Abstract
The C-terminal BAG domain is thought to play a key role in BAG-1-induced survival and proliferation by mediating protein-protein interactions, for example, with heat shock proteins HSC70 and HSP70, and with RAF-1 kinase. Here, we have identified thioflavin S (NSC71948) as a potential small-molecule chemical inhibitor of these interactions. NSC71948 inhibited the interaction of BAG-1 and HSC70 in vitro and decreased BAG-1:HSC70 and BAG-1:HSP70 binding in intact cells. NSC71948 also reduced binding between BAG-1 and RAF-1, but had no effect on the interaction between two unrelated proteins, BIM and MCL-1. NSC71948 functionally reversed the ability of BAG-1 to promote vitamin D3 receptor-mediated transactivation, an activity of BAG-1 that depends on HSC70/HSP70 binding, and reduced phosphorylation of p44/42 mitogen-activate protein kinase. NSC71948 can be used to stain amyloid fibrils; however, structurally related compounds, thioflavin T and BTA-1, had no effect on BAG-1:HSC70 binding, suggesting that structural features important for amyloid fibril binding and inhibition of BAG-1:HSC70 binding may be separable. We demonstrated that NSC71948 inhibited the growth of BAG-1 expressing human ZR-75-1 breast cancer cells and wild-type, but not BAG-1-deficient, mouse embryo fibroblasts. Taken together, these data suggest that NSC71948 may be a useful molecule to investigate the functional significance of BAG-1 C-terminal protein interactions. However, it is important to recognize that NSC71948 may exert additional “off-target” effects. Inhibition of BAG-1 function may be an attractive strategy to inhibit the growth of BAG-1-overexpressing cancers, and further screens of additional compound collections may be warranted.
Bcl-2-associated athanogene (BAG-1) is a multifunctional protein that interacts with multiple cellular targets and modulates a wide range of cellular processes (Townsend et al., 2003b). Overexpression of BAG-1 protects cells from many apoptotic stimuli, promotes autophagy, enhances proliferation and metastasis, and modulates the transcriptional activity of a variety of nuclear hormone receptors (NHRs) (Townsend et al., 2003b; Gurusamy et al., 2009). BAG-1 is essential for differentiation and survival of hematopoietic and neuronal cells (Götz et al., 2005). Functional and expression studies suggest that overexpression of BAG-1 may play an important role in diverse cancer types (Townsend et al., 2003b). BAG-1 is frequently overexpressed in cancer and can correlate with important clinical parameters (Cutress et al., 2003; Millar et al., 2009). Depletion of BAG-1 by small interfering RNA (siRNA) is sufficient to promote apoptosis in colorectal carcinoma cells (Clemo et al., 2008), and overexpression of a dominant negative form of BAG-1 decreases cell growth of breast cancer cells in vitro and in vivo (Kudoh et al., 2002). Antisense or siRNA-mediated depletion of BAG-1 also decreases cell growth and sensitizes to staurosporine or paclitaxel in HeLa cells (Takahashi et al., 2003; Xiong et al., 2003). BAG-1 haploinsufficiency impairs lung tumorigenesis (Götz et al., 2004). More recent findings have implicated BAG-1 in brain function and neurological disorders, where overexpression protects neuronal cells from staurosporine and thapsigargin and ameliorates motor defects in a mouse model of Huntington disease (Liman et al., 2005; Orr et al., 2008). BAG-1 is also a target for mood stabilizers and regulates recovery from manic-like and depression-like behavior (Maeng et al., 2008).
In human cells, BAG-1 exists as three major isoforms (BAG-1S, BAG-1M, and BAG-1L; Fig. 1A) derived by alternate translation initiation from a single mRNA. All BAG-1 isoforms contain a C-terminal, evolutionary conserved BAG domain (Takayama et al., 1999) and a central ubiquitin-like domain. The larger isoforms (M and L) have unique N-terminal extensions. The functional significance of these variable N-terminal regions is poorly understood. BAG-1L possesses a nuclear localization sequence and is a predominant nuclear protein, whereas the other isoforms partition between the cytoplasm and nucleus.
Fig. 1.
Screening for inhibitors of the BAG-1:HSC70 interaction. A, human BAG-1 isoforms. The structures of the three major human BAG-1 isoforms are shown, along with their size (amino acid residues). Translation of BAG-1L initiates at an upstream CUG codon, whereas BAG-1M and BAG-1S are AUG-derived. The position of the nuclear localization sequence (NLS), acidic repeats, ubiquitin-like domain (ULD), and BAG domain are shown. B, diagram to demonstrate the principle of the screening assay. Negative controls included GST in place of GST-BAG-1S and wells lacking HSC70. C, overview of screening strategy. A total of 3156 compounds were screened in two sets of primary assays. Secondary assays to determine IC50 values resulted in identification of 16 confirmed hits. IC50 values for inhibition of BAG-1:HSC70 binding are shown (mean values derived from two independent experiments each performed in duplicate ± S.D.). *, compound selected for further study. The structures of the compounds listed are available from the National Cancer Institute Discovery Services (NCI-DTP) website (http://dtp.nci.nih.gov/webdata.html).
The C-terminal BAG domain comprises a bundle of three α-helices, of which helices 2 and 3 mediate electrostatic interactions of BAG-1 with subdomains IB and IIB of the ATPase domain of the 70-kDa heat shock proteins, HSC70 and HSP70 (Briknarová et al., 2001; Sondermann et al., 2001). Although helix 1 is not directly involved in binding, it may contribute to intramolecular interactions that stabilize the overall structure of the BAG domain. BAG-1 acts as a cochaperone and stimulates nucleotide exchange of HSC70/HSP70 (Townsend et al., 2003b). The BAG domain is also important for interaction and activation of RAF-1, a key signaling molecule for cell survival and proliferation, potentially via binding sites within helices 1 and 2 (Song et al., 2001).
HSC70 and HSP70 play important roles in multiple cell processes, via effects on protein (re)folding and degradation, and expression and activity of NHR (Mayer and Bukau, 2005). BAG-1 binding to these multifunctional proteins may explain, at least in part, the multiple effects associated with BAG-1 overexpression. The BAG-1 ULD is required for the interaction of BAG-1 with the proteasome (Lüders et al., 2000), and BAG-1 may act to coordinate the function of chaperones and the proteasome in the degradation of specific target proteins (Arndt et al., 2007). Thus, BAG-1 can interact simultaneously with HSC70 and the proteasome (Lüders et al., 2000; Alberti et al., 2002), and its ability to influence chaperone function may facilitate the unloading of chaperone clients in the vicinity of the proteasome to enhance degradation. BAG-1 also interacts with CHIP, an E3 ubiquitin ligase that plays a key role in protein triage (i.e., degradation versus refolding) decisions (McDonough and Patterson, 2003). BAG-1 and CHIP cooperate to target the glucocorticoid receptor for proteasomal degradation (Demand et al., 2001). Conversely, overexpression of BAG-1 has been shown to decrease proteasomal degradation of Tau (Elliott et al., 2007).
Functional studies demonstrate that BAG-1 isoforms can promote the survival and proliferation of various cell types in vitro and in vivo. Mutational analysis demonstrates that suppression of apoptosis and regulation of NHR function is dependent on interaction with HSC70/HSP70 via the C-terminal BAG domain (Townsend et al., 2003b; Townsend et al., 2004). These data suggest that inhibition of the interaction between BAG-1 and HSC70/HSP70 by small chemical compounds is an attractive strategy to counter BAG-1 function. The aim of this work was to identify small molecule inhibitors of the BAG-1:HSC70 interaction.
Materials and Methods
Cell Culture and Plasmids
MCF7 and ZR-75-1 human breast cancer cells, and BAG-1-deficient and wild-type mouse embryo fibroblasts (MEFs) (kind gifts of S. Weisse, Ruhr-University, Germany) (Götz et al., 2005) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) fetal calf serum (FCS) (PAA Laboratories, Yeovil, United Kingdom) and penicillin and streptomycin (Invitrogen). H376 oral squamous carcinoma cells were cultured in DMEM: Ham's F-12 (1:1) Nutrient Mix medium (Invitrogen) containing 10% (v/v) FCS, 2 mM l-glutamine, and 0.5 μg/ml hydrocortisone. To produce stable clones overexpressing human BAG-1S, MCF7 cells were transfected with pcDNA3-BAG-1S expression construct (Townsend et al., 2003a) or empty pcDNA3 vector (Invitrogen) by use of calcium phosphate precipitation followed by single-cell cloning in the presence of G418. Wild-type BAG-1L and the BAG-1L H2 mutant (which has two point mutations within helix 2: E283A, K287A) were expressed by use of pcDNA3-derived plasmids (Townsend et al., 2003a; Lee et al., 2007). The luciferase reporter plasmid (DR3)4tkLUC was derived from the rat atrial natriuretic factor gene promoter and was a kind gift of Prof Carsten Carlberg (Department of Biochemistry, University of Kuopio, Finland).
Compounds
The mechanistic, diversity, challenge set and natural product compound collections were obtained from National Cancer Institute Developmental Therapeutics Program (NCI-DTP) [National Cancer Institute, Rockville, MD, (http://dtp.cancer.gov)]. Refills of selected compounds were also obtained from NCI-DTP and stored as 100 mM stocks in DMSO at −20°C. Thioflavin S, thioflavin T, and BTA-1 were obtained from Sigma Chemical (Poole, Dorset, UK). For experiments using MEFs, thioflavin S was prepared as a 10 mM stock in complete DMEM on the day of use.
BAG-1:HSC70 in Vitro Interaction Assay
Glutathione S-transferase (GST) and GST-BAG-1S were purified by use of glutathione-S-Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK) from bl21 Escherichia coli transformed with pGEX-2TK (GE Healthcare) or pGEX-BAG-1S as described previously (Brimmell et al., 1999). Wells of a 96-well plate were incubated overnight at 4°C with 10 ng of recombinant HSC70 (Cambridge BioScience, Cambridge, UK) in 100 μl of phosphate-buffered saline (PBS). The wells were washed three times with PBS, and GST or GST-BAG-1S (each 500 ng) was added. The plates were incubated for a further 20 min at room temperature. Unbound GST proteins were removed by washing in PBS. The 3.10 G3E2 BAG-1 specific monoclonal antibody (Brimmell et al., 1999; 1:1000 dilution of ascites in PBS) was added to the wells (200 μl/well) and incubated for 1 h at room temperature. Wells were washed, and 200 μl of horseradish peroxidase-conjugated anti-mouse immunoglobulin antibody (GE Healthcare) diluted (1:5000) in PBS was added to the wells and incubated for 1 h at room temperature. Wells were washed, and 200 μl of o-phenylenediamine substrate (Sigma Chemical) was added. The plate was incubated at 37°C for 10 min, and absorbance at 450 nm was measured with use of a plate reader. To test the ability of compounds to inhibit the BAG-1:HSC70 interaction, compounds were diluted in PBS and added to HSC70-coated wells for 4 h before the addition of GST-BAG-1S. The absorbance of wells with GST-BAG-1S and HSC70 (with no test compound present) was set at 100% interaction, and the absorbance of wells containing GST and HSC70 was set at 0%.
Counterscreens
Antibody Counterscreen.
To investigate whether compounds interfered with detection of BAG-1S, we measured the ability of compounds to interfere with detection of BAG-1S by the 3.10 G3E2 monoclonal antibody (Brimmell et al., 1999). Wells of a 96-well plate were incubated overnight at 4°C with 500 ng of recombinant GST-BAG-1S in 100 μl of PBS. The wells were washed three times in a bath of PBS, and nonspecific binding sites were blocked by incubation with PBS containing 0.1% (w/v) bovine serum albumin (BSA) (Sigma Chemical) for 1 h at room temperature. Compounds were added to the wells and incubated at room temperature for 4 h. The wells were washed in PBS, and 3.10 G3E2 BAG-1 specific monoclonal antibody (1:1000 dilution of ascites in PBS) was added to the wells (200 μl/well) and incubated for 1 h at room temperature. Bound antibody was detected as described above.
Luciferase Activity.
Compounds were incubated with purified luciferase (Sigma Chemical) (100 ng in 20 μl of luciferase lysis buffer; Promega, Southampton, UK). After 5 min, luciferase activity was detected after the addition of 100 μl of luciferase assay reagent (Promega) according to the manufacturer's instructions. Control luciferase assays were performed in the absence of compound.
BSA Counterscreen.
To identify compounds with high nonspecific protein-binding activity, we determined whether excess BSA interfered with the ability of compounds to interfere with BAG-1:HSC70 binding in the in vitro binding assay. The assay was performed as described above with the addition of BSA (0.1 mg/ml) to all buffers.
Interaction in Cells
BAG-1S:HSC70/HSP70.
To analyze the interaction of BAG-1 and endogenous HSC70/HSP70, MCF7 cells overexpressing BAG-1 (Clone BAG-1S D) were plated in medium containing serum and left to adhere for 4 to 5 h. Cells were then treated with compounds and were cultured for a further 16 h. Cells were resuspended in HMKEN buffer [10 mM HEPES, pH 7.2, 5 mM MgCl2, 142 mM KCl, 2 mM EGTA, 0.2% (v/v) Nonidet P40, 1:100 protease inhibitor cocktail (Sigma Chemical)] by trituration through a 21-gauge needle, lysed on ice for 30 min, and clarified by centrifugation (13,000 rpm for 30 min). One-thirtieth of the lysate was retained as a whole-cell lysate. The remaining sample was precleared by use of protein G-Sepharose beads for 30 min at 4°C. Protein G-Sepharose beads were removed by centrifugation. Lysates were incubated with the BAG-1-specific rabbit polyclonal antibody, TB3 (Brimmell et al., 1999) (5 μl per 1400 μl lysate), at 4°C for 16 h to analyze the interaction between BAG-1 and HSC70/HSP70. A further lysate was incubated with preimmune control serum (5 μl per 900 μl lysate) to control for nonspecific interactions. The immune complexes were incubated with protein G-Sepharose beads for 4 to 6 h and removed by centrifugation. The beads were washed five times by use of HMKEN buffer, resuspended in SDS-polyacrylamide gel electrophoresis sample buffer, and heated at 95°C for 5 min. Immunoblotting was performed as described previously (Packham et al., 1997). Primary antibodies used were 3.10 G3E2 hybridoma supernatant (Brimmell et al., 1999) for BAG-1, B6 (Santa Cruz Biotechnology, Santa Cruz, CA) for HSC70 and C92F3A5 (Cambridge BioScience) for HSP70.
MCL-1:BIM.
To analyze interaction of MCL-1 and BIM, MCF7 cells were plated in medium containing serum and left to adhere for 4 to 5 h. Cells were then treated with compound and were cultured for a further 16 h. Cells were resuspended in MCL-1:BIM coimmunoprecipitation buffer (142.5 mM KCl, 5 mM MgCl2, 20 mM Tris, pH 7.4, 1 mM EGTA, 0.2% (v/v) Nonidet P40), vortexed, lysed on ice for 30 min, and clarified by centrifugation (13,000 rpm for 30 min). One-tenth of the lysate was retained as a whole-cell lysate. The remaining sample was precleared by use of protein G-Sepharose beads for 30 min at 4°C. Protein G-Sepharose beads were removed by centrifugation. Lysates were incubated with the MCL-1-specific antibody (S19; Santa Cruz Biotechnology) (5 μl per 450 μl lysate) at 4°C for 16 h to analyze the interaction between MCL-1 and BIM. A further lysate was incubated with the epidermal growth factor receptor specific antibody (1005; Santa Cruz Biotechnology) (5 μl per 450 μl lysate) to control for nonspecific interactions. The immune complexes were incubated with protein G-Sepharose beads for 4 to 6 h and removed by centrifugation. The beads were washed five times with use of MCL-1:BIM coimmunoprecipitation buffer, resuspended in SDS-polyacrylamide gel electrophoresis sample buffer, and heated at 95°C for 5 min. Immunoblotting was performed as described previously (Packham et al., 1997). The primary antibodies used were S9 for MCL-1 and H-191 for BIM-1 (both from Santa Cruz Biotechnology).
Transcription Reporter Assays for Vitamin D Receptor
To determine the effects of compounds on the potentiation of the vitamin D receptor (VDR) by BAG-1L (Lee et al., 2007), H376 cells were plated at a density of 5000 in a 24-well plate. Cells were transfected 2 days later with (DR3)4tkLUC, and pcDNA3 BAG-1L, pcDNA3 BAG-1L-H2, or pCDNA3 control (Invitrogen) with use of Fugene 6 reagent (Roche, Lewes, UK). Cells were also transfected with pRL-tk-luc (Promega) to act as an internal control for transfection efficiency. After transfection, cells were treated with 1α,25-dihydroxyvitamin D3 at a concentration of 0.1 μM, or DMSO as a control. After 24 h, firefly and renilla luciferase activity was measured by use of the Dual Luciferase Assay System (Promega) according to the manufacturer's instructions. Results were analyzed as firefly luciferase activity divided by renilla luciferase activity to control for intersampling variations in transfection activity and cell number.
Effects of Compounds on ERK1/2 Phosphorylation
To determine the effect of compounds on ERK1/2 phosphorylation in MCF7 cells, cells were plated at a density of 2 × 106 cells in a 90-mm dish and incubated for 24 h before treatment with various concentrations of compounds. Cells were treated with compounds for 2 h before being harvested and radioimmunoprecipitation assay cell lysates being prepared. Cell lysates were normalized for protein concentration and samples were analyzed by Western blotting as described previously (Packham et al., 1997). Primary antibodies used were 9101 (Cell Signaling Technology, Danvers, MA) for T202/Y204 phosphorylated ERK1/2, 9102 (Cell Signaling Technology) for total ERK1/2 and PC10 (Cancer Research UK Research Services, London, UK) for proliferating cell nuclear antigen.
Cell Growth Assays
To measure growth inhibition, cells were plated at a density of 5000 cells per well of a 96-well plate. The next day, cells were treated with various concentrations of compounds. After 6 (for MCF7 or ZR-75-1 cells) or 3 (for MEFs) days, media were removed from each well and replaced with 200 μl of RPMI 1640 medium (Invitrogen) supplemented with 10% (v/v) FCS and 1% (v/v) penicillin/streptomycin/glutamine. CellTiter 96 AQueous One Solution Reagent (20 μl; Promega) was added to the wells, and the plate was incubated at 37°C for 1 to 4 h. Absorbance at 490 nm was measured by use of a Dynatech MR 5000 plate reader (Dynex Technologies, Chantilly, VA).
Results
Identification of NSC71948 as an Inhibitor of the BAG-1:HSC70 Interaction.
To identify potential chemical inhibitors of the BAG-1:HSC70 interaction, we screened the NCI-DTP mechanistic, diversity, natural product, and challenge sets with use of an in vitro BAG-1:HSC70-binding assay (Fig. 1B). We first screened the BAG-1:HSC70 interaction assay by use of the NCI-DTP discovery, natural product, and challenge sets by use of a 100 μM screening concentration and an activity cutoff of ≥70% inhibition to identify putative hits. We identified 12 compounds in this initial screen; however, two of these showed no activity in subsequent IC50 determinations. We also screened the mechanistic set by use of a 10 μM screening concentration and an activity cutoff of ≥50% inhibition. This identified six further hits, all of which demonstrated activity in subsequent IC50 determinations. Thus, from the 3156 compounds screened, we identified 16 confirmed hits (Fig. 1C). We obtained additional samples of the 12 most active compounds from the NCI-DTP and retested their activity in the same interaction assay. With the exception of NSC65828 and NSC652174, these compounds demonstrated inhibition of BAG-1:HSC70 binding. However, the activity of the second batch of compounds was less than that observed with the original compound collections.
A series of counterscreens were performed to exclude false positives or compounds with poor selectivity (Table 1). To determine whether the compounds interfered with the immunodetection system we investigated whether compounds inhibited direct detection of BAG-1S in an enzyme-linked immunosorbent assay-type assay (antibody counterscreen). NSC119911 significantly inhibited direct detection of BAG-1S (IC50 = 37 ± 11 μM) and was therefore excluded from further study. Two further counterscreens were performed to exclude chemical compounds (“frequent hitters”) that seem active in many assays because of nonselective inhibitory activity, for example, by nonselective protein binding and/or aggregation (McGovern et al., 2002); a luciferase assay and addition of excess of BSA to the BAG-1:HSC70-binding assay. Selective compounds would not be expected to inhibit luciferase activity, and their ability to inhibit BAG-1:HSC70 binding should not be significantly affected by BSA. For these assays, compounds were analyzed at approximately twice the IC50 for inhibition of BAG-1:HSC70 binding. The majority of the compounds tested showed a large degree of nonselectivity in at least one of the assays (i.e., >50% inhibition of luciferase, or >50% loss of activity in the presence of excess BSA) and were therefore excluded from further study. Only one compound, NSC71948, was considered “clean” in all counterscreens (Table 1) and was therefore selected for further study.
TABLE 1.
Results from counterscreens
This is a summary of the results obtained with the indicated compounds (refilled compounds) in the three counterscreen assays. “+“ indicates compound was considered active in relevant assay (i.e., >50% inhibition of luciferase activity or direct GST-BAG-1S detection, or >50% loss of activity in the presence of BSA).
| Compound | Direct GST-BAG-1S Detectiona | Luciferaseb | BSAc |
|---|---|---|---|
| NSC638352 | − | + | − |
| NSC71948 | − | − | − |
| NSC85561 | − | + | + |
| NSC73413 | − | − | + |
| NSC76027 | − | + | + |
| NSC59265 | − | + | − |
| NSC7333 | − | + | − |
| NSC7223 | − | − | + |
| NSC119911 | + | NDd | ND |
| NSC119913 | − | + | − |
Inhibition of direct immunodetection of GST-BAG-1S.
Inhibition of luciferase activity.
Inhibition of activity in the BAG-1:HSC70 interaction assay by BSA. For effects on direct detection of GST-BAG-1S, compounds were tested at 100 μM. For effects in the luciferase and BSA assays, compounds were tested at approximately two times their IC50 for inhibition of BAG-1S:HSC70 in vitro binding.
ND, not determined.
NSC71948 (Fig. 2A), also known as thioflavin S, is a mixture of components resulting from the methylation of dehydrothiotoluidine (Kelényi, 1967). The sample from the compound collections had an IC50 of 0.9 ± 0.1 μM in the in vitro BAG-1:HSC70 interaction assay, whereas the resupplied material had an IC50 of 13 ± 0.1 μM (Fig. 2B). Differences in precise composition may explain the differences in the potency of the materials. All further experiments were performed by use of refill material from the NCI-DTP, and some experiments were performed with use of thioflavin S obtained from Sigma Chemical. NSC71948 did not inhibit luciferase activity and its ability to inhibit HSC70:BAG-1 binding was only very modestly affected by excess BSA (Fig. 2, C and D).
Fig. 2.
Identification of NSC71948 as an inhibitor of the in vitro BAG-1:HSC70 interaction. A, structure of NSC71948 (thioflavin S). B, inhibition of the in vitro BAG-1:HSC70 interaction by NSC71948. The experiment shows the mean of duplicate determinations (± S.D.) of BAG-1:HSC70 interaction in the presence of the indicated concentrations of NSC71948 (refilled stock). Experiment is representative of two independent experiments. C, luciferase counterscreen. The activity of recombinant luciferase was measured in the presence of NSC71948 or NSC119913 (both at 30 μM). The activity of luciferase in the absence of added compound was set at 100%. Data shown are mean (± S.D.) luciferase activity derived from two separate experiments, each performed in duplicate. D, BSA counterscreen. The ability of NSC71948 (30 μM) or NSC73413 (15 μM) to inhibit in vitro BAG-1:HSC70 binding was measured in the presence or absence of excess (0.1 mg/ml) BSA. Data shown are remaining activity (mean ± S.D.) in the presence of BSA and are derived from two separate experiments, each performed in duplicate.
NSC71948 Interferes with the Interaction of BAG-1 and Heat Shock Proteins in Cells.
We determined whether NSC71948 also interfered with BAG-1 interactions in intact cells. MCF7 breast cancer cells, engineered to overexpress BAG-1S, were incubated with 100 μM NSC71948 and the interaction of BAG-1 with HSC70 or HSP70 was determined by coimmunoprecipitation/immunoblot analysis. NSC71948 consistently decreased the binding of BAG-1 to HSC70 and HSP70 by 50 to 60% but had no effect on expression of BAG-1 or its ability to be immunoprecipitated with the anti-BAG-1 antibody (Fig. 3, A and B). The inhibition of binding was statistically significant (p < 0.05, Student's t test) for BAG-1:HSC70 binding, but not BAG-1:HSP70, because of greater variability between individual determinations. A control compound NSC119913 had no effect on BAG-1:HSC70 or BAG-1:HSP70 binding in intact cells (data not shown). We also analyzed the effect of NSC71948 on the interaction of two unrelated proteins, MCL-1 and BIM. NSC71948 had no effect on the interaction of MCL-1 and BIM and is therefore a relatively selective inhibitor of BAG-1:HSC70/HSP70 binding in cells (Fig. 3C).
Fig. 3.
Effect of NSC71948 on BAG-1:HSC70/HSP70 binding in intact MCF7 cells. A, MCF7-BAG-1S cells were incubated with 100 μM NSC71948, or left untreated as a control. Immunoprecipitations were performed by use of BAG-1-specific antibody TB3 from cells cultured in the presence or absence of NSC71948, or with control preimmune sera (PI). BAG-1S, HSC70, and HSP70 were analyzed by immunoblotting. B, the amounts of BAG-1-bound HSC70 and HSP70 were quantified by digital imaging. The graph shows the interaction of BAG-1 and HSC70 or HSP70 in the presence NSC71948. The level of binding in the absence of NSC71948 was used to set the 100% value. Data are mean (± S.D.) binding derived from two independent experiments each performed in duplicate. *, p <0.05 compared with untreated cells (Student's t test). C, MCF7 cells were incubated with 100 μM NSC71948, or left untreated as a control, and the binding of MCL-1 and BIM determined by coimmunoprecipitation/immunoblotting. The amount of MCL-1-bound BIM was determined by digital imaging and was set at 100% in control cells. The graph shows mean (± S.D.) MCL-1:BIM binding in the presence or absence of NSC71948. The data are derived from two independent experiments, each performed in duplicate.
NSC71948 Interferes with BAG-1-Mediated Potentiation of Vitamin D3 Receptor Activity.
BAG-1L potentiates the activity of the VDR via an HSC70/HSP70-dependent mechanism (Lee et al., 2007). Because NSC71948 interferes with BAG-1:HSC70/HSP70 binding in cells, we determined whether NSC71948 suppressed BAG-1-mediated potentiation of the VDR. In control H376 cells without BAG-1L overexpression, vitamin D3 modestly increased luciferase gene expression from a vitamin D3-responsive reporter construct (Fig. 4A). As reported previously (Lee et al., 2007), the activity of the reporter construct was significantly enhanced by overexpression of wild-type BAG-1L, but was unaffected by BAG-1LH2 which is deficient in HSC70/HSP70 binding (Lee et al., 2007). It is noteworthy that NSC71948 interfered with BAG-1L activity in a dose-dependent manner, reducing BAG-1L activity almost to that of the vector-only control at 50 μM. NSC71948 did not alter the levels of BAG-1L overexpression in H376 cells (Fig. 4B). NSC119913, which did not interfere with BAG-1:HSC70 binding, did not affect BAG-1L-mediated VDR potentiation (data not shown).
Fig. 4.
Effect of NSC71948 on regulation of VDR by BAG-1L. A, H376 cells were transfected with a vitamin D3-responsive luciferase reporter construct and the indicated BAG-1L expression constructs, or pcDNA3 as a control. Cells were also transfected with a constant amount of renilla luciferase to control for transfection efficiency. After 2 days, cells were treated with NSC71948 as indicated, and cells were stimulated with vitamin D3 (100 nM) (■) or left untreated as a control (□). Firefly and renilla luciferase activity were determined after 24 h. The figure shows normalized luciferase activity relative to control, unstimulated, pCDNA3-transfected cells (set to 1.0). Data shown are the mean (± S.D.) of triplicate transfections and are representative of four individual experiments. B, immunoblot analysis of BAG-1L expression in H376 cells transfected with the BAG-1L expression plasmid, or pcDNA3 as a control. Cells were treated with NSC71948 or NSC119913 (50 μM) as indicated.
NSC71948 Interferes with ERK1/2 Activation.
Previous studies have demonstrated that BAG-1 activates RAF-1 kinase activity, independent of HSC70/HSP70 binding (Song et al., 2001). To determine whether NSC71948 also interfered with BAG-1-mediated RAF-1 activation, we analyzed the effects of NSC71948 on phosphorylation of ERK1/2, a downstream target of RAF-1. Incubation of MCF7 cells with NSC71948 (25–50 μM) reduced phosphorylation of ERK1/2 by 85 to 90%, with little effect on total levels of ERK1/2 (Fig. 5, A and B). BAG-1 interacts directly with RAF-1, and we therefore determined the effect of NSC71948 on this interaction. The BAG-1:RAF-1 interaction was decreased by approximately 50% in NSC71948-treated cells compared with DMSO-treated cells, but was not affected by NSC119913 treatment (Fig. 5C). However, a modest reduction in RAF-1 binding was observed compared with the decrease in ERK1/2 phosphorylation.
Fig. 5.
Effect of NSC71948 on ERK1/2 phosphorylation and BAG-1:RAF-1 binding. A, MCF7 cells were treated for 2 h with the indicated concentrations of NSC71948 or left untreated as a control. Expression of total and phosphorylated ERK1/2 was analyzed by immunoblotting. The data are representative of two experiments performed in duplicate. B, inhibition of ERK1/2 phosphorylation by NSC71948 was quantified by digital imaging. The data shown are mean (± S.D.) ERK1/2 phosphorylation derived from two independent experiments each performed in duplicate. C, MCF7 cells were treated with DMSO, NSC71948, or NSC119913 (both at 50 μM) for 16 h and immunoprecipitations performed by use of a preimmune (PI) or BAG-1-specific antisera (TB3). The expression of RAF-1 and BAG-1 in whole cell lysates (left) and immunoprecipitates (right) was detected by immunoblotting. The data shown are representative of four experiments.
Growth Inhibitory Activity of NSC71948.
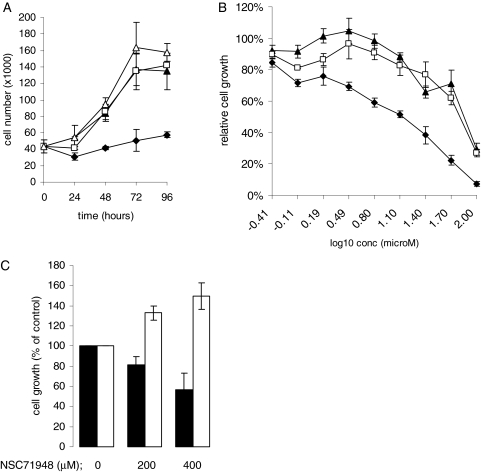
We treated ZR-75-1 breast cancer cells with NSC71948 or NSC119913 (both 50 μM) and determined their effect on cell proliferation (Fig. 6A). ZR-75-1 cells were selected for these studies because they express relatively high levels of BAG-1 compared with other breast cancer cell lines, and both overexpression and dominant negative experiments have suggested that BAG-1 plays an important role in the proliferation and survival of these cells (Brimmell et al., 1999; Kudoh et al., 2002). Whereas control cells reached confluence within 3 days, NSC71948 significantly decreased the rate of cell growth in ZR-75-1 cells. NSC119913 had no effect on cell growth. When measured in 6-day growth inhibition assays (Fig. 6B), the mean IC50 for NSC71948 was 19 ± 6 μM (mean derived from three independent experiments, each performed in triplicate ± S.D.). DMSO control-treated cells showed some growth inhibition in this assay at higher concentrations, but NSC119913 did not increase cell growth inhibition at any concentration relative to DMSO.
Fig. 6.
Growth inhibition by NSC71948. A, ZR-75-1 cells were plated at a density of 20,000 cells/well of a 24-well plate and allowed to recover for 24 h. Cell number was determined (time, 0 h) and the remaining wells were treated with 50 μM NSC71948 (♦), 50 μM NSC119913 (▴), DMSO (□), or left untreated as control (▵). Cell numbers were determined for up to 4 days. Results shown are mean of duplicate determinations (± S.D.). B, ZR-75-1 cells were treated with the indicated concentrations of NSC71948 (♦), NSC119913 (▴), or DMSO (□). After 6 days, cell number was determined by use of the CellTiter assay. Values obtained for untreated cells were set to 100%. Data are mean of triplicate determinations (± S.D.) and are representative of three independent experiments. C, wild-type (■) and BAG-1-deficient (□) MEFs were treated with 200 or 400 μM NSC71948, or were left untreated as a control. After 3 days, cell number was determined by use of the CellTiter assay. Values obtained for untreated cells were set to 100%. Data are mean of two experiments (± S.D.), each performed in triplicate.
We also investigated the effects of NSC71948 on wild-type and BAG-1-deficient MEFs (Götz et al., 2005). Wild-type MEFs were relatively resistant to growth inhibitory effects of NSC71948 compared with human ZR-75-1 breast cancer cells. The growth of wild-type MEFs was reduced when cells were treated with 200 or 400 μM NSC71948 compared with controls, but we were unable to define an IC50 in these cells because growth inhibition did not exceed 50% at any concentration tested (Fig. 6C). By contrast, NSC71948 did not inhibit the growth of BAG-1-deficient MEFs at these concentrations. In fact, the growth of these cells was modestly enhanced by NSC71948. These experiments were performed by use of NSC71948 stocks prepared with complete growth medium to avoid potential effects of DMSO at these higher compound concentrations. However, we were unable to test higher concentrations because of problems with poor solubility.
Effects of Structurally Related Compounds.
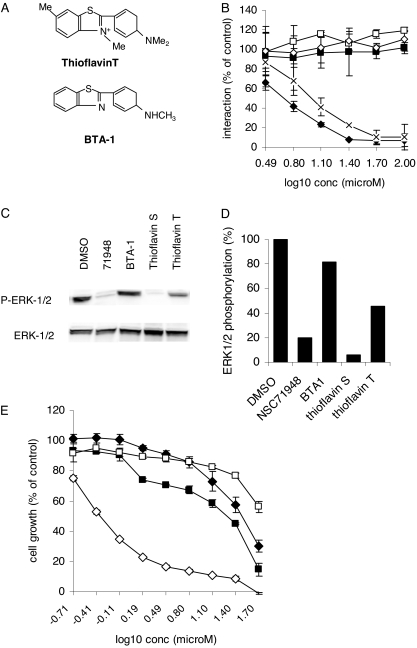
These data demonstrate that NSC71948 interferes with BAG-1 protein interactions. NSC71948 shows some selectivity toward the BAG-1:HSC70 interaction because it did not inhibit MCL-1:BIM binding in intact cells and was not active in any of the in vitro counterscreens. However, NSC71948 can be used as a stain to identify amyloid fibrils (Guntern et al., 1992) and is not therefore entirely specific for BAG-1/HSC70. Binding of NSC71948 to amyloid fibrils is thought to depend on interaction with β-sheets within amyloid proteins. Neither BAG-1 nor HSC70 contain β-sheet structures, suggesting that the effects of NSC71948 on these interactions may be mediated by structural features that are distinct from those required for binding to amyloid fibrils. To investigate the relationship between amyloid binding and inhibition of BAG-1:HSC70 binding, we analyzed the activity of a small series of structurally related proteins that can also be used to stain amyloid fibrils. We analyzed thioflavin T and BTA-1, an uncharged analog of thioflavin T (Fig. 7A). BTA-1, has a higher affinity for binding to Aβ(1–40) amyloid fibrils than thioflavin T (Ki 20 versus 890 nM) (Klunk et al., 2001). In these assays we also tested the effects of thioflavin S (from Sigma Chemical).
Fig. 7.
Analysis of structurally related compounds. (A) Structures of thioflavin S/NSC71948, thioflavin T and BTA1. (B) Inhibition of the in vitro BAG-1:HSC70 interaction. The experiment shows the mean (± S.D.) of duplicate determinations of BAG-1:HSC70 binding in the presence of the indicated concentrations of NSC71948 (♦), thioflavin S (×), thioflavin T (♢), BTA-1 (■), or DMSO (□). Binding in the absence of any added compound was set to 100%. The data are representative of two independent experiments. C, MCF7 cells were treated for 2 h with NSC71948, thioflavin S, thioflavin T, or BTA-1 (each at 50 μM), or DMSO as control. Expression of total and phosphorylated ERK1/2 was analyzed by immunoblotting. Data shown are representative of two experiments, each performed in duplicate. D, quantitation of effects of compounds (50 μM) on ERK1/2 phosphorylation. E, ZR-75-11 cells were treated with the indicated concentrations of NSC71948 (♦), thioflavin T (♢), BTA1 (■), or DMSO (□) as a control. After 6 days, cell number was determined by use of the CellTiter assay. Values obtained for untreated cells were set to 100%. Data are mean of triplicate determinations (± S.D.).
We first compared the effects of the compounds on in vitro interaction of BAG-1 with HSC70 (Fig. 7B). As before, binding was inhibited by NSC71948, with similar results obtained by use of an independent supply of thioflavin S. In contrast, the BAG-1:HSC70 interaction was completely unaffected by thioflavin T or BTA-1 at concentrations up to 100 μM. We also tested the effects of the compounds on ERK1/2 phosphorylation (Fig. 7, C and D). As before, NSC71948 (and the additional stock of thioflavin S) effectively decreased phosphorylation of ERK1/2. ERK1/2 phosphorylation was completely unaffected by BTA-1, even though BTA-1 would be expected to enter cells more readily than thioflavins. In addition thioflavin T modestly decreased ERK1/2 phosphorylation by approximately 50%. Finally, we tested the effects of the compounds on ZR-75-1 cell growth (Fig. 7E). BTA-1 (IC50 19 ± 1.4 μM; mean derived from three independent experiments each performed in triplicate ± S.D.) inhibited the growth of ZR-75-1 breast cancer cells in a manner similar to NSC71948 (IC50 31 ± 4 μM); however, thioflavin T showed potent growth inhibitory effects (IC50 1 ± 0.6 μM).
Discussion
Overexpression of BAG-1 enhances survival and proliferation in many cell lineages including primary cardiac myocytes, neuronal cells, and multiple cancer cell types. Moreover, BAG-1 is essential for the survival of hematopoietic and neuronal cells in vivo (Götz et al., 2005). Several studies have demonstrated that reducing expression or function of BAG-1 (by siRNA, antisense of overexpression of a dominant negative mutant) is sufficient to promote apoptosis and/or growth inhibition (Kudoh et al., 2002; Takahashi et al., 2003; Xiong et al., 2003; Clemo et al., 2008). Although the molecular mechanism of how BAG-1 promotes proliferation and survival of such a diverse range of cell types remains to be uncovered, overexpression studies have demonstrated that this activity frequently requires the C-terminal BAG domain that acts as a docking site for various proteins, including RAF-1, HSC70, HSP70, and CHIP. Therefore, chemical inhibitors of BAG-1 C-terminal protein binding would be valuable tools to investigate the functional significance of these interactions for endogenous BAG-1 and potentially as leads for the development of new therapeutics to interfere with BAG-1-mediated regulation of survival and NHR function in malignant cells.
Our work has taken the first steps toward the identification of chemical probes to investigate BAG-1 protein interactions. In general, protein-protein interactions are considered challenging targets for the development of small-molecule inhibitors, although there are some notable exceptions, such as ABT-737, which prevents interaction between prosurvival Bcl-2 family proteins and their proapoptotic BH3-only binding partners. In addition to these studies of small chemical inhibitors, we have also analyzed the activity of a series of overlapping peptides derived from the BAG domain to interfere with BAG-1S:HSC70 binding (submitted for publication). We identified a relatively small peptide (12 amino acids) derived from helix 2 of the BAG domain that was sufficient to bind HSC70 and block the BAG-1:HSC70 interaction. This suggests that there may be discrete “hot spots” on BAG-1 that play critical roles in mediating the interaction with HSC70/HSP70, consistent with the idea that the interactions may be inhibited by small chemical compounds.
We identified NSC71948 as a potent and potentially selective inhibitor of BAG-1 C-terminal interactions. NSC71948 inhibited BAG-1:HSC70 binding in vitro but promisingly did not show activity in any of the counterscreens. Moreover, NSC71948 inhibited binding of BAG-1 to HSC70/HSP70 and RAF-1 in intact cells, but had little effect on binding of two unrelated proteins. NSC71948 also interfered with the ability of BAG-1 to enhance transcriptional activation by vitamin D3, an activity that depends on C-terminal interactions (Lee et al., 2007) and decreased ERK1/2 phosphorylation, a downstream target of RAF-1. Because NSC71948 interfered with binding of BAG-1 to HSC70/HSP70 and RAF-1, we predict that it may interact directly with BAG-1. The binding site for NSC71948 may lie within helix 2 that is required for the interaction with both HSC70/HSP70 and RAF-1 (Briknarová et al., 2001; Sondermann et al., 2001; Song et al., 2001). Alternatively, NSC71948 may bind elsewhere within BAG-1 and elicit a conformational change in the BAG domain leading to loss of binding of multiple interaction partners.
Selectivity is a major concern with any chemical inhibitor. Indeed, the majority of the confirmed “hits” identified in primary screening were either false positives (i.e., they interfered with direct BAG-1S immunodetection) or seemed to act nonselectively (because they inhibited luciferase activity and/or their activity was significantly repressed by excess BSA). Although we have demonstrated a degree of selectivity for NSC71948 in its effects on BAG-1 interactions by use of both in vitro and cell based assays, it is important to recognize the clear potential for additional off-target effects. NSC71948 binds amyloid fibrils which demonstrates interaction with other protein targets; therefore, we analyzed structurally related compounds that stain amyloid fibrils to explore the potential structure features that mediate these effects. In particular, BTA-1 binds to Aβ(1–40) approximately 50-fold more effectively than thioflavin T (Klunk et al., 2001). However, neither thioflavin T or BTA-1 interfered with BAG-1:HSC70 binding in vitro, suggesting that the structural features of these molecules important for amyloid fibril binding are distinct from those of NSC71948 required for inhibition of BAG-1:HSC70 binding. Thus, it may be possible to synthesize NSC71948/thioflavin S-like molecules with enhanced BAG-1 binding activity and decreased binding to other targets.
In addition to its effects on VDR function and RAF-1 signaling, NSC71948 inhibited the growth of BAG-1 expressing ZR-75-1 human breast cancer cells. These results are consistent with previous studies demonstrating that BAG-1 is required for optimal cell proliferation and survival (Kudoh et al., 2002; Takahashi et al., 2003; Xiong et al., 2003; Götz et al., 2005; Clemo et al., 2008). To directly investigate the role of BAG-1 in mediating the growth inhibitory effects of NSC71948, we analyzed the effects of NSC71948 in wild-type and BAG-1-deficient MEFs. The growth of BAG-1-deficient MEFs was reduced compared with wild-type cells, consistent with the idea that endogenous levels of BAG-1 are required for optimal proliferation in these cells (data not shown). Moreover, NSC71948 inhibited the growth of wild-type, but not BAG-1-deficient MEFs. These results suggest that the growth inhibitory effects of NSC71948 are mediated, at least in part, via inactivation of BAG-1, although they do not discriminate whether these effects are due to inhibition of HSC70/HSP70 or RAF-1 function. We were surprised to find that the growth of BAG-1-deficient cells was modestly enhanced by NSC71948. This effect is clearly independent of BAG-1. However, the BAG domain is present in five distinct human proteins (Takayama and Reed, 2001), at least some of which also interact with HSC70/HSP70 and modulate cell growth/survival (Wang et al., 2008). Thus, NSC71948 may exert effects on other BAG proteins. Alternatively, this effect may reflect nonspecific activity, independent of BAG family proteins.
Despite these encouraging data, it is very important to recognize that the specificity of NSC71948 remains incompletely defined, and growth inhibitory effects may be only partially related to any effects on BAG-1. Indeed, thioflavin T demonstrated potent growth inhibitory activity, whereas effects on ERK1/2 phosphorylation were modest and it did not inhibit BAG-1:HSC70 binding. Presumably, the growth inhibitory effects of thioflavin T are exerted via an alternate pathway. In addition, BTA-1 induced growth inhibition to the same extent as NSC71984, although it did not alter BAG-1:HSC70 binding. Thus, the contribution of decreased BAG-1 function to NSC71948-induced growth inhibition remains unclear.
In summary, we have developed a robust assay to identify inhibitors of the in vitro BAG-1:HSC70 interaction and have identified NSC71948/thioflavin S as a potential inhibitor of BAG-1 C-terminal protein-protein interactions. NSC71948 may be a useful tool to investigate the functional role of BAG-1 interactions. However, NSC71948 is a mixture of compounds (Kelényi, 1967), and caution will be required in interpreting results from such studies because the selectivity profile of this compound is not fully known and off-target effects are possible. Clearly, NSC71948 is not an attractive starting point for medicinal chemistry, but further screens of additional libraries, especially those comprising more “drug-like” molecules, is warranted because inhibition of BAG-1 function may be an attractive strategy to reduce growth of BAG-1-overexpressing tumors.
Acknowledgments
We thank Prof. Carsten Carlberg (University of Kuopio, Finland) for the (DR3)4tkLUC plasmid, the NCI-DTP for providing compounds, and Prof. Stefan Wiese (Ruhr-University, Bochum, Germany) for supplying wild-type and BAG-1-deficient MEFs.
This work was supported by the Biotechnology and Biological Sciences Research Council [Grant BB/C005783/1]; and the Breast Cancer Campaign and Cancer Research UK [Grant C2750/A2740].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.153601
- BAG-1
- Bcl-2 associated athanogene
- ABT-737
- N-{4-[4-(4′-chloro-biphenyl-2-ylmethyl)-piperazin-1-yl]-benzoyl}-4-(3-dimethylamino-1-phenylsulfanylmethyl-propylamino)-3-nitro-benzenesulfonamide. BTA-1, 2-(4′-methylaminophenyl)benzothiazole
- CHIP
- carboxy terminus of Hsc70 interacting protein
- ERK1/2
- extracellular signal-regulated kinase 1/2
- NHR
- nuclear hormone receptors
- NCI-DTP
- National Cancer Institute Developmental Therapeutics Program
- DMEM
- Dulbecco's modified Eagle's medium
- FCS
- fetal calf serum
- MEF
- mouse embryo fibroblast
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- siRNA
- small interfering RNA
- VDR
- vitamin D receptor
- NLS
- nuclear localization sequence
- ULD
- ubiquitin-like domain
- thioflavin T
- 4-(3,6-dimethyl-1,3-benzothiazol-3-ium-2-yl)-N,N-dimethylaniline chloride.
References
- Alberti et al., 2002.Alberti S, Demand J, Esser C, Emmerich N, Schild H, Hohfeld J. (2002) Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem 277:45920–45927 [DOI] [PubMed] [Google Scholar]
- Arndt et al., 2007.Arndt V, Rogon C, Höhfeld J. (2007) To be, or not to be—molecular chaperones in protein degradation. Cell Mol Life Sci 64:2525–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briknarová et al., 2001.Briknarová K, Takayama S, Brive L, Havert ML, Knee DA, Velasco J, Homma S, Cabezas E, Stuart J, Hoyt DW, et al. (2001) Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat Struct Biol 8:349–352 [DOI] [PubMed] [Google Scholar]
- Brimmell et al., 1999.Brimmell M, Burns JS, Munson P, McDonald L, O'Hare MJ, Lakhani SR, Packham G. (1999) High level expression of differentially localized BAG-1 isoforms in some oestrogen receptor-positive human breast cancers. Br J Cancer 81:1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemo et al., 2008.Clemo NK, Collard TJ, Southern SL, Edwards KD, Moorghen M, Packham G, Hague A, Paraskeva C, Williams AC. (2008) BAG-1 is up-regulated in colorectal tumour progression and promotes colorectal tumour cell survival through increased NF-kappaB activity. Carcinogenesis 29:849–857 [DOI] [PubMed] [Google Scholar]
- Cutress et al., 2003.Cutress RI, Townsend PA, Sharp A, Maison A, Wood L, Lee R, Brimmell M, Mullee MA, Johnson PW, Royle GT, et al. (2003) The nuclear BAG-1 isoform, BAG-1L, enhances oestrogen-dependent transcription. Oncogene 22:4973–4982 [DOI] [PubMed] [Google Scholar]
- Demand et al., 2001.Demand J, Alberti S, Patterson C, Höhfeld J. (2001) Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol 11:1569–1577 [DOI] [PubMed] [Google Scholar]
- Elliott et al., 2007.Elliott E, Tsvetkov P, Ginzburg I. (2007) BAG-1 associates with Hsc70.Tau complex and regulates the proteasomal degradation of Tau protein. J Biol Chem 282:37276–37284 [DOI] [PubMed] [Google Scholar]
- Götz et al., 2004.Götz R, Kramer BW, Camarero G, Rapp UR. (2004) BAG-1 haplo-insufficiency impairs lung tumorigenesis. BMC Cancer 4:85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz et al., 2005.Götz R, Wiese S, Takayama S, Camarero GC, Rossoll W, Schweizer U, Troppmair J, Jablonka S, Holtmann B, Reed JC, et al. (2005) Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci 8:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntern et al., 1992.Guntern R, Bouras C, Hof PR, Vallet PG. (1992) An improved thioflavine S method for staining neurofibrillary tangles and senile plaques in Alzheimer's disease. Experientia 48:8–10 [DOI] [PubMed] [Google Scholar]
- Gurusamy et al., 2009.Gurusamy N, Lekli I, Gherghiceanu M, Popescu LM, Das DK. (2009) BAG-1 induces autophagy for cardiac cell survival. Autophagy 5:120–121 [DOI] [PubMed] [Google Scholar]
- Kelényi, 1967.Kelényi G. (1967) On the histochemistry of azo group-free thiazole dyes. J Histochem Cytochem 15:172–180 [DOI] [PubMed] [Google Scholar]
- Klunk et al., 2001.Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Mathis CA. (2001) Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci 69:1471–1484 [DOI] [PubMed] [Google Scholar]
- Kudoh et al., 2002.Kudoh M, Knee DA, Takayama S, Reed JC. (2002) Bag1 proteins regulate growth and survival of ZR-75–1 human breast cancer cells. Cancer Res 62:1904–1909 [PubMed] [Google Scholar]
- Lee et al., 2007.Lee SS, Crabb SJ, Janghra N, Carlberg C, Williams AC, Cutress RI, Packham G, Hague A. (2007) Subcellular localisation of BAG-1 and its regulation of vitamin D receptor-mediated transactivation and involucrin expression in oral keratinocytes: implications for oral carcinogenesis. Exp Cell Res 313:3222–3238 [DOI] [PubMed] [Google Scholar]
- Liman et al., 2005.Liman J, Ganesan S, Dohm CP, Krajewski S, Reed JC, Bähr M, Wouters FS, Kermer P. (2005) Interaction of BAG1 and Hsp70 mediates neuroprotectivity and increases chaperone activity. Mol Cell Biol 25:3715–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders et al., 2000.Lüders J, Demand J, Höhfeld J. (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 275:4613–4617 [DOI] [PubMed] [Google Scholar]
- Maeng et al., 2008.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, McCammon J, Schloesser RJ, Zhou R, Du J, et al. (2008) BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci U S A 105:8766–8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer and Bukau, 2005.Mayer MP, Bukau B. (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough and Patterson, 2003.McDonough H, Patterson C. (2003) CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern et al., 2002.McGovern SL, Caselli E, Grigorieff N, Shoichet BK. (2002) A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem 45:1712–1722 [DOI] [PubMed] [Google Scholar]
- Millar et al., 2009.Millar EK, Anderson LR, McNeil CM, O'Toole SA, Pinese M, Crea P, Morey AL, Biankin AV, Henshall SM, Musgrove EA, et al. (2009) BAG-1 predicts patient outcome and tamoxifen responsiveness in ER-positive invasive ductal carcinoma of the breast. Br J Cancer 100:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr et al., 2008.Orr AL, Huang S, Roberts MA, Reed JC, Li S, Li XJ. (2008) Sex-dependent effect of BAG1 in ameliorating motor deficits of Huntington disease transgenic mice. J Biol Chem 283:16027–16036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham et al., 1997.Packham G, Brimmell M, Cleveland JL. (1997) Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J 328 (Pt 3):807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann et al., 2001.Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I. (2001) Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291:1553–1557 [DOI] [PubMed] [Google Scholar]
- Song et al., 2001.Song J, Takeda M, Morimoto RI. (2001) Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol 3:276–282 [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 2003.Takahashi N, Yanagihara M, Ogawa Y, Yamanoha B, Andoh T. (2003) Down-regulation of Bcl-2-interacting protein BAG-1 confers resistance to anti-cancer drugs. Biochem Biophys Res Commun 301:798–803 [DOI] [PubMed] [Google Scholar]
- Takayama and Reed, 2001.Takayama S, Reed JC. (2001) Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol 3:E237–241 [DOI] [PubMed] [Google Scholar]
- Takayama et al., 1999.Takayama S, Xie Z, Reed JC. (1999) An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 274:781–786 [DOI] [PubMed] [Google Scholar]
- Townsend et al., 2004.Townsend PA, Cutress RI, Carroll CJ, Lawrence KM, Scarabelli TM, Packham G, Stephanou A, Latchman DS. (2004) BAG-1 proteins protect cardiac myocytes from simulated ischemia/reperfusion-induced apoptosis via an alternate mechanism of cell survival independent of the proteasome. J Biol Chem 279:20723–20728 [DOI] [PubMed] [Google Scholar]
- Townsend et al., 2003a.Townsend PA, Cutress RI, Sharp A, Brimmell M, Packham G. (2003a) BAG-1 prevents stress-induced long-term growth inhibition in breast cancer cells via a chaperone-dependent pathway. Cancer Res 63:4150–4157 [PubMed] [Google Scholar]
- Townsend et al., 2003b.Townsend PA, Cutress RI, Sharp A, Brimmell M, Packham G. (2003b) BAG-1: a multifunctional regulator of cell growth and survival. Biochim Biophys Acta 1603:83–98 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2008.Wang HQ, Liu HM, Zhang HY, Guan Y, Du ZX. (2008) Transcriptional upregulation of BAG3 upon proteasome inhibition. Biochem Biophys Res Commun 365:381–385 [DOI] [PubMed] [Google Scholar]
- Xiong et al., 2003.Xiong J, Chen J, Chernenko G, Beck J, Liu H, Pater A, Tang SC. (2003) Antisense BAG-1 sensitizes HeLa cells to apoptosis by multiple pathways. Biochem Biophys Res Commun 312:585–591 [DOI] [PubMed] [Google Scholar]