Abstract
Phosphodiesterase (PDE)-2 is a component of the nitric-oxide synthase (NOS)/guanylyl cyclase signaling pathway in the brain. Given recent evidence that pharmacologically induced changes in NO-cGMP signaling can affect anxiety-related behaviors, the effects of the PDE2 inhibitors (2-(3,4-dimethoxybenzyl)-7-det-5-methylimidazo-[5,1-f][1,2,4]triazin-4(3H)-one) (Bay 60-7550) and 3-(8-methoxy-1-methyl-2-oxo-7-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-5-yl)benzamide (ND7001), as well as modulators of NO, were assessed on cGMP signaling in neurons and on the behavior of mice in the elevated plus-maze, hole-board, and open-field tests, well established procedures for the evaluation of anxiolytics. Bay 60-7550 (1 μM) and ND7001 (10 μM) increased basal and N-methyl-d-aspartate- or detanonoate-stimulated cGMP in primary cultures of rat cerebral cortical neurons; Bay 60-7550, but not ND7001, also increased cAMP. Increased cGMP signaling, either by administration of the PDE2 inhibitors Bay 60-7550 (0.5, 1, and 3 mg/kg) or ND7001 (1 mg/kg), or the NO donor detanonoate (0.5 mg/kg), antagonized the anxiogenic effects of restraint stress on behavior in the three tests. These drugs also produced anxiolytic effects on behavior in nonstressed mice in the elevated plus-maze and hole-board tests; these effects were antagonized by the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (20 mg/kg). By contrast, the NOS inhibitor Nω-nitro-l-arginine methyl ester (50 mg/kg), which reduces cGMP signaling, produced anxiogenic effects similar to restraint stress. Overall, the present behavioral and neurochemical data suggest that PDE2 may be a novel pharmacological target for the development of drugs for the treatment of anxiety disorders.
Anxiety disorders are among the most prevalent of psychiatric illnesses, which has led to the search for their neurobiological bases as well as molecular targets for anxiolytic drug development. The limbic system and the hypothalamic-pituitary-adrenal axis are important mediators of anxiety and responses to stress (Chrousos and Gold, 1992; Ray et al., 1993). Exposure to aversive stimuli produces numerous physiological and behavioral effects in animals, including changes in endocrine function, food intake, body weight, body temperature, and goal-oriented behaviors (Dallman et al., 1992; Lang et al., 2000). Recent work has suggested that cGMP signaling may be involved in anxiety-related behavior (Masood et al., 2008).
Cyclic GMP formed by the action of the soluble isoform of guanylyl cyclase is hydrolyzed by enzymes of the PDE superfamily. Twenty-one phosphodiesterase (PDE) genes have been identified and classified into 11 families. PDE2, a cGMP-stimulated PDE, is a 105-kDa homodimer that exists in particulate and soluble forms. Although cGMP is the preferred substrate and effector molecule for this enzyme, PDE2 hydrolyzes both cGMP and cAMP with positively cooperative kinetics. At physiological concentrations of cyclic nucleotides, PDE2 responds to elevated cGMP with increased hydrolysis of cAMP (Manganiello et al., 1990). Therefore, PDE2 may be a link between cAMP- and cGMP-mediated signaling mechanisms (Hajjhussein et al., 2007).
PDE2 is highly expressed in the brain and adrenal gland (Boess et al., 2004; Nikolaev et al., 2005). A reduction in PDE2 activity through pharmacological inhibition of this enzyme would result in an increase in cGMP that could influence anxiety/stress-related events. Consistent with this, it has been found that Bay 60-7550, a selective inhibitor of PDE2, prevents the neurochemical and anxiogenic-like behavioral effects of oxidative stress in mice through a cGMP-mediated process (Masood et al., 2008). This may have relevance to neuropsychiatric disorders that involve oxidative stress (Gingrich, 2005).
NO has been implicated in neuroprotective and pathological states, including those involved in anxiety and responses to stress (Moncada et al., 1991; Thippeswamy et al., 2001). NO is generated through nitric-oxide synthase (NOS), which can be activated by Ca2+ influx through N-methyl-d-aspartate (NMDA) receptors on neurons. Pharmacological studies suggest that NO plays a role in anxiety- and stress-related processes. Intra-amygdalar or intrahippocampal administration of Nω-nitro-l-arginine methyl ester (l-NAME), a NOS inhibitor, induces anxiogenic effects in rats in the elevated plus-maze (Monzón et al., 2001). By contrast, l-arginine, an NO donor, produces anxiolytic effects, suggesting an antistress profile for NO in the central nervous system (Masood et al., 2003, 2004). In addition, mice deficient in cyclic cGMP kinase (i.e., cyclic cGMP kinase II), a downstream mediator of cGMP, exhibit an anxiogenic behavioral profile (Werner et al., 2004). Finally, inhibition of NOS, which reduces cGMP signaling, attenuates the behavioral effects of the benzodiazepine chlordiazepoxide (Elfline et al., 2004). Overall, these data suggest a role for NO-cGMP signaling in anxiety.
The present study investigated the effects of PDE2 inhibition on anxiety-related behaviors and responses to stress and assessed the involvement of cGMP signaling in their actions. The effects of two selective PDE2 inhibitors, Bay 60-7550 and ND7001, as well as NO modulators, were tested using anxiety-related behavioral models with stressed and nonstressed mice. Furthermore, neurochemical measures were obtained to determine the effects of PDE2 inhibition on cGMP and cAMP signaling in primary cultures of cerebral cortical neurons. It was found that PDE2 inhibition results in increased cGMP signaling and anxiolytic effects on behavior.
Materials and Methods
Animals
Male ICR mice weighing 28 to 35 g (Harlan, Indianapolis, IN) were used. Mice were housed in groups of three to four in a colony maintained at 21°C. Rodent chow and tap water were freely available. Standard laboratory conditions, with a 12-h light/12-h dark cycle (lights on at 7:00 AM) were maintained. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996) and were approved by the West Virginia University Institutional Animal Care and Use Committee.
Mice were subjected to a restraint stress procedure for 24 h at room temperature by immobilizing them in adjustable restrainers (Braintree Scientific, Braintree, MA) and then returned to their home cages before behavioral testing; nonstressed mice were left in their home cages. Mice exposed to restraint stress did not have access to food or water; nonstressed controls were treated similarly. This restraint stress protocol has been demonstrated to induce endocrinological, physiological, and behavioral effects indicative of anxiety (Ray et al., 1992; Choo et al., 2002). In the present study, it did not affect the overall health status of the mice, as indicated by unchanged body weights relative to nonstressed mice.
Drugs and Chemicals
Detanonoate, l-NAME, MK-801, erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA), NMDA, poly-l-lysine, plasma-derived horse serum, and DNase were purchased from Sigma-Aldrich (St. Louis, MO), and diazepam was from MedVet International (Mettawa, IL). Dulbecco's modified Eagle's medium (DMEM), penicillin G, streptomycin, and amphotericin B (Fungizone) were purchased from Invitrogen (Carlsbad, CA). Trypsin was purchased from Worthington Biochemicals (Freehold, NJ), and bicinchoninic acid protein assay kits from Pierce Chemical (Rockford, IL). Tetrodotoxin was purchased from Alexis Laboratories (San Diego, CA) and Calbiochem (San Diego, CA). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was purchased from Axxora (San Diego, CA). Bay 60-7550 (97% purity) was generously provided by Bayer AG (Wuppertal, Germany) and ND7001 (> 95% purity) was synthesized at the University of New Mexico (Albuquerque, NM). All other supplies and reagents were purchased from VWR (Willard, OH) or Thermo Fisher Scientific (Waltham, MA).
Drug Treatments
Bay 60-7550 (0.5, 1, and 3 mg/kg), ND7001 (0.5, 1.0, and 3 mg/kg), detanonoate (0.5 mg/kg), l-NAME (50 mg/kg), or diazepam (1 mg/kg) was administered after restraint stress and 30 min before behavioral testing. Mice also were treated with Bay 60-7550 (3 mg/kg), ND7001 (3 mg/kg), detanonoate, (0.5 mg/kg), l-NAME (50 mg/kg), or diazepam (1 mg/kg) in the absence of restraint stress; drugs were administered 30 min before the behavioral tests. Bay 60-7550 shows 50-fold selectivity for PDE2 compared with PDE1, 100-fold compared with PDE5, and greater than 200-fold compared with the other PDE families (Boess et al., 2004). ND7001 exhibits at 1east 100-fold selectivity for inhibition of PDE2 relative to other PDE families (Abarghaz et al., 2005). For antagonism tests to assess the role of cGMP signaling in the behavioral effects of the PDE2 inhibitors, ODQ, an inhibitor of soluble guanylyl cyclase (20 mg/kg; Li et al., 2004), was administered 20 min before Bay 60-7550 or ND7001.
Detanonoate and l-NAME were dissolved in distilled water, Bay 60-7550 and ODQ were dissolved in 50% dimethyl sulfoxide, and ND7001 was dissolved in 40% ethanol. Diazepam was suspended in distilled water with a drop of Tween 80. All drugs were prepared fresh and administered intraperitoneally in a volume of 2 ml/kg body weight.
PDE2 Activity Assay Using Recombinant Protein
COS-7 cells were maintained in complete DMEM (containing 10% fetal calf serum, 100 units/ml penicillin G, 100 mg/ml streptomycin, and 400 μM l-alanyl-l-glutamine) at 37°C in 5% CO2 atmosphere. A PDE2 expression plasmid (generously provided by Dr. Joe Beavo, University of Washington, Seattle, WA) was introduced into COS-7 cells using the FuGENE6 transfection reagent (Roche Diagnostics, Indianapolis, IN). Cells were lysed in solubilization buffer (275 mM NaCl, 1.5 mM MgCl2, 2 mM EGTA, 2% Triton X, 20% glycerol, and 40 mM Tris-HCl), and the cell lysates were used in the immunoprecipitation procedures. A protein A-agarose bead slurry (100 μl) was washed three times with ice-cold phosphate-buffered saline (100 mM NaCl, 2.7 mM KCl, 10.6 mM Na2HPO4, and 1.6 mM NaH2PO4) and mixed with the 5 μg of PDE2 antibody (Fabgennix, Frisco, TX) and 100 μl (2 μg/μl) of the lysate sample and rotated overnight at 4°C. The bead/sample mixture was then centrifuged at 1000g to separate the beads from the supernatant. The beads were resuspended in 100 μl of ice-cold lysis buffer (20 mM Tris, pH 7.4, 140 mM NaCl, 0.75 mM MgCl2, 1 mM EGTA, 1% Triton X-100, and 20% glycerol, containing protease and phosphatase inhibitors) to elute the PDE2 for use in the enzyme activity assays. The PDE2 activity assay was done by a modification of the two-step procedure of Thompson and Appleman (1971) (Hajjhussein et al., 2007). The recombinant PDE2 enzyme derived from COS-7 cell expression and diluted in KHEM buffer (50 mM KCl, 50 mM HEPES, 10 mM EGTA, and 1.9 mM MgCl2, pH 7.2) was mixed with different concentrations of PDE2 inhibitors (Bay 60-7550, ND7001, and EHNA) and [3H]cGMP/cGMP (5 μM) as the substrate. The mixture was then incubated for 30 min at 37°C (100 μl of reaction volume). To convert the [3H]GMP to [3H]guanosine, samples were incubated with snake venom from Crotalus atrox (Sigma-Aldrich) for 30 min at 37°C. The samples were then vortexed with a freshly prepared slurry of Dowex/water/ethanol [1:1:1, v/v] and then centrifuged for 10 min. [3H]Guanosine in the supernatant was then quantified by liquid scintillation counting. Bay 60-7550 was dissolved in dimethyl sulfoxide, EHNA was dissolved in distilled water, and ND7001 was dissolved in ethanol as 10 mM stocks and then diluted for use in assays with 20 mM Tris, pH 7.4; final concentrations of the respective solvents did not affect the assay. IC50 values at a single substrate concentration were determined by nonlinear regression analysis of the log concentration-response curves for each PDE2 inhibitor; Ki values were calculated using the method of Cheng and Prusoff (1973).
Measurement of cGMP and cAMP in Neuronal Cultures
Primary cultures of rat cerebral cortical neurons were prepared from the brains of newborn male and female rat pups as described previously (Suvarna and O'Donnell, 2002; Masood et al., 2008). Neuronal cultures were incubated at 37°C in a 5% CO2, 95% O2 atmosphere. On day 3, the media were replaced with fresh DMEM containing 10 μM β-cytosine arabinoside to diminish glial cell proliferation. On day 5, the β-cytosine arabinoside-containing media were aspirated, and regular DMEM was added to the cells. The cells were grown for seven more days before they were used on day 12 for cGMP and cAMP assays.
The cells were rinsed and then incubated for 50 min at 37°C in HEPES buffer (140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 100 nM glycine, 15 mM glucose, and 25 mM HEPES, pH 7.4) containing 1 μM tetrodotoxin. PDE2 inhibitors, MK-801, and l-NAME were added 10 min before the addition of NMDA or detanonoate. Fifteen minutes after the addition of NMDA or detanonoate, the cells were scraped into 100 μl of 0.1 N ice-cold hydrochloric acid. The cell preparations were then sonicated and centrifuged at 13,000g for 50 min at 4°C. Cyclic AMP and cGMP in supernatant were measured by enzyme-linked immunosorbent assay (Assay Designs, Ann Arbor, MI) and normalized to protein content (Smith et al., 1985; Bio-Rad Laboratories, Hercules, CA).
Behavioral Testing
Anxiogenic and anxiolytic effects on behavior were assessed using the elevated plus-maze, hole-board, and open-field tests; these tests have been shown to be sensitive to anxiolytic drugs from different pharmacological classes (Cryan and Holmes, 2005). Behavioral measures (see below) were recorded by a trained observer who was unaware of the treatment condition. Each behavioral test was carried over a period of 2 to 3 days, with treatments assessed in a random manner.
Elevated Plus-Maze Test.
The elevated plus-maze test was conducted as described previously; anxiolytic effects were inferred from increases in percentage of open-arm entries and percentage of open-arm time (Masood et al., 2008). The elevated plus-maze (San Diego Instruments, San Diego, CA) was constructed of white plastic and consisted of two open arms (30 × 5 cm) and two enclosed arms (30 × 5 × 15 cm) that extended from a central platform (5 × 5 cm). The maze was elevated 40 cm above the floor. Experiments began by placing a mouse on the central platform facing an open arm. During the first 5 min of free exploration, the number of entries into and the time spent in open and closed arms were recorded. An entry was defined as all four paws in an arm.
Hole-Board Test.
The hole-board test was conducted as described previously; anxiolytic effects were inferred from increases in the number of head-dips and the time spent head-dipping (Masood et al., 2008). The hole-board apparatus consisted of a Perspex box (60 × 60 × 35 cm) with four equidistant holes 4 cm in diameter in the floor. For the hole-board experiments, each animal was placed in the center of the hole-board and allowed to freely explore the apparatus for 5 min. The number of head-dips and total time spent in head-dipping were recorded.
Open-Field Test.
The open-field test was conducted as described previously; anxiolytic effects were inferred from a decrease in entry latency, i.e., the time to leave the start square and enter a new square, and an increase in ambulation and rearing (Masood et al., 2008). The open-field was made of white acrylic (50 × 50 cm) with 22-cm-high walls. The floor was divided into 16 squares by black parallel and intersecting lines. Mice were placed singly in one corner of the open-field and entry latency, ambulation, and rearing were recorded for 5 min.
Statistical Analysis
Data are expressed as means ± S.E.M. Data for the effects of each drug treatment, which were normally distributed, were analyzed by one-way analysis of variance followed by Bonferroni's post hoc tests. A p value <0.05 is considered statistically significant.
Results
Inhibition of PDE2 Activity by Bay 60-7550 and ND7001
The Ki values for the PDE2 inhibitors were calculated by nonlinear regression analysis of three replicates of the concentration-response curves for inhibition of PDE2-catalyzed hydrolysis of cGMP. Bay 60-7550 was the most potent, with a Ki value of 3.8 ± 0.2 nM; ND7001 and EHNA exhibited Ki values of 114 ± 24 nM and 10 ± 1 μM, respectively.
Effects of PDE2 Inhibitors on NMDA-Stimulated cGMP and cAMP in Neuronal Cultures
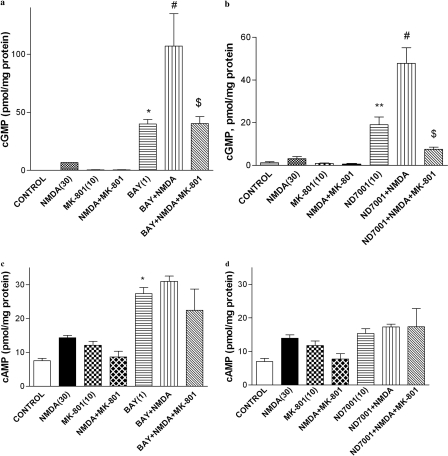
Effects of the PDE2 inhibitors Bay 60-7550 and ND7001 on NMDA receptor-mediated signaling were evaluated by measurement of cGMP and cAMP in primary cultures of rat cerebral cortical neurons (Fig. 1). Both Bay 60-7550 (1 μM) and ND7001 (10 μM) increased cGMP in the neuronal cultures compared with control [F(6,14) = 12.97, p < 0.05 and F(6,14) = 17.52, p < 0.01 for Bay 60-7550 and ND7001, respectively]. Bay 60-7550 and ND7001 in the presence of NMDA (30 μM) resulted in further increases in cGMP compared with NMDA alone. The NMDA receptor antagonist MK-801 (10 μM) blocked both Bay 60-7550 + NMDA- and ND7001 + NMDA-induced elevation in cGMP in neuronal cultures.
Fig. 1.
Effects of the PDE2 inhibitors Bay 60-7550 (BAY) and ND7001, alone and in combination with NMDA receptor modulators, on cGMP (a and b) and cAMP (c and d) in primary cultures of rat cerebral cortical neurons. Values are expressed as means ± S.E. (n = 3–4). *, p < 0.05 and **, p < 0.01 compared with control; #, p < 0.05 compared with Bay 60-7550; $, p < 0.05 compared with Bay 60-7550 or ND7001 in combination with NMDA.
Bay 60-7550 (1 μM) also increased cAMP, although the magnitude of this effect was markedly less than the increase in cGMP [F(6,14) = 12.05, p < 0.01)] (Fig. 1). A combination of Bay 60-7550 (1 μM) with NMDA (30 μM) increased cAMP compared with NMDA alone; MK-801 (10 μM) did not block the effect produced by this combination. In contrast, although there was an overall effect of treatment on cAMP in the neuronal cultures [F (6,14) = 3.31, p < 0.05], post hoc analysis showed that ND7001 (10 μM) did not increase cAMP, either alone or in combination with NMDA (Fig. 1). NMDA or MK-801, alone or in combination, did not produce any effect on cGMP or cAMP levels.
Effects of PDE2 Inhibitors on NO Donor-Stimulated cGMP in Neuronal Cultures
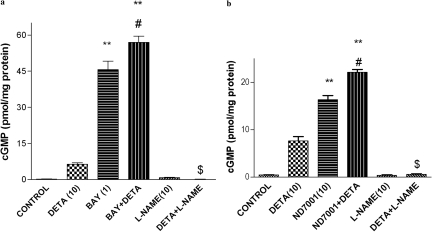
PDE2 inhibitors and NO modulators were evaluated for their effects on cGMP in neuronal cultures (Fig. 2); cGMP levels were significantly different across treatment groups [F(5,12) = 233.8 and F(5,12) = 272.7, p < 0.01 for Bay 60-7550- and ND7001-treated groups, respectively]. The NO donor detanonoate (10 μM) increased cGMP; a combination of detanonoate + Bay 60-7550 (1 μM) or ND7001 (10 μM) further increased cGMP compared with detanonoate alone, but the effect only was significant for the detanonoate + Bay 60-7550 treatment. The NOS inhibitor l-NAME (10 μM) blocked the detanonoate-induced increase in cGMP in the neuronal cultures.
Fig. 2.
Effects of the PDE2 inhibitors Bay 60-7550 (BAY; a) and ND7001 (b), alone and in combination with NO modulators, on cGMP in neuronal cultures. Values are expressed as means ± S.E. (n = 3–4). **, p < 0.01 compared with control; #, p < 0.05 compared with Bay 60-7550; $, p < 0.05 compared with detanonoate (DETA).
Effects of the PDE2 Inhibitors Bay 60-7550 and ND7001 in Anxiolytic Behavioral Tests in Stressed and Nonstressed Mice
Elevated Plus-Maze Test.
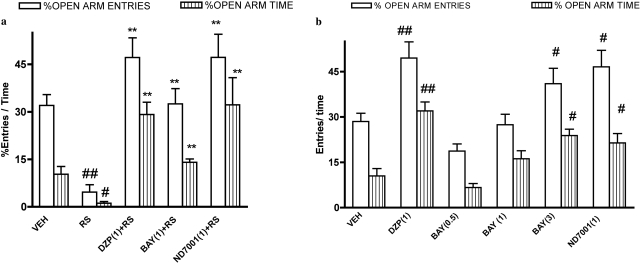
Overall analysis of elevated plus-maze data revealed that percentages of entries and time spent in open arms differed across treatment groups [F(4,26) = 13.59 (restraint stress) and F(5,33) = 9.81 (nonstressed), p < 0.01 for open-arm entries and F(4,26) = 16.13 (restraint stress) and F(5,33) = 10.60 (nonstressed), p < 0.01 for open-arm time, respectively]. Restraint stress reduced percentages of open-arm entries and open-arm time in the elevated plus-maze compared with the nonstressed condition, indicative of an anxiogenic effect (Fig. 3). The PDE2 inhibitors Bay 60-7550 (1 mg/kg) and ND7001 (1 mg/kg) reversed restraint stress-induced alterations in behavior, resulting in increased percentages of open-arm entries and open-arm time compared with the vehicle + restraint stress condition.
Fig. 3.
Effects of the PDE2 inhibitors Bay 60-7550 (BAY) and ND7001 on behavior in the elevated plus-maze in stressed (a) and nonstressed (b) mice. Increases in percentages open-arm entries and time indicate an anxiolytic effect, whereas decreases in these measures indicate an anxiogenic effect. Values are expressed as means ± S.E.M. (n = 6–8). #, p < 0.05 and ##, p < 0.01 compared with vehicle (VEH). **, p < 0.01 compared with restraint stress (RS). DZP, diazepam. Doses shown parenthetically are milligrams per kilogram intraperitoneally.
In nonstressed mice, Bay 60-7550 produced a dose-dependent increase in percentages of open-arm entries and open-arm time compared with the vehicle-treated group; significant increases were observed at a dose of 3 mg/kg (Fig. 3). ND7001 (1 mg/kg) produced a similar increase in percentages open-arm entries and open-arm time (Fig. 3).
The effects of the benzodiazepine anxiolytic drug diazepam on behavior in the elevated plus-maze in both stressed and nonstressed mice were similar to those of the PDE2 inhibitors (Fig. 3).
Overall analysis of the effects of NO-modulating drugs and PDE2 inhibitors showed that percentages open-arm entries and time were significantly different across treatment conditions [F(5,30) = 12.3 and F(5,30) = 11.76, p < 0.01, respectively]. The NO donor detanonoate (0.5 mg/kg), which like the PDE2 inhibitors increases cGMP signaling, produced an anxiolytic-like effect in the elevated plus-maze in stressed, but not nonstressed, mice (Table 1); the effects of detanonoate tended to be enhanced by cotreatment with the PDE2 inhibitors. In contrast, the NOS inhibitor l-NAME (50 mg/kg) produced an anxiogenic effect in nonstressed mice but did not alter behavior in mice subjected to restraint stress (Table 1).
TABLE 1.
Effects of the NOS inhibitor l-NAME and the NO donor detanonoate on behavior in anxiolytic tests
Values shown are means ± S.E. l-NAME (50 mg/kg) did not affect behavior in any of the tests in mice exposed to restraint stress (data not shown). Detanonoate (DETA; 0.5 mg/kg) did not affect behavior in the elevated plus-maze or hole-board in nonstressed mice; however, it did reduce entry latency and increase ambulation and rearing in nonstressed mice (p < 0.05; data not shown). Detanonoate + Bay 60-7550 (BAY; 1 mg/kg) increased percentage of open-arm time in the elevated plus-maze and reduced entry latency and increased ambulation in the open-field in nonstressed mice (p < 0.05; data not shown) but did not affect the other measures. Detanonoate + ND7001 increased percentage of open-arm time in the elevated plus-maze in nonstressed mice (p < 0.05; data not shown) but did not affect the other measures. The effect of Bay 60-7550 and ND7001 alone or in the presence of stress is shown in Figs. 4 to 6.
| Anxiolytic Test Behavioral Measure | Vehicle No Stress | l-NAME No Stress | Vehicle Stress | DETA Stress | DETA + BAY Stress | DETA + ND7001 Stress |
|---|---|---|---|---|---|---|
| Elevated plus-maze | ||||||
| % Open-arm entries | 32.0 ± 3.4 | 13.6 ± 2.3* | 4.6 ± 2.3** | 32.5 ± 4.8† | 39.6 ± 2.8†† | 40.1 ± 2.6†† |
| % Open-arm time | 10.8 ± 3.4 | 1.2 ± 1.3* | 0.8 ± 0.5* | 6.0 ± 5.4 | 20.2 ± 2.6†† | 29.0 ± 1.9†† |
| Hole-board | ||||||
| Head-dips | 17.7 ± 1.4 | 8.6 ± 1.8* | 5.2 ± 1.3* | 14.7 ± 1.2† | 15.5 ± 1.0†† | 22.9 ± 3.2†† |
| Head-dip time (s) | 29.6 ± 2.8 | 11.6 ± 1.7* | 6.4 ± 1.1** | 26.2 ± 2.9†† | 33.6 ± 4.7†† | 28.1 ± 4.0†† |
| Open-field | ||||||
| Entry latency (s) | 12.3 ± 2.2 | 28.6 ± 3.2 | 20.0 ± 3.0 | 4.6 ± 1.1† | 7.8 ± 4.3 | 6.6 ± 2.3† |
| Ambulation (crosses) | 153.5 ± 9.6 | 84.2 ± 6.4* | 108.3 ± 3.9* | 184.3 ± 19.5†† | 190.5 ± 13.9†† | 161.8 ± 12.1† |
| Rears | 50.5 ± 1.9 | 19.7 ± 3.1 | 26.2 ± 4.7* | 58.6 ± 3.4†† | 62.0 ± 2.0†† | 55.1 ± 3.5† |
Significantly different from vehicle + no stress, p < 0.05;
p < 0.01.
Significantly different from vehicle + restraint stress, p < 0.05;
p < 0.01.
For all of the tests, total arm entries did not differ significantly among the treatment groups compared with their respective controls (data not shown).
Hole-Board Test.
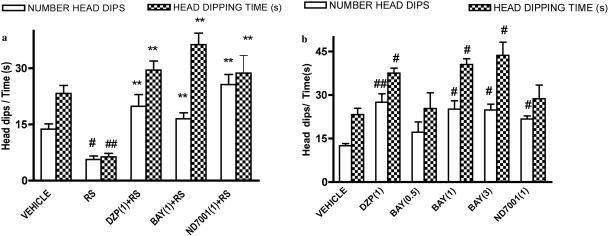
Overall analysis of the effects of PDE2 inhibitors showed that numbers of head-dips and time spent head-dipping differed across treatments [F(4,27) = 12.40 (restraint stress) and F(5,31) = 5.54 (nonstressed), p < 0.01 for number of head-dips and F(4,27) = 12.99 (stressed) and F(5,31) = 5.31 (nonstressed), p < 0.01 for head-dipping time, respectively]. Restraint stress reduced the number of head-dips and time spent head-dipping in the hole-board test compared with the nonstressed condition, indicative of an anxiogenic effect (Fig. 4). The PDE2 inhibitors Bay 60-7550 (1 mg/kg) and ND7001 (1 mg/kg) reversed the effects of restraint stress on the number of head-dips and time spent head-dipping.
Fig. 4.
Effects of the PDE2 inhibitors Bay 60-7550 (BAY) and ND7001 on behavior in the hole-board test in stressed (a) and nonstressed (b) mice. Increases in head-dips and time of head-dipping indicate an anxiolytic effect, whereas decreases in these measures indicate an anxiogenic effect. Values are expressed as means ± S.E.M. (n = 6–8). #, p < 0.05 and ##, p < 0.01 compared with vehicle (VEH); *,226, p < 0.01 compared with restraint stress (RS). DZP, diazepam. Doses shown parenthetically are milligrams per kilogram intraperitoneally.
In nonstressed mice, Bay 60-7550 increased, in a dose-dependent manner, the number of head-dips and time spent head-dipping, compared with vehicle-treated mice; significant increases were observed at doses of 1 and 3 mg/kg (Fig. 4). At a dose of 1 mg/kg, ND7001 increased the number of head-dips in nonstressed mice but did not affect time of head-dipping.
Diazepam (1 mg/kg) increased the number of head-dips and time spent head-dipping in the hole-board test in stressed mice and increased the number of head-dips in nonstressed mice (Fig. 4).
Overall analysis of the effects of NO-modulating drugs and PDE2 inhibitors showed that number of head-dips and time spent head-dipping were significantly different across treatments [F(5,30) = 7.71 and F(5,30) = 8.9, p < 0.01, with and without restraint stress, respectively]. The NO donor detanonoate (0.5 mg/kg) produced an anxiolytic-like effect in the hole-board test in stressed, but not nonstressed, mice (Table 1); the effects of detanonoate tended to be enhanced by cotreatment with the PDE2 inhibitors. By contrast, l-NAME (50 mg/kg) produced an anxiogenic effect in nonstressed mice but did not alter behavior in mice subjected to restraint stress (Table 1).
Open-Field Test.
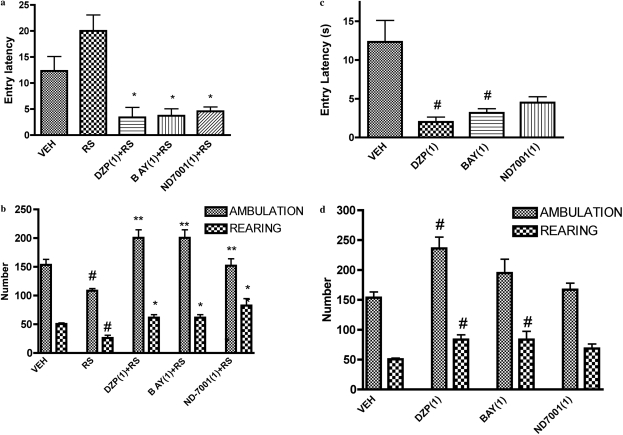
Overall analysis of open-field data showed that entry latency, ambulation, and rearing differed across treatments [F(4,26) = 12.06 (restraint stress) and F(3,20) = 9.77 (nonstressed), p < 0.01 for entry latency; F(4,26) = 11.91 (restraint stress) and F(3,20) = 4.80 (nonstressed), p < 0.01 for ambulation; and F(4,26) = 11.37 (restraint stress), F(3,20) = 3.17 (nonstressed), p < 0.01 for rearing]. Restraint reduced ambulation and rearing and increased entry latency (i.e., the time to move to an adjacent square from the start square) in the open-field compared with the nonstressed condition, indicative of an anxiogenic effect (Fig. 5). Bay 60-7550 (1 mg/kg) and ND7001 (1 mg/kg) reversed the effects of restraint stress on these measures.
Fig. 5.
Effects of the PDE2 inhibitors Bay 60-7550 (BAY) and ND7001 on behavior in the open-field test in stressed (a and b) and nonstressed (c and d) mice. Decreases in entry latency (i.e., the time to enter the rest of the open field from the start location) and increases in ambulation and rearing indicate an anxiolytic effect, whereas the opposite changes indicate an anxiogenic effect. Values are expressed as means ± S.E.M. (n = 6–8). #, p < 0.05 compared with vehicle (VEH); *, p < 0.05 and **, p < 0.01 compared with restraint stress (RS). DZP, diazepam. Doses shown parenthetically are milligrams per kilogram intraperitoneally.
In nonstressed mice, Bay 60-7550 (1 mg/kg) reduced entry latency and increased rearing but did not alter ambulation. ND7001 (1 mg/kg) did not affect any measure in the open-field test in nonstressed mice.
Diazepam (1 mg/kg) increased ambulation and rearing and reduced entry latency in the open-field test in both stressed and nonstressed mice (Fig. 5).
Overall analysis of open-field data for the effects of NO-modulating drugs showed that entry latency, ambulation, and rearing differed across treatments [F(5,30) = 9.8 for entry latency, F(5,30) = 15.77 for ambulation, and F(5,30) = 29.96 for rearing, p < 0.01]. The NO donor detanonoate (0.5 mg/kg) produced an anxiolytic-like effect in the open-field test in both stressed and nonstressed mice (Table 1); the effects of detanonoate tended to be enhanced by cotreatment with the PDE2 inhibitors. In contrast, l-NAME (50 mg/kg) tended to produce an anxiogenic effect in nonstressed mice (only the effect on ambulation was significant) but did not alter behavior in mice subjected to restraint stress (Table 1).
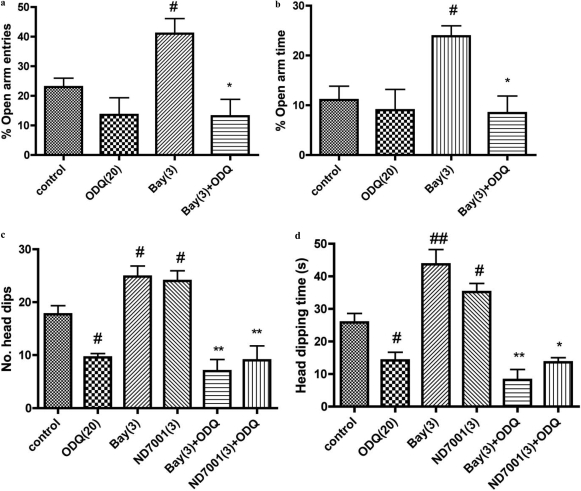
Effects of the Guanylyl Cyclase Inhibitor ODQ on the Anxiolytic Effects of Bay 60-7550 and ND7001
Overall analysis showed that the guanylyl cyclase inhibitor ODQ (20 mg/kg) antagonized the anxiolytic-like effects of Bay 60-7550 and ND7001 in the elevated plus-maze [F(3,29) = 6.96 and F(3,29) = 5.10, p < 0.01 for percentage open-arm entries and time, respectively] and hole-board tests [F(5,32) = 19.80 and F(5,32) = 27.61, p < 0.01 for number of head-dips and time of head-dipping, respectively] (Fig. 6). This was evidenced by reduced percentages of open-arm entries and time in the elevated plus-maze and reduced head-dips and time head-dipping in the hole-board test compared with values after Bay 60-7550 treatment. ODQ also antagonized the effects of ND7001 in the hole-board test; head-dips and time of head-dipping were reduced compared with values after ND7001 treatment. When administered alone, ODQ produced an anxiogenic effect in the hole-board test, reducing the number of head-dips and time of head-dipping but had no effect on behavior in the elevated plus-maze.
Fig. 6.
Antagonistic effects of the guanylyl cyclase inhibitor ODQ (20 mg/kg) on the anxiolytic effects of Bay 60-7550 (Bay) in the elevated plus-maze (a and b) and both Bay 60-7550 and ND7001 in the hole-board tests (c and d). See legends to Figs. 3 and 4 for details regarding the interpretation of behavioral effects. Values are expressed as means ± S.E.M. (n = 6–8). #, p < 0.05 and ##, p < 0.01 compared with control; *, p < 0.05 and **, p < 0.01 compared with Bay 60-7550 and ND7001. Doses shown parenthetically are milligrams per kilogram intraperitoneally.
Discussion
The PDE2 inhibitors Bay 60-7550 and ND7001, as well as the NO donor detanonoate, produced anxiolytic-like behavioral effects in both stressed and nonstressed mice; these drugs also increased cGMP signaling in neuronal cultures. By contrast, the NOS inhibitor l-NAME, which reduced cGMP signaling in neuronal cultures, produced anxiogenic effects on behavior. The anxiolytic effects resulting from PDE2 inhibition and increased NO-cGMP signaling were demonstrated using three behavioral models: elevated plus-maze, hole-board, and open-field tests. These behavioral models are well established for testing anxiolytic and anxiogenic effects on behavior and have been used to test both benzodiazepine and nonbenzodiazepine anxiolytics (Cryan and Holmes, 2005; Masood et al., 2008; Rizzi et al., 2008). Previously published data have shown that EHNA, Bay 60-7550, and ND7001 are selective inhibitors of PDE2 (van Staveren et al., 2001; Boess et al., 2004; Abarghaz et al., 2005). The present data show both Bay 60-7550 and ND7001 to be considerably more potent than the classic PDE2 inhibitor EHNA. Despite its lower potency in vitro compared with Bay 60-7550, ND7001 affected behavior at similar doses. This may be due to a limited ability of Bay 60-7550 to penetrate into the brain after peripheral administration (F. Menneti, personal communication). EHNA also is a potent inhibitor of adenosine deaminase (Rivet-Bastide et al., 1997), which limits its use in vivo as a PDE2 inhibitor. The present results show that Bay 60-7550 and ND7001 were particularly effective in reversing anxiogenic effects produced by an emotional stressor, i.e., restraint stress. Although both PDE2 inhibitors produced anxiolytic effects in the absence of restraint stress, as did the benzodiazepine anxiolytic diazepam, this was not observed for all measures. It is possible that pharmacokinetic factors could contribute to differential effects of PDE2 inhibitors in the presence and absence of restraint stress (Pollack et al., 1991; Watanabe et al., 2002).
Bay 60-7550 and ND7001 also tended to enhance the behavioral effects of the NO donor detanonoate. The anxiolytic effects probably result from enhanced NO-cGMP signaling because PDE2 inhibition is known to increase cGMP (Suvarna and O'Donnell, 2002), whereas detanonoate increases NO, which activates guanylyl cyclase, leading to an increase in cGMP (Cary et al., 2006). Consistent with this, it has been reported that mice deficient in cGMP-dependent protein kinase (i.e., cyclic cGMP kinase II), a mediator of the intracellular actions of cGMP, exhibit an anxiogenic-like behavioral phenotype (Werner et al., 2004). This kinase is highly expressed in cerebral cortex, basal forebrain, and amygdala, regions thought to be important regulators of emotionality. A previous study has reported that pretreatment with a cGMP PDE inhibitor antagonizes the anxiogenic effect of 15% N2O and enhances the anxiolytic effect of 25% N2O, implying that cGMP reduces anxiety (Li et al., 2004). Furthermore, inhibition of NOS attenuates the behavioral effects of chlordiazepoxide, which suggests some role for cGMP signaling in the mediation of the behavioral effects of benzodiazepine anxiolytics (Elfline et al., 2004). Another report also points to the importance of NO-cGMP signaling in controlling emotionality (Li et al., 2005).
The NOS inhibitor l-NAME produced anxiogenic effects on behavior in nonstressed mice. However, it did not produce an additive anxiogenic effect in mice exposed to restraint stress. This may be because restraint stress itself had pronounced effects on behavior, precluding any additional effect of l-NAME. In addition, because it has been reported that restraint leads to a decrease in NO levels in brain (Masood et al., 2004), blocking NOS with l-NAME in mice exposed to restraint stress may not lead to a further decrease in NO activity. In contrast to NOS inhibition, the NO donor detanonoate reversed the anxiogenic effects of restraint stress in mice but did not produce an anxiolytic effect on its own. Given that PDE2 inhibitors were effective, at least to some degree, in nonstressed mice, this suggests that PDE2 inhibition may produce greater or more targeted increases in cGMP signaling.
The present study showed that inhibiting cGMP synthesis using the guanylyl cyclase inhibitor ODQ reversed the anxiolytic effects of the PDE2 inhibitors Bay 60-7550 and ND7001; in the hole-board test ODQ produced an anxiogenic effect on its own. ODQ also antagonizes the effect of PDE2 inhibition on cGMP signaling in neurons (Suvarna and O'Donnell, 2002). These data indicate that the anxiolytic effects of PDE2 inhibitors depend on increased cGMP signaling. Further studies will be required to evaluate the relative importance of the effects of PDE2 inhibitors on downstream protein kinases, phosphoproteins, and cGMP-regulated ion channels that may mediate the behavioral effects.
Bay 60-7550 also has been shown to enhance memory (Boess et al., 2004; Rutten et al., 2007); this may result from the involvement of PDE2 in NMDA receptor-mediated signaling in the hippocampus (Suvarna and O'Donnell, 2002). This effect of Bay 60-7550 contrasts with those of benzodiazepine anxiolytics, which have deleterious effects on memory (Hu et al., 2006). Thus, PDE2 inhibitors, in contrast to benzodiazepines, may be able to exert anxiolytic effects without impairing memory. It remains to be determined whether the potential abuse liability of PDE2 inhibitors is favorable to those of benzodiazepines.
Previous work also has shown that Bay 60-7550 reverses the anxiogenic effects on behavior produced by oxidative stress, whereas diazepam has little effect under these conditions (Masood et al., 2008). Therefore, although PDE2-mediated increases in cGMP signaling may reverse both oxidative stress- and emotional stress-related anxiogenic behavioral effects, benzodiazepines may exhibit a somewhat different profile of effectiveness. Because long-term use of benzodiazepines in the treatment of generalized anxiety disorder and panic disorders is limited due to tolerance and dependence phenomena, studies examining the longer-term effects of PDE2 inhibitors are warranted.
NO has been shown to play a crucial role in central nervous system function (Zhang and Snyder, 1995). In the present study, the NO donor detanonoate mimicked the anxiolytic effects of diazepam in the three behavioral tests, whereas the NOS inhibitor l-NAME produced behavioral effects similar to those observed after restraint stress. These effects of NO donors and inhibitors are consistent with the results of earlier studies (De Oliveira et al., 1997; Vale et al., 1998; Monzón et al., 2001; Masood et al., 2003).
Both Bay 60-7550 and ND7001 increased cGMP in primary neuronal cultures. This was evident both for basal and NMDA-stimulated cGMP levels. Likewise, the NO donor detanonoate increased the levels of cGMP in neuronal cultures; this effect was enhanced by cotreatment with Bay 60-7550 or ND7001. These data, when considered in light of the ODQ antagonism data, suggest that anxiolytic effects on behavior can be achieved either by enhancing cGMP synthesis by increasing NO or by reducing cGMP degradation by inhibiting its hydrolysis by PDE2 (Masood et al., 2003, 2008).
Cyclic GMP signaling pathways are linked to cAMP and involve PDE2, PDE3, and protein kinase G (Vandecasteele et al., 2001). Thus, it is possible that cGMP may not be the only mediator of neurobehavioral factors associated with anxiety, but cAMP also might be playing an important role. In the present study, Bay 60-7550 increased cAMP, but this effect seemed not to be NMDA receptor-mediated. By contrast, ND7001 had minimal effects on cAMP signaling, suggesting that enhanced cGMP signaling may be sufficient to produce anxiolytic effects on behavior.
Overall, the results of the present study show that inhibition of PDE2 leads to anxiolytic behavioral effects due to increased cGMP signaling. Although PDE2 inhibitors may act via some common mechanism with benzodiazepine anxiolytics in stress disorders caused by disturbances in hypothalamic-pituitary-adrenal axis function, their pharmacological profile suggests some unique actions of PDE2 inhibitors, particularly in regards to memory function and neurodegenerative diseases involving oxidative stress (Boess et al., 2004; Masood et al., 2008). To date, only two selective PDE2 inhibitors, Bay 60-7550 and ND7001, have been shown to be suitable for in vivo use. Given knowledge of the three-dimensional structure of PDE2 and the binding profiles of the inhibitors (Iffland et al., 2005), this seems to be an area ripe for drug discovery and development efforts.
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grants R01-MH40694, R01-MH51175]; and the Kentucky Science and Engineering Foundation [Grant KSEP-925-RDE-008].
J.M.O. was supported by Memory Pharmaceuticals (Montvale, NJ), Lundbeck (Copenhagen, Denmark), and Wyeth (Collegeville, PA). C.-G.Z. has received gifted funds, consultation fees, and honoraria from Pfizer (Groton, CT), Eli Lilly (Indianapolis, IN), Reckitt Benckiser Pharmaceuticals (Berkshire, UK), Lexington Pharmaceuticals (Lexington, KY), and Lawrence Pharmaceuticals (Lexington, KY).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156729
- PDE
- phosphodiesterase
- Bay 60-7550
- (2-(3,4-dimethoxybenzyl)-7-det-5-methylimidazo-[5,1-f][1,2,4]triazin-4(3H)-one)
- NOS
- nitric-oxide synthase
- NMDA
- N-methyl-d-aspartate
- l-NAME
- Nω-nitro-l-arginine methyl ester
- ND7001
- 3-(8-methoxy-1-methyl-2-oxo-7-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-5-yl)benzamide
- MK-801
- 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate)
- EHNA
- erythro-9-(2-hydroxy-3-nonyl) adenine
- DMEM
- Dulbecco's modified Eagle's medium
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
References
- Abarghaz et al., 2005.Abarghaz M, Biondi S, Duranton J, Limanton E, Moddadori C, Wagner P. (2005), inventors; NEURO3D, assignee. Benzo[1,4]diazepin-2-one derivatives as phosphodiesterase PDE2 inhibitors, preparation and therapeutics use thereof. World Patent WO/2005/063723. 2005 July 14 [Google Scholar]
- Boess et al., 2004.Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G. (2004) Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47:1081–1092 [DOI] [PubMed] [Google Scholar]
- Cary et al., 2006.Cary SP, Winger JA, Derbyshire ER, Marletta MA. (2006) Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci 31:231–239 [DOI] [PubMed] [Google Scholar]
- Cheng and Prusoff, 1973.Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Choo et al., 2002.Choo D, Khwaja K, Nori K, Rewinski M, Zeff R, Perdrizet G. (2002) In vivo characterization of the molecular-genetic changes in gastric mucosa during the development of acute gastritis and stress ulceration. J Trauma 52:720–725; discussion 725–726 [DOI] [PubMed] [Google Scholar]
- Chrousos and Gold, 1992.Chrousos GP, Gold PW. (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252 [PubMed] [Google Scholar]
- Cryan and Holmes, 2005.Cryan JF, Holmes A. (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790 [DOI] [PubMed] [Google Scholar]
- Dallman et al., 1992.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker C, Strack AM, Cascio CS. (1992) Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–526 [DOI] [PubMed] [Google Scholar]
- De Oliveira et al., 1997.De Oliveira CL, Del Bel EA, Guimarães FS. (1997) Effects of L-NOARG on plus-maze performance in rats. Pharmacol Biochem Behav 56:55–59 [DOI] [PubMed] [Google Scholar]
- Elfline et al., 2004.Elfline GS, Branda EM, Babich M, Quock RM. (2004) Antagonism by NOS inhibition of the behavioral effects of benzodiazepine and GABAA receptor agonists in the mouse elevated plus-maze. Antagonism by NOS inhibition of the behavioral effects of benzodiazepine and GABAA receptor agonists in the mouse elevated plus-maze. Neuropsychopharmacology 29:1419–1425 [DOI] [PubMed] [Google Scholar]
- Gingrich, 2005.Gingrich JA. (2005) Oxidative stress is the new stress. Nat Med 11:1281–1282 [DOI] [PubMed] [Google Scholar]
- Hajjhussein et al., 2007.Hajjhussein H, Suvarna NU, Gremillion C, Chandler LJ, O'Donnell JM. (2007) Changes in NMDA receptor-induced cyclic nucleotide synthesis regulate the age-dependent increase in PDE4A expression in primary cortical cultures. Brain Res 1149:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al., 2006.Hu XD, Ge YX, Hu NW, Zhang HM, Zhou LJ, Zhang T, Li WM, Han YF, Liu XG. (2006) Diazepam inhibits the induction and maintenance of LTP of C-fiber evoked field potentials in spinal dorsal horn of rats. Neuropharmacology 50:238–244 [DOI] [PubMed] [Google Scholar]
- Iffland et al., 2005.Iffland A, Kohls D, Low S, Luan J, Zhang Y, Kothe M, Cao Q, Kamath AV, Ding YH, Ellenberger T. (2005) Structural determinants for inhibitor specificity and selectivity in PDE2A using wheat germ in vitro translation system. Biochemistry 44:8312–8325 [DOI] [PubMed] [Google Scholar]
- Lang et al., 2000.Lang PJ, Davis M, Ohman A. (2000) Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord 61:137–159 [DOI] [PubMed] [Google Scholar]
- Li et al., 2004.Li S, Chung E, Quock RM. (2004) Role of cyclic GMP in nitrous-oxide-induced anxiolytic-like behavior in the mouse light-dark exploration test. Behav Neurosci 118:648–652 [DOI] [PubMed] [Google Scholar]
- Li et al., 2005.Li S, Doss JC, Hardee EJ, Quock RM. (2005) Involvement of cyclic GMP-dependent protein kinase in nitrous oxide-induced anxiolytic-like behavior in the mouse light/dark exploration test. Brain Res 1038:113–117 [DOI] [PubMed] [Google Scholar]
- Manganiello et al., 1990.Manganiello VC, Tanaka T, Murshima S. (1990). Cyclic GMP-stimulated cyclic nucleotide phosphodiesterases, in Beavo J, Houslay M. (eds), pp 61–85, Cyclic Nucleotide Phosphodiesterases: Structure, Regulation and Drug Action, Wiley, New York: [Google Scholar]
- Masood et al., 2003.Masood A, Banerjee B, Vijayan VK, Ray A. (2003) Modulation of stress induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol 458:135–139 [DOI] [PubMed] [Google Scholar]
- Masood et al., 2004.Masood A, Banerji B, Vijayan VK, Ray A. (2004) Pharmacological and biochemical studies on the possible role of nitric oxide in stress adaptation in rats. Eur J Pharmacol 493:111–115 [DOI] [PubMed] [Google Scholar]
- Masood et al., 2008.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. (2008) Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther 326:369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada et al., 1991.Moncada S, Palmer RM, Higgs EA. (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142 [PubMed] [Google Scholar]
- Monzón et al., 2001.Monzón ME, Varas MM, De Barioglio SR. (2001) Anxiogenesis induced by nitric oxide synthase inhibition and anxiolytic effect of melanin-concentrating hormone (MCH) in rat brain. Peptides 22:1043–1047 [DOI] [PubMed] [Google Scholar]
- Nikolaev et al., 2005.Nikolaev VO, Gambaryan S, Engelhardt S, Walter U, Lohse MJ. (2005) Real-time monitoring of the PDE2 activity of live cells: hormone-stimulated cAMP hydrolysis is faster than hormone-stimulated cAMP synthesis. J Biol Chem 280:1716–1719 [DOI] [PubMed] [Google Scholar]
- Pollack et al., 1991.Pollack GM, Browne JL, Marton J, Haberer LJ. (1991) Chronic stress impairs oxidative metabolism and hepatic excretion of model xenobiotic substrates in the rat. Drug Metab Dispos 19:130–134 [PubMed] [Google Scholar]
- Ray et al., 1993.Ray A, Henke PG, Gulati K, Sen P. (1993) The amygdaloid complex, corticotropin releasing factor and stress-induced gastric ulcerogenesis in rats. Brain Res 624:286–290 [DOI] [PubMed] [Google Scholar]
- Ray et al., 1992.Ray A, Mediratta PK, Sen P. (1992) Modulation by naltrexone of stress-induced changes in humoral immune responsiveness and gastric mucosal integrity in rats. Physiol Behav 51:293–296 [DOI] [PubMed] [Google Scholar]
- Rivet-Bastide et al., 1997.Rivet-Bastide M, Vandecasteele G, Hatem S, Verde I, Bénardeau A, Mercadier JJ, Fischmeister R. (1997) cGMP-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. J Clin Invest 99:2710–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi et al., 2008.Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. (2008) Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol 154:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten et al., 2007.Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. (2007) Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4, and 5 inhibitors. Eur J Pharmacol 558:107–112 [DOI] [PubMed] [Google Scholar]
- Smith et al., 1985.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- Suvarna and O'Donnell, 2002.Suvarna NU, O'Donnell JM. (2002) Hydrolysis of N-methyl-d-aspartate receptor-stimulated cAMP and cGMP by PDE4 and PDE2 phosphodiesterases in primary neuronal cultures of rat cerebral cortex and hippocampus. J Pharmacol Exp Ther 302:249–256 [DOI] [PubMed] [Google Scholar]
- Thippeswamy et al., 2001.Thippeswamy T, Jain RK, Mumtaz N, Morris R. (2001) Inhibition of neuronal nitric oxide synthase results in neurodegenerative changes in the axotomised root ganglion neurons: evidence for a neuroprotective role of nitric oxide in vivo. Neurosci Res 40:37–44 [DOI] [PubMed] [Google Scholar]
- Thompson and Appleman, 1971.Thompson WJ, Appleman MM. (1971) Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry 10:311–316 [PubMed] [Google Scholar]
- Vale et al., 1998.Vale AL, Green S, Montgomery AM, Shafi S. (1998) The nitric oxide synthesis inhibitor L-NAME produces anxiogenic-like effects in the rat elevated plus-maze test, but not in the social interaction test. J Psychopharmacol 12:268–272 [DOI] [PubMed] [Google Scholar]
- Vandecasteele et al., 2001.Vandecasteele G, Verde I, Rücker-Martin C, Donzeau-Gouge P, Fischmeister R. (2001) Cyclic GMP regulation of the L-type Ca2+channel current in the human atrial myocytes. J Physiol 533:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staveren et al., 2001.van Staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J. (2001) The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res 888:275–286 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 2002.Watanabe K, Matsuka N, Okazaki M, Hashimoto Y, Araki H, Gomita Y. (2002) The effect of immobilization stress on the pharmacokinetics of omeprazole in rats. Acta Med Okayama 56:19–23 [DOI] [PubMed] [Google Scholar]
- Werner et al., 2004.Werner C, Raivich G, Cowen M, Strekalova T, Sillaber I, Buters JT, Spanagel R, Hofmann F. (2004) Importance of NO/cGMP signalling via cGMP-dependent protein kinase II for controlling emotionality and neurobehavioural effects of alcohol. Eur J Neurosci 20:3498–3506 [DOI] [PubMed] [Google Scholar]
- Zhang and Snyder, 1995.Zhang J, Snyder SH. (1995) Nitric oxide in the nervous system. Annu Rev Pharmacol Toxicol 35:213–233 [DOI] [PubMed] [Google Scholar]