Abstract
Repeated administration of opioids produces long-lasting changes in μ-opioid receptor (MOR) signaling that underlie behavioral changes such as tolerance. Mitogen-activated protein kinase (MAPK) pathways, including MAPK extracellular signal-regulated kinases (ERK1/2), are modulated by opioids and are known to produce long-lasting changes in cell signaling. Thus, we tested the hypothesis that ERK1/2 activation contributes to the development and/or expression of morphine tolerance mediated by the periaqueductal gray (PAG). Changes in phosphorylated ERK1/2 expression were assessed with confocal microscopy and compared to behavioral measures of tolerance to the antinociceptive effects of chronic morphine administration. Repeated microinjection of morphine into the PAG produced tolerance and caused a significant increase in ERK1/2 phosphorylation, an effect not evident with acute morphine microinjection. Microinjection of the MAPK/ERK kinase inhibitor, 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene ethanolate (U0126), into the PAG had no effect on antinociception produced by acute morphine administration. However, repeated coadministration of U0126 and morphine into the PAG blocked ERK1/2 phosphorylation and enhanced the development of morphine tolerance. Coadministration of U0126 with morphine only on the test day also enhanced the expression of morphine tolerance. Administration of the irreversible opioid receptor antagonist β-chlornaltrexamine blocked the activation of ERK1/2 caused by repeated morphine microinjections, demonstrating that ERK1/2 activation was a MOR-mediated event. In summary, these studies show that chronic morphine administration alters ERK1/2 signaling and that disruption of ERK1/2 signaling enhances both the development and expression of morphine tolerance. Contrary to expectations, these data indicate that ERK1/2 activation opposes the development of morphine tolerance.
Opioid receptor activation results in both acute and long-term changes in neuronal physiology. Tolerance, defined as a decrease in agonist potency with repeated administration, is an example of a long-term change caused by opioids. Although tolerance to the antinociceptive effect of morphine develops rapidly with repeated opioid administration (Bailey and Connor, 2005), the signaling mechanism underlying the loss of potency has not been elucidated. One problem with identifying the mechanism is that μ-opioid receptor (MOR) signaling is coupled to several downstream effectors, including inhibition of adenylyl cyclase, activation of K+ channels, inhibition of Ca2+ channels, and activation of p42/44 mitogen-activated protein kinases (MAPK) (Spencer et al., 1997; Standifer and Pasternak, 1997; Williams et al., 2001).
MAPK extracellular signal-regulated kinases (ERK1/2) are prime candidates for the cellular mechanism underlying tolerance because they are known to contribute to synaptic plasticity (Mazzucchelli et al., 2002; Pouyssegur and Lenormand, 2003). ERK1/2 signaling has been implicated in several types of synaptic plasticity, including long-term potentiation, long-term depression, long-term memory formation, including spatial learning and fear-conditioning, and in dendritic spine formation (Sweatt, 2004; Thomas and Huganir, 2004). Previous work demonstrates that MOR agonists may increase and/or decrease the MAPK pathway depending on the time course or system under investigation. For example, there is a lack of ERK1/2 activation following acute morphine treatment of cultured neurons or acute morphine administration in rats (Schulz and Höllt, 1998; Muller and Unterwald, 2004; Macey et al., 2006). These findings suggest that ERK1/2 does not contribute to the actions of acute morphine. However, activation of ERK1/2 has been demonstrated in dorsal root ganglion neurons and several brain regions in mice and rats following chronic morphine treatment (Ortiz et al., 1995; Berhow et al., 1996; Ma et al., 2001; Narita et al., 2002; Asensio et al., 2006), indicating that adaptations during prolonged exposure to morphine may induce MOR coupling to ERK1/2. On the other hand, phosphorylated ERK1/2 was down-regulated in human opiate addicts and rats following chronic morphine treatment (Ferrer-Alcón et al., 2004; Muller and Unterwald, 2004), indicating that in some cases chronic opiate administration is correlated with a decrease in ERK1/2 phosphorylation.
Although opiates act on many sites throughout the nervous system, the descending nociceptive modulatory system that runs from the periaqueductal gray (PAG) to rostral ventromedial medulla to the dorsal horn of the spinal cord seems to be particularly important in both morphine antinociception and tolerance. Microinjection of morphine into the ventrolateral PAG (vlPAG) produces antinociception, and repeated administration produces tolerance (Jacquet and Lajtha, 1976; Siuciak and Advokat, 1987; Tortorici et al., 1999; Lane and Morgan, 2005; Morgan et al., 2006). Furthermore, inactivation of opioid receptors in the vlPAG blocks the development of tolerance to repeated systemic administration of morphine (Lane and Morgan, 2005). Thus, the vlPAG is an ideal brain site to test whether ERK1/2 activation contributes to morphine tolerance. Given that ERK1/2 is known to contribute to neuronal plasticity, it is hypothesized that repeated administration of morphine into the vlPAG will activate ERK1/2 and that blocking ERK1/2 will also block morphine tolerance.
Materials and Methods
Subjects
Male Sprague-Dawley rats (200–350 g; Animal Technologies, Livermore, CA) were anesthetized with pentobarbital (60 mg/kg i.p.) and implanted with a guide cannula (23 gauge, 9 mm long) aimed at the vlPAG (AP: +1.7 mm, ML: ±0.6 mm, DV: −5.0 mm from lambda) using stereotaxic techniques. The guide cannula was attached to two screws in the skull by dental cement. At the end of the surgery, the rat received a prophylactic injection of the antibiotic cefazolin (15 mg/0.15 ml i.m.), and a stylet was inserted to plug the guide cannula. The rat was maintained under a heating lamp until awake.
After surgery, rats were housed individually. The animal housing room was maintained on a reverse light/dark schedule (lights off at 7:00 AM) so rats could be tested during the active dark phase. Food and water were available at all times except during testing. Rats were handled daily before and after surgery. Testing began at least 7 days after surgery. All procedures were conducted in accordance with the Institute of Laboratory Animal Resources (1996). Efforts were made to minimize the number of experimental subjects (e.g., using a within subjects design when possible).
Nociceptive Assessment
Nociception was assessed using the hot-plate test. The hot-plate test consisted of measuring the latency to lick the hind paw when placed on a 52.5°C plate. The rat was removed from the hot plate if no response occurred within 50 s.
Microinjections
Drugs were administered through a 31-gauge injection cannula inserted into and extending 2 mm beyond the tip of the guide cannula. Rats received a sham injection in which an injector was inserted into the guide cannula but no drug was administered. This procedure reduces confounds resulting from mechanical stimulation of neurons on the test day and habituates the rat to the microinjection procedure. Testing with drug administration began 1 day later. Drugs were microinjected in a volume of 0.4 μl and at a rate of 0.1 μl/10 s while the rat was gently restrained by hand. The injection cannula remained in place an additional 20 s to minimize backflow of the drug up the cannula track. After the injection, the stylet was replaced, and the rat was returned to its home cage.
A cumulative microinjection procedure was used to assess changes in morphine antinociceptive potency. Increasing cumulative doses of morphine sulfate (a gift from the National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD) in third log doses of 1, 2.2, 4.6, 10, and 22 μg/0.4 μl were injected into the PAG every 20 min. Nociception was assessed using the hot-plate test 15 min after each injection. This procedure has been shown to produce reliable dose-response curves (Morgan et al., 2005, 2006; Meyer et al., 2007).
Experiment 1: Does ERK1/2 Activation Contribute to the Development of Morphine Tolerance?
The development of tolerance to the antinociceptive effect of morphine and activation of ERK1/2 were assessed in morphine tolerant animals pretreated with the MEK inhibitor U0126 (Sigma-Aldrich, St. Louis, MO). Animals received microinjections of vehicle (saline or 20% DMSO) or the MEK inhibitor U0126 (4.7 μM) 20 min before receiving doses of morphine (5 μg/0.4 μl) or saline twice daily on days 1 and 2. Nociception was assessed before and 15 min after microinjection of morphine or saline after the first set of injections on day 1 but not after subsequent injections on days 1 and 2 to avoid confounds from repeated testing (Milne and Gamble, 1989; Lane and Morgan, 2005). U0126 (100 ng) was dissolved in saline or 20% DMSO and sonicated before microinjection. A fresh aliquot was used for each set of injections. The same shift in D50 values was observed whether U0126 was dissolved in saline or 20% DMSO; therefore, groups were collapsed for data analysis. Antinociceptive tolerance was assessed on day 3 using the morphine cumulative dose procedure described above. Animals were exposed to a lethal dose of halothane and perfused with formalin within 5 min after the last hot-plate test. The brain was removed, and PAG slices were prepared for ERK1/2 immunolabeling as described below.
The effect of blocking opioid receptors after tolerance had developed was assessed to determine whether ERK1/2 activation caused by repeated morphine administration was mediated through an opioid receptor-dependent mechanism. Animals received morphine (5 μg/0.4 μl) or saline microinjection twice a day for 2 days. On day 3, animals were injected twice with the irreversible opioid receptor antagonist β-CNA (0.270 μg/0.5 μl) (Sigma-Aldrich) or vehicle (0.1% ethanol) 6 h and 30 min before the morphine cumulative dose procedure as described above. The brain was removed for ERK1/2 immunolabeling as described previously.
Experiment 2: Does ERK1/2 Activation Contribute to the Expression of Morphine Tolerance?
The involvement of ERK1/2 on the expression of tolerance to the antinociceptive effect of morphine was assessed in morphine-tolerant animals treated with the MEK inhibitor U0126. Animals received twice daily microinjections of morphine (5 μg/0.4 μl) or saline on days 1 and 2. Nociception was assessed before and 15 min after microinjection with morphine or saline after the first set of injections on day 1. Tolerance was assessed on days 3 and 4. Animals were pretreated with U0126 (4.7 μM) or vehicle 20 min before the morphine cumulative dose procedure, as described above, to determine whether blocking ERK1/2 activation alters the expression of tolerance. A within-subjects design was used with saline and U0126 administered in a counterbalanced manner on days 3 and 4.
Histology
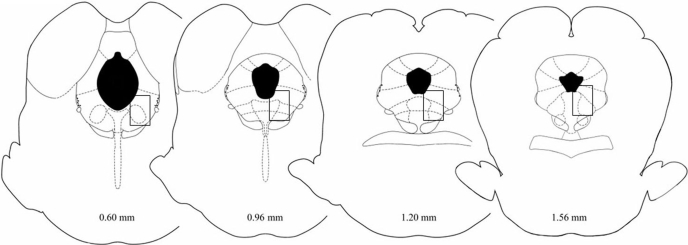
Rats were perfused with saline and then formalin (10% mixed with saline) within 5 min of the last hot-plate test. The brain was removed and placed in formalin (10%). At least 2 days later, the brain was sectioned coronally (60 μm) and the location of the injection site identified (Fig. 1) (Paxinos and Watson, 2005). Brains with correct placements from experiment 1 were processed for ERK1/2 immunolabeling.
Fig. 1.
Region of the vlPAG where microinjections were administered and ERK1/2 phosphorylation was assessed. Rectangles depict the region of the PAG where ERK1/2 activation was assessed using confocal microscopy [Reproduced with permission from Paxinos G and Watson SJ (2005) The Rat Brain in Stereotaxic Coordinates. Academic Press, New York. Copyright © Elsevier, 2005]. Slices imaged were 50 μm away adjacent to the site of injection. Morphine microinjections were in or immediately adjacent to this rectangle.
Immunohistochemistry
Coronal brain slices containing vlPAG were incubated in 5% goat serum (Sigma-Aldrich), 3% bovine serum albumin (Sigma-Aldrich), and 0.5% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature. All of the slices were then incubated in a cocktail of primary antibodies containing rabbit anti-phospho-ERK1/2 (1/300; Cell Signaling, Beverly, MA) and mouse anti-NeuN (1/500; Chemicon, Temecula, CA) overnight, washed, and then incubated for 2 h in a cocktail of secondary antibodies containing goat anti-rabbit IgG Alexa Fluor 488 (1/800; Invitrogen, Carlsbad, CA) and goat anti-mouse IgG Alexa Fluor 555 (1/800; Invitrogen) conjugates. Slices were washed, mounted onto a slide with Slowfade (Invitrogen), coverslipped, and imaged with a Zeiss LSM510 META confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY). The anti-NeuN antibody confirmed that activation of ERK1/2 was specific to neuronal cells.
Phospho-ERK1/2 expression was quantified in individual NeuN-labeled vlPAG neurons adjacent to the aqueduct using the Zeiss LSM Image Examiner program (Carl Zeiss MicroImaging, Inc.) (Fig. 1). Neurons used for analysis were selected based on abundant NeuN labeling in one optical slice through vlPAG. With the phospho-ERK1/2 channel turned off, each NeuN-labeled neuron in the optical slice was traced using the tracing tool in the LSM Image Examiner program. The experimenter was blind to treatments during analysis. A separate box was drawn in an area without labeling to measure background fluorescence for each optical slice per one animal. The phospho-ERK1/2 channel was then turned on, and the absolute frequencies [number of pixels/pixel intensity (0–255)] of phospho-ERK1/2 and background labeling were measured by LSM Image Examiner. The area of each neuron was also measured by LSM Image Examiner in square micrometers. The weighted intensities of phospho-ERK1/2 labeling and background labeling were calculated for each neuron as [sum of (absolute frequency × intensity (at each intensity))/square micrometers] (Excel; Microsoft, Redmond, WA). The weighted intensity of the background labeling was subtracted from that of the phospho-ERK1/2 labeling to obtain the final intensity/square micrometer of phospho-ERK1/2 labeling for each neuron. The average intensity of the mean (±S.E.M.) for the total number of neurons in each treatment group (n = 2–6 rats/group; 6–11 cells per rat) was then calculated. Statistical significance was determined by an ANOVA followed by Dunnett's post hoc test, p < 0.05 or p < 0.01 (Prism; GraphPad Software, Inc., La Jolla, CA).
Behavioral Data Analysis.
Hot-plate latencies were converted to maximal possible effect [%MPE = (test latency − baseline)/(50 s − baseline)]. The lower and upper limits for calculating D50 values were set at 0 and 100% MPE, respectively. Morphine dose-response curves and D50 values (dose for half-maximal antinociception) (Tallarida, 2001) were calculated using nonlinear regression (Prism; GraphPad Software, Inc.). Ninety-five percent confidence intervals (CI) were used to compare individual D50 values following a significant ANOVA (see “Experiments 1 and 2”). ANOVA also was used to determine whether morphine or U0126 had acute effects, and Tukey's post hoc test was used to compare specific groups when there was a significant main effect, p < 0.05. Only rats with injection cannula in or on the border of the vlPAG were included in data analysis.
Results
Experiment 1: Does ERK1/2 Activation Contribute to the Development of Morphine Tolerance?
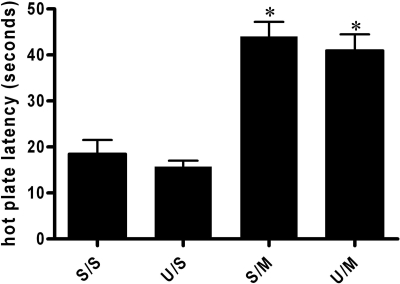
Baseline hot-plate latencies measured before injections on day 1 ranged from 12.7 to 15.4 s for the four groups. Microinjection of morphine into the vlPAG caused a significant increase in hot-plate latency compared with saline [F(3,39) = 22.82; p < 0.05; Fig. 2]. The effect of microinjecting morphine or saline was not altered by pretreatment of U0126.
Fig. 2.
Increase in hot-plate latency after acute microinjection of morphine into the vlPAG. There was no significant difference in hot-plate latency following saline treatment between U0126 (U/S; n = 10) and saline-pretreated (S/S; n = 9) animals. Acute microinjection of morphine (5 μg/0.4 μl) into the vlPAG caused a significant increase in hot-plate latencies for rats pretreated with saline (S/M; n = 10) or the MEK inhibitor U0126 (U/M; n = 14) [F(3,39) = 22.82; * p < 0.05]. These data indicate that U0126 has no effect on the acute antinociceptive effects of morphine.
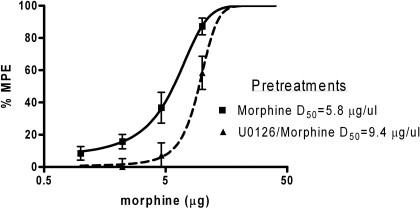
Twice daily microinjections of morphine induced a rightward shift in the morphine dose-response curve as expected with the development of tolerance (Table 1; Fig. 3). Analysis of variance revealed a significant difference in D50 values between groups [F(3,164) = 13.81, p < 0.05]. The D50 for morphine-tolerant rats was 5.8 μg (CI = 4.8–6.8 μg; n = 10). The D50 values for saline (D50 = 4.6 μg; n = 9) and U0126-treated animals (D50 = 4.2 μg; n = 10) were significantly different from morphine-pretreated rats as indicated by the 95% confidence intervals. Rats pretreated with morphine and U0126 on days 1 and 2 had a significantly larger rightward shift in the morphine dose-response curve (D50 = 9.4 μg; n = 14) compared with morphine-tolerant animals (Table 1; Fig. 3).
Table 1.
D50 values for development and expression of morphine tolerance
| Morphine Tolerance | D50 | CI | No. of Subjects |
|---|---|---|---|
| Development | |||
| Sal/Sal | 4.6 | 3.3–5.9 | 9 |
| U0126/Sal | 4.2 | 3.4–5.0 | 10 |
| Sal/MOR | 5.8 | 4.8–6.8 | 10 |
| U0126/MOR | 9.4 | 8.3–10.5 | 14 |
| Expression | |||
| Saline | 6.0 | 2.2–9.8 | 8 |
| U0126 | 10.8 | 6.2–15.3 | 8 |
Fig. 3.
ERK1/2 inhibition enhances morphine tolerance. Third-log cumulative microinjections of morphine into the vlPAG on day 3 produced dose-dependent increase in antinociception. This tolerance to the antinociceptive effect of morphine was enhanced by coadministration of morphine and U0126 (D50 = 9.4 μg; n = 14; U0126/Morphine) during the development of tolerance. Nociception was assessed using the hot-plate test, and data were converted to %MPE. Each point on the graph represents the mean hot plate latency score ± S.E.M. for every dose of morphine.
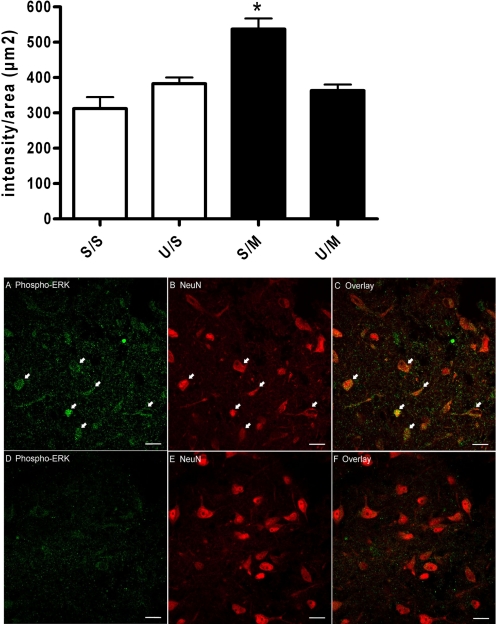
Immunohistochemical analysis of ERK1/2 activation revealed an overall group effect [F(3,84) = 14.22, p < 0.01; Fig. 4, graph] in the NeuN-containing cells in the vlPAG in animals that received repeated morphine administration. Repeated morphine administration (S/M) significantly increased ERK1/2 activation (537 ± 30 intensity/μm2; n = 26 neurons from three animals; Fig. 4, A and C) compared to saline pretreatment (S/S) (312 ± 32 intensity/μm2; n = 20 neurons from three animals, Dunnett's, p < 0.01; Fig. 4, graph). Furthermore, pretreatment with U0126 blocked the ERK1/2 activation produced by repeated morphine administration (U/M) (363 ± 17 intensity/μm2; n = 22 neurons from three animals; Fig. 4, D and F) and administration of U0126 in the absence of morphine did not increase ERK1/2 phosphorylation (U/S) (383 ± 18 intensity/μm2; n = 26 neurons from three animals; Fig. 4, graph). Neither group differed from saline-pretreated animals (Dunnett's test; p > 0.05). There were no significant differences in NeuN labeling (Fig. 4, B and E) between the four treatment groups, which suggest that differences in neuron density do not account for the changes in ERK activation (data not shown).
Fig. 4.
ERK1/2 activation following repeated morphine microinjections into the vlPAG. Graph, rats were given two pretreatment microinjections on days 1 and 2 of saline (S) or U0126 (U) and saline (S) or morphine (M). Phospho-ERK1/2 fluorescence intensity levels were significantly increased in morphine-pretreated rats (S/M; n = 26 neurons from three animals) compared with saline-pretreated rats (S/S; n = 20 neurons from three animals; Dunnett's, *, p < 0.01). ERK1/2 activation was blocked in morphine-pretreated rats by coadministration of the MEK inhibitor U0126 (U/M; n = 22 neurons from three animals; Dunnett's, p > 0.05) compared with morphine alone (S/M). ERK1/2 activation was not increased in U0126-pretreated, saline-treated rats (U/S; n = 26 neurons from three animals; Dunnett's, p > 0.05). Each bar represents the mean intensity ± S.E.M. A to F, neurons in the vlPAG were labeled with phospho-ERK1/2 (A and D) and the neuron marker NeuN (B and E). Overlays of phospho-ERK1/2 and NeuN images (C and F) show that activation of ERK1/2 was specific to neurons (arrows). Increased phospho-ERK1/2 is observed in a representative slice from a rat treated chronically with morphine (A to C). Coadministration of U0126 and morphine prevented ERK1/2 phosphorylation (D to F). Scale bar = 20 μm. Rats were euthanized, and the brain was removed for immunohistochemical analysis of ERK1/2 phosphorylation 5 min after the last morphine injection on day 3.
Levels of phospho-ERK1/2 in two morphine-tolerant animals that had cannula placements outside the vlPAG (196 ± 40 intensity/μm2, n = 10 neurons) were significantly lower than morphine-tolerant animals with correct vlPAG placements (537 ± 30 intensity/μm2) [(t(13) = 4.739, p < 0.05]. Phosphorylation of ERK1/2 was not observed in β-CNA-pretreated, morphine-tolerant rats (113 ± 38 intensity/μm2; n = 21 neurons from four animals).
Experiment 2: Does ERK1/2 Activation Contribute to the Expression of Morphine Tolerance?
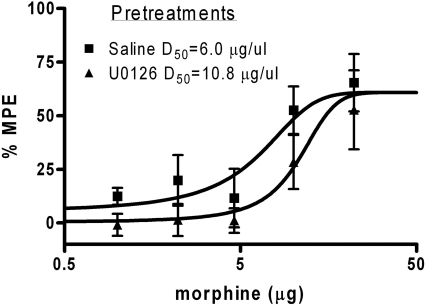
Morphine-tolerant rats given U0126 only before the assessment of tolerance (days 3 or 4) showed a significant rightward shift in the dose-response curve compared with morphine-tolerant rats tested with saline [F(1,76) = 4.3; p < 0.05]. That is, a single injection of U0126 in morphine-tolerant rats caused a further reduction in morphine potency compared to that produced by tolerance alone. The D50 for morphine-tolerant rats in the absence of U0126 was 6.0 ± 1.9 μg (n = 8) compared with a D50 of 10.8 ± 2.3 μg in the presence of U0126 (n = 8) (Table 1; Fig. 5).
Fig. 5.
Inhibition of ERK1/2 phosphorylation enhanced the expression of morphine tolerance. Cumulative dose microinjections of morphine into the vPAG produced a dose-dependent increase in antinociception in morphine-tolerant rats (D50 = 6.0 μg; n = 8; Saline). Pretreatment with U0126 on Trial 5 before assessment of tolerance to morphine caused a rightward shift in the dose-response curve (D50 = 10.8 μg; n = 8; U0126) compared with rats given saline before morphine (* p < 0.05). A within-subjects design was used with saline, and U0126 was administered in a counterbalanced manner on test days (test days were days 3 and 4). Nociception was assessed using the hot-plate test, and data were converted to %MPE. Each point on the graph represents the mean hot-plate latency score ± S.E.M. for every dose of morphine.
Discussion
These experiments demonstrate that a single treatment of morphine is not sufficient to activate ERK1/2 signaling in the vlPAG, but repeated morphine administration induces significant ERK1/2 phosphorylation in neurons within the vlPAG. In addition, blockade of ERK1/2 signaling in the vlPAG by the MEK inhibitor UO126 has no effect on hot-plate latency when administered alone but shifts the morphine dose-response curve to the right in tolerant rats pretreated with morphine (i.e., blocking ERK1/2 enhances morphine tolerance). This enhanced tolerance is observed both when the MEK inhibitor U0126 is administered during the development (experiment 1) and expression (experiment 2) of tolerance. Contrary to the hypothesis of this study, these data indicate that direct activation of ERK1/2 attenuates the development of morphine tolerance.
The lack of ERK1/2 activation with an acute treatment (single dose) of morphine is consistent with previous work demonstrating that morphine in striatal cultures did not activate ERK1/2 (Macey et al., 2006) and in vivo studies showing no activation of ERK1/2 following acute subcutaneous morphine administration in several brain regions, including the locus coeruleus and caudate putamen (Schulz and Höllt, 1998; Muller and Unterwald, 2004). One study observed a significant decrease in ERK1/2 activation at 4 h following acute administration of morphine in the nucleus accumbens but not at the shorter time periods (Muller and Unterwald, 2004), suggesting that time-dependent activation of ERK1/2 may occur after acute morphine microinjections. In addition, an increase in the levels of phospho-ERK1/2 in the cerebral cortex and striatum was reported following an acute, high dose of morphine (100 mg/kg) injected intraperitoneally in rats but not with lower doses of morphine (10 and 30 mg/kg i.p.) (Asensio et al., 2006). This result is consistent with observations that ERK1/2 activation is increased with acute administration of the more efficacious agonist fentanyl in striatal neurons (Macey et al., 2006) and with the full MOR agonist, [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin acetate salt, in MOR-transfected cells (Belcheva et al., 1998), suggesting that ERK1/2 activation by MOR may be preferentially stimulated by high-efficacy agonists.
Our finding that ERK1/2 phosphorylation occurs in rats following repeated morphine administration indicates that activation of MOR by opioid agonists is altered with repeated injections. Increased phosphorylation of ERK1/2 following repeated morphine exposure also has been shown in cultured dorsal root ganglion neurons (Ma et al., 2001) and in other brain regions, including the ventral tegmental area and pons/medulla following chronic, subcutaneous morphine treatment (Ortiz et al., 1995; Berhow et al., 1996; Narita et al., 2002; Asensio et al., 2006). Although it is possible that an acute, higher dose of morphine may activate ERK1/2 in the vlPAG, our previous data suggest that repeated administration of morphine induces an increase in potency of opioid agonists, as measured by an increase in G protein-coupled inwardly rectifying potassium channel signaling in the vlPAG (Ingram et al., 2008). This enhanced activation of MOR may be responsible for increased ERK1/2 activation following repeated morphine administration and could explain the activation by acute administration of high-efficacy agonists compared with acute administration of morphine.
The regulation of ERK1/2 is a candidate for understanding the cellular mechanism of tolerance due to its role in synaptic plasticity. The activation of ERK1/2 during the development and expression of tolerance is dependent on the tissue, agonist, and time investigated. In this study, blockade of ERK1/2 phosphorylation by the MEK inhibitor U0126 in the vlPAG shifts the morphine dose-response curve to the right (enhanced tolerance), suggesting that direct activation of ERK1/2 may oppose tolerance. This is contrary to the prevalent view that ERK1/2 activation with repeated administration of morphine leads to an increase in tolerance but is consistent with the observation that chronic morphine increases MOR potency in the PAG, stimulates phosphorylation of MOR (Schmidt et al., 2000), and increases MOR desensitization (Ingram et al., 2008). Therefore, MOR-mediated activation of ERK1/2 may play a role in the counteradaptations that occur during the development and expression of antinociceptive tolerance.
Although ERK1/2 is known to contribute to other forms of plasticity, the ability of ERK1/2 to counteract morphine tolerance has not been reported previously. In fact, a recent study showed that the intraperitoneal injection of the MEK inhibitor SL327 in mice had no effect on morphine tolerance (Mouledous et al., 2007). The MEK inhibitor U0126 at higher concentrations inhibits ERK5, another MAPK family member that responds to oxidative stress and epidermal growth factor (Mody et al., 2001). However, we did not use a high concentration of U0126 and do not expect that the regulation of ERK5 changed during morphine tolerance. Again, our data demonstrate a direct behavioral correlation of enhanced tolerance following inhibition of MEK signaling.
The increase in ERK1/2 activation following chronic morphine administration correlates with a wide range of other neuronal changes associated with the development of tolerance. Changes within the vlPAG include an increase in morphine potency (Ingram et al., 2008), the apparent mobilization of δ-opioid receptors (DOR) to the plasma membrane (Commons et al., 2001; Hack et al., 2005), and increased signaling of second messengers such as cAMP (Ingram et al., 1998; Bagley et al., 1999). The modulation of ERK1/2 via MOR activation by second messengers (Belcheva et al., 2005) suggests the up-regulation of various second messengers, including cAMP, after chronic morphine treatment (Ingram et al., 1998) could be the mechanism by which ERK1/2 activation is induced. An additional pathway that may lead to ERK1/2 activation may include the activation of cAMP through the DOR. Whether DOR and cAMP up-regulation are part of the same or different pathways is unclear, but both changes drive ERK1/2 activation after chronic morphine treatment. For example, the formation of MOR-DOR heterodimers has been shown to activate ERK1/2 (Rozenfeld and Devi, 2007). These results are provocative in that enhancement of ERK1/2 signaling may be a mechanism by which a drug could attenuate tolerance to morphine.
Acknowledgments
Morphine sulfate was a gift from the National Institutes of Health National Institute on Drug Abuse.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grants DA023318, DA015498] (to T.A.M. and M.M.M., respectively); the National Institutes of Health National Institute of Dental and Craniofacial Research [Grant DE012640] (to S.A.A.); the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant T32-NS045553] (to D.M.H.); and the National Institutes of Health National Center for Research Resources [Grant RR016858].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.152157
- MOR
- μ-opioid receptor
- ERK
- extracellular signal-regulated kinase
- ANOVA
- analysis of variance
- cAMP
- cyclic AMP
- CI
- confidence intervals
- DOR
- δ-opioid receptors
- MAPK
- mitogen-activated protein kinase
- MEK
- MAPK/ERK kinase
- PAG
- periaqueductal gray
- vlPAG
- ventrolateral PAG
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene ethanolate
- DMSO
- dimethyl sulfoxide
- %MPE
- maximal possible effect
- β-CNA
- β-chlornaltrexamine
- SL327
- α[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile.
References
- Asensio et al., 2006.Asensio VJ, Miralles A, García-Sevilla JA. (2006) Stimulation of mitogen-activated protein kinase kinases (MEK1/2) by mu-, delta- and kappa-opioid receptor agonists in the rat brain: regulation by chronic morphine and opioid withdrawal. Eur J Pharmacol 539:49–56 [DOI] [PubMed] [Google Scholar]
- Bagley et al., 1999.Bagley EE, Vaughan CW, Christie MJ. (1999) Inhibition by adenosine receptor agonists of synaptic transmission in rat periaqueductal grey neurons. J Physiol 516:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey and Connor, 2005.Bailey CP, Connor M. (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68 [DOI] [PubMed] [Google Scholar]
- Belcheva et al., 2005.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. (2005) Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem 280:27662–27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva et al., 1998.Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. (1998) Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem 70:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow et al., 1996.Berhow MT, Hiroi N, Nestler EJ. (1996) Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci 16:4707–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons et al., 2001.Commons KG, Beck SG, Rudoy C, Van Bockstaele EJ. (2001) Anatomical evidence for presynaptic modulation by the delta opioid receptor in the ventrolateral periaqueductal gray of the rat. J Comp Neurol 430:200–208 [PubMed] [Google Scholar]
- Ferrer-Alcón et al., 2004.Ferrer-Alcón M, García-Fuster MJ, La Harpe R, García-Sevilla JA. (2004) Long-term regulation of signalling components of adenylyl cyclase and mitogen-activated protein kinase in the pre-frontal cortex of human opiate addicts. J Neurochem 90:220–230 [DOI] [PubMed] [Google Scholar]
- Hack et al., 2005.Hack SP, Bagley EE, Chieng BC, Christie MJ. (2005) Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci 25:3192–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram et al., 2008.Ingram SL, Macey TA, Fossum EN, Morgan MM. (2008) Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology 33:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram et al., 1998.Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. (1998) Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18:10269–10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, 1996.Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC: [Google Scholar]
- Jacquet and Lajtha, 1976.Jacquet YF, Lajtha A. (1976) The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res 103:501–513 [DOI] [PubMed] [Google Scholar]
- Lane and Morgan, 2005.Lane DA, Morgan MM. (2005) Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res 1047:65–71 [DOI] [PubMed] [Google Scholar]
- Ma et al., 2001.Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. (2001) Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci 14:1091–1104 [DOI] [PubMed] [Google Scholar]
- Macey et al., 2006.Macey TA, Lowe JD, Chavkin C. (2006) Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem 281:34515–34524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli et al., 2002.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pagès G, Valverde O, et al. (2002) Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34:807–820 [DOI] [PubMed] [Google Scholar]
- Meyer et al., 2007.Meyer PJ, Fossum EN, Ingram SL, Morgan MM. (2007) Analgesic tolerance to microinjection of the mu-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology 52:1580–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne and Gamble, 1989.Milne RJ, Gamble GD. (1989) Habituation to sham testing procedures modifies tail-flick latencies: effects on nociception rather than vasomotor tone. Pain 39:103–107 [DOI] [PubMed] [Google Scholar]
- Mody et al., 2001.Mody N, Leitch J, Armstrong C, Dixon J, Cohen P. (2001) Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett 502:21–24 [DOI] [PubMed] [Google Scholar]
- Morgan et al., 2006.Morgan MM, Fossum EN, Levine CS, Ingram SL. (2006) Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav 85:214–219 [DOI] [PubMed] [Google Scholar]
- Morgan et al., 2005.Morgan MM, Tierney BW, Ingram SL. (2005) Intermittent dosing prolongs tolerance to the antinociceptive effect of morphine microinjection into the periaqueductal gray. Brain Res 1059:173–178 [DOI] [PubMed] [Google Scholar]
- Moulédous et al., 2007.Moulédous L, Díaz MF, Gutstein HB. (2007) Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav 88:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller and Unterwald, 2004.Muller DL, Unterwald EM. (2004) In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 310:774–782 [DOI] [PubMed] [Google Scholar]
- Narita et al., 2002.Narita M, Ioka M, Suzuki M, Narita M, Suzuki T. (2002) Effect of repeated administration of morphine on the activity of extracellular signal regulated kinase in the mouse brain. Neurosci Lett 324:97–100 [DOI] [PubMed] [Google Scholar]
- Ortiz et al., 1995.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. (1995) Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Watson, 2005.Paxinos G, Watson SJ. (2005) The Rat Brain in Stereotaxic Coordinates Academic Press, New York: [Google Scholar]
- Pouysségur and Lenormand, 2003.Pouysségur J, Lenormand P. (2003) Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Eur J Biochem 270:3291–3299 [DOI] [PubMed] [Google Scholar]
- Rozenfeld and Devi, 2007.Rozenfeld R, Devi LA. (2007) Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt et al., 2000.Schmidt H, Schulz S, Klutzny M, Koch T, Händel M, Höllt V. (2000) Involvement of mitogen-activated protein kinase in agonist-induced phosphorylation of the mu-opioid receptor in HEK 293 cells. J Neurochem 74:414–422 [DOI] [PubMed] [Google Scholar]
- Schulz and Höllt, 1998.Schulz S, Höllt V. (1998) Opioid withdrawal activates MAP kinase in locus coeruleus neurons in morphine-dependent rats in vivo. Eur J Neurosci 10:1196–1201 [DOI] [PubMed] [Google Scholar]
- Siuciak and Advokat, 1987.Siuciak JA, Advokat C. (1987) Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res 424:311–319 [DOI] [PubMed] [Google Scholar]
- Spencer et al., 1997.Spencer RJ, Jin W, Thayer SA, Chakrabarti S, Law PY, Loh HH. (1997) Mobilization of Ca2+ from intracellular stores in transfected neuro2a cells by activation of multiple opioid receptor subtypes. Biochem Pharmacol 54:809–818 [DOI] [PubMed] [Google Scholar]
- Standifer and Pasternak, 1997.Standifer KM, Pasternak GW. (1997) G proteins and opioid receptor-mediated signalling. Cell Signal 9:237–248 [DOI] [PubMed] [Google Scholar]
- Sweatt, 2004.Sweatt JD. (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317 [DOI] [PubMed] [Google Scholar]
- Tallarida, 2001.Tallarida RJ. (2001) Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–872 [PubMed] [Google Scholar]
- Thomas and Huganir, 2004.Thomas GM, Huganir RL. (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183 [DOI] [PubMed] [Google Scholar]
- Tortorici et al., 1999.Tortorici V, Robbins CS, Morgan MM. (1999) Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci 113:833–839 [DOI] [PubMed] [Google Scholar]
- Williams et al., 2001.Williams JT, Christie MJ, Manzoni O. (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343 [DOI] [PubMed] [Google Scholar]