Abstract
Antigen-presenting cells (APC) are thought to play an important role in the pathogenesis of drug-induced immune reactions. Various pathological factors can activate APC and therefore influence the immune equilibrium. It is interesting that several diseases have been associated with an increased rate of drug allergy. The aim of this project was to evaluate the impact of such “danger signals” on sulfamethoxazole (SMX) metabolism in human APC (peripheral blood mononuclear cells, Epstein-Barr virus-modified B lymphocytes, monocyte-derived dendritic cells, and two cell lines). APC were incubated with SMX (100 μM–2 mM; 5 min–24 h), in the presence of pathological factors: bacterial endotoxins (lipopolysaccharide and staphylococcal enterotoxin B), flu viral proteins, cytokines [interleukin (IL)-1β, IL-6, IL-10; tumor necrosis factor-α; interferon-γ; and transforming growth factor-β], inflammatory molecules (prostaglandin E2, human serum complement, and activated protein C), oxidants (buthionine sulfoximine and H2O2), and hyperthermia (37.5–39.5°C). Adduct formation was evaluated by enzyme-linked immunosorbent assay and confocal microscopy. SMX-protein adduct formation was time- and concentration-dependent for each cell type tested, in both physiological and danger conditions. A danger environment significantly increased the formation of SMX-protein adducts and significantly shortened the delay for their detection. An additive effect was observed with a combination of danger signals. Dimedone (chemical selectively binding cysteine sulfenic acid) and antioxidants decreased both baseline and danger-enhanced SMX-adduct formation. Various enzyme inhibitors were associated with a significant decrease in SMX-adduct levels, with a pattern varying depending on the cell type and the culture conditions. These results illustrate that danger signals enhance the formation of intracellular SMX-protein adducts in human APC. These findings might be relevant to the increased frequency of drug allergy in certain disease states.
Drug hypersensitivity reactions are thought to result from an abnormal immune reaction triggered by a drug or its metabolites. According to the hapten hypothesis, drugs are too small to stimulate the immune system, and effective immune activity is directly related to drug-protein complex formation. For most drugs, metabolism is required to generate an electrophilic intermediate that can attack nucleophilic residues on proteins. These drug-protein adducts provide antigenic determinants for the immune response, whereas additional signals, often referred to as “danger signals,” determine the outcome between immunological tolerance and immune reaction (Matzinger, 1998). Modifications of critical proteins through drug haptenation, drug-associated oxidative stress, and drug-induced cell death are drug-dependent events associated with danger signaling. Non–drug-dependent factors such as disease-induced oxidative stress or bacterial and viral infections have also been identified as potential danger signals (Gallucci and Matzinger, 2001).
Antigen-presenting cells (APC) take up and process drug-protein adducts for presentation to specific T lymphocytes. APC also seem to play an important role in the balance between immune tolerance and immune reactivity through modulation of the expression of costimulatory or coinhibitory molecules (e.g., CD expression and cytokine secretion) after danger signaling (Turley, 2002). Dendritic cells are powerful APC that are efficient at antigen uptake and processing in their immature state, whereas costimulatory signals trigger their maturation associated with functions essential for effective antigen presentation.
Sulfamethoxazole (SMX) is an inexpensive sulfonamide antimicrobial that has a broad spectrum of action and a wide tissue distribution. Sulfonamides are used to treat bacterial and protozoal infections and to prevent opportunistic infections in immunocompromised patients, such as HIV-positive individuals or transplanted patients. The use of sulfonamides, however, has been limited by the occurrence of potentially life-threatening hypersensitivity reactions. It is important to remember that most drugs are given to a patient because of a disease state in the first place, implying that drugs are usually not exposed to physiological conditions, especially not in the case of antibiotics. Moreover, the incidence of certain drug allergies, such as SMX allergy, seems increased in some disease states, such as viral infections like HIV (Slatore and Tilles, 2004), or cystic fibrosis (Wills et al., 1998).
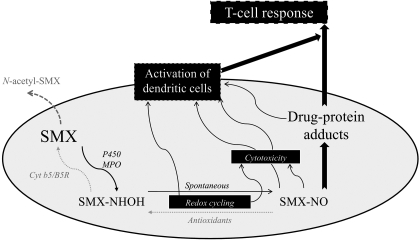
SMX is normally metabolized to an inert N-acetyl metabolite, but a small fraction can be oxidized to a hydroxylamine-metabolite (SMX-HA) that redox cycles with a toxic nitroso-intermediate (SMX-NO) (Vyas et al., 2005; Lavergne et al., 2006) (see Fig. 6). SMX-NO has been shown to be directly toxic (Naisbitt et al., 2002; Lavergne et al., 2006), to bind proteins covalently (Summan and Cribb, 2002; Callan et al., 2009), to activate dendritic cells (Sanderson et al., 2007), and to be immunogenic in animal models (Naisbitt et al., 2001; Farrell et al., 2003). SMX-protein adducts are thought to play a role in the pathogenesis of SMX hypersensitivity (Naisbitt et al., 2002; Cheng et al., 2008). It is interesting that human APC, such as monocytes (Cribb et al., 1990) and dendritic cells (Sieben et al., 1999; Sanderson et al., 2007), metabolize SMX to a nitroso intermediate that forms T-cell-stimulating intracellular SMX-protein adducts (S. N. Lavergne, B. K. Park, and D. J. Naisbitt, unpublished observation).
Fig. 6.
Scheme showing sulfamethoxazole metabolism in immune cells and its role in drug-specific T-cell activation.
Certain factors, such as lipopolysaccharide (LPS) (Yadav et al., 2006), phorbol 12-myristate 13-acetate (PMA) (Asseffa et al., 1993), cytokines (Chomarat et al., 2003), and oxidative stress (Rutault et al., 1999) have been shown to modify the phenotype and functions of dendritic cells and other APC. Such danger signals have also been showed to modify the oxidation status of cysteine-containing proteins (Carballal et al., 2003). Patients treated with SMX are, by definition, carriers of such pathological factors. Thus, the aim of this study was to evaluate the impact of danger signal on the formation of intracellular SMX-protein adduct by human APC.
Materials and Methods
Human Antigen-Presenting Cells
Peripheral Blood Mononuclear Cells.
PBMC were isolated from fresh blood of healthy volunteers collected in heparinized tubes using a Ficoll gradient separation protocol. They were then suspended in F1 media (RPMI 1640 medium, 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 mM HEPES).
Monocyte-Derived Dendritic Cells.
Monocyte-derived dendritic cells were grown from monocyte using an established method in DC media (F1 media complemented with 0.8 U/ml human granulocyte macrophage–colony-stimulating factor and human IL-4) (Sanderson et al., 2007).
Dendritic Cell-Like Cell Lines.
Commercially available THP1 and HL60 cell lines (European Collection of Cell Cultures, Salisbury, UK) were cultured in F1 media.
Epstein-Barr Virus-Modified B Lymphocytes.
EBV-modified B lymphocytes were developed using PBMC from three healthy volunteers and three SMX allergic patients and cultured in F1 media.
Drug Exposure
Nonadherent cell suspension (106 cells/ml) or monocyte-derived dendritic cells (grown from a PBMC suspension of 2 × 106 cells/ml) were incubated with SMX (0.05–2 mM), for 5 min to 24 h. Cells were then washed three times with phosphate-buffered saline before processing for confocal microscopy or before freezing for ELISA.
Generation of a Danger Environment for Human APC
Cells were exposed to danger signals aiming to mimic various pathological conditions encountered by patients treated with SMX. LPS (100 ng/ml) and SEB (2 μg/ml) were used as bacterial pathogenic factors, for Gram-negative and Gram-positive bacteria, respectively, whereas inactivated flu virus (1 μg/ml; A/Jap/305/57 virus [H2N2]) served as a model of viral infection.
In certain experiments, cells were coincubated with cytokines such as IL-1β (10 ng/ml), IL-6 (0.1 μg/ml), IL-10 (1 ng/ml), TGF-β (2 ng/ml), TNF-α (25 ng/ml), and IFN-γ (1 μg/ml). In addition, cells were coincubated with markers of inflammatory conditions, such as PGE2 (10 μM), human serum complement (1 mg/ml), and activated protein C (10 μg/ml).
Buthionine sulfoximine (BSO; 1 mM), a suicide inhibitor of γ-glutamylcysteine synthetase, the rate-limiting enzyme in glutathione (GSH) biosynthesis, and H2O2 (18 μM), a powerful oxidant, were also added to certain incubations.
Finally, experiments were performed in parallel at various temperatures (37–39.5°C) to evaluate the effect of hyperthermia associated with fever. PMA (5 ng/ml) was used as a nonspecific immune activator. All pathological factors were used at concentrations that have been shown to have an in vitro effect on immune cells (activation, maturation, proliferation, or stress) without significant cell toxicity.
Enzyme Inhibition Assays
To confirm that SMX-protein adduct formation is due to the enzymatic oxidation of SMX, experiments were repeated in the presence of various inhibitors. Cells were incubated for 1 h with 1 mM of the following enzyme inhibitors before SMX was added with or without danger signal. Aminobenzotriazole (ABT) was used as a P450 inhibitor, methimazole (MTZ) was used as an inhibitor of peroxidases and flavin-monooxygenases, aspirin and indomethacin were used as cyclooxygenase (COX) 1 inhibitors, ketoprofen and ibuprofen were used as nonselective COX1/2 inhibitors, and 4-aminobenzoic hydrazide (ABH) and salicylhydroxamic acid were used as myeloperoxidase (MPO) inhibitors. Results are presented as percentage of inhibition [100 × (inhibited sample blanked OD − baseline blanked OD)].
Antioxidant Assay
To evaluate the effect of antioxidants on the formation of SMX-protein adducts in human APC, experiments were performed with cells preincubated for 1 h with GSH, ascorbic acid (AA), or tocopherol (vitamin E) at 250 μM to 4 mM concentrations, before the addition of SMX (1 mM) with or without danger signal.
Dimedone Assay
Dimedone is a reactive chemical that binds to oxidized cysteine (sulfenic acid) residues on proteins (Saurin et al., 2004), reducing levels of in vitro SMX adduct formation (Callan et al., 2009). Cells were incubated for 1 h with dimedone (5 mM) before adding SMX (2 mM) to evaluate the existence of a sulfenic acid intermediate in APC.
Generation of a Specific Rabbit Anti-SMX Antiserum
The oxidative metabolites of SMX (SMX-HA and SMX-NO) were synthesized according to a method published previously (Naisbitt et al., 1996). SMX-conjugated keyhole limpet hemocyanin was synthesized according to a protocol described previously. This conjugate was used to raise rabbit anti-SMX antiserum according to an established method (Lavergne et al., 2006). Its specificity was tested against human serum albumin and SMX-human serum albumin conjugate (generated with the same protocol than SMX-keyhole limpet hemocyanin), using a specific rabbit anti-SMX antiserum kindly provided by Dr. Michael Rieder (London, ON, Canada) as a positive control. The specificity of this antiserum was further demonstrated using hapten inhibition (SMX at 2 mM) in the ELISA detection system described below (Supplemental Fig. 1).
Detection of Sulfamethoxazole-Protein Adducts by ELISA
Wells were coated overnight with cell lysate (25 μg) in the refrigerator. After phosphate-buffered saline/0.01% Tween washes, and blocking with 2.5% milk, samples were incubated overnight in the refrigerator with rabbit anti-SMX antisera (1:2000). Samples were then incubated for an additional 2 h with alkaline phosphatase-conjugated anti-rabbit IgG (1:1000) at room temperature. Finally, the plate was read at 405 nm, after a 30-min incubation with alkaline phosphatase substrate (Sigma-Aldrich, Gillingham, UK).
Detection of Sulfamethoxazole-Protein Adducts by Confocal Microscopy
APC were fixed with 4% paraformaldehyde for 30 min, permeabilized with NH4Cl buffer (0.16 M) for 10 min, followed by 0.1% Triton X-0.1% bovine serum albumin for 30 min, and finally blocked with F1 media for 1 h. Samples were then incubated overnight with rabbit anti-SMX antibody (1:500), before incubation with fluorescein isothiocyanate-anti-rabbit IgG for 2 h. Slides were finally mounted in Vectashield H-1200 (Vector Laboratories, Peterborough, UK).
Detection of Sulfamethoxazole-Protein Adducts by Immunoblotting
APC (1.5 × 106) were incubated with DMSO (as a negative control), SMX-NO (20 μM) (as a positive control), and SMX (2 mM) with or without LPS (100 ng/ml). After electrophoresis on a 12% SDS gel using a protocol described previously (Callen et al., 2009), samples were transferred onto a polyvinylidene difluoride membrane that was then blocked with 5% milk. The membrane then was incubated overnight with rabbit anti-SMX antiserum (up to 1:50), washed, and finally incubated with a peroxidase-conjugated anti-rabbit secondary antibody.
Statistical Analysis
Data were analyzed with a Student's t test. Each cell experiment was conducted three to seven times. Each of these experiments led to an ELISA in which samples were analyzed in duplicate. Duplicate OD readings were first averaged for each sample. For each ELISA, the average OD was compared with the average OD of the DMSO control with a paired t test to ensure that the sample readings were significantly different from the ELISA background signal. Furthermore, the average OD of the DMSO control from each experiment was subtracted from the average OD of each sample, leading to “blanked” OD values. An average of blanked OD was calculated for each sample from the different ELISA. Finally, average blanked OD values were compared with the SMX baseline sample of the corresponding assay using a paired t test. To ensure a more stringent analysis of the inhibition, each Student's t test was performed on blanked OD values (on paired conditions), and on the percentage of inhibition and the percentage of remaining signal. A similar control of the statistical analysis was performed with percentage of increase in the activation assays. In all cases, p < 0.05 was considered as statistically significant.
Results
PMA and LPS Significantly Increase Intracellular Sulfamethoxazole-Protein Adduct Formation in Human APC.
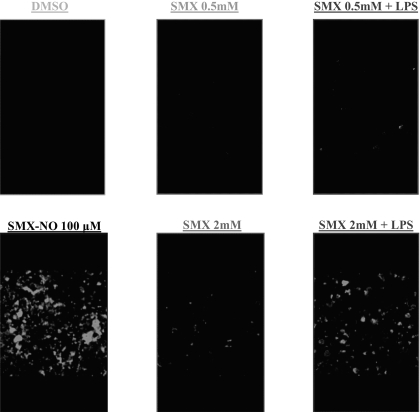
Using confocal microscopy, LPS treatment was found to increase SMX-protein adduct levels detected in human PBMC exposed to SMX (500 μM and 2 mM; Fig. 1).
Fig. 1.
Formation of intracellular SMX-protein adducts in human PBMC is enhanced by LPS. Human PBMC were incubated with SMX (500 μM and 2 mM) in the presence or absence of LPS (100 ng/ml) for 24 h, before being processed for confocal microscopy. LPS-stimulated PBMC showed a higher level of SMX-protein adducts than PBMC incubated in physiological conditions.
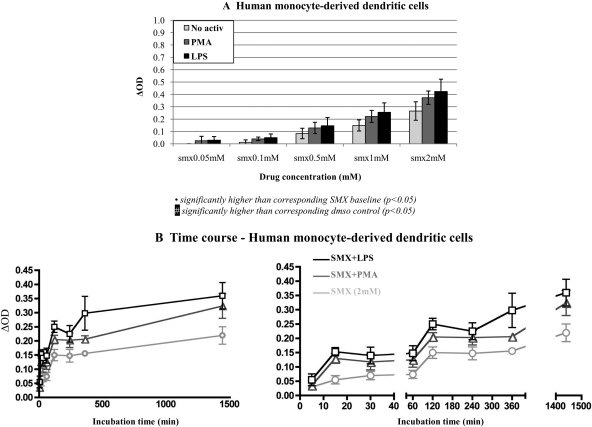
Using ELISA, PMA (5 ng/ml) and LPS (100 ng/ml) were found to significantly increase SMX-protein adduct formation in human monocyte-derived dendritic cells (Fig. 2A). Similar results were found in human PBMC, dendritic cell-like cell lines, and volunteer and allergic patient EBV-modified B lymphocytes exposed to titrated concentrations of SMX for 24 h (data not shown). Over a SMX concentration range of 10 μM to 2 mM, statistical significance was reached at 100 μM (with the exception of PMA-stimulated monocyte-derived dendritic cells for which the significance was only reached at 500 μM). Basal- and activator-induced SMX adduct formation in EBV-modified B lymphocytes was not statistically different between volunteer and allergic patients.
Fig. 2.
PMA and LPS significantly increase the formation of SMX-protein adducts in human dendritic cells. Human monocyte-derived dendritic cells were coincubated with SMX (0.01–2 mM) and PMA (5 ng/ml) or LPS (100 ng/ml). Both PMA and LPS significantly increased the formation of SMX-protein adducts in human PBMC from SMX 100 μM (n = 3; A). Over a period of 24 h, SMX-protein adducts increased gradually in cells incubated with SMX (2 mM), with significant levels detected as early as 5 min with LPS-stimulated cells (n = 4; B). OD in DMSO control was 0.29 ± 0.05. Similar results were observed with PBMC dendritic cells lines, and EBV-modified lymphocytes from volunteers and SMX allergic patients (data not shown).
Over a period of 24 h, PMA- and LPS-enhanced SMX-adduct formation was statistically significant at 5 min in monocyte-derived dendritic cells incubated with SMX (2 mM), compared with 15 min in cells in physiological conditions (Fig. 2B). Similar results were obtained with other human APC (data not shown).
Blanked OD values observed with cells incubated with SMX (with or without danger signal) were in the range of values for which the ELISA signal increased linearly with levels of adducts obtained with the protein reactive SMX-NO (used as a positive control). Adduct formation was not detected by immunoblotting when APC were incubated with SMX in the presence or absence of LPS (Supplemental Figure S2).
Other Danger Signals Increase Sulfamethoxazole-Adduct Formation in Human APC.
SEB (1 μg/ml), a major component of Gram-positive Staphylococcus bacteria, and flu virus (JAP strain; 1 μg/ml) also significantly increased levels of SMX-protein adducts formed by human monocyte-derived dendritic cells exposed to SMX (2 mM) (p = 0.0008 and p = 0.014, respectively; Table 1). Likewise, cytokines (IL-1β, IL-6, TNF-α, IFN-γ, IL-10, and TGF-β), inflammatory molecules (PGE2, human serum complement, and activated protein C), and oxidative stress inducers (BSO and H2O2) were associated with a statistically significant increase in levels of SMX-protein adducts in human monocyte-derived dendritic cells (Table 1). A similar pattern of results was obtained with dendritic cell lines (HL60 and THP1; Table 1) and EBV-modified B lymphocytes (data not shown).
Table 1.
Effect of various pathological factors on SMX-protein adduct formation in human dendritic cells
Data are presented as the average percentage of increase in adduct formation ± S.D. (n = 5 for Mo-DC and n = 4 for THP1 and HL60): [100 × (ΔODpatho − ΔODbaseline) − 100]. DMSO control OD average: 0.34 ± 0.06 in Mo-DC, 0.29 ± 0.03 in HL60, and 0.28 ± 0.03 in THP1.
| SEB | Flu Virus | IL-1β | PGE2 | IFN-γ | IL-6 | TNF-α | TGF-β | IL-10 | CRP | C3 | BSO | H2O2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo-DC | 47.1 ± 26.4 | 27.3 ± 8.2 | 68.1 ± 39.1 | 50.4 ± 30.4 | 96.5 ± 13.4 | 52.3 ± 20.8 | 45.1 ± 33.0 | 36.5 ± 18.5 | 24.4 ± 7.5 | 25.6 ± 10.1 | 24.3 ± 14.2 | 94.0 ± 62.1 | 96.5 ± 30.2 |
| (p = 0.008) | (p = 0.014) | (p = 0.004) | (p = 0.001) | (p = 0.0004) | (p = 0.0001) | (p = 0.006) | (p = 0.038) | (p = 0.015) | (p = 0.0003) | (p = 0.004) | (p = 0.004) | (p = 0.004) | |
| HL60 | NE | NE | 48.8 ± 21.4 | 55.2 ± 35.2 | 38.2 ± 26.8 | 52.0 ± 41.4 | 70.4 ± 49.4 | 20.6 ± 13.4 | 38.5 ± 31.4 | 89.3 ± 34.4 | 48.9 ± 21.2 | 71.8 ± 18.1 | NE |
| (p = 0.010) | (p = 0.026) | (p = 0.033) | (p = 0.020) | (p = 0.017) | (p = 0.020) | (p = 0.046) | (p = 0.002) | (p = 0.029) | (p = 0.002) | ||||
| THP1 | NE | NE | 39.4 ± 29.1 | 37.2 ± 5.0 | 45.0 ± 25.5 | NE | 26.7 ± 7.3 | 22.2 ± 9.9 | 45.4 ± 24.3 | 53.1 ± 29.9 | 38.5 ± 22.5 | NE | NE |
| (p = 0.037) | (p = 0.003) | (p = 0.019) | (p = 0.003) | (p = 0.030) | (p = 0.017) | (p = 0.019) | (p = 0.021) |
C3, human serum complement; NE, not examined; CRP, C reactive protein.
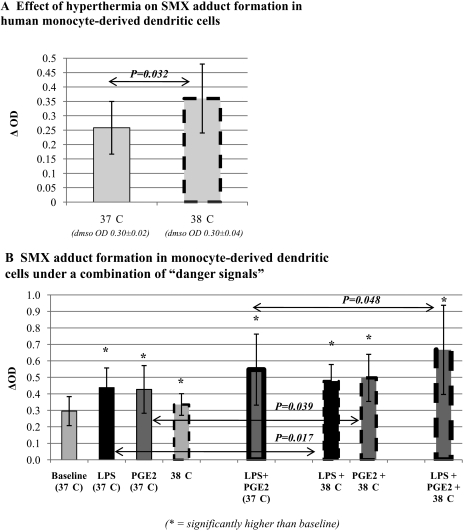
Increasing temperature (37–38.5°C) significantly increased both baseline and danger-enhanced SMX-adduct formation in dendritic cell lines (Fig. 3A). Temperatures above 38.5°C were associated with decreased SMX-adducts and decreased cell viability (data not shown). Used in combination, certain danger signals had a synergistic effect on SMX adduct formation in human monocyte-derived dendritic cells compared with their use alone (Fig. 3B).
Fig. 3.
Hyperthermia, alone or in combination with other danger signals, significantly increases SMX-protein adduct formation in human dendritic cells. Human monocyte-derived dendritic cells were incubated with SMX (2 mM) at 37 or 38°C, which significantly increased SMX adduct formation (n = 6; A). Finally, monocyte-derived dendritic cells were incubated with SMX (2 mM) in different combinations of danger signals, showing a significant enhancement in SMX adduct levels when hyperthermia was combined with LPS or PGE2 (n = 4; B).
Formation of Sulfamethoxazole-Adducts in Human Dendritic Cells Relies on a Sulfenic Acid Intermediate.
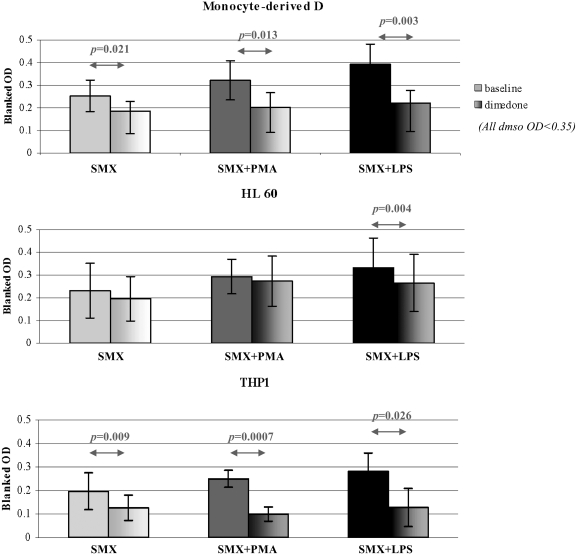
Dimedone binds covalently to oxidized cysteine residues on protein (e.g., cysteine sulfenic acid). Dimedone significantly decreased SMX-protein adduct formation in monocyte-derived dendritic cells, both in physiological (31.2 ± 22.8% inhibition, p = 0.036) and danger conditions (32.4 ± 12.1% inhibition for PMA, p = 0.006; 31.8 ± 22.5% inhibition for LPS, p = 0.033) (Fig. 4). Similar results were observed with THP1 cells (34.2 ± 22.6% inhibition for baseline, p = 0.007; 60.6 ± 6.6% inhibition for PMA, p = 0.002; 50.0 ± 37.0% inhibition for LPS, p = 0.019). However, the decrease only reached statistical significance in LPS-activated HL60 cells (13.5 ± 18.8% inhibition for baseline, p = 0.053; 10.7 ± 9.3% inhibition for PMA, p = 0.42; 22.1 ± 12.8% inhibition for LPS, p = 0.004).
Fig. 4.
Dimedone significantly decreases the formation of SMX-protein adducts in human dendritic cells. Human dendritic cells [monocyte-derived cells (n = 4) or cell lines HL60 (n = 7) and THP1 (n = 6)] were incubated with SMX (2 mM) in the presence or absence of dimedone (5 mM) that binds covalently to sulfenic acid cysteine, in both a physiological or danger conditions (PMA, 5 ng/ml or LPS, 100 ng/ml). Dimedone did not affect the average OD in DMSO controls but significantly reduced SMX-protein adduct formation in monocyte-derived dendritic cells and THP1, both in physiological and danger conditions. Conversely, dimedone only significantly decreased SMX adduct levels in LPS-stimulated HL60 cells. Results are presented as the average of blanked OD values from four independent experiments.
Danger-Enhanced Formation of Sulfamethoxazole-Protein Adducts Is Reduced by Enzyme Inhibitors in Human APC.
The coincubation of human APC with SMX (with or without danger signal) and enzyme inhibitors significantly decreased SMX-protein adduct formation (Table 2).
Table 2.
Inhibition of SMX-protein adduct formation by enzyme inhibitors in human APC in physiological conditions or activated by PMA or LPS
Monocyte-derived dendritic cells, HL60 cells, THP1 cells, and B-LCL cells were incubated with various enzyme inhibitors for 1 h before addition of SMX (2 mM) for 24 h in the presence or absence of PMA or LPS. Cell lysates were then analyzed by ELISA to detect SMX-protein adducts. Percentage of inhibition data ([100 × (inhibited sample blanked OD − baseline blanked OD]) are presented.
| Inhibitor |
ABT |
MTZ |
KetoP |
IbuP |
Aspirin |
IndoM |
ABH |
Sali.ac. |
|---|---|---|---|---|---|---|---|---|
| Enzyme inhibited | P450s | Peroxidases, FMOs | COX1/2 | COX1/2 | COX1 | COX1 | MPO | MPO |
| SMX | ||||||||
| Mo-DC | 11.1 ± 6.3* | 13.1 ± 4.6* | 9.8 ± 17.0 | 0 ± 0 | 11.1 ± 5.4* | 11.1 ± 6.3* | 22.1 ± 20.8 | 4.0 ± 6.9 |
| (p = 0.047) | (p = 0.02) | (p = 0.013) | (p = 0.047) | |||||
| HL60 | 12.0 ± 10.3 | 31.5 ± 18.7* | 29.4 ± 28.8 | 20.0 ± 23.1 | 6.7 ± 11.6 | 0 ± 0 | 32.6 ± 23.4* | 13.3 ± 12.6 |
| (p = 0.02) | (p = 0.03) | |||||||
| THP1 | 58.7 ± 40.1* | 43.8 ± 41.7* | 28.2 ± 16.7* | 4.4 ± 9.9 | 15.7 ± 23.7 | 7.1 ± 8.2 | 48.7 ± 22.1* | 28.9 ± 34.8 |
| (p = 0.015) | (p = 0.01) | (p = 0.009) | (p = 0.01) | |||||
| B-LCL | 19.3 ± 8.4* | 33.2 ± 11.0* | NE | NE | 26.3 ± 3.2* | NE | 26.3 ± 22.8 | NE |
| (p = 0.029) | (p = 0.018) | (p = 0.002) | ||||||
| SMX + PMA | ||||||||
| Mo-DC | 14.5 ± 8.6* | 30.6 ± 17.5* | 23.0 ± 26.1 | 8.7 ± 9.9 | 20.5 ± 17.8* | 16.3 ± 10.7* | 45.7 ± 22.0* | 2.8 ± 2.7 |
| (p = 0.022) | (p = 0.02) | (p = 0.03) | (p = 0.028) | (p = 0.035) | ||||
| HL60 | 20.7 ± 22.9 | 29.1 ± 22.2* | 30.8 ± 6.8* | 37.3 ± 19.4* | 9.5 ± 9.5 | 0 ± 0 | 37.7 ± 27.6* | 9.3 ± 3.2* |
| (p = 0.02) | (p = 0.001) | (p = 0.016) | (p = 0.036) | (p = 0.005) | ||||
| THP1 | 48.7 ± 9.9* | 46.0 ± 33.3* | 25.2 ± 18.7* | 6.4 ± 14.2 | 25.7 ± 20.1* | 20.0 ± 17.3 | 49.9 ± 11.1* | 34.1 ± 33.4* |
| (p = 0.0002) | (p = 0.003) | (p = 0.02) | (p = 0.007) | (p = 0.0015) | (p = 0.04) | |||
| B-LCL | 42.7 ± 10.1* | 51.7 ± 2.2* | NE | NE | 50.8 ± 7.4* | NE | 46.3 ± 0.1* | NE |
| (p = 0.009) | (p = 0.0003) | (p = 0.003) | (p = 0.018) | |||||
| SMX + LPS | ||||||||
| Mo-DC | 35.1 ± 14.8* | 45.4 ± 22.7* | 35.5 ± 11.1* | 32.4 ± 6.4* | 28.8 ± 27.7* | 30.9 ± 22.5* | 49.0 ± 21.0* | 22.6 ± 3.7* |
| (p = 0.009) | (p = 0.005) | (p = 0.016) | (p = 0.003) | (p = 0.041) | (p = 0.036) | (p = 0.028) | (p = 0.004) | |
| HL60 | 24.8 ± 16.4* | 46.5 ± 22.7* | 35.7 ± 14.8* | 40.7 ± 7.7* | 11.5 ± 8.7* | 3.6 ± 7.2 | 36.8 ± 26.4* | 18.9 ± 14.8* |
| (p = 0.014) | (p = 0.005) | (p = 0.009) | (p = 0.009) | (p = 0.039) | (p = 0.03) | (p = 0.04) | ||
| THP1 | 65.7 ± 35.6* | 64.5 ± 37.4* | 41.7 ± 23.0* | 29.0 ± 23.7* | 43.8 ± 23.3* | 31.4 ± 14.2* | 49.2 ± 12.5* | 46.8 ± 38.2* |
| (p = 0.007) | (p = 0.0009) | (p = 0.008) | (p = 0.026) | (p = 0.001) | (p = 0.01) | (p = 0.002) | (p = 0.026) | |
| B-LCL | 50.1 ± 9.5* | 54.3 ± 9.7* | NE | NE | 53.3 ± 5.4* | NE | 50.2 ± 8.2* | NE |
| (p = 0.006) | (p = 0.005) | (p = 0.002) | (p = 0.004) |
FMO, flavin-containing monooxygenases; IbuP, ibuprofen; IndoM, indomethacin; KetoP, ketoprofen; NE, not examined; Sali.ac., salicylhydroxamic acid.
It is interesting that a danger environment altered the effect of some enzyme inhibitors on the amount of SMX-adduct detected. Indeed, certain inhibitors that were not efficient in physiological conditions significantly inhibited danger-enhanced SMX-adduct formation. For example, aspirin did not modify the baseline formation of SMX-adducts, but it significantly decreased it in the presence of PMA and LPS in THP1 cells. Likewise, COX2 inhibitors did not significantly decrease SMX-adduct formation in HL60 cells unless they were stimulated. It is noteworthy that when an inhibitor was efficient in all conditions, this inhibition was significantly enhanced in the danger environment.
Antioxidants Decreased Both Baseline and Danger-Enhanced Sulfamethoxazole-Adduct Formation in Human APC.
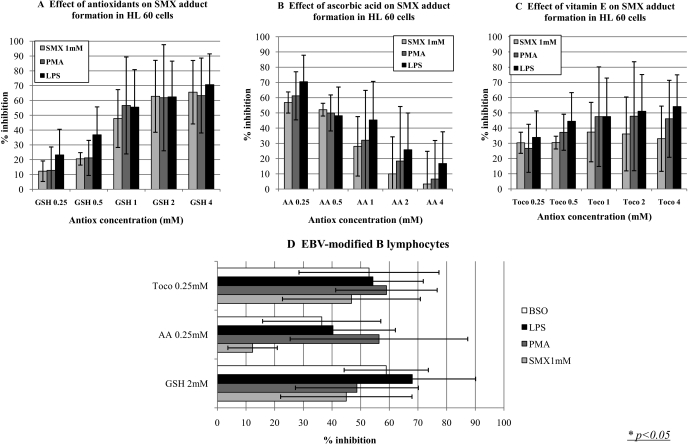
GSH, AA, and vitamin E significantly inhibited the formation of SMX-protein adducts in human APC (Fig. 5). Similar results were obtained with SMX-adduct formation enhanced by danger signals, including the mitogen PMA, the bacterial endotoxin LPS, and the GSH synthesis inhibitor BSO.
Fig. 5.
Antioxidants significantly reduce the levels of SMX-adducts in human antigen presenting cells in both physiological and danger conditions. HL60 cells were incubated with SMX (1 mM) and an increasing concentration of GSH (A), AA (B), or tocopherol (Toco; C), in the presence or absence of PMA (5 ng/ml) or LPS (100 ng/ml). GSH (≥500 μM) significantly decreased both baseline and danger-enhanced SMX adduct formation. Similar results were observed with AA (≤1 mM) and tocopherol (250 μM–4 mM). GSH (2 mM), AA (250 μM), and tocopherol (250 μM) had a similar effect on EBV-modified B cells incubated with SMX (1 mM) in the presence or absence of PMA (5 ng/ml), LPS (100 ng/ml), and BSO (1 mM) (D). Results are presented as the average of three independent experiments.
It is interesting that the effect of GSH increased from 0.25 to 2 mM, whereas the effect of AA and vitamin E was maximal at 0.25 mM and decreased until 1 mM for AA and 4 mM for vitamin E, concentrations at which these antioxidants had no inhibitory effect (Fig. 5, A–C).
Discussion
APC play an important role in immunity differentiating between tolerogenic and immunogenic responses. In the context of SMX hypersensitivity, metabolism by APC and formation of intracellular adducts stimulates pathways leading to costimulatory signaling (Sanderson et al., 2007) and generates unique antigenic determinants for T cells.
SMX is used to treat common bacterial and protozoal infections and to prevent opportunistic infections in immunocompromised patients. The danger signals tested in our study can each be found in patients receiving SMX: LPS or SEB in bacterial infection; IL-1β and PGE2 in inflammation, flu virus or IFN-γ in viral infections; hyperthermia, IL-1β, and PGE2 in fever; and BSO or H2O2 to mimic oxidative stress. Under these various pathological conditions, there was a significant increase the formation of SMX-adducts in human APC (Figs. 1 and 2A; Table 1). It is interesting that SMX-protein adducts reached statistically significant levels after only 5 min when dendritic cells were stimulated with PMA and LPS, whereas it required an extra 10 min to observe significant levels of SMX-adducts in cells incubated under physiological conditions (Fig. 2B). Adduct formation was detected by confocal microscopy and ELISA but not by immunoblotting. These findings are in line with our recent study comparing the sensitivity of Western blot, ELISA, and mass spectrometry for the detection of SMX-NO adducts on the model protein human serum albumin (Callan et al., 2009). ELISA was found to be more sensitive than mass spectrometric and immunoblotting methods. The limit of detection in the Western blot assay was only 10 μg/lane of albumin incubated with 4 μM SMX-NO, which is not achievable in in vitro cell culture systems containing the parent drug.
These results corroborate findings from Cribb et al. (1990) who showed that PMA significantly increases SMX-HA production in human and dog monocytes and that liver microsomes from LPS-treated rats display a decreased capacity to reduce SMX-HA back to SMX (Cribb et al., 2001). It is interesting that Khan et al. (2006) showed an increase in SMX-HA formation in human keratinocytes exposed to TNF-α (100 ng/ml) and LPS (500 ng/ml) but failed to demonstrate a concomitant increase in SMX-adducts despite higher concentrations of danger signal. Because the authors showed that the concentrations they used were not toxic to these cells, it is possible that the discrepancy between their results and ours is because APC, as sentinels of the immune system, are probably more sensitive to danger than keratinocytes.
It is noteworthy that SMX up to 2 mM and the danger signals at the concentrations tested in this study did not affect cell viability, as evaluated by trypan blue exclusion. Cell toxicity can therefore not explain the observed increase in SMX-protein adducts formation.
SMX-NO binds selectively to cysteine residues in glutathione, model peptides, and protein (Cribb et al., 1991; Callan et al., 2009). Cysteine residues exist in various oxidative forms, including cysteine sulfenic acids (Reddie and Carroll, 2008). We have recently shown that SMX-NO binds preferentially to oxidized cysteine on model proteins, generating an N-hydroxy sulfinamide and sulfonamide adducts (Callan et al., 2009). It is interesting that under pathological conditions, a greater number of cysteine residues are oxidized (Carballal et al., 2003; Saurin et al., 2004), providing a possible explanation for the observed increased level of SMX adduct formation in APC exposed to pro-oxidative conditions (BSO and H2O2). Dimedone, a compound that forms selective adducts with cysteine sulfenic acid, decreases the direct binding of SMX-NO to model proteins (Callan et al., 2009) and signaling in T cells (Michalek et al., 2007). In this study, dimedone also significantly decreased the amount of SMX-protein adducts detected (Fig. 4). These results suggest that the oxidative pathway leading to SMX-NO formation, and its subsequent binding to proteins, is dependent, at least partially, on the presence of oxidized cysteine residues in cellular proteins. It is interesting that dimedone only had a statistically significant effect on SMX adduct formation in HL60 cells when they were activated by LPS. This could be because LPS has been shown to oxidize proteins (Mehlhase et al., 2000).
Fever is a pathological state characterized by an increased body temperature, associated with increased systemic levels of inflammatory mediators, such as PGE2, IL-1β, IL-6, TNF-α, and IFN-γ (Dalal and Zhukovsky, 2006). Exposure of dendritic cells to fever and/or hyperthermia has been shown to affect their phenotype (CD expression and cytokine secretion) and function (migration and T-cell stimulation) (Ostberg and Repasky, 2006). Fever and hyperthermia have also been found to impair in vitro and in vivo drug metabolism (Kihara et al., 1998). In our study, increased temperatures from 37°C to 38.5°C augmented SMX-adduct formation in APC. In contrast, a further temperature increase was associated with decreased levels adducts in APC, which is probably related to secondary hyperthermia-induced cell toxicity (data not shown). These findings are of particular interest because several infectious states requiring SMX therapy can be associated with fever. It is noteworthy that when hyperthermia was combined with LPS and/or PGE2, the effect was significantly higher than with these danger signals present individually (Fig. 3B).
Soluble antioxidants prevent SMX-HA auto-oxidation (Cribb et al., 1991; Naisbitt et al., 1999) and reduce SMX-NO back to SMX-HA (Cribb et al., 1991; Lavergne et al., 2006), limiting SMX-NO protein binding and the T-cell stimulatory capacity of SMX-NO protein adducts (Naisbitt et al., 2002). The antioxidant-associated decrease (Fig. 4) and oxidative stress-induced increase (Table 1) in SMX-protein adduct formation in human APC have several clinical implications. Indeed, acute and chronic infections that can be treated with SMX can require antioxidant supplementation (Back et al., 2004; Fawzi et al., 2007). Of particular interest are the decreased antioxidant levels and/or a perturbed redox status found in plasma and cells from HIV-infected patients (Buhl et al., 1989) who have develop a higher number of hypersensitivity reactions after SMX exposure. Collectively, our data support the theory that localized oxidative stress within APC may be a risk factor for SMX hypersensitivity.
Several enzymes are known to be involved in SMX metabolism. N-Acetyltransferase can detoxify SMX (Cribb et al., 1993), whereas cytochromes P450, such as CYP2C9 in human liver, and peroxidase, such as MPO, can metabolize it to a stable hydroxylamine (SMX-HA) (Cribb et al., 1990; Roychowdhury et al., 2007) that oxidizes spontaneously to SMX-NO (Fig. 6). It is also been proposed that cyclooxygenases could oxidize SMX as these enzymes can oxidize arylamines (Goebel et al., 1999); however, the in vitro molecular system used in this study failed to show a direct role of these enzymes in SMX oxidation (Vyas et al., 2006a). In addition, flavin-containing monooxygenases have been suggested as enzymatic pathways involved in SMX-adduct formation. This was confirmed in normal human epidermal keratinocytes (Vyas et al., 2006b). APC have been shown to express the majority of cytochromes P450, myeloperoxidase, and cyclooxygenases (Sieben et al., 1999; Sanderson et al., 2007). Indeed, SMX-protein adducts have been detected in human DC (Roychowdhury et al., 2007; Sanderson et al., 2007). However, SMX metabolism in a range of APC exposed to physiological versus pathological conditions, and the effect of enzyme inhibitors on adduct formation, had not been studied to date.
In the present study, formation of SMX-protein adducts in human APC was decreased using various enzyme inhibitors (Table 2), confirming that SMX-protein adduct formation is a complex enzymatic phenomenon. Although MTZ significantly decreased levels of SMX adducts in all cell types and in all culture conditions, suggesting a key role of peroxidases and/or flavin-containing monooxygenases in SMX oxidation in human antigen-presenting cells, other enzyme inhibitors had different effects depending on the type of APC. In danger conditions (Table 2), the pattern of enzyme inhibition changed, implying that a danger signal can modify the enzymatic pathway involved in SMX-protein adduct formation.
These results are to be taken with caution because enzyme inhibitors are rarely strictly specific, and in this study, ELISA measurements of drug-protein adducts were used as surrogate measurements for SMX oxidation rather than direct detection of oxidized metabolites. For example, the 11% decrease in SMX adduct formation in monocyte-derived dendritic cells incubated with ABT was statistically significant but might not be biologically relevant.
In conclusion, although it is important to remember that in vitro danger signals cannot perfectly mimic an in vivo pathological state, our study showed that danger signals commonly encountered in diseases associated with SMX therapy could significantly increase the formation of SMX-protein adducts in APC. Immunological and metabolic assays, aiming to understand the pathogenesis of SMX allergy, are normally conducted in physiological conditions. It is therefore possible that previous studies have underestimated levels of SMX metabolism and therefore only partly characterized the mechanisms leading to SMX allergy. Further work is required to determine how this danger-modified formation of drug-protein adducts affects drug-specific proliferation of lymphocytes from SMX allergic patients, and drug-induced immunization in animal models of immunogenicity (Fig. 6). These data also raise the question of the reliability of pharmacokinetic and toxicological data in preclinical studies, because they are usually performed in physiological conditions.
Supplementary Material
Acknowledgments
We thank Dr. M. Rieder for providing some rabbit anti-SMX antisera that allowed us to validate our ELISA assay. We thank Dr. S. Marshall-Clarke (University of Liverpool, School of Biomedical Sciences, UK) for providing us with flu virus.
This work was supported by the Wellcome Trust [Grant 078598/Z/05/Z] (as part of the Centre for Drug Safety Science supported by the Medical Research Council [Grant G0700654]).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.155374
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- APC
- antigen-presenting cells
- SMX
- sulfamethoxazole
- HIV
- human immunodeficiency virus
- SMX-HA
- sulfamethoxazole-hydroxylamine
- SMX-NO
- sulfamethoxazole-nitroso
- Mo-DC
- monocyte-derived dendritic cells
- LPS
- lipopolysaccharide
- PMA
- phorbol 12-myristate 13-acetate
- PBMC
- peripheral blood mononuclear cells
- IL
- interleukin
- EBV
- Epstein-Barr virus
- ELISA
- enzyme-linked immunosorbent assay
- SEB
- staphylococcal enterotoxin B
- TNF
- tumor necrosis factor
- TGF
- transforming growth factor
- IFN
- interferon
- PGE
- prostaglandin E
- BSO
- buthionine sulfoximine
- GSH
- glutathione
- ABT
- aminobenzotriazole
- MTZ
- methimazole
- COX
- cyclooxygenase
- ABH
- aminobenzoic hydrazide
- MPO
- myeloperoxidase
- OD
- optical density
- AA
- ascorbic acid
- DMSO
- dimethyl sulfoxide.
References
- Asseffa et al., 1993.Asseffa A, Dickson LA, Mohla S, Bremner TA. (1993) Phorbol myristate acetate-differentiated THP-1 cells display increased levels of MHC class I and class II mRNA and interferon-gamma-inducible tumoricidal activity. Oncol Res 5:11–18 [PubMed] [Google Scholar]
- Back et al., 2004.Back EI, Frindt C, Nohr D, Frank J, Ziebach R, Stern M, Ranke M, Biesalski HK. (2004) Antioxidant deficiency in cystic fibrosis: when is the right time to take action?. Am J Clin Nutr 80:374–384 [DOI] [PubMed] [Google Scholar]
- Buhl et al., 1989.Buhl R, Holroyd KJ, Mastrangeli A, Cantin AM, Jaffe HA, Wells FB, Saltini C, Crystal RG. (1989) Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet 2:1294–1298 [DOI] [PubMed] [Google Scholar]
- Callan et al., 2009.Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. (2009) Multiple adduction reactions of nitroso sulfamethoxazole with cysteinyl residues of peptides and proteins: implications for hapten formation. Chem Res Toxicol 22:937–948 [DOI] [PubMed] [Google Scholar]
- Carballal et al., 2003.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, Alvarez B. (2003) Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 42:9906–9914 [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2008.Cheng L, Stewart BJ, You Q, Petersen DR, Ware JA, Piccotti JR, Kawabata TT, Ju C. (2008) Covalent binding of the nitroso metabolite of sulfamethoxazole is important in induction of drug-specific T-cell responses in vivo. Mol Pharmacol 73:1769–1775 [DOI] [PubMed] [Google Scholar]
- Chomarat et al., 2003.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. (2003) TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol 171:2262–2269 [DOI] [PubMed] [Google Scholar]
- Cribb et al., 2001.Cribb AE, McQuaid T, Renton KW. (2001) Effect of lipopolysaccharide (LPS)-evoked host defense activation on hepatic microsomal formation and reduction of sulfamethoxazole hydroxylamine in the rat. Biochem Pharmacol 62:457–459 [DOI] [PubMed] [Google Scholar]
- Cribb et al., 1991.Cribb AE, Miller M, Leeder JS, Hill J, Spielberg SP. (1991) Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab Dispos 19:900–906 [PubMed] [Google Scholar]
- Cribb et al., 1990.Cribb AE, Miller M, Tesoro A, Spielberg SP. (1990) Peroxidase-dependent oxidation of sulfonamides by monocytes and neutrophils from humans and dogs. Mol Pharmacol 38:744–751 [PubMed] [Google Scholar]
- Cribb et al., 1993.Cribb AE, Nakamura H, Grant DM, Miller MA, Spielberg SP. (1993) Role of polymorphic and monomorphic human arylamine N-acetyltransferases in determining sulfamethoxazole metabolism. Biochem Pharmacol 45:1277–1282 [DOI] [PubMed] [Google Scholar]
- Dalal and Zhukovsky, 2006.Dalal S, Zhukovsky DS. (2006) Pathophysiology and management of fever. J Support Oncol 4:9–16 [PubMed] [Google Scholar]
- Farrell et al., 2003.Farrell J, Naisbitt DJ, Drummond NS, Depta JP, Vilar FJ, Pirmohamed M, Park BK. (2003) Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J Pharmacol Exp Ther 306:229–237 [DOI] [PubMed] [Google Scholar]
- Fawzi et al., 2007.Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, Wei R, Hunter D. (2007) Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr 85:1335–1343 [DOI] [PubMed] [Google Scholar]
- Gallucci and Matzinger, 2001.Gallucci S, Matzinger P. (2001) Danger signals: SOS to the immune system. Curr Opin Immunol 13:114–119 [DOI] [PubMed] [Google Scholar]
- Goebel et al., 1999.Goebel C, Vogel C, Wulferink M, Mittmann S, Sachs B, Schraa S, Abel J, Degen G, Uetrecht J, Gleichmann E. (1999) Procainamide, a drug causing lupus, induces prostaglandin H synthase-2 and formation of T cell-sensitizing drug metabolites in mouse macrophages. Chem Res Toxicol 12:488–500 [DOI] [PubMed] [Google Scholar]
- Khan et al., 2006.Khan FD, Roychowdhury S, Nemes R, Vyas PM, Woster PM, Svensson CK. (2006) Effect of pro-inflammatory cytokines on the toxicity of the arylhydroxylamine metabolites of sulphamethoxazole and dapsone in normal human keratinocytes. Toxicology 218:90–99 [DOI] [PubMed] [Google Scholar]
- Kihara et al., 1998.Kihara T, Toda A, Umesue I, Ono N, Shigematsu H, Soeda S, Shimeno H. (1998) Effect of interleukin 1 beta-induced fever on hepatic drug metabolism in rat. Xenobiotica 28:559–569 [DOI] [PubMed] [Google Scholar]
- Lavergne et al., 2006.Lavergne SN, Kurian JR, Bajad SU, Maki JE, Yoder AR, Guzinski MV, Graziano FM, Trepanier LA. (2006) Roles of endogenous ascorbate and glutathione in the cellular reduction and cytotoxicity of sulfamethoxazole-nitroso. Toxicology 222:25–36 [DOI] [PubMed] [Google Scholar]
- Matzinger, 1998.Matzinger P. (1998) An innate sense of danger. Semin Immunol 10:399–415 [DOI] [PubMed] [Google Scholar]
- Mehlhase et al., 2000.Mehlhase J, Gieche J, Ullrich O, Sitte N, Grune T. (2000) LPS-induced protein oxidation and proteolysis in BV-2 microglial cells. IUBMB Life 50:331–335 [DOI] [PubMed] [Google Scholar]
- Michalek et al., 2007.Michalek RD, Nelson KJ, Holbrook BC, Yi JS, Stridiron D, Daniel LW, Fetrow JS, King SB, Poole LB, Grayson JM. (2007) The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J Immunol 179:6456–6467 [DOI] [PubMed] [Google Scholar]
- Naisbitt et al., 2002.Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. (2002) Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol 62:628–637 [DOI] [PubMed] [Google Scholar]
- Naisbitt et al., 2001.Naisbitt DJ, Gordon SF, Pirmohamed M, Burkhart C, Cribb AE, Pichler WJ, Park BK. (2001) Antigenicity and immunogenicity of sulphamethoxazole: demonstration of metabolism-dependent haptenation and T-cell proliferation in vivo. Br J Pharmacol 133:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt et al., 1999.Naisbitt DJ, Hough SJ, Gill HJ, Pirmohamed M, Kitteringham NR, Park BK. (1999) Cellular disposition of sulphamethoxazole and its metabolites: implications for hypersensitivity. Br J Pharmacol 126:1393–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt et al., 1996.Naisbitt DJ, O'Neill PM, Pirmohamed M, Park BK. (1996) Synthesis and reactions of nitroso sulphamethoxazole with biological nucleophiles: implications for immune-mediated toxicity. Bioorg Med Chem Lett 6:1511–1516 [Google Scholar]
- Ostberg and Repasky, 2006.Ostberg JR, Repasky EA. (2006) Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother 55:292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddie and Carroll, 2008.Reddie KG, Carroll KS. (2008) Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol 12:746–754 [DOI] [PubMed] [Google Scholar]
- Roychowdhury et al., 2007.Roychowdhury S, Vyas PM, Svensson CK. (2007) Formation and uptake of arylhydroxylamine-haptenated proteins in human dendritic cells. Drug Metab Dispos 35:676–681 [DOI] [PubMed] [Google Scholar]
- Rutault et al., 1999.Rutault K, Alderman C, Chain BM, Katz DR. (1999) Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med 26:232–238 [DOI] [PubMed] [Google Scholar]
- Sanderson et al., 2007.Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. (2007) Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol 178:5533–5542 [DOI] [PubMed] [Google Scholar]
- Saurin et al., 2004.Saurin AT, Neubert H, Brennan JP, Eaton P. (2004) Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci U S A 101:17982–17987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben et al., 1999.Sieben S, Baron JM, Blömeke B, Merk HF. (1999) Multiple cytochrome P450-isoenzymes mRNA are expressed in dendritic cells. Int Arch Allergy Immunol 118:358–361 [DOI] [PubMed] [Google Scholar]
- Slatore and Tilles, 2004.Slatore CG, Tilles SA. (2004) Sulfonamide hypersensitivity. Immunol Allergy Clin North Am 24:477–490, vii [DOI] [PubMed] [Google Scholar]
- Summan and Cribb, 2002.Summan M, Cribb AE. (2002) Novel non-labile covalent binding of sulfamethoxazole reactive metabolites to cultured human lymphoid cells. Chem Biol Interact 142:155–173 [DOI] [PubMed] [Google Scholar]
- Turley, 2002.Turley SJ. (2002) Dendritic cells: inciting and inhibiting autoimmunity. Curr Opin Immunol 14:765–770 [DOI] [PubMed] [Google Scholar]
- Vyas et al., 2006a.Vyas PM, Roychowdhury S, Svensson CK. (2006a) Role of human cyclooxygenase-2 in the bioactivation of dapsone and sulfamethoxazole. Drug Metab Dispos 34:16–18 [DOI] [PubMed] [Google Scholar]
- Vyas et al., 2006b.Vyas PM, Roychowdhury S, Koukouritaki SB, Hines RN, Krueger SK, Williams DE, Nauseef WM, Svensson CK. (2006b) Enzyme-mediated protein haptenation of dapsone and sulfamethoxazole in human keratinocytes: II. Expression and role of flavin-containing monooxygenases and peroxidases. J Pharmacol Exp Ther 319:497–505 [DOI] [PubMed] [Google Scholar]
- Vyas et al., 2005.Vyas PM, Roychowdhury S, Woster PM, Svensson CK. (2005) Reactive oxygen species generation and its role in the differential cytotoxicity of the arylhydroxylamine metabolites of sulfamethoxazole and dapsone in normal human epidermal keratinocytes. Biochem Pharmacol 70:275–286 [DOI] [PubMed] [Google Scholar]
- Wills et al., 1998.Wills R, Henry RL, Francis JL. (1998) Antibiotic hypersensitivity reactions in cystic fibrosis. J Paediatr Child Health 34:325–329 [DOI] [PubMed] [Google Scholar]
- Yadav et al., 2006.Yadav R, Zammit DJ, Lefrancois L, Vella AT. (2006) Effects of LPS-mediated bystander activation in the innate immune system. J Leukoc Biol 80:1251–1261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.