Abstract
Oxidative stress underlies diverse vascular diseases, but its management remains elusive, in part because of our inability to selectively detoxify reactive oxygen species (ROS) in pathological sites and our limited understanding which species need to be eliminated. The antioxidant enzymes (AOEs) superoxide dismutase (SOD) and catalase (which decompose  and H2O2, respectively), conjugated with an antibody to platelet-endothelial cell adhesion molecule-1 (PECAM-1), bind to endothelial cells and alleviate oxidative stress in cell culture models. Here, we studied the effects of these antioxidant conjugates in mouse models of vascular oxidative stress. Anti-PECAM/catalase and anti-PECAM/SOD conjugates, in contrast to control IgG/AOE conjugates, accumulated in the lungs and vascularized organs after intravenous injection in wild-type, but not PECAM KO mice. Anti-PECAM/catalase, but not anti-PECAM/SOD, protected mice from lung injury induced by H2O2 produced by glucose oxidase deposited in the pulmonary vasculature. Anti-PECAM/catalase also reduced alveolar edema and attenuated decline in arterial oxygen in mice that underwent unilateral lung ischemia/reperfusion, whereas anti-PECAM/SOD was not effective, implying the key role of H2O2 in tissue damage in this pathology. In contrast, anti-PECAM/SOD, but not anti-PECAM/catalase prevented oxidation of tetrahydrobiopterin and normalized vasoreactivity in the vessels of mice rendered hypertensive by pretreatment with angiotensin-II. This outcome agrees with reports implicating superoxide and peroxynitrite in altered endothelium-dependent vasodilatation in hypertension. Therefore, the use of endothelial cell-targeted antioxidants identifies the key specific species of ROS involved in various forms of vascular disease and holds promise for the mechanistically tailored treatment of these pathologies.
and H2O2, respectively), conjugated with an antibody to platelet-endothelial cell adhesion molecule-1 (PECAM-1), bind to endothelial cells and alleviate oxidative stress in cell culture models. Here, we studied the effects of these antioxidant conjugates in mouse models of vascular oxidative stress. Anti-PECAM/catalase and anti-PECAM/SOD conjugates, in contrast to control IgG/AOE conjugates, accumulated in the lungs and vascularized organs after intravenous injection in wild-type, but not PECAM KO mice. Anti-PECAM/catalase, but not anti-PECAM/SOD, protected mice from lung injury induced by H2O2 produced by glucose oxidase deposited in the pulmonary vasculature. Anti-PECAM/catalase also reduced alveolar edema and attenuated decline in arterial oxygen in mice that underwent unilateral lung ischemia/reperfusion, whereas anti-PECAM/SOD was not effective, implying the key role of H2O2 in tissue damage in this pathology. In contrast, anti-PECAM/SOD, but not anti-PECAM/catalase prevented oxidation of tetrahydrobiopterin and normalized vasoreactivity in the vessels of mice rendered hypertensive by pretreatment with angiotensin-II. This outcome agrees with reports implicating superoxide and peroxynitrite in altered endothelium-dependent vasodilatation in hypertension. Therefore, the use of endothelial cell-targeted antioxidants identifies the key specific species of ROS involved in various forms of vascular disease and holds promise for the mechanistically tailored treatment of these pathologies.
Oxidative stress induced by an excess of reactive oxygen species (ROS) plays an important role in a number of vascular pathologies including hypertension, ischemia, stroke, acute myocardial infarction, and inflammation (Cai et al., 2003; Krause and Bedard, 2008). To improve management of these conditions, intense efforts are being focused on the development of ROS-detoxifying interventions. For example, nonenzymatic antioxidants, including scavengers of ROS or donors of reducing equivalents (e.g., glutathione precursors), may help alleviate subtle chronic oxidative stress, but these consumable agents provide rather marginal protection against severe oxidative stresses (Dikalov et al., 2007; Porkert et al., 2008).
The use of enzymes that serve as antioxidant catalysts capable of decomposing unlimited copies of ROS may be more promising (McCord, 2002). Two examples are superoxide dismutase (SOD) (which converts superoxide anion to hydrogen peroxide, H2O2) and catalase (which detoxifies H2O2 to water and oxygen). Unfortunately, they currently have no medical utility because of our inability to adequately deliver them to their key therapeutic targets, in particular, the endothelial cells that both generate ROS and suffer oxidative injury (Terada et al., 1992; Houston et al., 1999; Muzykantov, 2001; Cai et al., 2003; Guo et al., 2007). Thus, effective management of acute vascular oxidative stress still remains elusive (Muzykantov, 2001).
SOD variants with affinity to anionic endothelial glycocalyx are being designed, and show promising protective effects in some models of vascular oxidative stress (Gao et al., 2003). Another approach, namely, enzyme conjugation to antibodies directed to endothelium-specific proteins, including cell adhesion molecules expressed by inflamed endothelial cells, offers the possibility to target endothelium and provide the intracellular delivery of antioxidant enzymes (Muzykantov et al., 1996, 1999). We and others have previously shown that antibody/catalase conjugates targeted to the endothelium provide superior protection versus that afforded by nontargeted catalase in animal models of acute vascular oxidative stress (Sweitzer et al., 2003; Nowak et al., 2007).
A particularly attractive endothelial-specific protein for targeting antioxidants is platelet-endothelial adhesion cell molecule-1, CD31 (PECAM-1) (Muzykantov et al., 1999; Li et al., 2000). This molecule is stably expressed on the endothelial lumen at a level of approximately one million copies per cell and is involved in the migration of activated leukocytes across endothelium in inflammation (Newman, 1997). Inhibition of leukocyte transmigration by blocking PECAM may provide a secondary benefit in the context of vascular oxidative stress (Matthay et al., 2003). Endothelial cells internalize anti-PECAM/conjugates (Muzykantov et al., 1999; Li et al., 2000). Anti-PECAM/catalase conjugates protect against lung injury in animal models involving endothelial generation of H2O2 by glucose oxidase sequestered in the pulmonary vasculature (Christofidou-Solomidou et al., 2003; Kozower et al., 2003).
Superoxide anion produced by vascular cells, including endothelial cells, has also been implicated in the vascular oxidative stress in hypertension, ischemia, hyperoxia, stroke, and other conditions (Bonaventura and Gow, 2004; Loomis et al., 2005). Superoxide contributes to vascular disorders and vasoconstriction by inactivating NO and forming the strong oxidant peroxynitrite, among other mechanisms (Cai et al., 2003). Peroxynitrite can inactivate enzymes that produce vasodilators such as prostacyclin (Zou, 2007) and also oxidizes tetrahydrobiopterin (BH4) a critical cofactor for the nitric-oxide synthases (Kuzkaya et al., 2003), thus reducing endothelial NO production and promoting further superoxide production. Therefore, selective scavenging of superoxide will inhibit peroxynitrite formation and may help preserve endothelial NO production. Accordingly, we have designed anti-PECAM/SOD conjugates and found that endothelial targeting of anti-PECAM/SOD protects against cell damage caused by either extracellular or intracellular superoxide anion production in cell culture (Shuvaev et al., 2007b).
In this study, we have taken advantage of our ability to deliver high levels of specific and effective AOEs using anti-PECAM/catalase and anti-PECAM/SOD to the endothelium in mice to: i) define the role of endothelial superoxide and H2O2 in specific forms of vascular oxidative stress in vivo including glucose oxidase-induced pulmonary vascular injury, lung ischemia-reperfusion, and angiotensin II-induced hypertension; and ii) design prototypes for specific antioxidant treatment of acute vascular oxidative stress.
Materials and Methods
Conjugate Preparation.
Conjugates were prepared by use of amino-based cross-linking chemistry as described previously (Shuvaev et al., 2007b). Number of corresponding reactive groups introduced per molecule of IgG, antibody, and AOE (catalase or SOD) has been adjusted individually for each type of conjugate to maintain functional activity of both antibody and AOE moieties and produce conjugates with diameter of ∼300 nm. The size of prepared conjugates was measured by dynamic light scattering (DLS) apparatus 90Plus Particle Sizer (Brookhaven Instruments Corp., Holtsville, NY). Our previous studies in vitro and in vivo documented that anti-PECAM conjugates of this size optimally bind to endothelial cells, enter cells via cell adhesion molecule-mediated endocytosis, circulate in vivo, and deliver cargoes to endothelium in the vasculature (Muzykantov et al., 1999; Christofidou-Solomidou et al., 2003; Kozower et al., 2003; Shuvaev et al., 2007b).
Biodistribution of anti-PECAM/AOE Conjugates in Vivo.
Animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. 125I-radiolabeled anti-PECAM/SOD and IgG/SOD conjugates were prepared as described previously (Shuvaev et al., 2007b) with final conjugate labeling at a density of ∼10,000 cpm/μg total SOD. Ten micrograms of radiolabeled immunoconjugated SOD were injected intravenously in normal C57BL/65 mice (The Jackson Laboratory, Bar Harbor, ME) via tail vein. After 1 h, the internal organs were harvested, rinsed with saline, blotted dry, and weighed. Tissue radioactivity in organs and 100-μl samples of blood was determined in a Wallac 1470 Wizard gamma counter (PerkinElmer Life and Analytical Sciences–Wallac Oy, Turku, Finland). The results of 125I measurements in the organs were used to calculate four parameters characterizing the total and relative SOD uptake, the anti-PECAM/SOD conjugate biodistribution, and targeting. First, the percentage of injected dose in an organ (%ID) measures the total amount of antibody uptake in an organ, showing biodistribution and effectiveness of antibody uptake. Total AOE dose circulating in blood was calculated considering that mouse blood volume normally equals to 7.2% of animal weight. Second, the percentage of injected dose per gram of tissue (%ID/g) permits comparisons of the antibody targeting to different organs, evaluating tissue selectivity of antibody uptake.
A Model of Pulmonary Oxidative Stress Caused by Glucose Oxidase Sequestered in the Pulmonary Vasculature in Mice Exposed to Elevated Oxygen Level (GOX)/Hyperoxia.
In this model, pulmonary vascular oxidative stress was induced by intravenous injection of glucose oxidase conjugated with thrombomodulin antibody (anti-TM/GOX conjugate) in anesthetized C57BL/6 mice (The Jackson Laboratory). Anti-TM/GOX accumulates in the mouse lungs and generates H2O2 from glucose, thereby causing an acute edematous lung injury at anti-TM/GOX dose of 1.0 μg/g (Shuvaev et al., 2007a). To enhance injury by increasing the GOX substrate supply, animals were subjected to 80% O2 in hyperoxic chamber for 4 h. Our previous study showed that this treatment aggravates oxidative stress and lung tissue injury in mice that received injections of anti-TM/GOX (Shuvaev et al., 2007a). The lungs were harvested and allocated for histopathology and immunostaining, and bronchoalveolar lavage (BAL) to test BAL fluid protein concentration. For histopathological studies, the lungs were instilled before removal from the animal with 0.75 ml of buffered formalin through a 20-gauge angiocatheter placed in the trachea, immersed in buffered formalin overnight, and then processed for conventional paraffin histology. Sections were stained with hematoxylin and eosin, and examined by light microscopy.
Unilateral in Situ Lung Ischemia/Reperfusion Injury in Mice.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The technique is described in detail elsewhere (Lee et al., 2008). In brief, mice were anesthetized and tracheostomy and mechanical ventilation were performed (fraction of inspired oxygen, 21%; tidal volume; 10 ml/kg; respiratory rate, 130 min−1). Saline, an anti-PECAM/AOE or IgG/AOE conjugate was injected intravenously and allowed to circulate for 30 min. Thoracotomy was performed, and the left pulmonary hilum was clamped for 60 min. The clamp was then removed and reperfusion allowed for an additional 60 min. Sham animals [undergoing thoracotomy, but not ischemia/reperfusion (I/R)] were used as controls. Immediately at sacrifice, we have determined PaO2 level in arterial blood and protein level in the bronchoalveolar lavage fluid (BALF) collected post mortem. Malondialdehyde (MDA), a reactive carbonyl compound formed upon decomposition of polyunsaturated fatty acid peroxides, was measured as an indicator of oxidative stress (Kinniry et al., 2006). Lipid peroxidation in homogenized lung tissues was detected by use of a commercially available kit (OXIS Research, Inc., Portland, OR) according to manufacturer's recommendations. The results were expressed as micromoles of MDA per gram of wet lung tissue. The percentage increase of MDA level compared with control mice (no ischemia-reperfusion injury), was calculated and expressed graphically.
Angiotensin II-Induced Vascular Dysfunction.
Animal experiments were approved by the Emory University Animal Care and Use Committee. At 12 weeks of age the C57BL/6 mice were anesthetized with Avertin 2.5% (0.3 ml per 25 g body weight i.p.) and osmotic minipumps (Alzet model 2002; Alzet Corp., Cupertino, CA) were implanted to permit subcutaneous infusion of angiotensin II (0.7 mg/kg per day for 14 days) as described earlier (Widder et al., 2007). Sham-operated animals underwent an identical surgical procedure, except that either no pump or an empty osmotic pump was implanted. Two weeks later, the animals were briefly anesthetized with Ketamine (100 mg/kg) and xylazine (10 mg/kg) and were given an intravenous injection of anti-PECAM/enzyme conjugates. Animals were euthanized 15 min, 1 h, or 3 h after conjugate injections, and the aorta was removed for isometric tension studies. To test vascular reactivity of blood vessels obtained from angiotensin-treated mice, 3-mm aortic ring segments were suspended in organ chambers as described previously (Landmesser et al., 2003), and passive tension was adjusted to 1 g. Vessels were preconstricted to equal levels with PGF2α and relaxations to cumulative concentrations of acetylcholine and nitroglycerin were examined as we described.
Determining Vascular Level of H2O2,  , and Biopterin (BH4) in the Blood Vessels Obtained from Angiotensin II-Treated Mice.
, and Biopterin (BH4) in the Blood Vessels Obtained from Angiotensin II-Treated Mice.
Hypertension is associated with vascular overproduction of reactive oxygen species. To determine vascular  and H2O2 levels, the oxidation of dihydroethidium to 2-hydroxyethidium and dichlorofluorescein was examined in the homogenates of the blood vessels as described previously (Dikalov et al., 2007). Tetrahydrobiopterin, BH4, is a critical cofactor for the NO synthases, and in its absence these enzymes become “uncoupled,” producing ROS rather than NO. Level of oxidized biopterin in aortas was analyzed by use of high-performance liquid chromatography and a differential oxidation method as performed previously (Fukushima and Nixon, 1980).
and H2O2 levels, the oxidation of dihydroethidium to 2-hydroxyethidium and dichlorofluorescein was examined in the homogenates of the blood vessels as described previously (Dikalov et al., 2007). Tetrahydrobiopterin, BH4, is a critical cofactor for the NO synthases, and in its absence these enzymes become “uncoupled,” producing ROS rather than NO. Level of oxidized biopterin in aortas was analyzed by use of high-performance liquid chromatography and a differential oxidation method as performed previously (Fukushima and Nixon, 1980).
Administration of Antioxidant Interventions.
Equimolar doses of catalase (100 μg per animal) or SOD (12.5 μg per animal) conjugated with either control IgG (i.e., IgG/enzyme) or with anti-PECAM (i.e., anti-PECAM/enzyme) were injected intravenously in mice 15 min before initiation of an insult, unless a different time interval is specified otherwise.
Analysis of Pulmonary Edematous Injury by the Protein Level in Bronchoalveolar Lavage Fluid.
BAL was performed by exposing and cannulating the trachea with a 20-gauge angiocatheter (BD Biosciences, Sandy, UT) and then lavaging three times with 0.5 ml of phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) at 10 μl/ml (Kinniry et al., 2006). Recovery of infused fluid was >90%. The lavage fluid was spun at 2000 rpm for 3 to 4 min; the supernatant was collected, aliquoted, and frozen at −70°C. Protein concentrations were later measured in the thawed supernatant of the BALF by use of a standard BCA assay (Pierce Chemicals; Rockford, IL). Protection against edematous lung injury was calculated using following equation: Protection (%) = (BALPOX+AOE − BALPsham)/(BALPOX − BALPsham) × 100%, where BALP is protein concentrations in bronchoalveolar lavage fluid of control animals (sham) or animals subjected to oxidative stress (either GOX or I/R model) alone (OX) or in the presence of antioxidant enzyme treatment (OX + AOE).
Statistical Analysis.
Statistical difference among groups was determined using one-way analysis of variance. When statistically significant differences were found (p < 0.05) individual comparisons were made by use of the Bonferoni/Dunn test and the Student's t test (Statview 4.0; SAS Institute, Cary, NC).
Results
Vascular Targeting of Anti-PECAM Conjugates in Mice.
In accord with previous reports from us and other laboratories (Muzykantov, 2001), 125I-labeled unconjugated SOD and catalase were rapidly cleared from the bloodstream after intravenous injection in mice (T1/2 5–7 min and ∼10–15 min, respectively) without significant uptake in organs except the kidney and liver, the sites of clearance of SOD and catalase, respectively (data not shown). In contrast, both SOD (Fig. 1A) and catalase (Fig. 1B) conjugated with anti-PECAM accumulated to a similar extent in the lungs after intravenous injection. Anti-PECAM/catalase and anti-PECAM/SOD also accumulated in other vascularized organs, e.g., in the heart (Fig. 1). This result is consistent with previous data showing that PECAM-targeted conjugates and fusion proteins accumulate after intravenous injection in highly vascularized organs: first, in the lungs, because the pulmonary vasculature receives the entire cardiac venous blood output and represents approximately 30% of the total endothelial surface in the body (Muzykantov, 2005).
Fig. 1.
Vascular targeting of antioxidant enzymes conjugated with PECAM antibody. Distribution of 125I-labeled SOD, catalase anti-PECAM, or IgG conjugates 1 h after intravenous injection in mice. A, 125I-SOD conjugated with anti-PECAM ( ) or control IgG (■). B, 125I-catalase conjugated with anti-PECAM in the organs of wild-type C57BL/6 (
) or control IgG (■). B, 125I-catalase conjugated with anti-PECAM in the organs of wild-type C57BL/6 ( ) or PECAM−/− (■) mice. The data are shown as mean ± S.D, n = 3. *, P < 0.05 versus the corresponding control (■) group. WT, wild type; KO, knockout.
) or PECAM−/− (■) mice. The data are shown as mean ± S.D, n = 3. *, P < 0.05 versus the corresponding control (■) group. WT, wild type; KO, knockout.
In contrast, neither IgG/SOD nor IgG/catalase accumulated in the lungs, showing uptake of 6.1 and 9.7%ID/g, respectively. The results indicate that the enzymes conjugated with control IgG did not accumulate in the lungs and heart (Fig. 1A), indicating that uptake of anti-PECAM conjugates in vascularized organs was not due to nonspecific uptake, i.e., entrapment in the capillaries. Furthermore, anti-PECAM/catalase injected in PECAM−/− mice did not accumulate in the lungs and heart, ultimately confirming the specificity of endothelial targeting (Fig. 1B). The blood level of anti-PECAM/enzyme conjugates was lower than that of control counterparts (Fig. 1, A and B), reflecting depletion of the circulating pool of anti-PECAM conjugates as these bind to the endothelium.
Anti-PECAM/Catalase, but Not Anti-PECAM/SOD Protects against Acute Oxidative Lung Injury Induced Hydrogen Peroxide Generated by Endothelium-Targeted Glucose Oxidase.
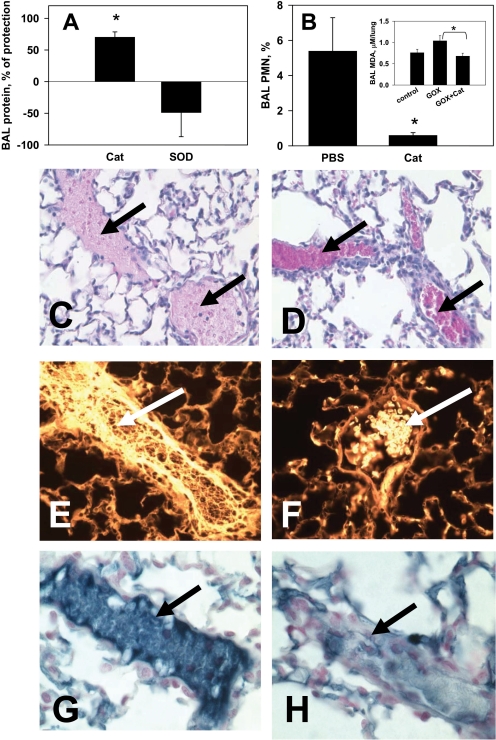
Intravenous injection of glucose oxidase conjugated with thrombomodulin antibody (anti-TM/GOX) leads to anti-TM/GOX uptake in the pulmonary vasculature, generation of H2O2 in endothelium, and acute edematous lung injury in mice (Christofidou-Solomidou et al., 2003). Exposure of mice injected with anti-TM/GOX to elevated levels of oxygen (98% O2, hyperoxia) further aggravates lung injury (Shuvaev et al., 2007b). This “double-hit” model imitates pulmonary pathological conditions caused by vascular ROS toxicity aggravated by iatrogenic effects of oxygen ventilation that may happen, for example, in treatment of acute lung injury (Matthay et al., 2003). Analysis of BALF protein and neutrophil levels (reflecting alveolar edema and infiltration, respectively) showed that anti-PECAM/catalase, but not anti-PECAM/SOD, protected against this type of lung injury (Fig. 2, A and B). This was probably due to direct detoxification of H2O2 produced by endothelium-bound glucose oxidase. The inset in Fig. 2B shows that anti-PECAM/catalase normalizes BALF level of MDA, a marker of lipid peroxidation. Microscopy analysis of lung tissue sections showed that anti-PECAM/catalase attenuated tissue damage (Fig. 2, C and D), intravascular hemolysis (Fig. 2, E and F) and thrombosis (Fig. 2, G and H) in this model of acute pulmonary oxidative stress caused by H2O2 production in the endothelium.
Fig. 2.
Anti-PECAM/catalase, but not anti-PECAM/SOD, attenuates acute lung injury and thrombosis in a mouse model of “double-hit” oxidative stress caused by H2O2 production and hyperoxia (“GOX”). Mice received intravenous injections of equimolar doses of anti-PECAM/catalase or anti-PECAM/SOD or a mixture of both conjugates. Ten minutes later, 0.75 μg/g i.v. anti-TM/GOX was injected and mice were placed in 80% O2. Four hours later, mice were sacrificed, BALF obtained, and the lungs harvested and analyzed. A, degree of protection against lung edema tested by BALF protein level. B, attenuation of neutrophil alveolar transmigration and level of oxidative stress marker MDA in BALF (inset). *, P < 0.05 versus the corresponding control group. C and D, hematoxylin and eosin staining of lung tissue sections of GOX/hyperoxia challenged mice treated with saline (C) or anti-PECAM/catalase (D). Arrows in C show vessels with intravascular hemolysis and fibrin deposition (pink background), and arrows in D show congested vessels with intact red blood cells. E and F, autofluorescence of lung sections viewed via epifluorescence 4,6-diamidino-2-phenylindole/orange filter (380.0 nm/750.0 nm) show hemolyzed erythrocytes (arrow) in the vessels of PBS-treated mice challenged with GOX (E), and intact RBCs in anti-PECAM/catalase-treated mice challenged with GOX (F). G and H, anti-PECAM/catalase protects against pulmonary thrombosis caused by GOX/hyperoxia vascular oxidative stress. Blue color shows immunostaining for mouse fibrinogen. Cat, catalase; PMN, polymorphonuclear neutrophil.
Anti-PECAM/Catalase, but Not Anti-PECAM/SOD, Protects against in Situ Lung Ischemia-Reperfusion Injury.
To test effects of AOE targeting in a model of acute lung oxidative stress initiated not solely by H2O2, we injected conjugates IV in mice 15 min before in situ I/R of ventilated mouse lungs. In this model, diverse injurious agents including ROS, such as superoxide anion and H2O2 produced by vascular and blood cells, may contribute to the pathological outcome (Guo and Ward, 2007). Despite this multifactorial pathogenesis of I/R injury, anti-PECAM/catalase, but not anti-PECAM/SOD conjugate protected against alveolar edema (Fig. 3A) and attenuated the decline in the arterial PaO2 in mice that underwent I/R (Fig. 3B), reflecting pulmonary functional improvement. Control IgG/enzyme conjugates provided no protective effects, confirming the necessity of specific endothelial targeting of catalase.
Fig. 3.
Anti-PECAM/catalase, but not anti-PECAM/SOD, protects lungs against I/R. Mice were anesthetized and received intravenous injections of equimolar doses of catalase or SOD conjugates 30 min before left pulmonary artery clamping. Animals were subjected to ischemia (1 h) and then reperfusion (1 h) of the left lung. A, BAL proteins shown as percentage of protection versus sham-operated animals calculated as described in Materials and Methods. B, blood oxygenation in animals preinjected with conjugates. Upper and lower dash lines indicate blood oxygenation in sham and untreated I/R groups, respectively. #, P < 0.05, conjugate-treated group versus control I/R group; *, P < 0.05, anti-PECAM group versus control IgG group.
Anti-PECAM/SOD, but Not Anti-PECAM/Catalase, Prevents Alteration of Endothelium-Dependent Vasodilatation Caused by Angiotensin II.
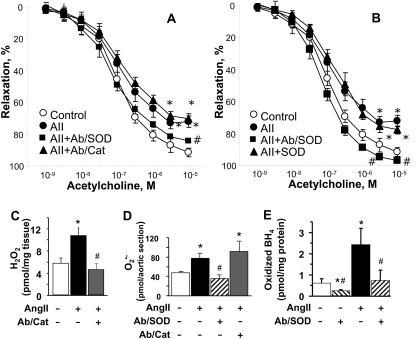
Finally, we tested the effects of endothelial targeting of catalase or SOD in a mouse model of AngII-induced vasoconstriction. In agreement with the literature, AngII treatment reduced endothelium-dependent vasodilatation evoked by acetylcholine (Fig. 4A). Anti-PECAM/catalase reduced the aortic level of H2O2, but had no effect on vasodilatory response (Fig. 4A). In contrast, anti-PECAM/SOD normalized vascular relaxation in response to acetylcholine. This was accompanied by a reduction in the level of superoxide (Fig. 4D). A particularly important consequence of superoxide formation is oxidation of tetrahydrobiopterin. In keeping with this, oxidation of tetrahydrobiopterin was prevented by anti-PECAM/SOD in vessels obtained from AngII-treated animals (Fig. 4E). A mixture of unconjugated SOD and anti-PECAM had no such effects (Fig. 4, B and E), revealing the necessity of targeted delivery of SOD to endothelium.
Fig. 4.
Anti-PECAM/SOD, but not anti-PECAM/catalase normalizes endothelial dysfunction induced by angiotensin II treatment. A and B, relaxation induced by acetylcholine was measured on preconstricted aortic rings obtained from mice from the indicated experimental groups: control untreated mice (○), AngII-treated mice (●), and AngII-treated mice received injections 15 min before aorta harvesting of anti-PECAM/catalase (▴ in A) or anti-PCEAM/SOD (■), or a mixture of unconjugated anti-PECAM and SOD (▴ in B). In C to E, aortas extracted from mice that underwent treatments as indicated have been analyzed for the level of: H2O2 (C), superoxide anion  (D), and oxidized BH4 (E). *, P < 0.05 versus control; #, P < 0.05 versus AngII, n = 4–9.
(D), and oxidized BH4 (E). *, P < 0.05 versus control; #, P < 0.05 versus AngII, n = 4–9.
To define the potential contribution of PECAM blocking in protective effects of the conjugates, we have injected a mixture of nonconjugated anti-PECAM and antioxidant enzymes of interest. However, injection of anti-PECAM and catalase mixture produced no protective effect in TM/GOX and I/R models, whereas the mixture of anti-PECAM and SOD produced no significant effect in the AngII model (not shown).
Discussion
Vascular oxidative stress (VOS) is a collective term that refers to an abnormal surplus of reactive oxygen and nitrogen species (ROS and RNS) in the vasculature in conditions with diverse etiologies. The cells responsible for VOS are also diverse and include leukocytes, platelets, endothelial cells, smooth muscle cells, macrophages, and cells in the extravascular compartments (Andreadis et al., 2003). VOS has been implicated in the pathogenesis of a plethora of cardiovascular, cerebral, and pulmonary maladies. Oxidant injury in any organ, and in particular VOS, is notoriously difficult to treat, in part, because of an incomplete understanding of the pathogenic roles of a given ROS in specific cell types in a given disease and the inability to detoxify the culprit ROS in the proper target cell where ROS production is increased.
Pharmacological, genetic, and imaging evidence indicate that endothelial cells represent one of the key therapeutic targets in VOS. In theory, the targeting of specific antioxidants to endothelium could help both identify pathogenic roles of specific endothelial ROS in given types of VOS and design more effective and selective antioxidant interventions aimed at treatment of these pathologies.
To achieve this goal, we studied the effects of anti-PECAM/catalase and anti-PECAM/SOD in several mouse models of VOS characterized by distinct locations, mechanisms, and extent of the pathological ROS surplus. Based on our prior work, we know that these conjugates specifically bind to endothelial cells and blunt oxidative stress caused by both intra- and extracellular superoxide and H2O2 generated in endothelial cell cultures (Sweitzer et al., 2003; Shuvaev et al., 2007b). The current study shows that in vivo administration of anti-PECAM/catalase and anti-PECAM/SOD allows their delivery to the endothelium equally, effectively, and specifically, to an extent unattainable by nontargeted control SOD and catalase or conjugates themselves in PECAM-deficient animals (Fig. 1). These data imply that failure of a given conjugate to provide protection is not due to lack of its functionality, but rather reflects the role of the corresponding endothelial ROS in a specific form of VOS.
We first examined the highly defined anti-TM/glucose oxidase conjugate that specifically produces H2O2 in the endothelium in hyperoxia-exposed mice. Glucose oxidase has been used extensively for modeling local oxidative stress in the airways, joints, and other injectible compartments. Targeting glucose oxidase to the pulmonary endothelium using antibodies to thrombomodulin, an endothelial glycoprotein specifically enriched in this vascular bed, provides an opportunity to study VOS specifically induced by H2O2 in this cell type (Christofidou-Solomidou et al., 2003). Hyperoxia alone is rather innocuous over this short time frame, requiring several days to cause oxidative lung injury (Matthay et al., 2003; Perkowski et al., 2006). However, in this “double-hit” model system, we believe it provides additional influx of oxygen substrate to GOX and thereby augments lung injury (Shuvaev et al., 2007a). Although we know that high levels of H2O2 are produced and play an important role in inflicting maximal edematous lung injury within 4 h in this model (Shuvaev et al., 2007a), the role of superoxide had never been examined. This is important, because superoxide-mediated mechanisms, including NO consumption and production of highly injurious oxidants like peroxynitrite and hydroxyl radical in reactions with NO and H2O2, respectively, could contribute to tissue injury in the anti-TM/GOX-hyperoxia model. The lack of anti-PECAM/SOD protection, however, implies that these mechanisms are of relatively minor importance in this model and confirms that augmentation of anti-TM/GOX-induced injury by hyperoxia (Shuvaev et al., 2007a) is primarily due to enhancement of enzymatic activity of endothelium-bound GOX via increased supply of the rate-limiting substrate, oxygen. These results indicate that conversion of surplus superoxide into H2O2 does not alleviate this type of oxidative injury, inflicted predominantly by H2O2, a more diffusible and long-living ROS that gives rise to highly injurious hydroxyl radical and hypochlorous acid. However, detoxification of endothelial H2O2 by anti-PECAM/catalase alleviates vascular injury and its immediate complications, including hemolysis and thrombosis, thus, for the first time, demonstrating the protective effects of catalase targeting in this model of “double-hit” acute lung injury (Fig. 2).
Models of pulmonary ischemia/reperfusion are related to lung transplantation and cardiopulmonary bypass. The nature of this injury is very complex (Ovechkin et al., 2007), and several studies have implicated superoxide in its pathogenesis (Goswami et al., 2007). Therefore, we were somewhat surprised that anti-PECAM/SOD provided no protection in the I/R model. The similarity of pulmonary targeting of anti-PECAM/catalase and anti-PECAM/SOD (Fig. 1), the protective activity of anti-PECAM/SOD in endothelial cells in culture (Shuvaev et al., 2007b), and its protective effects in AngII model (Fig. 4) all argue against failure of the conjugate functionality. It seems more likely that the more diffusible and toxic H2O2 is the main culprit in tissue injury in I/R, and the facilitation of its production from superoxide is not protective unless an effective mechanism for H2O2 detoxification is in place. The difference observed with other studies that have reported protective effects of superoxide detoxification (Kennedy et al., 1989) may be explained by differences in animal species, protocols, and readouts of injury used in these studies. It also seems plausible that some subtle mechanisms mediated by superoxide are overshadowed by high acuity and amplitude of the injury in this highly traumatic model.
The varying effects of the conjugates on AngII-mediated vascular dysfunction (Fig. 4) also emphasize the necessity of targeting specific ROS. Our current data support the concept that abnormal endothelium-dependent vasodilatation is mediated by superoxide rather than H2O2 as it was ameliorated by anti-PECAM/SOD and not by anti-PECAM/catalase delivery, despite the fact that the latter increased local degradation of H2O2. This negative outcome agrees with reports that H2O2 functions as a vasodilator rather than as a vasoconstrictor in some types of vessels (Cai, 2005; Saitoh et al., 2007). In contrast, accumulation of SOD in endothelium, achieved by anti-PECAM/SOD, but not the unconjugated counterpart, quenched superoxide and protected BH4 (and, presumably, NO), thus enhancing endothelium-dependent vasodilatation.
Neither conjugate was effective in all models. The fact that anti-PECAM/SOD had no effect in models of I/R, and that anti-PECAM/catalase had no effect in the AngII-induced endothelial tonic disorder, implicates a specific type of endothelial ROS in each of these pathologies. Systematic comparative analysis of the conjugate effects in animal models presented in this study supports the conclusion that every type of vascular oxidative stress, even in relatively oversimplified animal models, has a unique profile of the pathological role of ROS and, as a consequence, the efficacy of different treatments varies. Theoretically, PECAM blocking by the conjugates could contribute to their protective effects, e.g., via inhibition of leukocyte adhesion and transmigration into the pulmonary tissue. The fact that we did not observe protective effects in control experiments in which we injected a mixture of nonconjugated anti-PECAM and antioxidant enzymes indicates that PECAM blocking itself does not afford a significant protection in our models. Furthermore, only one of the conjugates, i.e., either anti-PECAM/catalase or anti-PECAM/SOD, was effective in any given model. This outcome confirms the specificity of antioxidant interventions and clearly indicates that targeted delivery of catalase or SOD to PECAM is necessary to achieve protection in the animal models used in our study. It should be noted, however, that in most cases leukocyte transmigration and secondary proinflammatory damage develops several hours after initial insults, whereas our models had been terminated 4 h after the insults. Therefore, it was difficult to expect anti-inflammatory effects of the conjugates in our models.
Our current findings are somewhat reminiscent of findings observed in animal models of hypertension and atherosclerosis and in clinical trails. In animal models of hypertension, various forms of membrane-targeted SOD lower blood pressure, and in keeping with the current findings, improve endothelium-dependent vasodilatation. In contrast, overexpression of SOD alone in transgenic mice has no effect on atherosclerosis development, whereas overexpression of catalase is protective (Yang et al., 2004).
In conclusion, the results shown in this article demonstrate for the first time that endothelial delivery of catalase and SOD targeted to the same endothelial specific surface protein, PECAM-1, offers unique advantages in detoxification of specific ROS implicated in particular forms of vascular oxidative stress. Nontargeted counterparts have no effects, whereas endothelium-targeted antioxidants attain effective and multifaceted protection against specific types of oxidative stress implicated in pathogenesis of ischemia/reperfusion and vascular dysfunction. This study focused on the short-term effects of the conjugates in animal models of very acute oxidative injury that developed in the course of several hours. Ongoing and projected studies are aimed at determining how improvement of these short-term readouts translates into recovery and survival benefits. Conceivably, duration of activity in the lung tissue of enzyme conjugates described in this study may be suboptimal for long-term interventions. Therefore, the present data justify further efforts to translate prototype conjugates presented in this study into clinically applicable recombinant fusion proteins and polymer nanocarriers, providing prolonged effects of encapsulated antioxidant enzymes. This strategy holds promise to both improve pharmacological management of vascular oxidative stress and allow us to more precisely understand the pathological and/or signaling role of specific surface proteins.
This work was supported by the National Institutes of Health [Grant R01-HL073940, P01-HL793063] (to V.R.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156877
- ROS
- reactive oxygen species
- AOE
- antioxidant enzymes
- BH4
- tetrahydrobiopterin
- SOD
- superoxide dismutase
- PECAM-1
- platelet-endothelial cell adhesion molecule-1
- GOX
- glucose oxidase
- TM
- thrombomodulin
- BAL
- bronchoalveolar lavage
- BALF
- bronchoalveolar lavage fluid
- I/R
- ischemia/reperfusion
- VOS
- vascular oxidative stress
- AngII
- angiotensin II
- MDA
- malondialdehyde.
References
- Andreadis et al., 2003.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. (2003) Oxidative and nitrosative events in asthma. Free Radic Biol Med 35:213–225 [DOI] [PubMed] [Google Scholar]
- Bonaventura and Gow, 2004.Bonaventura J, Gow A. (2004) NO and superoxide: opposite ends of the seesaw in cardiac contractility. Proc Natl Acad Sci U S A 101:16403–16404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, 2005.Cai H. (2005) Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 68:26–36 [DOI] [PubMed] [Google Scholar]
- Cai et al., 2003.Cai H, Griendling KK, Harrison DG. (2003) The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24:471–478 [DOI] [PubMed] [Google Scholar]
- Christofidou-Solomidou et al., 2003.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. (2003) PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol 285:L283–L292 [DOI] [PubMed] [Google Scholar]
- Dikalov et al., 2007.Dikalov S, Griendling KK, Harrison DG. (2007) Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima and Nixon, 1980.Fukushima T, Nixon JC. (1980) Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem 102:176–188 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2003.Gao B, Flores SC, Leff JA, Bose SK, McCord JM. (2003) Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol 284:L917–L925 [DOI] [PubMed] [Google Scholar]
- Goswami et al., 2007.Goswami SK, Maulik N, Das DK. (2007) Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann Med 39:275–289 [DOI] [PubMed] [Google Scholar]
- Guo et al., 2007.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. (2007) Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther 321:18–27 [DOI] [PubMed] [Google Scholar]
- Guo and Ward, 2007.Guo RF, Ward PA. (2007) Role of oxidants in lung injury during sepsis. Antioxid Redox Signal 9:1991–2002 [DOI] [PubMed] [Google Scholar]
- Houston et al., 1999.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. (1999) Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem 274:4985–4994 [DOI] [PubMed] [Google Scholar]
- Kennedy et al., 1989.Kennedy TP, Rao NV, Hopkins C, Pennington L, Tolley E, Hoidal JR. (1989) Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest 83:1326–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniry et al., 2006.Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, Carter J, Christofidou-Solomidou M. (2006) Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr 136:1545–1551 [DOI] [PubMed] [Google Scholar]
- Kozower et al., 2003.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. (2003) Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 21:392–398 [DOI] [PubMed] [Google Scholar]
- Krause and Bedard, 2008.Krause KH, Bedard K. (2008) NOX enzymes in immuno-inflammatory pathologies. Semin Immunopathol 30:193–194 [DOI] [PubMed] [Google Scholar]
- Kuzkaya et al., 2003.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. (2003) Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278:22546–22554 [DOI] [PubMed] [Google Scholar]
- Landmesser et al., 2003.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. (2003) Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111:1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2008.Lee JC, Bhora F, Sun J, Cheng G, Arguiri E, Solomides CC, Chatterjee S, Christofidou-Solomidou M. (2008) Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 294:L255–L265 [DOI] [PubMed] [Google Scholar]
- Li et al., 2000.Li S, Tan Y, Viroonchatapan E, Pitt BR, Huang L. (2000) Targeted gene delivery to pulmonary endothelium by anti-PECAM antibody. Am J Physiol Lung Cell Mol Physiol 278:L504–L511 [DOI] [PubMed] [Google Scholar]
- Loomis et al., 2005.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. (2005) Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 315:1058–1064 [DOI] [PubMed] [Google Scholar]
- Matthay et al., 2003.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, et al. (2003) Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167:1027–1035 [DOI] [PubMed] [Google Scholar]
- McCord, 2002.McCord JM. (2002) Superoxide dismutase in aging and disease: an overview. Methods Enzymol 349:331–341 [DOI] [PubMed] [Google Scholar]
- Muzykantov, 2001.Muzykantov VR. (2001) Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release 71:1–21 [DOI] [PubMed] [Google Scholar]
- Muzykantov, 2005.Muzykantov VR. (2005) Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv 2:909–926 [DOI] [PubMed] [Google Scholar]
- Muzykantov et al., 1996.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. (1996) Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci U S A 93:5213–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov et al., 1999.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. (1999) Streptavidin facilitates internalization and pulmonary targeting of an antiendothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A 96:2379–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, 1997.Newman PJ. (1997) The biology of PECAM-1. J Clin Invest 99:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak et al., 2007.Nowak K, Weih S, Metzger R, Albrecht RF, 2nd, Post S, Hohenberger P, Gebhard MM, Danilov SM. (2007) Immunotargeting of catalase to lung endothelium via anti-ACE antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol 293:L162–L169 [DOI] [PubMed] [Google Scholar]
- Ovechkin et al., 2007.Ovechkin AV, Lominadze D, Sedoris KC, Robinson TW, Tyagi SC, Roberts AM. (2007) Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem 113:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkowski et al., 2006.Perkowski S, Scherpereel A, Murciano JC, Arguiri E, Solomides CC, Albelda SM, Muzykantov V, Christofidou-Solomidou M. (2006) Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol 291:L1050–L1058 [DOI] [PubMed] [Google Scholar]
- Porkert et al., 2008.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, et al. (2008) Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens 22:401–407 [DOI] [PubMed] [Google Scholar]
- Saitoh et al., 2007.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. (2007) Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol 293:H3720–H3725 [DOI] [PubMed] [Google Scholar]
- Shuvaev et al., 2007a.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, Simone E, Arguiri E, Tliba S, Pick J, Kennel S, Albelda SM, Muzykantov VR. (2007a) Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J Control Release 118:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev et al., 2007b.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. (2007b) Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther 323:450–457 [DOI] [PubMed] [Google Scholar]
- Sweitzer et al., 2003.Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR. (2003) PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med 34:1035–1046 [DOI] [PubMed] [Google Scholar]
- Terada et al., 1992.Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. (1992) Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A 89:3362–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder et al., 2007.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. (2007) Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol 27:762–768 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2004.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. (2004) Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res 95:1075–1081 [DOI] [PubMed] [Google Scholar]
- Zou, 2007.Zou MH. (2007) Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat 82:119–127 [DOI] [PubMed] [Google Scholar]