Abstract
7-Chloro-5-(4-hydroxyphenyl)-1-methyl-3-(napthalen-2-ylmetyl)-4,5,-dihydro-1H-benzo[b][1,4]diazepin-2(3H)-one (Bz-423) is a proapoptotic 1,4-benzodiazepine that potently suppresses disease in the murine model of lupus by selectively killing pathogenic lymphocytes. In MRL/MpJ-Faslpr (MRL-lpr) mice, Bz-423 overcomes deficient expression of the Fas death receptor and hyperactivation of antiapoptotic phosphatidylinositol 3-kinase (PI3K)-Akt signaling to specifically kill pathogenic CD4+ T cells. Bz-423 binds to the oligomycin-sensitivity-conferring protein component of the mitochondrial F0F1-ATPase, which modulates the enzyme leading to formation of superoxide by the mitochondrial respiratory chain. Scavenging this reactive oxygen species blocks all subsequent components of the apoptotic cascade. To gain insight into how apoptotic signaling activated by Bz-423-induced superoxide contributes to the selective depletion of MRL-lpr CD4+ T cells, we characterized the death mechanism in a CD4+ T cell leukemia line (Jurkat). Although Bz-423-induced superoxide indirectly inactivates Akt, this response is not required for T cell death. Apoptosis instead results from parallel increases in levels of the proapoptotic Bcl-2 proteins Noxa and Bak leading to specific activation of Bak, mitochondrial outer membrane permeabilization, and a commitment to apoptosis. By directly up-regulating proteins that trigger loss of mitochondrial outer membrane integrity, Bz-423 bypasses defective Fas function and antiapoptotic PI3K-Akt signaling in MRL-lpr CD4+ T cells. Moreover, because disease-associated abnormalities should sensitize autoreactive CD4+ T cells to transcriptional up-regulation of Noxa by redox signals and to Bak-dependent apoptosis, the apoptotic mechanism elucidated in Jurkat cells provides important clues into the cell-type- and disease-selective effects of Bz-423 in MRL-lpr mice.
Human and murine lupus is mediated by autoreactive lymphocytes that survive abnormally and are chronically activated because of persistent stimulation with endogenous autoantigens (Hoffman, 2004; Santiago-Raber et al., 2004). Bz-423 is a proapoptotic 1,4-benzodiazepine with therapeutic properties in murine models of lupus that are linked to specific apoptosis of disease-causing lymphocytes (Blatt et al., 2002; Bednarski et al., 2003). In MRL/MpJ-Faslpr (MRL-lpr) mice, Bz-423 selectively kills splenic CD4+ T cells, which is the lymphoid subset responsible for disease in this model (Bednarski et al., 2003). Treatment is not associated with opportunistic infections, and Bz-423 does not reduce lymphocyte numbers or alter immune function in normal mice, which indicates that this agent is not globally immunosuppressive.
Bz-423 binds to the oligomycin-sensitivity-conferring protein subunit of the mitochondrial F0F1-ATPase, which induces a state 3-to-state 4 respiratory transition and promotes formation of superoxide (O2∸) by the mitochondrial respiratory chain (Johnson et al., 2005). Bz-423-induced O2∸ functions as the proximal signal in an apoptotic cascade marked by release of proapoptotic proteins (e.g., cytochrome c) from the mitochondria intermembrane space (MIS), collapse of the mitochondrial electrochemical gradient (Δψm), and activation of caspases (Blatt et al., 2002). In isolated mitochondria, Bz-423 increases O2∸, but collapse of Δψm and cytochrome c release are not observed (Blatt et al., 2002, 2008). These data demonstrate that extramitochondrial factors couple Bz-423-induced O2∸ to cytochrome c release.
Differences in the factors that mediate return of the apoptotic signal to mitochondria should contribute to the selectivity of Bz-423 for pathogenic versus normal lymphocytes and other cell types. In relatively resistant cell types, such as mouse embryonic fibroblasts (MEFs), apoptosis signal-regulating kinase1 (ASK1) was identified as a critical upstream cellular redox sensor linking Bz-423-induced O2∸ to apoptosis (Blatt et al., 2008). Activation of ASK1 initiates a mitogen-activated protein (MAP) kinase cascade culminating in the activation of c-Jun N-terminal kinase (JNK). Activated JNK is then necessary for activation of the proapoptotic Bcl-2 proteins Bax and Bak resulting in mitochondrial outer membrane permeabilization (MOMP) and a commitment to apoptotic cell death, as a small-molecule JNK inhibitor prevents these steps. In contrast, Bz-423-induced O2∸ does not activate the ASK1-JNK pathway in more sensitive Ramos B cells (Blatt et al., 2009). Instead, degradation of the antiapoptotic Bcl-2 family protein Mcl-1 and functional activation of proapoptotic BH3-only proteins link the O2∸ signal to activation of Bax and Bak in this B cell line.
Accumulation of activated CD4+ T cells in MRL-lpr mice results from defects in Fas-death receptor signaling along with hyperactivation of the prosurvival PI3K-Akt pathway (Barber et al., 2005). To understand how signaling activated by Bz-423 overcomes antiapoptotic signals in MRL-lpr CD4+ T cells and leads to their selective depletion, we elucidated the death mechanism in a CD4+ T cell leukemia line (Jurkat). We selected Jurkat cells for these studies because they are amenable to genetic manipulation and constitutive activation of Akt in this line mimics hyperactivated PI3K-Akt signaling in MRL-lpr CD4+ T cells (Astoul et al., 2001). Although Bz-423-induced O2∸ indirectly inactivates Akt and activates JNK in Jurkat cells, neither response is required for cell death. Rather, Bz-423-induced T cell death depends on a parallel apoptotic cascade marked by increased levels of the proapoptotic Bcl-2 proteins Noxa and Bak leading to preferential activation of Bak. Collectively, these data suggest that selective killing is mediated by the induction of Noxa and Bak in sensitive cell types and not in cells that are resistant to Bz-423, such as fibroblasts. Integrating knowledge of the apoptotic signaling network activated by Bz-423 in T cells with properties of autoreactive T lymphocytes provides new insight into why modulation of the F0F1-ATPase with Bz-423 leads to specific apoptosis of MRL-lpr CD4+ T cells.
Materials and Methods
Reagents.
Manganese(III)meso-tetrakis(4-benzoic acid)porphyrin (MnTBAP) was purchased from Alexis Biochemicals (San Diego, CA). Dihydroethidium (DHE) was obtained from Invitrogen (Carlsbad, CA). SP600125 was purchased from EMD Biosciences (San Diego, CA). Bz-423 was synthesized as described previously (Bunin et al., 1994). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Lines and Culture.
Jurkat cells were provided by V. Castle (University of Michigan), and were maintained in RPMI 1640 medium supplemented with fetal bovine serum (10%), penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (300 μg/ml). In vitro experiments were conducted in media containing 2% FBS. Organic compounds were dissolved in media containing 0.5% dimethyl sulfoxide.
Transient Transfections.
Small interfering RNA molecules (siRNAs) targeting Bax, Bak, or Noxa were purchased from Dharmacon RNA Technologies (Layfayette, CO) as siGENOME SMARTpool reagents. J. C. Rathmell (Duke University Medical School) provided the plasmid-encoding myrAkt. Cells were washed once with ice-cold phosphate-buffered saline (PBS), suspended in electroporation buffer T (Amaxa Biosystems, Gaithersburg, MD) at a density of 7.5 × 106 cells/ml, and subjected to electroporation with the desired siRNA (2 μg) or plasmid (8 μg) with an Amaxa Nucleofector apparatus and a 2.0-mm electroporation cuvette with use of program G-16. Cells were transfected once daily for 2 days. Three days after the initial transfection, cellular extracts were prepared or cells were incubated with Bz-423.
Detection of Cell Death, DNA Content, O2∸, and ATP Levels.
Cell viability and apoptotic DNA fragmentation were assessed as described previously (Blatt et al., 2002). To detect intracellular O2∸, cells were incubated with DHE (2 μM) for 30 min at 37°C and ethidium fluorescence was measured by flow cytometry. For analysis of ATP content, cells were washed with ice-cold PBS, and lysed in a solution containing trichloroacetic acid (1% w/v) and EDTA (2 mM). Cell debris was removed by centrifugation (13,000 rpm, 5 min), and resulting supernatants were neutralized with a Tris-acetate buffer (25 mM, pH 7.75) and diluted 20× with distilled water. ATP content in neutralized samples was quantified by use of ENLITEN rLuciferase/Luciferin reagent (Promega, Madison, WI) according to the manufacturer's instructions.
Preparation of Cellular Extracts.
Cellular lysates were prepared as described previously (Blatt et al., 2002). Mitochondrial and cytosolic extracts were isolated by digitonin permeabilization followed by differential centrifugation as described previously (Blatt et al., 2002). Immunoblotting with antibodies specific for the cytosolic protein GAPDH or the mitochondrial proteins Hsp60 or the β-subunit of the F0F1-ATPase (V-β) was used to evaluate the purity of cytosolic and mitochondrial fractions. Total protein content in cellular lysates or mitochondrial/cytosolic fractions was evaluated by the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA).
Immunoblot Analysis.
Cellular lysates or mitochondrial/cytosolic fractions were separated by SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes as described previously (Blatt et al., 2002). Membranes were blocked by use of skim milk powder (5% w/v) in PBS containing Tween (0.05% v/v) and probed with antibodies specific for cytochrome c, Hsp60, Bad, and Bcl-xL (BD Biosciences, San Jose, CA); Smac/DIABLO (Alexis Biochemicals); Bax, Bak, and GAPDH (Millipore, Billerica, MA); Bcl-2 (DakoCytomation, Glostrup, Denmark); Bik, Mcl-1, and PARP (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-Akt Ser473, pan-Akt, phospho-JNK Thr183 / Tyr185, pan-JNK, and Bid (Cell Signaling, Beverly, MA); Bmf (Abcam, Cambridge, MA); Puma and Noxa (EMD Biosciences); or the β-subunit of the F0F1-ATPase (Invitrogen). Primary antibodies were detected by use of horseradish peroxidase-linked donkey anti-rabbit IgG or sheep anti-mouse IgG (GE Healthcare, Piscataway, NJ) and visualized with the enhanced chemiluminescence detection system (GE Healthcare). Band intensities were quantified by use of ImageJ (National Institutes of Health, Bethesda, MD).
Fluorescence Microscopy.
Cells were fixed to glass slides with PBS containing paraformaldehyde (2% v/v), and probed for activated Bax and Bak with N-terminal specific antibodies (Millipore) suspended in PBS containing saponin (0.05% w/v) and FBS (5% v/v). Primary antibodies were visualized with biotin-anti-rabbit (The Jackson Laboratory, Bar Harbor, ME) and fluorescein-avidin (Vector Laboratories, Burlingame, CA). Samples were examined by use of a Leica DM-LB microscope interfaced with a SPOT RS slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI) interfaced to a Macintosh PC. Percentage of Bax or Bak activation was determined by scoring >100 cells in triplicate for the presence of bright, punctate staining.
Statistical Analysis.
Where indicated, statistical significance was assessed by a Student's t test. p values are two-tailed. Error bars represent ± 1 S.D. from the mean of three replicate cultures. All figures contain data that are representative of at least three independent experiments.
Results
Bz-423-Induced O2∸ Signals Apoptosis in T Cells.
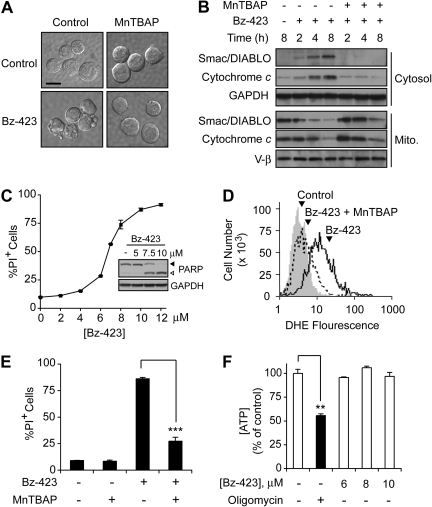
Treating Jurkat cells with Bz-423 for 12 h results in shrinkage, cytoplasmic vacuolization, and membrane blebbing (Fig. 1A), which are morphological indicators of apoptosis. Release of cytochrome c and Smac/DIABLO from mitochondria is observed in Jurkat cells treated with Bz-423 for 4 h (Fig. 1B), which is consistent with activation of the intrinsic (mitochondrial) apoptotic pathway. Once in the cytosol, cytochrome c and Smac/DIABLO promote caspase activation by initiating apoptosome formation or displacing inhibitor of apoptosis proteins from caspases, respectively (Danial and Korsmeyer, 2004). As predicted by release of these proapoptotic MIS proteins, cleavage of the caspase-3 substrate PARP is detected at 8 h (Fig. 1C, inset). These events are followed by apoptotic DNA fragmentation (data not shown) and a loss of plasma membrane integrity indicative of cell death (Fig. 1C).
Fig. 1.
Bz-423-induced O2∸ signals apoptosis in Jurkat cells. A, digital interference contrast micrographs (630×) of Jurkat cells treated with vehicle or Bz-423 (10 μM, 12 h) with or without preincubation with MnTBAP (100 μM, 30 min). Scale bar, 10 μm. B, Jurkat cells were preincubated with MnTBAP (100 μM, 30 min) and then treated with Bz-423 (10 μM) for indicated times. Release of cytochrome c or Smac/DIABLO was assessed by immunoblotting cytosolic and mitochondrial fractions. C, Bz-423-induced cell death was measured by propidium iodide (PI) exclusion after 24 h of total culture. Inset, PARP cleavage was assessed by immunoblotting lysates from Jurkat cells treated (8 h) with the indicated concentrations of Bz-423. ◀, full-length PARP; ▷, cleaved PARP. D, Jurkat cells were preincubated with MnTBAP (100 μM, 30 min, dashed histogram) or vehicle (black histogram) before incubation with Bz-423 (10 μM, 1 h) or vehicle (gray histogram) and cellular O2∸ levels were detected by staining with DHE using the FL2 channel. E, Jurkat cells treated with vehicle or Bz-423 (10 μM, 24 h) with or without preincubation with MnTBAP (100 μM, 30 min) and cell viability was determined by PI exclusion. F, ATP was measured in Jurkat cells treated (1 h) with the indicated concentrations of Bz-423 or oligomycin (0.5 μM) with use of a luciferin-luciferase assay. **, p < 0.01; ***, p < 0.001.
Preincubation of Jurkat cells with MnTBAP, a cell-permeable porphyrin possessing superoxide dismutase activity (Ohse et al., 2001), reduces Bz-423-induced O2∸ (Fig. 1D), blocks release of cytochrome c and Smac/DIABLO (Fig. 1B), and inhibits cell death (Fig. 1, A and E). The fact that MnTBAP prevents Bz-423-induced apoptosis argues that, unlike some inhibitors of the F0F1-ATPase (e.g., oligomycin), which significantly deplete ATP and trigger necrosis (Watabe and Nakaki, 2007), modulation of the F0F1-ATPase by Bz-423 primarily affects O2∸. Consistent with this expectation, Bz-423 did not alter ATP levels before the release of proapoptotic MIS proteins (Fig. 1F). Given the essential role of O2∸ in Bz-423-induced T cell apoptosis, antioxidant levels and mechanisms should affect the sensitivity of T cells to this agent. Hence, the reduced levels of glutathione present in MRL-lpr CD4+ T cells relative to normal murine T cells provides one possible basis to explain their sensitivity to Bz-423 (Bobé et al., 2006).
Bak Activation Is Essential for Bz-423-Induced T Cell Apoptosis.
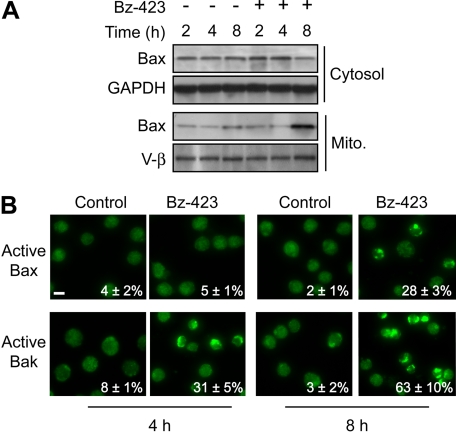
Because release of proapoptotic MIS proteins is a key apoptotic checkpoint (Danial and Korsmeyer, 2004), differences in the mediators coupling O2∸ to loss of mitochondrial outer membrane integrity should also contribute to the selectivity of Bz-423. MOMP is a process often mediated by two multidomain proapoptotic Bcl-2 proteins, Bax and Bak (Youle and Strasser, 2008). In its inactive state, Bax is present in the cytosol, whereas inactive Bak is localized to the mitochondrial outer membrane (Youle and Strasser, 2008). In response to apoptotic stimuli, Bax translocates to mitochondria, and undergoes a conformational change that leads to homo-oligomerization and formation of high-molecular-weight channels in the mitochondrial outer membrane (Youle and Strasser, 2008). Immunoblotting cytosolic and mitochondrial fractions from Bz-423-treated Jurkat cells reveals redistribution of Bax from the cytosol to the mitochondria (Fig. 2A). This change occurs after 8 h, which is significantly delayed relative to appearance of cytochrome c and Smac/DIABLO in the cytosol at 4 h (Fig. 1B). Although Bz-423 induces Bax translocation in Jurkat cells, it cannot account for the observed MOMP.
Fig. 2.
Bz-423 activates Bax and Bak. A, subcellular distribution of Bax was determined by immunoblotting mitochondrial and cytosolic fractions from Jurkat cells treated with Bz-423 (10 μM) for the indicated times. B, Jurkat cells were treated with vehicle or Bz-423 (10 μM) for indicated times followed by detection of activated Bax or Bak by immunofluorescence microscopy (400×). Scale bar, 10 μm.
In contrast, activation of Bak does not involve subcellular redistribution, but is associated with a conformational change and exposure of a cryptic N-terminal domain that can be detected by immunofluorescence (Youle and Strasser, 2008). Bak activation is observed in 31 ± 5% of cells within 4 h and 63 ± 10% of cells by 8 h (Fig. 2B). In agreement with the immunoblotting data, active Bax was detected by immunofluorescence only after 8 h, at which time 28 ± 3% of cells were positive (Fig. 2B). Pretreating Jurkat cells with MnTBAP blocked activation of both Bax and Bak (Supplemental Fig. 1). These data indicate that Bz-423-induced O2∸ signals activation of Bak in a time frame consistent for it to cause MOMP.
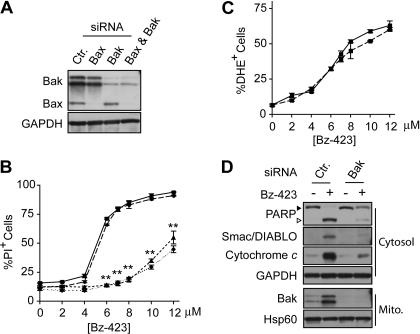
To test whether either Bax or Bak are necessary for Bz-423-induced T cell apoptosis, expression of these proteins was reduced by use of siRNAs (Fig. 3A). Knockdown of Bax (>99%) did not block Bz-423-induced cell death, whereas reduction of Bak expression (>90%) blunted apoptosis, increasing the EC50 for Bz-423-induced cell death >2-fold (Fig. 3B). Knockdown of Bax along with Bak provided no additional protection compared with reduction of only Bak (Fig. 3B). Intracellular O2∸ levels were increased equivalently by Bz-423 in Bak knockdown and control cells after 1 h (Fig. 3C), which indicates that reducing Bak levels does not prevent Bz-423-induced O2∸ production. In contrast, release of cytochrome c and Smac/DIABLO, as well as the downstream cleavage of PARP was suppressed in Jurkat cells with reduced levels of Bak (Fig. 3D). Together, these results indicate that activation of Bak links Bz-423-induced O2∸ with loss of mitochondrial outer membrane integrity and apoptosis.
Fig. 3.
Knockdown of Bak inhibits Bz-423-induced T cells apoptosis. A, immunoblot of lysates prepared from Jurkat cells transiently transfected with siRNAs targeting Bax, Bak, or a noncoding sequence (Ctr). B, Jurkat cells were transfected with siRNAs targeting Bax (circles, long dashes), Bak (triangles, short dashes), Bax and Bak (diamonds, dotted line), or a noncoding sequence (squares, solid line) followed by treatment with indicated concentrations of Bz-423. Viability was determined after 24 h by PI exclusion. C, Jurkat cells were transfected with siRNAs targeting Bak (dashed line) or a noncoding sequence (solid line) and percentage increase in cellular O2∸ was measured with use of DHE after 1 h. D, release of cytochrome c and Smac/DIABLO or PARP cleavage were assessed by immunoblotting cytosolic fractions from Jurkat cells transfected with siRNAs targeting Bak or noncoding sequence (Ctr), and then treated with vehicle or Bz-423 (10 μM, 8 h). Reduction of Bak expression was confirmed by immunoblotting of mitochondrial fractions. ▶, full-length PARP; ▷, cleaved PARP; **, p < 0.01.
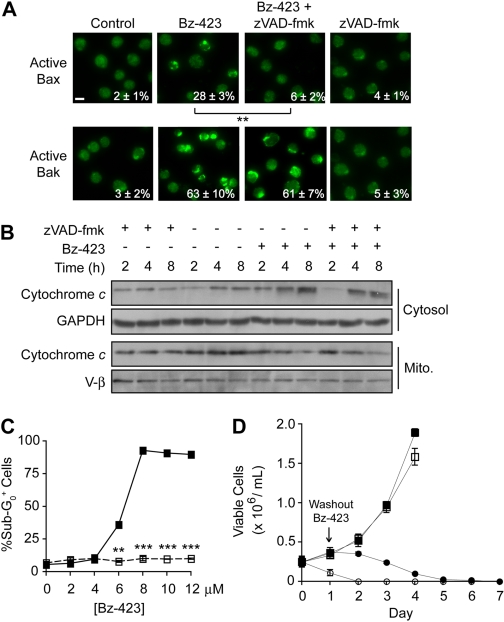
Recent studies show that activation of Bax can be caspase-dependent (Kepp et al., 2007; Lakhani et al., 2006). Because Bak is required for Bz-423-induced MOMP and caspase activation, we hypothesized that Bax activation might be blocked by knockdown of Bak. As expected, reduction of Bak expression blocked Bax activation, whereas Bax knockdown increased the fraction of cells staining positive for active Bak (Supplemental Fig. 2). In addition, activation of Bax is blocked by preincubation of Jurkat cells with the pan-caspase inhibitor zVAD-fmk, whereas Bak activation and cytochrome c release are still observed (Fig. 4, A and B). These data support a hierarchical model in which Bak mediates MOMP, whereas Bax is activated by a subsequent, caspase-dependent process. Although cytochrome c release is still observed, zVAD-fmk prevents downstream consequences of caspase activation such as apoptotic DNA fragmentation (Fig. 4C). To determine whether inhibiting caspase activation blocks Bz-423-induced killing, Jurkat cells were treated with a concentration of Bz-423 >EC50 for cell death along with zVAD-fmk for 24 h, at which point Bz-423 was removed and cultures replenished with zVAD-fmk. Although caspase inhibition prevented Bz-423-induced cell death (i.e., loss of plasma membrane integrity) after 24 h, no viable cells remained after 5 days (Fig. 4D). Thus, given that cell death occurs in the absence of caspase activity, Bak-dependent MOMP represents the point at which Jurkat cells commit to die in response to Bz-423.
Fig. 4.
Caspase activation is not required for Bz-423-induced cell death. A, Jurkat cells were preincubated with zVAD-fmk (50 μM, 30 min) then treated with Bz-423 (10 μM, 8 h) and activated Bax or Bak were detected by immunofluorescence microscopy (400×). Scale bar, 10 μm. B, release of cytochrome c and Smac/DIABLO was measured in Jurkat cells after treatment with Bz-423 (10 μM) for indicated times with or without preincubation with zVAD-fmk (50 μM, 30 min) by immunoblotting mitochondrial and cytosolic fractions. C, Jurkat cells were preincubated with zVAD-fmk (50 μM, 30 min, ■) or vehicle (□), treated with the indicated concentrations of Bz-423 for 24 h, and caspase activation was assessed by identifying cells with sub-G0 DNA content. D, Jurkat T cells were treated with zVAD-fmk (50 μM; ●, ■) or vehicle (○, □) for 30 min and then incubated with Bz-423 (10 μM; ●, ○) or vehicle (■, □) for 24 h, at which point Bz-423 was removed, cells were maintained in media containing zVAD-fmk (50 μM), and the number of viable cells was determined daily. **, p < 0.01; ***, p < 0.001.
Effects of Bz-423-Induced O2∸ on Akt and JNK.
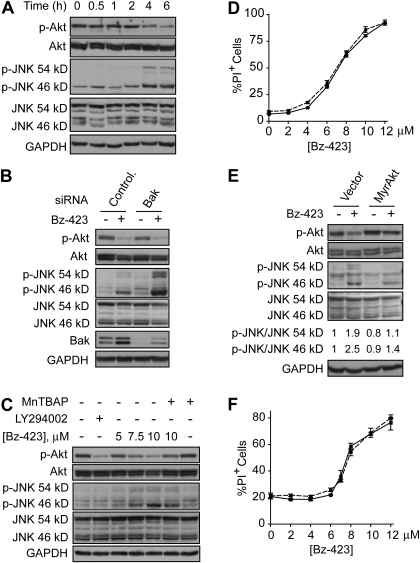
The next series of experiments were conducted to identify apoptotic signaling linking Bz-423-induced O2∸ to activation of Bak. In MEFs, activation of the cytosolic redox-sensor ASK1 is the response most proximal to the O2∸ signal (Blatt et al., 2008). Activated ASK1 then initiates a MAP kinase cascade that culminates in phosphorylation and activation of JNK, a proapoptotic kinase that promotes Bax and Bak activation through several mechanisms (Weston and Davis, 2007). In contrast, Bz-423-induced O2∸ does not activate ASK1-JNK signaling in B cells (Blatt et al., 2009). To determine whether the ASK1-JNK pathway is engaged in T cells, phospho-JNK was measured in Bz-423-treated Jurkat cells. Although Bz-423 activates JNK in Jurkat cells (increasing phospho-JNK levels as in Fig. 5A), it occurs significantly later relative to the kinetics observed in MEFs (4 h versus 0.5 h). These results suggest that Jurkat cells actively restrict activation of JNK by ROS.
Fig. 5.
Bz-423 inactivates Akt and activates JNK. A, lysates prepared from Jurkat cells treated with Bz-423 (10 μM) were immunoblotted to detect total and phosphorylated Akt or JNK. B, lysates prepared from Jurkat cells transfected with siRNAs targeting Bak or noncoding sequence (Ctr) treated with vehicle or Bz-423 (10 μM, 6 h) were immunoblotted to detect total and phospho-Akt or -JNK. C, Jurkat cells were preincubated with MnTBAP (100 μM, 30 min) before incubation with indicated concentrations of Bz-423 or the PI3K inhibitor LY294002 (20 μM) for 6 h, and levels of phosphorylated and total Akt and JNK were analyzed by immunoblot. D, after preincubation with SP600125 (30 min, 10 μM, dashed line) or vehicle (solid line), Jurkat cells were incubated with Bz-423 and viability (24 h) was assessed. E, levels of total and phospho-Akt or -JNK were evaluated by immunoblotting lysates prepared from Jurkat cells treated with Bz-423 (10 μM, 6 h) 24 h after transfection with plasmid-encoding myrAkt or the empty vector. F, Jurkat cells transfected with myrAkt (dashed line) or empty vector (solid line) were incubated with Bz-423 and viability was measured after 24 h.
The prosurvival kinase Akt is constitutively active in Jurkat cells as an indirect consequence of defects in the expression of the lipid phosphatases, phosphatase and tensin homolog and SH2-containing inositol phosphatase, which render PI3K activity unopposed, increasing levels of phosphatidylinositol triphosphates (Astoul et al., 2001). Binding of Akt to phosphatidylinositol triphosphates results in a conformational change that promotes phosphorylation and activation of Akt (Hanada et al., 2004). O2∸-dependent inactivation (dephosphorylation) of Akt occurs in Jurkat cells treated with 2-methoxyestradiol, an estrogen-metabolite proposed to inhibit superoxide dismutases (Gao et al., 2005). Likewise, Bz-423-induced O2∸ also caused a decrease in phospho-Akt levels that coincides with JNK activation at 4 h (Fig. 5A).
The timing of Bz-423-induced Akt dephosphorylation and JNK activation coincides with MOMP, allowing for the possibility that these responses may signal Bak activation. To explore this hypothesis, we first excluded that these changes are downstream of and dependent on MOMP (Fig. 5B). Despite the inability to undergo apoptosis, Bz-423-induced Akt dephosphorylation and JNK activation were observed to an equivalent or greater extent, respectively, in Bak knockdown cells versus control (Fig. 5B). Preincubation with MnTBAP blocked changes in phospho-Akt levels and suppressed JNK activation (Fig. 5C). Unlike MEFs, pretreating Jurkat cells with a JNK-selective kinase inhibitor (SP600125; Bennett et al., 2001), which prevented phosphorylation of the JNK substrate activating transcription factor 2 (data not shown), did not block Bz-423-induced apoptosis (Fig. 5D). This finding indicates that in Jurkat cells, Bz-423-induced O2∸ activates other proapoptotic mechanisms that run in parallel with JNK signaling.
Because Akt promotes survival by inhibitory phosphorylation of proapoptotic proteins, including the BH3-only protein Bad, caspase-9, and ASK1 (Hanada et al., 2004), inhibition of Akt can cause cell death (Maurer et al., 2006). Given that Bz-423 inactivates Akt, we asked whether Akt inhibition is necessary for Bz-423-induced T cell apoptosis. To test this hypothesis, Jurkat cells were transiently transfected with a dominant, constitutively active myristoylated form of Akt (myrAkt) that bypasses the need for upstream activating signals that are redox-sensitive (Gao et al., 2005). Phospho-Akt levels were unaltered in transfected cells in the presence of Bz-423, whereas JNK activation was reduced by >60% (Fig. 5E), which argues that inhibition of Akt by Bz-423 is necessary for JNK activation. Despite preserving Akt activity, the myrAkt construct failed to block Bz-423-induced apoptosis (Fig. 5F). Thus, although Bz-423 inactivates Akt and activates JNK, other parallel processes couple O2∸ to Bak-mediated MOMP in Jurkat cells.
Activation of Bak during Bz-423-Induced T Cell Apoptosis.
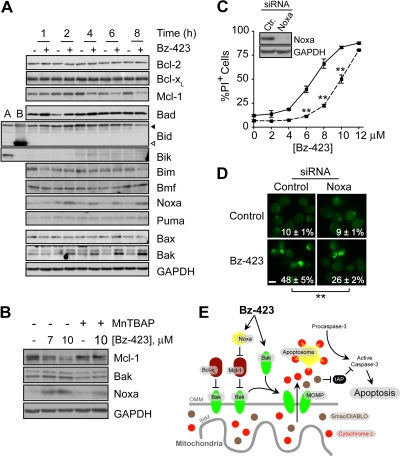
Bak activation is restrained by binding to the antiapoptotic Bcl-2 proteins Bcl-xL and Mcl-1 (Willis et al., 2005). Because Bz-423-induced O2∸ signals degradation of Mcl-1 in B cells (Blatt et al., 2009), we sought to determine whether a similar mechanism contributes to activation of Bak in Jurkat cells. Although levels of Bcl-xL were stable, Mcl-1 was reduced by Bz-423 to <50% of control levels after 4 h, and continued to fall to <10% by 8 h (Fig. 6A). The decrease in Mcl-1 is concentration-dependent, and is blocked by MnTBAP (Fig. 6B). As in B cells, Bz-423 causes O2∸-dependent reduction in Mcl-1 within a time frame coincident with activation of Bak in T cells.
Fig. 6.
Bz-423-induced Bak activation. A, lysates from Jurkat cells treated with Bz-423 (10 μM) for indicated times were analyzed by immunoblot for Bcl-2 family proteins. Lane A, Ramos B cells; Lane B, recombinant human tBid; ◀, full-length Bid; ◁, tBid. B, Jurkat cells were preincubated with MnTBAP (100 μM, 30 min) before incubation with the indicated concentrations of Bz-423 (6 h), and levels of Mcl-1, Bak, and Noxa were evaluated by immunoblot. C, Jurkat cells were transfected with siRNAs targeting Noxa (dashed line) or a noncoding sequence (solid line) and treated with Bz-423. Cell viability was evaluated after 24 h. Inset, levels of Noxa were evaluated in lysates from Jurkat cells transfected with siRNAs targeting Noxa or a noncoding sequence (Ctr) by immunoblot. D, Jurkat cells transfected with siRNAs targeting Noxa or a noncoding sequence (Control) were treated with Bz-423 (10 μM, 8 h) followed by immunofluorescence microscopy (400×). Scale bar, 10 μm. E, proposed mechanism of Bak activation during Bz-423-induced T cell apoptosis. Binding of Noxa to Mcl-1 releases Bak, which combined with increased Bak translation, saturates the inhibitory capacity of Bcl-xL. Accumulation of unbound (active) Bak triggers MOMP, release of cytochrome c and Smac/DIABLO from the MIS, which results in caspase activation. IAP, inhibitor of apoptosis protein. **, p < 0.01.
Mcl-1 is controlled by a variety of transcriptional and post-translational mechanisms (Warr and Shore, 2008). For example, binding of the BH3-only protein Noxa to Mcl-1 displaces Bak and targets Mcl-1 for proteasomal degradation (Willis et al., 2005). Levels of Noxa are elevated >3-fold in Jurkat cells treated with Bz-423 for 2 h (Fig. 6A). It is interesting that siRNA knockdown of Noxa by >99% increased the EC50 for cell death by ∼40% (Fig. 6C), and decreased Bak activation by ∼50% (Fig. 6D). These data indicate that the increase in Noxa levels contributes to, but is not solely responsible for, activation of Bak.
Forced expression of Noxa can be insufficient to trigger apoptosis unless combined with a BH3-only protein (e.g., Bad) capable of disrupting Bak · Bcl-xL complexes (Willis et al., 2005). Therefore, we tested whether Bz-423 changes the concentration of other BH3-only proteins that could contribute to Bak activation by neutralizing Bcl-xL. Bz-423 did not increase levels of the BH3-only proteins Bad, Bik, Bim, Bmf, and Puma or promote activation of Bid (Fig. 6A).
Although Bak is typically activated by increases in levels of BH3-only proteins relative to antiapoptotic Bcl-2 proteins (Youle and Strasser, 2008), increased Bak expression induced by some proapoptotic stimuli including ROS can also contribute to Bak activation (Chirakkal et al., 2006; Fei et al., 2008). As seen in Fig. 6A, Bak levels increase >2-fold within 2 h of Bz-423 treatment, and continue to rise to >5-fold above control levels at 8 h, whereas Bax levels remain unchanged. The increase in both Noxa and Bak is blocked by MnTBAP (Fig. 6B), which directly links these responses to O2∸. In sum, these data suggest a model in which activation of Bak results from neutralization of Mcl-1 by increased Noxa, which, together with an absolute increase in Bak protein levels, overcomes Bcl-xL-dependent cell survival (Fig. 6E).
Discussion
The apoptotic mechanism of Bz-423 was characterized in Jurkat cells as a means to understand the cell-type- and disease-selective apoptosis in MRL-lpr mice. In all cell types examined, activation of Bax and/or Bak is required for Bz-423-induced O2∸ to cause cytochrome c release. However, the signaling pathway leading to activation of these proapoptotic Bcl-2 family proteins differs between resistant cell types (e.g., MEFs) and lymphocytes. In fibroblasts, Bz-423-induced apoptosis depends on signals transmitted through JNK, which is activated within 30 min via a MAP kinase cascade initiated by ASK1 (Blatt et al., 2008). Bz-423-induced O2∸ does not engage the ASK1-JNK pathway in Ramos B cells; instead Bax/Bak-dependent apoptosis is mediated by functional activation of BH3-only proteins in this cell type (Blatt et al., 2009). Although JNK is activated in Bz-423-treated Jurkat cells, it is not necessary for apoptosis and its activation is delayed relative to the timing defined in MEFs. We reason that absence of an early JNK response in Jurkat cells probably results from suppression of ASK1 activation by constitutively active Akt (Astoul et al., 2001; Zhang et al., 2005). Consistent with this hypothesis, constitutive activation of Akt by use of a myrAkt construct suppresses JNK activation in Jurkat cells. The fact that neither JNK activation nor Akt inhibition are necessary for Jurkat cell death argues that, in T lymphocytes, Bz-423 activates additional proapoptotic signaling in parallel to these responses.
The pathway leading to apoptosis in Jurkat cells is associated with increased levels of Noxa and Bak. These increases are first detected at 2 h, which is before both MOMP and JNK activation. This observation suggests that up-regulation of Noxa and Bak are key components of the JNK-independent apoptotic signaling that links Bz-423-induced O2∸ to MOMP in Jurkat cells. Because free, uncomplexed Bak adopts an active conformation once available binding sites on Bcl-xL and Mcl-1 are saturated (Willis et al., 2007), further increase of Bak levels causes MOMP. Noxa potentiates this process by both displacing Bak from Mcl-1 and targeting Mcl-1 for proteasomal degradation (Willis et al., 2005). Hence, neutralization of Mcl-1 by Noxa in the absence of increased Bcl-xL levels, allows Bak to overcome Bcl-xL-mediated cell survival on the basis of its increased stoichiometry (Fig. 6E). This model is consistent with several recent reports demonstrating that neutralization of Mcl-1 and Bcl-xL is sufficient to activate Bak and trigger apoptosis even in the presence of high levels of Bcl-2 (Kepp et al., 2007; Kutuk et al., 2009; Willis et al., 2005, 2007). As such, apoptotic stimuli like Bz-423 represent a potential means to bypass elevated expression of Bcl-2 in tumors and autoimmune lymphocytes.
Changes in the Noxa, Bak, and Mcl-1 are not observed in Bz-423-treated fibroblasts (unpublished observations). Although Bz-423-induced O2∸ also signals down-regulation of Mcl-1 in B cells (Blatt et al., 2009), this response is not accompanied by an increase in Noxa and Bak and does not lead to preferential activation of Bak as observed in T cells. These cell-type-specific differences in apoptotic signaling potentially result from variations in levels of redox-regulated transcription factors between fibroblasts, B cells, and T cells (see below). Overall, these studies demonstrate the ability of O2∸ produced during a mitochondrial respiratory transition to activate cell-type-specific apoptotic signaling. Selective killing of pathogenic lymphocytes in Bz-423-treated mice is proposed to result from the intersection of mitochondrial-derived O2∸ with disease-specific apoptotic signaling in the target cell population.
The apoptotic mechanism uncovered in Jurkat cells provides several lines of reasoning to understand the basis for the selectivity of Bz-423 against autoreactive T cells in MRL-lpr mice. First, because MRL-lpr CD4+ T cells possess aberrantly low glutathione levels (Bobé et al., 2006), they have a reduced capacity to decompose Bz-423-induced O2∸ compared with normal murine T cells. Moreover, lymphocytes display low activities of antioxidant enzymes (e.g., superoxide dismutases) relative to most other cells types (Van Remmen et al., 1999). Together, these effects predict increased sensitivity to Bz-423 because all components of the death cascade are O2∸-dependent.
Second, defective Fas signaling and chronic T cell antigen receptor stimulation in MRL-lpr lymphocytes can lead to increased transcriptional induction of Noxa by redox signals and enhanced sensitivity to Bak-dependent apoptosis, respectively. MRL-lpr CD4+ T cells express supraphysiological levels of Fas ligand (FasL) because of failed negative regulation (Bobé et al., 1997). A key transcriptional activator of both FasL and Noxa is forkhead box 3a, a forkhead transcription factor that is activated by ROS (Kavurma and Khachigian, 2003; Nakamura and Sakamoto, 2008; Obexer et al., 2007). Hence, elevated basal levels of forkhead box 3a in MRL-lpr CD4+ T cells should cause exaggerated transcriptional up-regulation of Noxa in response to Bz-423-induced O2∸. Moreover, persistent stimulation of lupus T cells by endogenous autoantigens down-regulates costimulatory CD28 signaling thereby limiting expression of Bcl-xL (Bertsias et al., 2009; Vallejo, 2005). The combination of low levels of Bcl-xL and increased Noxa should selectively sensitize MRL-lpr CD4+ T cells to Bz-423 relative to both normal lymphocytes and resistant cell types such as MEFs. Moreover, earlier findings that Bz-423 selectively reduces CD4+ T cells without affecting CD8+ T cells in MRL-lpr mice are consistent with the fact that Noxa does not mediate activation-induced cell death in CD8+ T cells (Fischer et al., 2008).
Finally, inactivation of Akt by Bz-423 blocks a primary pathway required for abnormal survival and activation of T lymphocytes in MRL-lpr mice. Previous work has shown that the PI3K-Akt signaling axis is hyperactivated in MRL-lpr CD4+ T cells and inhibition of this pathway specifically reduces this lymphoid subset via increased apoptosis, similar to the effects of Bz-423 (Barber et al., 2005). Because inactivation Akt is not required for Bz-423-induced apoptosis in Jurkat cells, other signaling mechanisms must couple O2∸ to activation of Bak in this model. Nevertheless, these results do not exclude the possibility that inactivation of Akt contributes to the selective killing of CD4+ T cells by Bz-423 in MRL-lpr mice.
Modulation of the mitochondrial F0F1-ATPase leads to potent therapeutic responses in murine models of lupus linked to specific apoptosis of disease-causing lymphocytes. Intersections between the apoptotic-signaling network activated by modulation of the F0F1-ATPase with Bz-423 in Jurkat cells and specific disease-associated characteristics of autoreactive T cells provides an understanding of the basis for selective killing of pathogenic CD4+ T cells in MRL-lpr mice. Other immune diseases and certain neoplastic conditions possess similar phenotypic changes that should sensitize them to the mechanism identified here (Rommel et al., 2007; Samuels et al., 2004). Such observations suggest that development of small molecule modulators of the F0F1-ATPase based on the Bz-423 pharmacophore may have broad therapeutic potential (Hong and Pedersen, 2008).
Supplementary Material
Acknowledgments
We thank J. C. Rathmell for the plasmid-encoding myrAkt and for helpful advice.
This study was supported by the National Institutes of Health [Grants AI47450, CA10456].
G.D.G. and A.W.O. acknowledge stock ownership and consulting compensation from a corporation that has licensed certain commercial rights to Bz-423.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156422
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- Bz-423
- 7-chloro-5-(4-hydroxyphenyl)-1-methyl-3-(napthalen-2-ylmetyl)-4,5,-dihydro-1H-benzo[b][1,4]diazepin-2(3H)-one
- ASK1
- apoptosis signal-regulating kinase1
- DHE
- dihydroethidium
- GAPDH
- glyceradehyde-3-phosphate dehydrogenase
- JNK
- c-Jun N-terminal kinase
- MAP
- mitogen-activated protein
- MEF
- mouse embryonic fibroblast
- MnTBAP
- manganese (III) tetrakis (4-benzoic acid)porphyrin
- MIS
- mitochondrial intermembrane space
- MOMP
- mitochondrial outer membrane permeabilization
- MRL/MpJ-Faslpr
- MRL-lpr
- myrAkt
- myristoylated Akt
- O2∸
- superoxide
- PI
- propidium iodide
- PBS
- phosphate-buffered saline
- PI3K
- phosphatidylinositol 3-kinase
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- SP600125
- anthra[1–9cd]pyrazol-6(2H)-one
- V-β
- β-subunit of the F0F1-ATPase
- zVAD-fmk
- benzyloxycarbonyl-valine-alanine-aspartic acid fluoromethyl ketone.
References
- Astoul et al., 2001.Astoul E, Edmunds C, Cantrell DA, Ward SG. (2001) PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol 22:490–496 [DOI] [PubMed] [Google Scholar]
- Barber et al., 2005.Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Rückle T, Schwarz MK, Rodríguez S, et al. (2005) PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11:933–935 [DOI] [PubMed] [Google Scholar]
- Bednarski et al., 2003.Bednarski JJ, Warner RE, Rao T, Leonetti F, Yung R, Richardson BC, Johnson KJ, Ellman JA, Opipari AW, Jr, Glick GD. (2003) Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum 48:757–766 [DOI] [PubMed] [Google Scholar]
- Bennett et al., 2001.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 98:13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsias et al., 2009.Bertsias GK, Nakou M, Choulaki C, Raptopoulou A, Papadimitraki E, Goulielmos G, Kritikos H, Sidiropoulos P, Tzardi M, Kardassis D, et al. (2009) Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum 60:207–218 [DOI] [PubMed] [Google Scholar]
- Blatt et al., 2002.Blatt NB, Bednarski JJ, Warner RE, Leonetti F, Johnson KM, Boitano A, Yung R, Richardson BC, Johnson KJ, Ellman JA, et al. (2002) Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J Clin Invest 110:1123–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt et al., 2008.Blatt NB, Boitano AE, Lyssiotis CA, Opipari AW, Jr, Glick GD. (2008) Bz-423 superoxide signals apoptosis via selective activation of JNK, Bak, and Bax. Free Radic Biol Med 45:1232–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt et al., 2009.Blatt NB, Boitano AE, Lyssiotis CA, Opipari AW, Jr., Glick GD. (2009) Bz-423 superoxide signals B cell apoptosis via Mcl-1, Bak, and Bax. Biochem Pharmacol 78:966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobé et al., 2006.Bobé P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. (2006) Arsenic trioxide: a promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood 108:3967–3975 [DOI] [PubMed] [Google Scholar]
- Bobé et al., 1997.Bobé P, Bonardelle D, Reynès M, Godeau F, Mahiou J, Joulin V, Kiger N. (1997) Fas-mediated liver damage in MRL hemopoietic chimeras undergoing lpr-mediated graft-versus-host disease. J Immunol 159:4197–4204 [PubMed] [Google Scholar]
- Bunin et al., 1994.Bunin BA, Plunkett MJ, Ellman JA. (1994) The combinatorial synthesis and chemical and biological evaluation of a 1,4-benzodiazepine library. Proc Natl Acad Sci U S A 91:4708–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirakkal et al., 2006.Chirakkal H, Leech SH, Brookes KE, Prais AL, Waby JS, Corfe BM. (2006) Upregulation of BAK by butyrate in the colon is associated with increased Sp3 binding. Oncogene 25:7192–7200 [DOI] [PubMed] [Google Scholar]
- Danial and Korsmeyer, 2004.Danial NN, Korsmeyer SJ. (2004) Cell death: critical control points. Cell 116:205–219 [DOI] [PubMed] [Google Scholar]
- Fei et al., 2008.Fei Q, McCormack AL, Di Monte DA, Ethell DW. (2008) Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J Biol Chem 283:3357–3364 [DOI] [PubMed] [Google Scholar]
- Fischer et al., 2008.Fischer SF, Belz GT, Strasser A. (2008) BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci U S A 105:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al., 2005.Gao N, Rahmani M, Dent P, Grant S. (2005) 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene 24:3797–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada et al., 2004.Hanada M, Feng J, Hemmings BA. (2004) Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim Biophys Acta 1697:3–16 [DOI] [PubMed] [Google Scholar]
- Hoffman, 2004.Hoffman RW. (2004) T cells in the pathogenesis of systemic lupus erythematosus. Clin Immunol 113:4–13 [DOI] [PubMed] [Google Scholar]
- Hong and Pedersen, 2008.Hong S, Pedersen PL. (2008) ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev 72:590–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 2005.Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW, Jr, Glick GD. (2005) Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol 12:485–496 [DOI] [PubMed] [Google Scholar]
- Kavurma and Khachigian, 2003.Kavurma MM, Khachigian LM. (2003) Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ 10:36–44 [DOI] [PubMed] [Google Scholar]
- Kepp et al., 2007.Kepp O, Rajalingam K, Kimmig S, Rudel T. (2007) Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J 26:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuk et al., 2009.Kutuk O, Arisan ED, Tezil T, Shoshan MC, Basaga H. (2009) Cisplatin overcomes Bcl-2-mediated resistance to apoptosis via preferential engagement of Bak: critical role of Noxa-mediated lipid peroxidation. Carcinogenesis doi: 10.1093/carcin/bgp165 [DOI] [PubMed] [Google Scholar]
- Lakhani et al., 2006.Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer et al., 2006.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. (2006) Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21:749–760 [DOI] [PubMed] [Google Scholar]
- Nakamura and Sakamoto, 2008.Nakamura T, Sakamoto K. (2008) Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol 281:47–55 [DOI] [PubMed] [Google Scholar]
- Obexer et al., 2007.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. (2007) FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ 14:534–547 [DOI] [PubMed] [Google Scholar]
- Ohse et al., 2001.Ohse T, Nagaoka S, Arakawa Y, Kawakami H, Nakamura K. (2001) Cell death by reactive oxygen species generated from water-soluble cationic metalloporphyrins as superoxide dismutase mimics. J Inorg Biochem 85:201–208 [DOI] [PubMed] [Google Scholar]
- Rommel et al., 2007.Rommel C, Camps M, Ji H. (2007) PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7:191–201 [DOI] [PubMed] [Google Scholar]
- Samuels et al., 2004.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304:554 [DOI] [PubMed] [Google Scholar]
- Santiago-Raber et al., 2004.Santiago-Raber ML, Laporte C, Reininger L, Izui S. (2004) Genetic basis of murine lupus. Autoimmun Rev 3:33–39 [DOI] [PubMed] [Google Scholar]
- Vallejo, 2005.Vallejo AN. (2005) CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 205:158–169 [DOI] [PubMed] [Google Scholar]
- Van Remmen et al., 1999.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. (1999) Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys 363:91–97 [DOI] [PubMed] [Google Scholar]
- Warr and Shore, 2008.Warr MR, Shore GC. (2008) Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med 8:138–147 [DOI] [PubMed] [Google Scholar]
- Watabe and Nakaki, 2007.Watabe M, Nakaki T. (2007) ATP depletion does not account for apoptosis induced by inhibition of mitochondrial electron transport chain in human dopaminergic cells. Neuropharmacology 52:536–541 [DOI] [PubMed] [Google Scholar]
- Weston and Davis, 2007.Weston CR, Davis RJ. (2007) The JNK signal transduction pathway. Curr Opin Cell Biol 19:142–149 [DOI] [PubMed] [Google Scholar]
- Willis et al., 2005.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19:1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis et al., 2007.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859 [DOI] [PubMed] [Google Scholar]
- Youle and Strasser, 2008.Youle RJ, Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2005.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. (2005) Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 24:3954–3963 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.