Abstract
Very little is known about constitutive activity in vivo. This study examined whether constitutive activity and inverse agonism contribute to discriminative stimulus effects of drugs acting at serotonin (5-HT)2A receptors. Rats were trained to discriminate between saline and either 0.56 mg/kg 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), 1.0 mg/kg 5-HT2A receptor antagonist ketanserin, or 0.1 mg/kg purported 5-HT2A receptor inverse agonist (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol (MDL100907). Discriminative control was established with each drug after 33 to 35 sessions. MDL100907 and ketanserin did not occasion DOM lever responding but attenuated the discriminative stimulus effects of DOM. DOM did not occasion responding on the drug-associated lever in rats discriminating MDL100907 or ketanserin, but attenuated the discriminative stimulus effects of both drugs. Ketanserin and ritanserin occasioned MDL100907-lever responding, whereas rats discriminating ketanserin responded only partially on the drug-associated lever after receiving MDL100907, ritanserin, or the α1-adrenergic antagonist prazosin. Combining prazosin with MDL100907 or ritanserin resulted in near-complete ketanserin-lever responding, indicating that the ketanserin stimulus involves both 5-HT2A and α1-adrenergic receptors. Administration of p-chlorophenylalanine methyl ester, then fenfluramine, significantly decreased cortical 5-HT, enhanced sensitivity to the discriminative stimulus effects of DOM, and occasioned partial MDL100907-lever responding. Collectively, these results show that DOM and MDL100907 discriminative stimulus effects are mediated by 5-HT2A receptors and that ketanserin discriminative stimulus effects involve both 5-HT2A and α1-adrenergic receptors. Results in 5-HT-depleted rats further suggest that the discriminative stimulus effects of MDL100907 might involve antagonism of endogenous 5-HT and/or inverse agonism at 5-HT2A receptors.
In classic receptor theory, receptor ligands are characterized by affinity and intrinsic activity (Ariens, 1954), with the latter varying from no (zero) activity (i.e., antagonist) to high activity (efficacy; e.g., full agonist). Two observations prompted revision of this conceptualization of ligand/receptor interactions. First, under some conditions receptor signaling occurs in the absence of ligand binding, suggesting constitutive activity of unbound receptors. Second, some drugs that are presumed to be antagonists (zero efficacy) decrease receptor signaling that occurs in the absence of endogenous or exogenous agonist. Thus, a third class of receptor ligands was proposed—inverse agonists (Kenakin, 1996; Strange 2000; Costa and Herz, 1989; Parra and Bond, 2007); in a constitutively active (i.e., ligand-independent) system, receptor signaling is increased by agonists, decreased by inverse agonists, and not affected by antagonists.
Serotonin (5-HT)2 receptors are constitutively active under some conditions (for review, see Aloyo et al., 2009). In particular, 5-HT2C receptors are constitutively active in several in vitro systems (Barker et al., 1994; Berg et al., 1999; Niswender et al., 1999; Schlag et al., 2004); however, there has been only limited evidence for constitutive activity and inverse agonism in vivo (e.g., Walker et al., 2005). Studies examining the effects of drugs on conditioned responses provide some of the strongest support for constitutive activity and inverse agonism at 5-HT2A receptors in vivo. Thus, 5-HT2A receptor agonists (e.g., quipazine) enhance, purported 5-HT2A receptor inverse agonists (e.g., ritanserin) retard, and 5-HT2A receptor neutral antagonists (e.g., ketanserin) have no effect on acquisition of a conditioned response (Romano et al., 1991, 2000; Alhaider et al., 1993; Welsh et al., 1998; Harvey et al., 1999; Harvey, 2003). The generality of those findings to other measures of 5-HT receptor activation is not known, despite the potential importance of constitutive activity and inverse agonism in understanding pathology and in developing new pharmacotherapies (e.g., Weiner et al., 2001).
Drug discrimination is a highly selective assay for examining ligand/receptor interactions in vivo, and there is a large literature on the discriminative stimulus effects of drugs acting at 5-HT2A receptors. The current study attempted to establish two-choice (drug versus saline) discrimination procedures in separate groups of rats either with a drug known to have agonist actions at 5-HT2A receptors (DOM), a drug known to have antagonist actions at 5-HT2A receptors (ketanserin), or a drug thought to have inverse agonist actions at 5-HT2A receptors (MDL100907). Several laboratories have established stimulus control with DOM (e.g., Glennon et al., 1983). Stimulus control also has been established with MDL100907 (Dekeyne et al., 2002), although this discrimination has not been used to explore possible inverse agonism by MDL100907; and stimulus control has been established between ketanserin and another drug (e.g., quipazine; Smith et al., 1995, 2002), although there are no reports of a discrimination between ketanserin and vehicle.
The purpose of the current study was to test the following hypotheses: 1) ketanserin is a neutral antagonist at 5-HT2A receptors and, therefore, can not be established as a discriminative stimulus in a simple two-choice (i.e., drug versus vehicle) discrimination procedure (if a discrimination can be established with ketanserin it is the result of ketanserin antagonizing endogenous 5-HT, of the inverse agonist actions of ketanserin, of actions of ketanserin at receptors other than 5-HT2A receptors, or of a combination of these mechanisms); 2) the discriminative stimulus effects of DOM (agonist) and MDL100907 (inverse agonist) are mutually antagonized; 3) as a neutral antagonist ketanserin antagonizes the discriminative stimulus effects of DOM and those of MDL100907; and 4) depletion of 5-HT increases sensitivity to the discriminative stimulus effects of 5-HT2A receptor agonists and also possibly to inverse agonists (5-HT depletion will mimic the discriminative stimulus effects of drugs that result exclusively from antagonism of endogenous 5-HT).
Materials and Methods
Subjects.
Thirty male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually on a 12/12-h light/dark cycle (behavioral experiments were conducted during the light period) with free access to water in the home cage. Twenty-four rats were used in the discrimination study, and six rats were used as controls for studies on 5-HT content. Rats in the discrimination study were maintained at 340 to 360 g by providing rodent chow (Rodent sterilizable diet; Harlan Teklad, Madison, WI) postsession in the home cage where they also had free access to water. Before discrimination training rats were randomly assigned to three groups of eight rats each. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Apparatus.
Experiments were conducted with commercially available chambers (model ENV-008CT; MED Associates Inc., St. Albans, VT) located within sound-attenuating, ventilated cubicles (model ENV-022M; MED Associates Inc.). The chambers were equipped with two levers, associated stimulus lights, and a food hopper. Data were collected by use of MED-PC IV software (MED Associates Inc.), an interface, and a computer.
Discrimination Procedure.
Different groups of eight rats each were trained to discriminate between saline and either 0.56 mg/kg DOM, 1.0 mg/kg ketanserin, or 0.1 mg/kg MDL100907 while responding under a fixed ratio (FR) schedule of food presentation. The training doses of DOM, ketanserin, and MDL100907 were based on published reports of these drugs when they were used as training drugs or to antagonize drugs presumed to be acting at 5-HT2A receptors (Schreiber et al., 1994; Dekeyne et al., 2002; Smith et al., 2002; Li et al., 2007). Daily sessions consisted of a 30-min timeout period, during which the chamber was dark and lever presses had no programmed consequence, followed by a response period, during which stimulus lights above both levers were illuminated and an FR 5 schedule of food presentation was active. Saline or a training drug (DOM, ketanserin, or MDL100907) was injected intraperitoneally at the start of the timeout period, 30 min before the response period during which rats could receive food (45 mg; Research Diets, New Brunswick, NJ) by making five consecutive responses on the active lever. When rats received saline, only responding on the saline-associated lever resulted in food delivery, and when they received their respective training drug only responding on the other (i.e., drug-associated) lever resulted in food delivery. A response on the incorrect (inactive) lever reset the FR requirement on the correct lever with the response period ending after 15 min or the delivery of 50 food pellets, whichever occurred first. Sessions were conducted 6 or 7 days per week and the order of training sessions was generally double alternation (e.g., saline, saline, drug, drug…).
Rats were considered to be under adequate stimulus control for testing when the following criteria were satisfied for five consecutive or six of seven consecutive sessions: at least 90% of the total responses were made on the correct lever and fewer than five responses (one FR) were made on the incorrect lever before delivery of the first food pellet. Thereafter, tests were conducted whenever the same criteria were satisfied for two consecutive days and minimally for one saline-training session and one drug-training session.
Test sessions were identical to training sessions, with the exception that five consecutive responses on either lever resulted in the delivery of food and different doses of drugs (alone or in combination) were administered. Rats were tested with saline and different doses of DOM (0.1–0.56 mg/kg), ketanserin (0.1–1.0 mg/kg), MDL100907 (0.01–1.0 mg/kg), morphine (0.32–10.0 mg/kg), ketamine (0.32–10.0 mg/kg), and phencyclidine (0.1–3.2 mg/kg) to assess the relative selectivity of each discrimination procedure. In general, drugs were studied up to doses that occasioned at least 90% responding on the drug-associated lever or to doses that decreased the rate of responding.
Next, antagonism studies were conducted with selected drugs administered in combination. In particular, to examine whether a common receptor mediated the effects of all three training drugs, for each group, a dose of the training drug that occasioned at least 90% responding on the drug lever was studied in combination with different doses of a second compound (see Results for details) up to a dose that decreased drug lever responding to less than 20%. Thus, a fixed dose of DOM was studied with different doses of ketanserin [combinations of MDL100907 with DOM in rats discriminating DOM have been published previously (Li et al., 2007)] and fixed doses of MDL100907 and ketanserin were studied with different doses of DOM. The order of tests varied nonsystematically across subjects. Ritanserin (0.032–3.2 mg/kg), reported to be an antagonist or inverse agonist at 5-HT2A receptors, also was studied in rats discriminating MDL100907 and in rats discriminating ketanserin. Ritanserin and MDL100907 each occasioned partial drug lever responding in rats discriminating ketanserin. Because only partial effects were obtained with these drugs and because ketanserin is known to have α1-adrenergic receptor antagonist effects (Orallo et al., 2000), the α1-adrenergic antagonist prazosin was studied alone (0.032–3.2 mg/kg), in combination with MDL100907, and in combination with ritanserin. This experiment examined whether the combination of a 5-HT2A receptor antagonist (ritanserin) and an α1-adrenergic receptor antagonist (prazosin) occasioned more ketanserin-lever responding than either drug alone.
Next, discriminative stimulus effects were evaluated after temporary discontinuation of training and after treatments to deplete 5-HT. Training was temporarily suspended for 3 days; rats remained in the home cage and received a daily injection of saline. Over the next 4 days rats were tested with vehicle and three different doses of their respective training drug; the order of testing was randomized across animals. When training was suspended temporarily, stimulus control with DOM and MDL100907 was stable, whereas stimulus control with ketanserin deteriorated further (data not shown). Moreover, stimulus control with DOM and MDL100907 increased slightly over the course of these studies, whereas stimulus control with ketanserin decreased slightly (see Table 1 and Results). Thus, the final study on whether 5-HT depletion affects the discriminative stimulus effects of training drugs was conducted only in rats discriminating DOM or MDL100907 [however, all 24 rats, including those trained to discriminate ketanserin, received p-chlorophenylalanine methyl ester (PCPA) and fenfluramine and contributed to studies of 5-HT concentrations in brain]. Three-day suspension of training followed by tests over four consecutive days provided a control for the final study when rats were treated for 3 days with an injection of 160 mg/kg i.p. PCPA on the first and second days and an injection of 10 mg/kg i.p. fenfluramine on the third day (Prinssen et al., 2002); this final suspension of training and drug treatment occurred only after rats again satisfied the testing criteria for two consecutive days. The same tests (i.e., saline and three doses of the respective training drug) were conducted over four consecutive days. Rats were sacrificed, brains were rapidly removed, and the cortex was dissected, sealed, and frozen at −80°C. Six drug-naive, age-matched rats received daily intraperitoneal injections of saline before sacrifice and collection of brain tissue.
TABLE 1.
Discrimination performances in drug- and vehicle-training sessions at the beginning and end of the study
| Training Drug | Beginning of Study / End of Study |
|
|---|---|---|
| Drug-Training Session | Vehicle-Training Session | |
| DOM | 15.5 ± 0.7 / 17.4 ± 0.8* | 16.1 ± 1.0 / 18.6 ± 0.4 |
| MDL100907 | 11.0 ± 1.1 / 13.9 ± 1.5 | 12.8 ± 0.9 / 14.4 ± 1.1 |
| Ketanserin | 9.6 ± 1.0 / 11.0 ± 1.5 | 12.1 ± 1.7 / 10.6 ± 1.8 |
The average number of drug- and vehicle-training sessions (± S.E.M.) in which rats satisfied the testing criteria: in the first 20 training sessions after the test criteria were satisfied (Beginning); and in the last 20 sessions of the study (i.e., immediately before 5-HT depletion study; End). Values are for eight rats in each group.
For assessing 5-HT concentration, brain samples were thawed and a portion of the frontal or occipital cortex was isolated (100–200 mg) and weighed. Ten volumes of a solution (0.4 M perchloric acid, 0.1% sodium metabisulfite, 0.02% cysteine, and 0.01% EDTA) were added to the samples, which were homogenized by use of a Polytron homogenizer (Kinematica, Littau-Lucerne, Switzerland). After centrifugation, the supernatants were collected and the concentration of 5-HT was measured by use of high-performance liquid chromatography. The concentration of 5-HT was expressed in nanograms of 5-HT/g wet weight of brain tissue. The high-performance liquid chromatography system consisted of a model 584 pump (ESA Laboratories, Inc., Chelmsford, MA), model 542 autosampler (ESA), an Alltima C18 analytical column (Alltech Associates, Inc., Deerfield, IL), and a Coulochem II detector (ESA) equipped with a 5011 detector cell (D1 = 220 mV; ESA).
Drugs.
The compounds used in this study were as follows: DOM, morphine sulfate, phencyclidine hydrochloride (all from Research Technology Branch, National Institute on Drug Abuse, Rockville, MD); MDL100907 was synthesized as described previously (Ullrich and Rice, 2000); ketanserin tartrate, ritanserin, prazosin hydrochloride, PCPA, and fenfluramine hydrochloride (all purchased from Sigma-Aldrich, St Louis, MO); and ketamine hydrochloride (purchased from Vetus Animal Health, MFA Inc., Columbia, MO). MDL100907 was dissolved in (v/v) 20% dimethyl sulfoxide and all other compounds were dissolved in sterile 0.9% saline. Injection volumes were 0.1 to 1.0 ml i.p.
Data Analyses.
For the drug discrimination data, the percentage of responses on the drug-associated lever during the response period is plotted as a function of dose. Rate of lever pressing on both levers during the response period is expressed as responses per second. Discrimination data were not used to calculate means ± S.E.M. when a rat failed to respond at a rate that was at least 20% of its individual control (vehicle) rate (average rate from the five previous saline-training days). All response rate data were used to calculate means and S.E.M. ED50 values and 95% confidence limits were estimated by use of linear regression. The effect of a drug on response rate was considered significant when the mean rate of responding was outside the 95% confidence limits of the mean control (vehicle) response rate, defined as the mean response rate of 10 preceding vehicle-training sessions in which the testing criteria were satisfied. A curve-fitting method was used to examine the dose-response function of DOM in rats discriminating DOM before and after treatment with PCPA and fenfluramine. For this method, the differences among dose-response curves were analyzed by simultaneously fitting straight lines to the individual dose-response data by means of GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). Straight lines were fitted to the linear portion of dose-response curves, which comprised doses with effects immediately below and above 50%, and included not more than one dose with more than 75% effect and not more than one dose with less than 25% effect. The slopes and intercepts of dose-response functions for each condition were compared with an F-ratio test using GraphPad; the two conditions were considered to be significantly different when the data sets could not be described with a single line.
Results
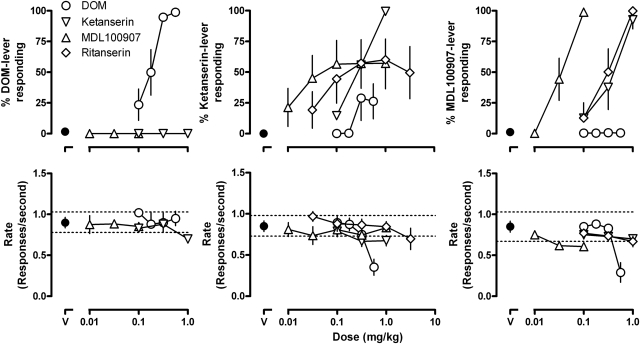
Adequate stimulus control was established with DOM, ketanserin, and MDL100907 after an average (± S.E.M.) of 33 ± 3, 35 ± 5, and 33 ± 2 training sessions, respectively. After administration of the training dose of DOM, ketanserin, or MDL100907, rats responded an average of 98.8 ± 0.7%, 99.4 ± 0.2%, and 98.9 ± 0.6% on the drug-associated lever, respectively (Fig. 1). DOM [ED50 (95% CL) = 0.186 (0.132, 0.239) mg/kg], ketanserin [ED50 = 0.344 (0.195, 0.493) mg/kg], and MDL100907 [ED50 = 0.039 (0.026, 0.052) mg/kg] each dose dependently increased responding on the training-drug associated lever (Fig. 1, upper) without markedly affecting rate of responding (Fig. 1, lower). The frequency of rats satisfying the testing criteria in training sessions (Table 1) and, therefore, the frequency with which tests were conducted, was greatest for rats discriminating DOM and least for rats discriminating ketanserin.
Fig. 1.
Effects of DOM, ketanserin, MDL100907, and ritanserin in rats discriminating between saline and either 0.56 mg/kg DOM (left), 1.0 mg/kg ketanserin (center), or 0.1 mg/kg MDL100907 (right). Ordinates: upper, percentage of responses on the drug-associated lever; lower, rate of responding in responses per second. Abscissae: dose in mg/kg body weight; points above “V” represent data obtained after the administration of saline vehicle. Each entry is the mean ± S.E.M. for eight rats. Lower, dashed lines represent the 95% confidence limits of the mean rate of responding over the preceding 10 vehicle-training sessions in which the test criteria were satisfied.
Neither ketanserin nor MDL100907 occasioned responding on the drug-associated lever in rats discriminating DOM (Fig. 1, upper left), and DOM occasioned little responding on the drug-associated lever in rats discriminating ketanserin (Fig. 1, ○, upper center) and no responding on the drug-associated lever in rats discriminating MDL100907 (Fig. 1, ○, upper right). MDL100907 occasioned partial responding on the drug-associated lever in rats discriminating ketanserin with a dose of 1.0 mg/kg MDL100907 occasioning a maximum 57% ketanserin-lever responding (Fig. 1, ▵, upper center). In contrast, ketanserin [ED50 = 0.472 (0.346, 0.597) mg/kg] produced near-complete responding on the drug-associated lever in rats discriminating MDL100907 (Fig. 1, ▿, upper right). Like MDL100907, ritanserin occasioned only partial responding on the drug-associated lever in rats discriminating ketanserin (maximum 60% at a dose of 1.0 mg/kg; Fig. 1, ◊, upper center) and complete responding on the drug-associated lever in rats discriminating MDL100907 [ritanserin ED50 = 0.362 (0.210, 0.514) mg/kg; Fig. 1, ◊, upper right]. The same doses of ketanserin occasioned responding on the drug-associated lever in rats discriminating ketanserin and in rats discriminating MDL100907 (compare ▿, upper center and right in Fig. 1). Moreover, the same doses of ketanserin and ritanserin occasioned responding on the drug-associated lever in rats discriminating MDL100907 (Fig. 1, upper right). With the exception of modest rate-decreasing effects observed after administration of 0.56 mg/kg DOM in rats discriminating ketanserin or MDL100907, none of these drugs markedly altered the rate of responding.
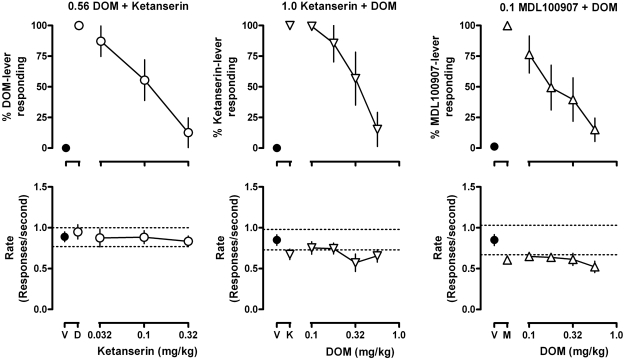
The discriminative stimulus effects of the training dose of DOM (0.56 mg/kg) were attenuated in a dose-related manner by ketanserin (Fig. 2, upper left) with a dose of 0.32 mg/kg ketanserin decreasing responding on the drug-associated lever to not more than 15% [ketanserin ED50 (95% CL) = 0.137 (0.071, 0.203) mg/kg]. The discriminative stimulus effects of ketanserin (Fig. 2, upper center) and those of MDL100907 (Fig. 2, upper right) each were attenuated in a dose-related manner by DOM [ED50 = 0.330 (0.239, 0.421) mg/kg with ketanserin; ED50 = 0.262 (0.160, 0.364) mg/kg with MDL100907] with a dose of 0.56 mg/kg DOM decreasing responding on the drug-associated lever to not more than 15% in each group.
Fig. 2.
Effects of the training dose of DOM in combination with increasing doses of ketanserin (left), and the training doses of ketanserin (center) and MDL100907 (right) each in combination with increasing doses of DOM. Points above “D”, “K,” and “M” represent effects obtained with the training dose of DOM, ketanserin, and MDL100907, respectively. See Fig. 1 for other details.
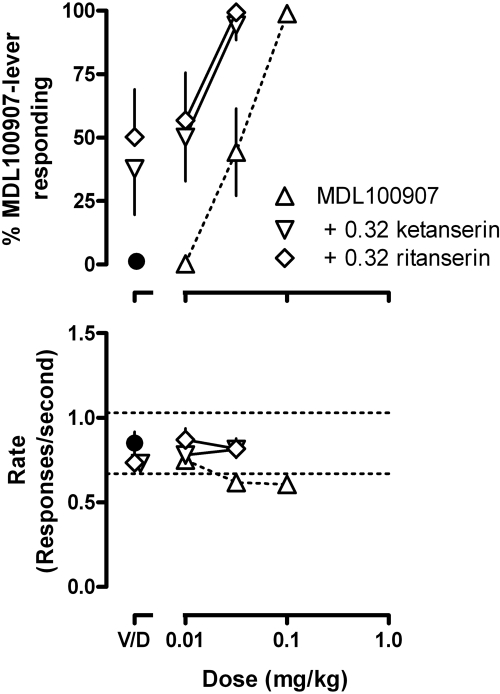
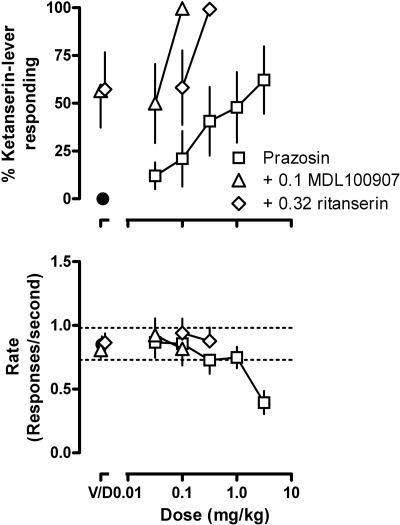
A dose (0.32 mg/kg) of ketanserin or ritanserin that occasioned only partial responding on the drug-associated lever in rats discriminating MDL100907 (Fig. 1, upper right, and Fig. 3, points above “V”) shifted the MDL100907 discrimination dose-response curve leftward (Fig. 3, upper) without affecting rate of responding (Fig. 3, lower). In rats discriminating ketanserin, prazosin occasioned a maximum of 62% responding on the drug-associated lever at a dose (3.2 mg/kg) that decreased the average rate or responding to 0.39 ± 0.09 responses per second (i.e., 40% of the control rate); up to a same dose (3.2 mg/kg), prazosin occasioned little (23 ± 15%) responding on the drug-associated lever in rats discriminating MDL100907 (data not shown). When prazosin was administered with a dose of MDL100907 (0.1 mg/kg) or ritanserin (0.32 mg/kg) that alone occasioned only partial ketanserin-lever responding (Fig. 1, upper center), rats responded exclusively on the ketanserin-associated lever (Fig. 4, upper) at rates that were not different from control (Fig. 4, lower).
Fig. 3.
Effects of MDL100907 alone and in combination with 0.32 mg/kg ketanserin or 0.32 mg/kg ritanserin in rats discriminating between saline and 0.56 mg/kg MDL100907. See Fig. 1 for other details.
Fig. 4.
Effects of prazosin alone and in combination with 0.1 MDL100907 or 0.32 mg/kg ritanserin in rats discriminating between saline and 1.0 mg/kg ketanserin. See Fig. 1 for other details.
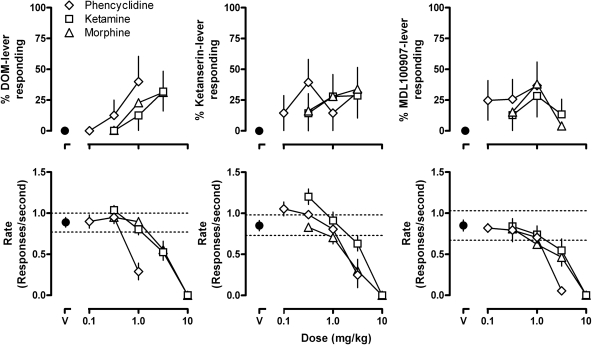
Phencyclidine, ketamine, and morphine occasioned partial responding on the drug-associated lever in all three group of rats (Fig. 5, upper), up to doses of each compound that markedly decreased or eliminated lever pressing (Fig. 5, lower). The magnitude of responding on the drug-associated lever obtained with these three compounds was similar across the three different groups of rats.
Fig. 5.
Effects of phencyclidine, morphine, and ketamine in rats discriminating between saline and either 0.56 mg/kg DOM (left), 1.0 mg/kg ketanserin (center), or 0.1 mg/kg MDL100907 (right). Each data point is the mean ± S.E.M. for 6–8 rats. See Fig. 1 for other details.
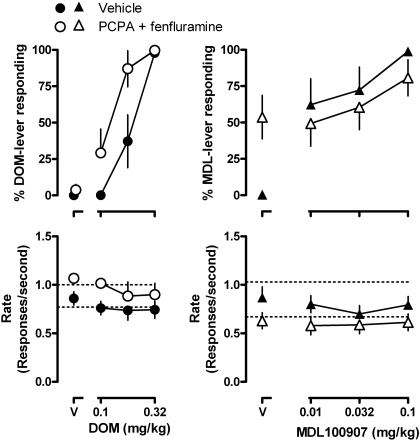
As measured by the number of training sessions when rats satisfied the testing criteria, and comparing the first 20 sessions with the last 20 training sessions, discrimination performance improved slightly over the course of these studies for rats discriminating DOM and for those discriminating MDL100907 and worsened slightly for rats discriminating ketanserin (Table 1). When saline and three different doses of DOM were examined over four consecutive days (i.e., after temporary discontinuation of training), rats responded exclusively on the saline-associated lever after receiving saline and increasingly on the drug-associated lever after receiving DOM (Fig. 6, ●, upper left). The DOM discrimination dose-response curve determined over consecutive days, and after temporary discontinuation of training, was similar to the DOM dose-response curve determined earlier in the study when training sessions occurred between consecutive tests (compare Figs. 1 and 6, ●, upper left). After a second temporary discontinuation of training during which rats were treated with PCPA and fenfluramine, DOM, but not saline, increased responding on DOM-associated lever (Fig. 6, ○, upper) [F(3,21) = 35.82, p < 0.0001]; DOM was significantly more potent as a discriminative stimulus in rats treated with PCPA and fenfluramine, as reflected by a shift leftward in the DOM dose-response curve [F(1,7) = 24.24, p < 0.005 for treatment, no significant interaction between treatment and dose; F(3,21) = 3.04, p = 0.051]. Post hoc analysis revealed that the effect of 0.178 mg/kg DOM was significantly increased after treatment with PCPA and fenfluramine (p < 0.05; Fig. 6, upper left).
Fig. 6.
Effects of DOM (left) and MDL100907 (right) after a 3-day suspension of training and treatment with either vehicle (●, ▴) or PCPA and fenfluramine (○, ▵). See Fig. 1 for other details.
When saline and three different doses of MDL100907 were examined over four consecutive days (i.e., after temporary discontinuation of training), rats responded exclusively on the saline-associated lever after receiving saline and increasingly on the drug-associated lever after receive MDL100907 (Fig. 6, ▴, upper right). However, the MDL100907 dose-response curve determined after temporary discontinuation of training was shifted upward (i.e., more drug lever responding with 0.01 and 0.032 mg/kg) compared with the MDL100907 dose-response curve determined earlier in the study when training sessions occurred between consecutive tests (compare Figs. 1 and 6, upper right). After treatment with PCPA and fenfluramine, saline occasioned an average of 55.5 ± 13.9% responding on the drug-associated lever in rats discriminating MDL100907, with three of eight rats responding greater than 90% on the MDL100907-associated lever; subsequent administration of MDL100907 further increased responding on the drug-associated lever with a dose of 0.1 mg/kg occasioning 85% responding on the MDL100907-associated lever.
Although stimulus control in rats discriminating ketanserin was not adequate for assessing possible changes in sensitivity to discriminative stimulus effects after discontinuation of training, all 24 rats (i.e., all three discrimination groups) received PCPA and fenfluramine and were used to measure 5-HT concentrations in brain. Treatment with PCPA and fenfluramine significantly decreased 5-HT content in the frontal and occipital cortex. Compared with saline-treated control rats, 5-HT was decreased more than 93% in the frontal cortex (control = 194.0 ± 36.5 ng/g; treated = 12.2 ± 1.3 ng/g) and in the occipital cortex (control = 114.0 ± 29.7 ng/g; treated = 7.8 ± 1.2 ng/g).
Discussion
Constitutive activity and inverse agonism have been demonstrated for several receptors in vitro (e.g., Bond and Ijzerman, 2006). Little is known about constitutive activity and inverse agonism in vivo, in part, because of difficulties (e.g., endogenous ligand binding) that are inherent to studies in vivo (e.g., Negus, 2006). Notwithstanding a paucity of in vivo data, there is evidence for constitutive activity of 5-HT2A receptors and for inverse agonist actions of drugs acting at these receptors (for reviews, see Harvey, 2003; Aloyo et al., 2009). The current study examined whether constitutive activity and inverse agonism might be examined by use of drug discrimination with separate groups of rats trained to discriminate different drugs that are known or presumed to have different actions at 5-HT2A receptors: agonism (DOM), antagonism (ketanserin), and inverse agonism (MDL100907). For each drug, stimulus control was established within 35 sessions and the results of substitution studies support the view that all three drugs act, at least in part, at 5-HT2A receptors.
The discriminative stimulus effects of DOM and other 5-HT2A receptor agonists have been studied extensively in rats (Silverman and Ho, 1980; Glennon et al., 1982, 1983; Fiorella et al., 1995; Li et al., 2007) and much less in non-human primates (Li et al., 2008). Consistent with those studies, discriminative stimulus effects of DOM in this study were pharmacologically selective with other compounds failing to occasion substantial responding on the DOM-associated lever. Evidence that this effect of DOM is mediated by 5-HT2A receptors was a dose-related antagonism of DOM by ketanserin. Previous studies showed that the 5-HT2A receptor-selective antagonist MDL100907 also antagonizes the discriminative stimulus effects of DOM in rats (e.g., Li et al., 2007) and in non-human primates (Li et al., 2008). Together with the published studies, these results confirm a prominent role of 5-HT2A receptors in the discriminative stimulus effects of DOM across a range of training doses (0.56–1.5 mg/kg), and they provide a comparison for effects obtained with drugs that are presumed to be antagonists or inverse agonists at 5-HT2A receptors.
This study tested four hypotheses, the first being that a bona fide “neutral” 5-HT2A receptor antagonist (e.g., ketanserin) could not be established as a discriminative stimulus, unless it was blocking the effects of an endogenous agonist or had actions on other receptors. Although ketanserin had been used in a drug-versus-drug discrimination (Smith et al., 1995), there was no report of ketanserin being trained in a simple drug-versus-vehicle discrimination. In fact, stimulus control was established with ketanserin after approximately the same number of training sessions required for establishing stimulus control with DOM or MDL100907. The ketanserin discriminative stimulus was not fully mimicked by any of the compounds studied. Although ketanserin antagonized the discriminative stimulus effects of DOM and substituted for the MDL100907 discriminative stimulus, it was not clear why other drugs that are presumed to have antagonist actions at 5-HT2A receptors (ritanserin and MDL100907) did not substitute for ketanserin. In addition to antagonism at 5-HT2A receptors, ketanserin has antagonist actions at α1-adrenergic receptors (Orallo et al., 2000). Like ritanserin and MDL100907, the α1-adrenergic receptor antagonist prazosin occasioned partial responding on the ketanserin-associated lever. When prazosin was combined with ritanserin or MDL100907, rats responded exclusively on the ketanserin-associated lever, suggesting that the discriminative stimulus effects of ketanserin result from actions at both 5-HT2A and α1-adrenergic receptors. That MDL100907 did not substitute fully for ketanserin could be due to MDL100907 not acting at α1-adrenergic receptors or to differences in the intrinsic activity of MDL100907 and ketanserin (e.g., inverse agonism versus antagonism) at 5-HT2A receptors.
The second hypothesis was that the effects of DOM (agonist) and MDL100907 (inverse agonist) are mutually antagonized. A previous study established a discrimination in rats with MDL100907 and showed that drugs with high affinity (and presumably little or no efficacy) for 5-HT2A receptors substitute for MDL100907 (Dekeyne et al., 2003). In the current study two drugs with high affinity and presumably little or no efficacy at 5-HT2A receptors (ketanserin and ritanserin) substituted for MDL100907, and, when administered as pretreatments, both drugs enhanced the effects of MDL100907. Moreover, doses of MDL100907 and ketanserin that had discriminative stimulus effects in rats discriminating MDL100907 were the same as those antagonizing the discriminative stimulus effects of DOM in rats discriminating DOM (Li et al., 2007; Fig. 2, this study). Conversely, DOM antagonized the discriminative stimulus effects of MDL100907 and those of ketanserin, being slightly more potent in antagonizing MDL100907 compared with ketanserin. Mutual antagonism between DOM and MDL100907 supports the second hypothesis and confirms the role of 5-HT2A receptors in the discriminative stimulus effects of all three drugs.
The third hypothesis was that ketanserin (neutral antagonist) antagonizes the discriminative stimulus effects of DOM (agonist) and MDL100907 (inverse agonist). Mutual antagonism between DOM and ketanserin is consistent with this hypothesis; however, ketanserin alone substituted for MDL100907 and it shifted the MDL100907 dose-response curve leftward. Failure to support this hypothesis could indicate that both ketanserin and MDL100907 are inverse agonists at 5-HT2A receptors and/or that both drugs are acting by blocking endogenous 5-HT.
The MDL100907 discriminative stimulus was pharmacologically selective; neither DOM, phencyclidine, ketamine, or morphine substituted for MDL100907. Although a maximum of 80% responding on the MDL100907-associated lever was obtained with prazosin in rats discriminating 0.16 mg/kg MDL100907 (Dekeyne et al., 2002), in the current study prazosin occasioned little (23 ± 15%) responding on the MDL100907-associated lever in rats discriminating 0.1 mg/kg MDL100907. These studies differed as well in the number of training sessions required to establish stimulus control: 70 ± 11 with 0.16 mg/kg MDL100907 (Dekeyne et al., 2002) and 33 ± 2 with 0.1 mg/kg (current study). Differences between that previous study and the current study might result from the use of different strains of rats (Wistar and Sprague-Dawley, respectively), different schedules of reinforcement (FR 10 and 5, respectively), or different pretreatment times (15 and 30 min, respectively). Together with the results of previous studies, these data support the view that the discriminative stimulus effects of MDL100907 are due to actions at 5-HT2A receptors.
The fourth hypothesis was that depletion of 5-HT increases sensitivity to the discriminative stimulus effects of DOM (agonists) and possibly to MDL100907 (inverse agonist). Confirming other studies (e.g., Prinssen et al., 2002), treatment with PCPA, then fenfluramine, significantly depleted 5-HT in the cortex. After 5-HT depletion, rats were more sensitive to the discriminative stimulus effects of DOM, as indicated by a leftward shift in the DOM dose-response curve. This result is consistent with 5-HT depletion increasing sensitivity to the discriminative stimulus effects of other 5-HT receptor agonists (Browne and Ho, 1975; White et al., 1980; Fiorella et al., 1995). A different method for depleting 5-HT (i.e., 5,7-dihydroxytryptamine) did not change the ability of lysergic acid diethylamide (5-HT2A receptor agonist) and MDL 11939 (5-HT2A receptor inverse agonist) to retard associative learning (Romano et al., 2006). If the discriminative stimulus effects of a drug are due exclusively to the blockade of endogenous 5-HT, then depletion of 5-HT might mimic the drug stimulus. If the discriminative stimulus effects of a drug are due exclusively to inverse agonist actions at 5-HT receptors, then depletion of 5-HT might increase sensitivity to discriminative stimulus effects, as is the case with 5-HT2A receptor agonists. After 5-HT depletion, rats responded 55% on the MDL100907-associated lever in the absence of drug; this responding does not seem to reflect a loss of stimulus control because these rats responded exclusively on the vehicle-associated lever in the absence of drug when training was discontinued and rats received saline and not PCPA and fenfluramine. The testing order of the four conditions (vehicle and different doses of MDL100907) varied among rats and it is possible that the magnitude of 5-HT depletion varied across 4 days of testing (e.g., increased variance among rats in a group). Nevertheless, substantial responding on the MDL100907-associated lever in the absence of MDL100907 and further increases in responding on the MDL100907-associated lever after administration of MDL100907 in 5-HT-depleted rats are consistent with the notion that the MDL100907 discriminative stimulus involves antagonism of endogenous 5-HT and possibly inverse agonism at 5-HT2A receptors. Methods for rapidly determining full dose-response curves (e.g., cumulative dosing) would facilitate a more precise characterization of the effects of 5-HT depletion on discriminative stimulus effects of drugs.
In summary, rats were trained to discriminate either the 5-HT2A receptor agonist DOM, the 5-HT2A receptor antagonist ketanserin, or the purported 5-HT2A receptor inverse agonist MDL100907. The results confirm a role for 5-HT2A receptors in the discriminative stimulus effects of all three drugs, although it seems as though α1-adrenergic receptors play a role in the discriminative stimulus effects of ketanserin. Enhanced discriminative stimulus effects of DOM after depletion of 5-HT might reflect increased receptor sensitivity. Data obtained with MDL100907 are consistent with the possibility of both antagonism and inverse agonism at 5-HT2A receptors, although further studies are warranted with procedures that can rapidly assess sensitivity to drugs in 5-HT-depleted rats.
Acknowledgments
We thank Christopher Cruz, Daniel Mojica, Olivia Dominguez, Jennifer Kite, and Jesus Sanchez for expert technical assistance.
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse; and by the National Institutes of Health National Institute on Drug Abuse [Grant K05-DA17918] (Senior Scientist Award to C.P.F.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.157560
- 5-HT
- serotonin
- DOM
- 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane
- MDL100907
- (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol
- FR
- fixed ratio
- PCPA
- p-chlorophenylalanine methyl ester
- MDL 11939
- a-phenyl-1-(2-phenylethyl)-4-piperidinemethanol.
References
- Alhaider et al., 1993.Alhaider AA, Ageel AM, Ginawi OT. (1993) The quipazine- and TFMPP-increased conditioned avoidance response in rats: role of 5-HT1C/5-HT2 receptors. Neuropharmacology 32:1427–1432 [DOI] [PubMed] [Google Scholar]
- Aloyo et al., 2009.Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. (2009) Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121:160–173 [DOI] [PubMed] [Google Scholar]
- Ariens, 1954.Ariens EJ. (1954) Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch Int Pharmacodyn Ther 99:32–49 [PubMed] [Google Scholar]
- Barker et al., 1994.Barker EL, Westphal RS, Schmidt D, Sanders-Bush E. (1994) Constitutively active 5-hydroxytryptamine 2C receptors reveal novel inverse agonist activity of receptor ligands. J Biol Chem 269:11687–11690 [PubMed] [Google Scholar]
- Berg et al., 1999.Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. (1999) Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol 55:863–872 [PubMed] [Google Scholar]
- Bond and Ijzerman, 2006.Bond RA, Ijzerman AP. (2006) Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96 [DOI] [PubMed] [Google Scholar]
- Browne and Ho, 1975.Browne RG, Ho BT. (1975) Role of serotonin in the discriminative stimulus properties of mescaline. Pharmacol Biochem Behav 3:429–435 [DOI] [PubMed] [Google Scholar]
- Costa and Herz, 1989.Costa T, Herz A. (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A 86:7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekeyne et al., 2002.Dekeyne A, Iob L, Hautefaye P, Millan MJ. (2002) The selective serotonin(2A) receptor antagonist, MDL100,907, elicits a specific interoceptive cue in rats. Neuropsychopharmacology 26:552–556 [DOI] [PubMed] [Google Scholar]
- Dekeyne et al., 2003.Dekeyne A, Iob L, Millan MJ. (2003) Generalization of clozapine as compared to other antipsychotic agents to a discriminative stimulus elicited by the serotonin (5-HT)2A antagonist, MDL100,907. Neuropharmacology 44:604–615 [DOI] [PubMed] [Google Scholar]
- Fiorella et al., 1995.Fiorella D, Helsley S, Lorrain DS, Rabin RA, Winter JC. (1995) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology (Berl) 121:364–372 [DOI] [PubMed] [Google Scholar]
- Glennon et al., 1982.Glennon RA, Young R, Rosecrans JA. (1982) Discriminative stimulus properties of DOM and several molecular modifications. Pharmacol Biochem Behav 16:553–556 [DOI] [PubMed] [Google Scholar]
- Glennon et al., 1983.Glennon RA, Young R, Rosecrans JA. (1983) Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol 91:189–196 [DOI] [PubMed] [Google Scholar]
- Harvey, 2003.Harvey JA. (2003) Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem 10:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey et al., 1999.Harvey JA, Welsh SE, Hood H, Romano AG. (1999) Effect of 5-HT2 receptor antagonists on a cranial nerve reflex in the rabbit: evidence for inverse agonism. Psychopharmacology (Berl) 141:162–168 [DOI] [PubMed] [Google Scholar]
- Kenakin, 1996.Kenakin T. (1996) The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev 48:413–463 [PubMed] [Google Scholar]
- Li et al., 2007.Li JX, Rice KC, France CP. (2007) Behavioral effects of dipropyltryptamine in rats: evidence for 5-HT1A and 5-HT2A agonist activity. Behav Pharmacol 18:283–288 [DOI] [PubMed] [Google Scholar]
- Li et al., 2008.Li JX, Rice KC, France CP. (2008) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) in rhesus monkeys. J Pharmacol Exp Ther 324:827–833 [DOI] [PubMed] [Google Scholar]
- Negus, 2006.Negus SS. (2006) Some implications of receptor theory for in vivo assessment of agonists, antagonists and inverse agonists. Biochem Pharmacol 71:1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender et al., 1999.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. (1999) RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem 274:9472–9478 [DOI] [PubMed] [Google Scholar]
- Orallo et al., 2000.Orallo F, Rosa E, García-Ferreiro T, Campos-Toimil M, Cadavid MI, Loza MI. (2000) Cardiovascular effects of ketanserin on normotensive rats in vivo and in vitro. Gen Pharmacol 35:95–105 [DOI] [PubMed] [Google Scholar]
- Parra and Bond, 2007.Parra S, Bond RA. (2007) Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol 7:146–150 [DOI] [PubMed] [Google Scholar]
- Prinssen et al., 2002.Prinssen EP, Assié MB, Koek W, Kleven MS. (2002) Depletion of 5-HT disrupts prepulse inhibition in rats: dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology 26:340–347 [DOI] [PubMed] [Google Scholar]
- Romano et al., 1991.Romano AG, Bormann NM, Harvey JA. (1991) A unique enhancement of associative learning produced by methylenedioxyamphetamine. Behav Pharmacol 2:225–231 [PubMed] [Google Scholar]
- Romano et al., 2000.Romano AG, Hood H, Harvey JA. (2000) Dissociable effects of 5-HT2A antagonist mianserin on associative learning and performance in the rabbit. Pharmacol Biochem Behav 67:103–110 [DOI] [PubMed] [Google Scholar]
- Romano et al., 2006.Romano AG, Quinn JL, Liu R, Dave KD, Schwab D, Alexander G, Aloyo VJ, Harvey JA. (2006) Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology 184:173–181 [DOI] [PubMed] [Google Scholar]
- Schlag et al., 2004.Schlag BD, Lou Z, Fennell M, Dunlop J. (2004) Ligand dependency of 5-hydroxytryptamine 2C receptor internalization. J Pharmacol Exp Ther 310:865–870 [DOI] [PubMed] [Google Scholar]
- Schreiber et al., 1994.Schreiber R, Brocco M, Millan MJ. (1994) Blockade of the discriminative stimuluseffects of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol 264:99–102 [DOI] [PubMed] [Google Scholar]
- Silverman and Ho, 1980.Silverman PB, Ho BT. (1980) The discriminative stimulus properties of 2,5-dimethoxy-4-methylamphetamine (DOM): differentiation from amphetamine. Psychopharmacology (Berl) 68:209–215 [DOI] [PubMed] [Google Scholar]
- Smith et al., 1995.Smith RL, Barrett RJ, Sanders-Bush E. (1995) Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. J Pharmacol Exp Ther 275:1050–1057 [PubMed] [Google Scholar]
- Smith et al., 2002.Smith RL, Gresch PJ, Barrett RJ, Sanders-Bush E. (2002) Stimulus generalization by fenfluramine in a quipazine-ketanserin drug discrimination is not dependent on indirect serotonin release. Pharmacol Biochem Behav 72:77–85 [DOI] [PubMed] [Google Scholar]
- Strange, 2000.Strange PG. (2000) Agonist binding to G-protein coupled receptors. Br J Pharmacol 129:820–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich and Rice, 2000.Ullrich T, Rice KC. (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorg Med Chem 8:2427–2432 [DOI] [PubMed] [Google Scholar]
- Walker et al., 2005.Walker EA, Kohut SJ, Hass RW, Brown EK, Jr, Prabandham A, Lefever T. (2005) Selective and nonselective serotonin antagonists block the aversive stimulus properties of MK212 and m-chlorophenylpiperazine (mCPP) in mice. Neuropharmacology 49:1210–1219 [DOI] [PubMed] [Google Scholar]
- Weiner et al., 2001.Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. (2001) 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics J Pharmacol Exp Ther 299:268–276 [PubMed] [Google Scholar]
- Welsh et al., 1998.Welsh S, Romano A, Harvey J. (1998) Effects of serotonin 5HT2A/2C antagonists on associative learning in the rabbit. Psychopharmacology (Berl) 137:157–163 [DOI] [PubMed] [Google Scholar]
- White et al., 1980.White FJ, Simmons MA, West KB, Holohean AM, Appel JB. (1980) The effect of serotonin depletion on the discriminability of LSD. Pharmacol Biochem Behav 13:569–574 [DOI] [PubMed] [Google Scholar]