Institute of General Medical Sciences [Grant GM48677] (to J.M.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.155457
- VTA
- ventral tegmental area
- NAc
- nucleus accumbens
- nAChR
- nicotinic acetylcholine receptor
- LC
- locus coeruleus
- MII[H9A;L15A]
- α-conotoxin H9A;L15A
- CPP
- conditioned place preference
- CPA
- conditioned place aversion
- MPE
- maximal possible effect.
References
- Benwell and Balfour, 1992.Benwell ME, Balfour DJ. (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson et al., 2006.Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. (2006) Genetic dissociation of two behaviors associated with nicotine addiction: β2-containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology 187:189–199 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel and Markou, 2004.Bruijnzeel AW, Markou A. (2004) Adaptations in cholinergic transmission associated with the affective signs of nicotine withdrawal. Neuropharmacology 47:572–579 [DOI] [PubMed] [Google Scholar]
- Champtiaux et al., 2002.Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. (2002) Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci 22:1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux et al., 2003.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23:7820–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall et al., 1992.Corrigall WA, Franklin KB, Coen KM, Clarke PB. (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107:285–289 [DOI] [PubMed] [Google Scholar]
- Corrigall et al., 1994.Corrigall WA, Coen KM, Adamson KL. (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284 [DOI] [PubMed] [Google Scholar]
- Damaj et al., 2003.Damaj MI, Kao W, Martin BR. (2003) Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther 307:526–534 [DOI] [PubMed] [Google Scholar]
- Dani and Heinemann, 1996.Dani JA, Heinemann S. (1996) Molecular and cellular aspects of nicotine abuse. Neuron 16:905–908 [DOI] [PubMed] [Google Scholar]
- Dizgah et al., 2005.Dizgah IM, Karimian SM, Zarrindast MR, Sohanaki H. (2005) Attenuation of morphine withdrawal signs by a D1 receptor agonist in the locus coeruleus of rats. Neuroreport 16:1683–1686 [DOI] [PubMed] [Google Scholar]
- Drenan et al., 2008.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, et al. (2008) In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron 60:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff et al., 1986.Grenhoff J, Aston-Jones G, Svensson TH. (1986) Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand 128:351–358 [DOI] [PubMed] [Google Scholar]
- Hildebrand et al., 1998.Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. (1998) Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res 779:214–225 [DOI] [PubMed] [Google Scholar]
- Jackson et al., 2008.Jackson KJ, Martin BR, Changeux JP, Damaj MI. (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen and Ahtee, 2004.Janhunen S, Ahtee L. (2004) Comparison of the effects of nicotine and epibatidine on the striatal extracellular dopamine. Eur J Pharmacol 494:167–177 [DOI] [PubMed] [Google Scholar]
- Janhunen et al., 2005.Janhunen S, Linnervuo A, Svensk M, Ahtee L. (2005) Effects of nicotine and epibatidine on locomotor activity and conditioned place preference in rats. Pharmacol Biochem Behav 82:758–765 [DOI] [PubMed] [Google Scholar]
- Klink et al., 2001.Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota et al., 2007.Kota D, Martin BR, Robinson SE, Damaj MI. (2007) Nicotine dependence and reward differ between adolescent and adult mice. J Pharmacol Exp Ther 322:399–407 [DOI] [PubMed] [Google Scholar]
- Lai et al., 2005.Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. (2005) Long-term nicotine treatment decreases striatal α6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol 67:1639–1647 [DOI] [PubMed] [Google Scholar]
- Laviolette and van der Kooy, 2003.Laviolette SR, van der Kooy D. (2003) Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8:50–59 [DOI] [PubMed] [Google Scholar]
- Le Novère et al., 1996.Le Novère N, Zoli M, Changeux JP. (1996) Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci 8:2428–2439 [DOI] [PubMed] [Google Scholar]
- le Novère et al., 1999.le Novère N, Zoli M, Léna C, Ferrari R, Picciotto MR, Merlo-Pich E, Changeux JP. (1999) Involvement of alpha6 nicotinic receptor subunit in nicotine-elicited locomotion, demonstrated by in vivo antisense oligonucleotide infusion. Neuroreport 10:2497–2501 [DOI] [PubMed] [Google Scholar]
- Léna et al., 1999.Léna C, de Kerchove D'Exaerde A, Cordero-Erausquin M, Le Novère N, del Mar Arroyo-Jimenez M, Changeux JP. (1999) Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci U S A 96:12126–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu and Jin, 2004.Liu ZH, Jin WQ. (2004) Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport 15:1479–1481 [DOI] [PubMed] [Google Scholar]
- Marubio et al., 1999.Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398:805–810 [DOI] [PubMed] [Google Scholar]
- Matta et al., 1995.Matta SG, McCoy JG, Foster CA, Sharp BM. (1995) Nicotinic agonists administered into the fourth ventricle stimulate norepinephrine secretion in the hypothalamic paraventricular nucleus: an in vivo microdialysis study. Neuroendocrinology 61:383–392 [DOI] [PubMed] [Google Scholar]
- McIntosh et al., 2004.McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. (2004) Analogs of α-conotoxin MII are selective for α6-containing nicotinic acetylcholine receptors. Mol Pharmacol 65:944–952 [DOI] [PubMed] [Google Scholar]
- Picciotto et al., 1998.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. (1998) Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177 [DOI] [PubMed] [Google Scholar]
- Pons et al., 2008.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. (2008) Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits in the ventral tegmental area in systemic nicotine self-administration. J Neurosci 28:12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri et al., 1996.Pontieri FE, Tanda G, Orzi F, Di Chiara G. (1996) Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382:255–257 [DOI] [PubMed] [Google Scholar]
- Rada et al., 2001.Rada P, Jensen K, Hoebel BG. (2001) Effects of nicotine and mecamylamine-induced withdrawal in extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology 157:105–110 [DOI] [PubMed] [Google Scholar]
- Salminen et al., 2004.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65:1526–1535 [DOI] [PubMed] [Google Scholar]
- Tapper et al., 2004.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. (2004) Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032 [DOI] [PubMed] [Google Scholar]
- Tritto et al., 2004.Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ. (2004) Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha 7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res 6:145–158 [DOI] [PubMed] [Google Scholar]
- Wada et al., 1990.Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. (1990) The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res 526:45–53 [DOI] [PubMed] [Google Scholar]
- Walters et al., 2006.Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. (2006) The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology 184:339–344 [DOI] [PubMed] [Google Scholar]
- Zoli et al., 2002.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. (2002) Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 22:8785–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]