Abstract
Single-molecule manipulation studies have revealed that double-stranded DNA undergoes a structural transition when subjected to tension. At forces that depend on the attachment geometry of the DNA (65 pN or 110 pN), it elongates ≈1.7-fold and its elastic properties change dramatically. The nature of this overstretched DNA has been under debate. In one model, the DNA cooperatively unwinds, while base pairing remains intact. In a competing model, the hydrogen bonds between base pairs break and two single DNA strands are formed, comparable to thermal DNA melting. Here, we resolve the structural basis of DNA overstretching using a combination of fluorescence microscopy, optical tweezers, and microfluidics. In DNA molecules undergoing the transition, we visualize double- and single-stranded segments using specific fluorescent labels. Our data directly demonstrate that overstretching comprises a gradual conversion from double-stranded to single-stranded DNA, irrespective of the attachment geometry. We found that these conversions favorably initiate from nicks or free DNA ends. These discontinuities in the phosphodiester backbone serve as energetically favorable nucleation points for melting. When both DNA strands are intact and no nicks or free ends are present, the overstretching force increases from 65 to 110 pN and melting initiates throughout the molecule, comparable to thermal melting. These results provide unique insights in the thermodynamics of DNA and DNA-protein interactions.
Keywords: DNA melting, fluorescence microscopy, optical trapping, single-molecule techniques, single-stranded DNA
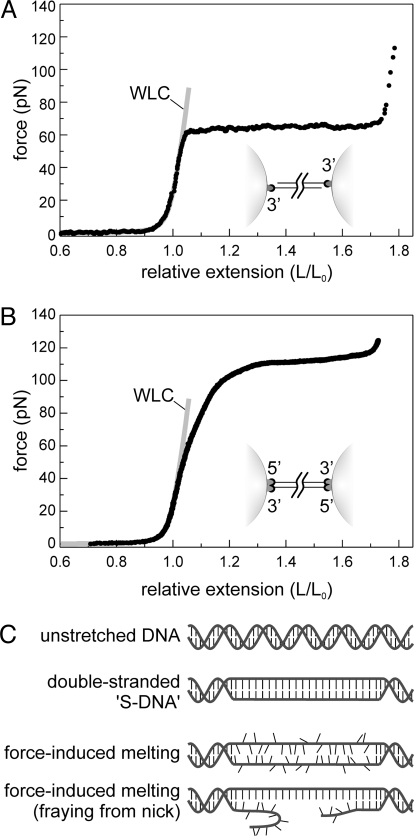
The elastic properties of DNA affect a wide variety of cellular processes, such as protein-induced DNA bending, twisting, or looping, but also genome compaction. Ever since the first single-molecule stretching experiments were performed on double-stranded DNA (dsDNA) (1), many efforts have been made to understand its elastic behavior. A striking feature of dsDNA elasticity is the overstretching (OS) transition: at forces of around 65 pN, DNA gains about 70% of contour length, while the force only increases slightly (Fig. 1A) (2, 3). A similar transition is observed when the DNA is torsionally constrained (4, 5), then occurring at a force of around 110 pN (Fig. 1B). Two qualitatively different models have been put forward for the molecular mechanism of the overstretching transition. The first one assumes that it comprises a gradual conversion to a double-stranded conformation, structurally different from Watson and Crick's B-DNA (6), named S-DNA (3). The structure of S-DNA is usually depicted as a partially unwound ladder with base pairing intact (Fig. 1C). The second model assumes force-induced melting, causing the hydrogen bonds between the two strands to gradually break, yielding single-stranded DNA (ssDNA) similar to thermal melting (7, 8). This latter interpretation is supported by molecular dynamics simulations (9) and thermodynamic modeling (10), explaining dependencies on pH, salt, temperature (7, 8, 11, 12), and ssDNA-specific ligands (13–15). However, four major arguments have been put forward against the force-induced melting interpretation. First, it has been observed that the two DNA strands do not immediately separate when pulled beyond the OS transition at 65 pN (see Fig. 1A) (2). Second, using similar DNA constructs, experiments at higher forces have revealed an additional transition at 150 to 300 pN, resulting in strand separation (16). Therefore, it was concluded that no melting occurs in the first transition at 65 pN (16). Third, the elastic properties of DNA beyond the 65 pN OS transition appear to differ from ssDNA (17). Fourth, studies of torsionally constrained DNA suggested that S-DNA has a helicity of ≈35 base pairs per turn (4, 5). Unambiguous discrimination between the two models would require direct visualization of the structure of DNA during overstretching.
Fig. 1.
The OS transition of dsDNA under tension. (A) Typical force-extension curve of a 3′-3′ attached DNA, with free 5′ ends (schematically represented in the inset). The elastic properties of DNA below the OS force of 65 pN are well described by the extensible worm-like chain model (WLC, gray line). At 65 pN, the DNA molecule undergoes the OS transition, during which the intrinsic contour length of the DNA increases from 100% to about 170%. (B) In a 3′5′-5′3′ attachment geometry, where all four strand ends are linked to the microspheres (schematically represented in the inset), the OS force raises to 110 pN. The elastic properties of this DNA construct is well described by the extensible worm-like chain model (WLC, gray line) up to forces of 60 pN. (C) Schematic representation of two models explaining the nature of the OS transition. In the first model, the transition is a result of gradual cooperative unwinding of the DNA double helix resulting in a base-paired structure, “S-DNA,” resembling a parallel ladder. In the second model, force-induced melting of the two strands causes the transition.
Results
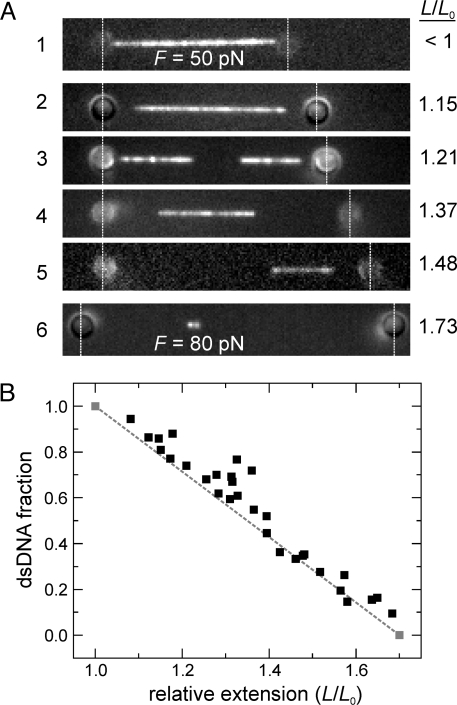
To visualize the structural changes of an individual DNA undergoing the overstretching transition, we used a combination of optical tweezers, fluorescence microscopy, and microfluidics (18). Using two optically trapped microspheres linked to the ends of a double-stranded DNA, we hold and extend the molecule. In our initial set of experiments, focusing on the overstretching transition occuring at 65 pN, we use lambda DNA that is linked to the microspheres with one of the strands on either side of the DNA (designated 3′-3′ attached DNA) (see Fig. 1A). We set out to specifically visualize these double-stranded DNA segments by transferring the overstretched DNA to a channel in our microfluidic flow chamber containing YOYO, a dsDNA-specific dye (19). To prevent YOYO from influencing the overstretching transition, we kept the incubation time to the minimum required for visualization, resulting in at most one dye per 500 base pairs [see supporting information (SI) Fig. S1]. Furthermore, each individual DNA was used for one measurement only and then discarded. Fig. 2a shows fluorescence images of six different DNA molecules extended as indicated and then exposed to YOYO. At extensions L up to the contour length L0, the molecule is stained along its full length (image 1). In contrast, at extensions beyond the contour length (i.e., in the OS transition), only a discrete fraction of the molecule is stained (images 2–6). This result indicates that overstretching at 65 pN involves a gradual conversion of double-stranded B-DNA into a conformation to which YOYO cannot bind. This behavior was observed for monovalent salt concentrations from <5 mM to 150 mM, at which similar images and force-extension curves were obtained. Interestingly, the regions at the extremities, close to the beads, remained unstained. Hence, we conclude that for the 3′-3′ attached DNA construct, the ends of dsDNA are favorable nucleation points for the transition. In a few cases, an additional unlabeled segment between two labeled dsDNA segments was observed (e.g., image 3), which may imply overstretching initiated here from a nick, a break in one of the two DNA strands. Image 6 was taken beyond the OS transition at a force of 80 pN and, strikingly, it still contained a short segment that was labeled. Apparently, some dsDNA is still present beyond the transition. We further analyzed the images by calculating the relative amount of base pairs that were still in a dsDNA conformation, for each extension (see Fig. 2B). For comparison, the expected behavior of a first-order phase transition is shown: a linear decrease of the dsDNA fraction (dashed line) (20). The data lie close to this line, implying that the YOYO-labeled fraction is a largely continuous double-stranded segment. If local overstretched patches, too small to be optically resolved, would be present, one would have expected an overestimation of the dsDNA fraction, which is not apparent from Fig. 2B. Taken together, our data reveal that the overstretching transition at 65 pN, observed for the 3′-3′ attached DNA construct, is processive and initiates from DNA ends.
Fig. 2.
Specific staining of 3′-3′ attached dsDNA stretches with the intercalating dye YOYO shows that the DNA overstretching transition at 65 pN is a nucleation-limited, first-order phase transition. (A) Fluorescence images of different, extended dsDNA molecules exposed to YOYO show a binary subdivision of the DNA in labeled and unlabeled segments. The vertical dashed lines highlight the locations of the optically trapped beads. Image 1 is taken before the OS transition (L/L0 < 1) at a force of 50 pN, where the DNA is labeled along its full length. At higher extensions (L/L0 > 1), where the DNA undergoes the OS transition at a force of 65 pN, discrete unlabeled segments appear at the expense of labeled, double-stranded segments. This subdivision in large labeled and unlabeled segments reveals that the OS transition is nucleation limited. Image 3 shows an unlabeled segment halfway, suggesting that another OS nucleation took place at a nick. Image 6 is taken beyond the OS transition at a force of 80 pN, yet shows that the two strands are still connected by a short YOYO-labeled segment. (B) The fraction of dsDNA plotted as a function of DNA extension. This fraction was obtained from the length of YOYO-labeled segments in fluorescence images such as in (A), assuming them to have the same length as B-form dsDNA at this low degree of labeling (see Fig. S1). The gray dashed line connecting the two gray points indicates the behavior expected for a first-order phase transition from 100% dsDNA at the start of the OS transition (L/L0 = 1) to no dsDNA at the end (L/L0 = 1.7).
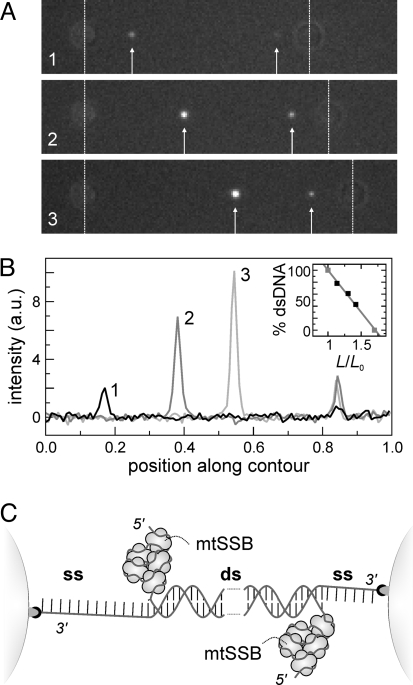
Labeling overstretched DNA with YOYO alone does not allow discrimination between ssDNA and S-DNA. To test whether ssDNA is actually generated, we incubated overstretched DNA with Alexa-555-labeled human mitochondrial single-stranded DNA-binding proteins (mtSSB), which binds with high specificity to ssDNA (21). First, we confirmed that mtSSB does not affect the OS-transition (Fig. S2), similar to the related bacteriophage T4 gene 32 protein (13, 14, 22). Next, we recorded fluorescence images of overstretched 3′-3′ attached DNA, incubated with mtSSB. Fig. 3A shows that binding of mtSSB results in the appearance of two isolated fluorescent spots. We interpret that these spots are caused by mtSSB bound to relaxed, melted ssDNA, which has a radius of gyration on the order of the diffraction limit for the DNA lengths involved here (23). When the DNA is extended further, the intensity of the fluorescent spots increases (Fig. 3 A and B). We assign this increase to the binding of more and more mtSSB to relaxed ssDNA at the interface between dsDNA and ssDNA. From these observations for 3′-3′ attached DNA, two conclusions can be drawn right away. First, during the overstretching transition at 65 pN ssDNA is formed, which is incompatible with the S-DNA model for overstretching. Second, mtSSB does not bind to the partially melted strand that is under tension, as depicted in Fig. 3C. We independently determined that mtSSB cannot bind to ssDNA under tensions exceeding ≈40 pN, consistent with the wrapped binding mode of mtSSB (24). We next calculated the relative amount of dsDNA from the distance between the spots in these images. The inset in Fig. 3B shows that our data are consistent with the double-stranded fraction of DNA decreasing linearly with relative extension, analogous to the results obtained with YOYO labeling (see Fig. 2).
Fig. 3.
The DNA overstretching transition at 65 pN is a melting transition. (A) Three consecutive fluorescence images of overstretched 3′-3′ attached DNA exposed to mitochondrial single-stranded binding protein (mtSSB) labeled with Alexa-555. MtSSB accumulates in two spots (white arrows) that brighten and translocate upon further extension. (B) The intensity in the moving spot scales linearly with the distance moved from the bead, showing that ssDNA accumulates during overstretching. The spot to the right remains stationary relative to the right bead and does not brighten. The inset shows the fraction of base-paired nucleotides as a function of the relative extension, L/L0. The fraction is obtained from the distance between the mtSSB spots, assuming dsDNA in between. (C) Interpretation of images of fluorescent mtSSB bound to overstretched DNA (A). Overstretching initiates on fraying DNA extremities near the beads. MtSSB binds to the melted DNA strand that is not connected to the bead and consequently not under tension.
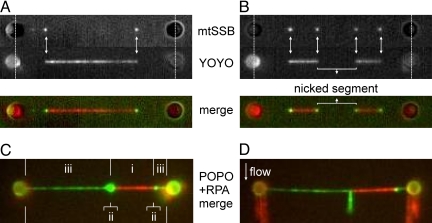
To find out how the results with YOYO and mtSSB relate, we performed two-color fluorescence measurements using both fluorescent markers. We partly overstretched 3′-3′ attached DNA in the presence of mtSSB (as in Fig. 3A) and subsequently exposed it to a buffer containing YOYO, while keeping the extension fixed (see, for example, Fig. 2A). Fig. 4 A and B show two fluorescence images of two different DNA molecules, obtained by exciting Alexa555-mtSSB or YOYO, respectively. The mtSSB spots, indicating partly melted ssDNA, coincide with the edges of a YOYO-labeled dsDNA segment (white arrows in Fig. 4 A and B). Fig. 4B shows a molecule in which the OS transition nucleated not only at its extremities, as in Fig. 3C, but also from nicks in the dsDNA. If the central, nonstained segment shown in Fig. 4B were nick-free, both single-stranded DNA strands would be under high tension and no ssDNA would accumulate at the transition interface. As a final confirmation, we performed two-color fluorescence experiments using a different set of probes: the bis-intercalating dye POPO-3 as dsDNA label and eGFP-tagged Replication Protein A (RPA) as ssDNA label. In contrast to mtSSB, RPA binds to ssDNA without wrapping it (25). Consequently, we inferred that it would bind to both relaxed and stretched ssDNA. In independent experiments we confirmed that RPA binds to ssDNA at forces up to at least 70 pN. In two-color fluorescence images (see Fig. 4C) of partly overstretched POPO-3 and RPA-labeled 3′-3′ attached DNA, three regions can be discerned: (i) a dsDNA region where only POPO-3 binds is flanked by (ii) two bright spots of RPA bound to relaxed ssDNA strands, which are in turn flanked by (iii) regions of RPA-bound, stretched ssDNA connected to the beads. This third region, ssDNA under tension, was not observed in the mtSSB experiments (see Fig. 4 A and B). To confirm that the fluorescent spots at the ssDNA-dsDNA interface indeed correspond to relaxed ssDNA, we used our microfluidic device to apply a gentle flow perpendicular to the overstretched DNA. As can be seen in Fig. 4D, the fluorescent spot is stretched out along the flow direction, as expected for a relaxed piece of ssDNA. These experimental results show that the OS transition, occurring at 65 pN for 3′-3′ attached DNA, is a force-induced melting transition that nucleates from free ends or nicks.
Fig. 4.
Concomitant labeling of dsDNA and ssDNA segments for the 3′-3′ attached DNA shows that, during the overstretching transition at 65 pN, dsDNA is converted into ssDNA. (A and B) Images of overstretched 3′-3′ attached DNA labeled with Alexa-555-mtSSB and YOYO. (Top) Separate emission channels. (Bottom) Merged image (green: mtSSB, red: YOYO). The images show that mtSSB dots coincide with the edges of YOYO-segments (white arrows). In (A) two melting fronts are observed, leading to two relaxed ssDNA segments forming dots, in line with conversion from dsDNA to ssDNA being nucleation limited. In (B) two additional melting fronts can be discerned, originating from a nick. (C) Images of overstretched DNA labeled sequentially with eGFP-RPA (green), and dsDNA-intercalator POPO (red). Three segments can be readily identified: (i) a homogeneous dsDNA segment, (ii) two dots caused by relaxed ssDNA at the intersection between ssDNA and dsDNA, (iii) two stretched ssDNA segments connected to the beads. (D) Image of the same DNA molecule shown in (C), with the application of a gentle buffer flow perpendicular to the DNA, extending the relaxed ssDNA segments.
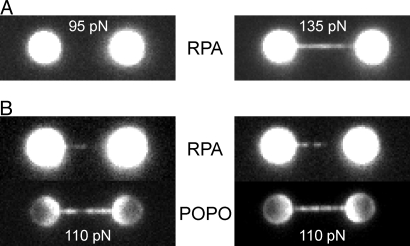
To study the molecular mechanism of the overstretching transition in the absence of such favorable nucleation points, we designed a DNA construct that was linked to the beads via both strands on either side (designated 3′5′-5′3′ attached DNA) (see Fig. 1B). We found that the overstretching plateau for this construct occurs at substantially higher forces, ≈110 pN (see Fig. 1B), in agreement with previous studies using similarly attached DNA constructs (4). Exposing 3′5′-5′3′ attached DNA to a buffer containing RPA did not result in binding of RPA at tensions below the overstretching force of 110 pN (Fig. 5A). Homogeneous RPA binding was, however, clearly observed at forces above 110 pN (see Fig. 5A), indicating that ssDNA is generated. In contrast to the 3′-3′ attached DNA (see Fig. 4), where binding of RPA resulted in three clearly distinguishable regions on the DNA, spatial separation in distinct regions was far less clear at a tension of 110 pN, within the overstretching transition (Fig. 5B). For the 3′5′-5′3′ attached DNA construct, it appears that nucleation of force-induced melting occurs throughout the DNA molecule. This result was confirmed by subsequent exposure of RPA-labeled, overstretched DNA to POPO-3 to visualize dsDNA (see Fig. 5B). In these experiments, regions of dsDNA were resolved throughout the DNA molecule. The intensity distribution of POPO-3 along the DNA molecule appeared to be anticorrelated with that of RPA, suggesting spatial separation of regions of ssDNA and dsDNA. However, this spatial separation cannot be totally resolved because of the limited resolution of our instrument (≈250 nm, 750 bp). In some cases, one of the attachments between the beads and the 3′5′-5′3′ DNA broke during the overstretching experiments. This resulted in a sharp drop in the force from 110 to 65 pN (Fig. S3). Subsequent fluorescent labeling showed that, after rupture, melting nucleated from the broken end, as expected from our results on 3′-3′ attached DNA. Taken together, these experimental results, using a combination of multicolor fluorescence imaging, optical tweezers, and microfluidics, demonstrate directly that the OS transition, which occurs at 110 pN for 3′5′-5′3′ attached DNA, is a force-induced melting transition that nucleates throughout the DNA molecule.
Fig. 5.
The DNA overstretching transition at 110 pN is a melting transition. (A) Images of two different 3′5′-5′3′ attached DNA molecules labeled with the ssDNA marker eGFP-RPA, one held at a tension of 95 pN (before the onset of the OS transition) and the other at 135 pN (beyond the transition). At force below the transition, no eGFP-RPA binding is observed. Beyond the transition, eGFP-RPA covers the DNA homogeneously and completely. (B) Images of two different 3′5′-5′3′ attached DNA molecules at a tension of 110 pN labeled sequentially with eGFP-RPA (Top), and dsDNA-intercalator POPO (Bottom). eGFP-RPA binds throughout the DNA molecules and no unique nucleation point can be observed.
Discussion
Our experiments have confirmed the dependence of the elastic behavior of DNA on the details of its terminal attachment (4): the overstretching transition occurs at different forces, depending on whether a nick or free end is available (3′-3′ attached DNA) or not (3′5′-5′3′ attached DNA). We probed the structure of DNA in the overstretching transition by taking snapshots after applying specific single-stranded and double-stranded DNA probes. For both attachment geometries, the experiments demonstrated the occurrence of ssDNA, confirming that DNA gradually melts during the overstretching transition.
In our experiments on the overstretching transition at 65 pN of 3′-3′ attached DNA, we observed that overstretching initiates at the extremities of the DNA. On each DNA end, only one of the strands of the double helix is attached to the microsphere, such that each strand is attached to only one bead. Apparently dsDNA extremities form a favorable nucleation point: in other words, the overstretching transition at 65 pN is cooperative force-induced fraying (26). In some cases, we observed additional nucleation within a DNA molecule (see Fig. 2A image 3, and Fig. 4B). Our experiments combining YOYO and mtSSB staining (see Fig. 4B) revealed that this is caused by single-stranded nicks. The remarkable processivity of overstretching we observed along the heterogeneous sequence of thousands of base pairs has not been anticipated in earlier studies, where overstretching was suggested to initiate in small AT-rich domains throughout the DNA molecule (8, 10, 27). Our observations rule out the large-scale occurrence of such interior single-stranded melting “bubbles” when a free end is available for nucleation. Apparently, the energy penalty for a new nucleation bubble is larger than a gradual progress of the melting front from the ends or from a nick, regardless of the sequence. This can be understood by realizing that melting at these locations allows one of the newly formed ssDNA strands to relax and release elastic energy.
The situation is quite different for 3′5′-5′3′ attached DNA, which lacks energetically preferred nucleation points on DNA ends. For this DNA, the nucleation of force-induced melting more closely resembles thermal melting (see Fig. 5B), which was found to initiate predominantly from small, interior (AT-rich) regions (28). Indeed, we show that RPA and POPO-3 bind throughout the overstretched DNA (see Fig. 5B). In addition, we measured that the 3′5′-5′3′ attached DNA overstretches at forces of 110 pN, in agreement with previous studies on torsionally and end-constrained DNA (4). Notably, when the DNA is fully overstretched (see Fig. 5A, Right), RPA binds homogeneously along the DNA, in contrast to earlier predictions that the structure of fully overstretched torsionally constrained DNA is biphasic to conserve the linking number (29). The most straightforward explanation of homogeneous RPA binding is that the new structure, generated during overstretching at 110 pN, consists of two single DNA strands lacking hydrogen bonds between the bases, wrapped around each other with a linking number close to that of relaxed dsDNA. In line with this hypothesis, one could speculate that the DNA structure with one turn per ≈35 bases that is obtained when underwound torsionally constrained DNA was stretched to ≈50 pN (4), is also composed of two melted and wrapped ssDNA strands. To resolve how these experiments relate to our findings, further studies that combine fluorescence imaging, force control, and torsional control of the DNA will be required.
As mentioned in the introduction, an argument against the interpretation of force-induced melting for 3′-3′ attached DNA molecules has been the reported stability of DNA beyond the transition at 65 pN. Our approach of direct visualization yielded a surprising observation that allows us to straightforwardly explain this paradox. As seen in image 6 of Fig. 2A, at forces beyond the overstretching transition, a small, transiently stable YOYO-labeled and thus double-stranded segment remains. We hypothesize that the mechanism of overstretching is qualitatively similar to mechanical DNA unzipping, where DNA is melted by pulling apart the two strands on one side of a dsDNA (29). In these experiments, it was demonstrated that the energy to open the DNA duplex increases with the decrease of the stiffness of the construct. The same takes place during the overstretching transition because of the increasing fraction of softer ssDNA. Apparently, it takes more time to overcome the higher energy barriers to melt the last hundreds of base pairs and the transition is no longer in equilibrium on experimental time scales (17). This can explain why the DNA molecule can at least transiently remain intact at forces above 65 pN. A similar conclusion was drawn from thermodynamic modeling (10), which indicated that the DNA is held together by several small dsDNA regions. Interestingly, however, we find that only a single nonmelted region keeps the DNA strands connected. This region appears to be consistently positioned asymmetrically along the molecule (rather than in the center), which might suggest that the GC-rich half of lambda DNA (2) is the last part to melt, as expected. In accordance with a small dsDNA stretch remaining, the elastic properties of DNA beyond the OS-transition can be described by a linear combination of ssDNA and a single, slowly vanishing fraction of dsDNA, indicating out-of-equilibrium behavior (Fig. S4).
In conclusion, we have unveiled that, independent of the details of strand attachment, DNA overstretching unambiguously comprises a gradual conversion of dsDNA to ssDNA. We directly visualized dsDNA using intercalating dyes and ssDNA using single-stranded binding proteins, concurrent with force-extension measurements. Our interpretation therefore does not rely on thermodynamic, mechano-chemical or other models and assumptions. Moreover, the insensitivity of the observed force-induced melting to ionic strength leads us to conclude that the generic process governing the OS transition is the melting of base pairs. We anticipate that our results will provide a basis for the fundamental understanding of the thermodynamics of DNA and DNA-protein interactions.
Materials and Methods
The combined fluorescence and dual-beam optical trapping instrument was adapted from the one described previously (18). Enhancements made to the fluorescence excitation and imaging are described here. For fluorescence excitation of either YOYO or Alexa-555, the linearly polarized light of a 473-nm laser (Cobolt Blues, 25 mW cw, Cobolt AB) or 532-nm laser (GCL-025-L, 25 mW cw, Crystalaser), respectively, was decollimated for widefield excitation and coupled into the microscope using a dichroic mirror [DM3: z473rdc (YOYO), z532rdc (Alexa-555), or z488/532/633rdc (triple-band), Chroma]. Fluorescence was band-pass filtered [EM1: hq540/80m-2p (YOYO), hq575/50m (Alexa-555), or z488/532/633m (triple-band), Chroma] and imaged onto a sensitive electron multiplying CCD camera (EM-CCD: Cascade 512B, Princeton Instruments). Camera readout was externally triggered using a transistor-transistor logic signal for time-lapsed data acquisition. A custom-built flow cell with multiple parallel, laminar channels (30) was used for swift buffer exchange.
To generate the DNA construct with biotin labels on one of the strands on either side of the DNA (3′-3′ attached DNA), we used a protocol to terminally label lambda DNA, as previously described (30, 31). The DNA construct that possesses biotin-labels at both strands on either end (3′5′-5′3′ attached DNA) was generated as follows. First, a 3′ overhang was introduced into the 8,393-bp plasmid pKYB1. Biotin-labled oligos (29 nt) were ligated to both ends, yielding a DNA construct with biotinylated 5′ overhangs on both ends. Both 3′ ends where biotin-labeled by incorporation of biotinylated dATPs (NEB) along with unlabeled nucleotides, by Klenow exo– DNA polymerase. YOYO (YOYO-1) and POPO (and POPO-3) were purchased from Molecular Probes. For fluorescent labeling of human mitochondrial single-stranded DNA-binding protein, a cysteine residue was introduced at the C-terminal end by PCR. The PCR fragment was cloned into pBacPAK9 and used to generate recombinant Autographa californica nuclear polyhedrosis virus (BacPAK, Clontech). The protein was expressed in Spodoptera frugiperda (Sf9) cells and purified as previously described (32), with an additional purification step (before the hydroxyapatite column) using a 1-ml HiTrap SP column (GE Healthcare). The purified mtSSB cysteine variant was stored in 10 mM KPO4 pH 7.2, 0.1 M NaCl and 10% glycerol, and labeled with maleimide Alexa-555 dye (Molecular Probes). Unreacted dye was removed from the sample with size-exclusion spin-columns (Sephadex G-25, GE Healthcare).
To obtain fluorescent RPA, a DNA fragment encoding a polyhistidine-tagged variant of the enhanced GFP (eGFP) was inserted in a frame at the 3′ end of the cDNA encoding the large subunit of human RPA in the expression plasmid p11d-tRPA (33). Fluorescent hRPA-eGFP was produced in Escherichia coli and purified by chromatography through a Histrap FF column followed by chromatography through Hitrap SP HP and Hitrap Q HP columns (GE Healthcare Life Science). The protein, in 200 mM KCl, 20 mM Tris pH 7.5, 1 mM DTT, 0.5 mM EDTA and 10% glycerol, was snap frozen in liquid nitrogen and stored at –80 °C. The eGFP tagged hRPA bound ΦX174 ssDNA with the same affinity as untagged hRPA.
DNA molecules were captured between two optically trapped beads (1.87 μm streptavidin-coated polystyrene beads, Spherotech) using the multichannel laminar flow cell and stretched by increasing the distance between the optical traps. Fluorescence and force-extension data were recorded in a synchronized manner. For experiments involving YOYO labeling, we first partially overstretched DNA in a buffer without YOYO, before exposing it to a YOYO-containing buffer by a rapid and complete buffer exchange (≈0.5 sec). This ensured that the progress of the phase transition itself cannot be influenced. During this buffer exchange, fluorescence was recorded. Every partly overstretched DNA molecule was exposed to a 10-mM Tris buffer (pH 7.4–7.8) containing 10–50 nM YOYO and either 5, 50, or 150 mM NaCl, while fluorescence was recorded. Measurements of the labeled fraction were performed only on the first few frames where YOYO appeared. Control experiments showed that after prolonged exposure to YOYO the dsDNA length increases slightly under our experimental conditions, but that this effect is negligible initially, when the labeling is already bright enough for high-resolution imaging (see Fig. S1). From the small initial tension drop caused by YOYO-induced lengthening (34) we estimate that in this case the dye:base-pair ratio does not exceed 1:500. DNA molecules were always discarded after being exposed to YOYO. All stretching experiments were performed in a 10-mM Tris buffer (pH 7.4–7.8) containing 50 mM NaCl. For experiments with Alexa-555 labeled mtSSB, a protein concentration of 50 nM was used. Fluorescence snapshots were taken in a flow channel without mtSSB to avoid background fluorescence caused by unbound protein. For two-color experiments, mtSSB-labeled DNA molecules were briefly exposed to a 10-nM YOYO buffer. Alternatively, we used eGFP-labeled RPA (20 nM) to label ssDNA segments of an overstretched DNA molecule (both, the 3′-3′ attached DNA and the 3′5′-5′3′ attached DNA), followed by exposure to a 50 to 70 nM POPO solution to label dsDNA segments. To extend frayed ssDNA segments for the 3′-3′ attached DNA, we then imposed a lateral flow to the DNA.
Supplementary Material
Acknowledgments.
This work is part of the research program of the Stichting voor Fundamenteel Onderzoek der Materie, which is financially supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek. P.G. is supported by Atlas, a European Commission-funded Marie Curie early stage-training network. This work was supported in part by an Access grant of Laserlab Europe, the integrated initiative of European Laser Infrastructures, financed by the European Commission.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 18047.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904322106/DCSupplemental.
References
- 1.Smith SB, Finzi L, Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992;258:1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- 2.Smith SB, Cui Y, Bustamante C. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- 3.Cluzel P, et al. DNA: An extensible molecule. Science. 1996;271:792–794. doi: 10.1126/science.271.5250.792. [DOI] [PubMed] [Google Scholar]
- 4.Léger JF, et al. Structural transitions of a twisted and stretched DNA molecule. Phys Rev Lett. 1999;83:1066. [Google Scholar]
- 5.Bryant Z, et al. Structural transitions and elasticity from torque measurements on DNA. Nature. 2003;424:338–341. doi: 10.1038/nature01810. [DOI] [PubMed] [Google Scholar]
- 6.Watson JD, Crick FHC. Molecular structure of nucleic acids—a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 7.Williams MC, Wenner JR, Rouzina L, Bloomfield VA. Effect of pH on the overstretching transition of double-stranded DNA: Evidence of force-induced DNA melting. Biophys J. 2001;80:874–881. doi: 10.1016/S0006-3495(01)76066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenner JR, Williams MC, Rouzina I, Bloomfield VA. Salt dependence of the elasticity and overstretching transition of single DNA molecules. Biophys J. 2002;82:3160–3169. doi: 10.1016/S0006-3495(02)75658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piana S. Structure and energy of a DNA dodecamer under tensile load. Nucleic Acids Res. 2005;33:7029–7038. doi: 10.1093/nar/gki1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouzina I, Bloomfield VA. Force-induced melting of the DNA double helix. 1. Thermodynamic analysis. Biophys J. 2001;80:882–893. doi: 10.1016/S0006-3495(01)76067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouzina I, Bloomfield VA. Force-induced melting of the DNA double helix. 2. Effect of solution conditions. Biophys J. 2001;80:894–900. doi: 10.1016/S0006-3495(01)76068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams MC, Wenner JR, Rouzina I, Bloomfield VA. Entropy and heat capacity of DNA melting from temperature dependence of single molecule stretching. Biophys J. 2001;80:1932–1939. doi: 10.1016/S0006-3495(01)76163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pant K, Karpel RL, Rouzina I, Williams MC. Mechanical measurement of single-molecule binding rates: Kinetics of DNA helix-destabilization by T4 gene 32 protein. J Mol Biol. 2004;336:851–870. doi: 10.1016/j.jmb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Pant K, Karpel RL, Williams MC. Kinetic regulation of single DNA molecule denaturation by T4 gene 32 protein structural D÷omains. J Mol Biol. 2003;327:571–578. doi: 10.1016/s0022-2836(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 15.Shokri L, McCauley MJ, Rouzina I, Williams MC. DNA overstretching in the presence of glyoxal: structural evidence of force-induced DNA melting. Biophys J. 2008;95:1248–1255. doi: 10.1529/biophysj.108.132688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rief M, Clausen-Schaumann H, Gaub HE. Sequence-dependent mechanics of single DNA molecules. Nat Struct Biol. 1999;6:346–349. doi: 10.1038/7582. [DOI] [PubMed] [Google Scholar]
- 17.Cocco S, et al. Overstretching and force-driven strand separation of double-helix DNA. Phys Rev E. 2004;70 doi: 10.1103/PhysRevE.70.011910. 011910. [DOI] [PubMed] [Google Scholar]
- 18.van Mameren J, et al. Counting RAD51 proteins disassembling from nucleoprotein filaments under tension. Nature. 2009;457:745–748. doi: 10.1038/nature07581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rye HS, et al. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes—properties and applications. Nucleic Acids Res. 1992;20:2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vladescu ID, McCauley MJ, Rouzina I, Williams MC. Mapping the phase diagram of single DNA molecule force-induced melting in the presence of ethidium. Phys Rev Lett. 2005;95:158102. doi: 10.1103/PhysRevLett.95.158102. [DOI] [PubMed] [Google Scholar]
- 21.Mignotte B, Barat M, Mounolou JC. Characterization of a mitochondrial protein-binding to single-stranded-DNA. Nucleic Acids Res. 1985;13:1703–1716. doi: 10.1093/nar/13.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberts BM, Frey L. T4 bacteriophage gene 32: A structural protein in replication and recombination of DNA. Nature. 1970;227:1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- 23.Tinland B, Pluen A, Sturm J, Weill G. Persistence length of single-stranded DNA. Macromolecules. 1997;30:5763–5765. [Google Scholar]
- 24.Yang C, Curth U, Urbanke C, Kang CH. Crystal structure of human mitochondrial single stranded DNA binding protein at 2.4 angstrom resolution. Nat Struct Biol. 1997;4:153–157. doi: 10.1038/nsb0297-153. [DOI] [PubMed] [Google Scholar]
- 25.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 26.Jose D, Datta K, Johnson NP, von Hippel PH. Spectroscopic studies of position-specific DNA “breathing” fluctuations at replication forks and primer-template junctions. Proc Nat Acad Sci USA. 2009;106:4231–4236. doi: 10.1073/pnas.0900803106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stigter D. An electrostatic model of B-DNA for its stability against unwinding. Biophys Chem. 1998;75:229–233. doi: 10.1016/s0301-4622(98)00211-7. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Montrichok A, Zocchi G. Bubble nucleation and cooperativity in DNA melting. J Mol Biol. 2004;339:67–75. doi: 10.1016/j.jmb.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 29.Bockelmann U, Essevaz-Roulet B, Heslot F. Molecular stick-slip motion revealed by opening DNA with piconewton forces. Phys Rev Lett. 1997;79:4489–4492. [Google Scholar]
- 30.Noom MC, van den Broek B, van Mameren J, Wuite GJL. Visualizing single DNA-bound proteins using DNA as a scanning probe. Nat Methods. 2007;4:1031–1036. doi: 10.1038/nmeth1126. [DOI] [PubMed] [Google Scholar]
- 31.van den Broek B, Noom MC, Wuite GJL. DNA-tension dependence of restriction enzyme activity reveals mechanochemical properties of the reaction pathway. Nucleic Acids Res. 2005;33:2676–2684. doi: 10.1093/nar/gki565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korhonen JA, Gaspari M, Falkenberg M. TWINKLE has 5 ′-> 3 ′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J Biol Chem. 2003;278:48627–48632. doi: 10.1074/jbc.M306981200. [DOI] [PubMed] [Google Scholar]
- 33.Henricksen LA, Umbricht CB, Wold MS. Recombinant replication protein A: Expression, complex formation, and functional characterization [published erratum appears in J Biol Chem 1994 Jun 10;269(23):16519] J Biol Chem. 1994;269:11121–11132. [PubMed] [Google Scholar]
- 34.Berman HM, Young PR. The interaction of intercalating drugs with nucleic-acids. Annu Rev Biophys Bio. 1981;10:87–114. doi: 10.1146/annurev.bb.10.060181.000511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.