Abstract
Plants and their herbivores constitute more than half of the organisms in tropical forests. Therefore, a better understanding of the evolution of plant defenses against their herbivores may be central for our understanding of tropical biodiversity. Here, we address the evolution of antiherbivore defenses and their possible contribution to coexistence in the Neotropical tree genus Inga (Fabaceae). Inga has >300 species, has radiated recently, and is frequently one of the most diverse and abundant genera at a given site. For 37 species from Panama and Peru we characterized developmental, ant, and chemical defenses against herbivores. We found extensive variation in defenses, but little evidence of phylogenetic signal. Furthermore, in a multivariate analysis, developmental, ant, and chemical defenses varied independently (were orthogonal) and appear to have evolved independently of each other. Our results are consistent with strong selection for divergent defensive traits, presumably mediated by herbivores. In an analysis of community assembly, we found that Inga species co-occurring as neighbors are more different in antiherbivore defenses than random, suggesting that possessing a rare defense phenotype increases fitness. These results imply that interactions with herbivores may be an important axis of niche differentiation that permits the coexistence of many species of Inga within a single site. Interactions between plants and their herbivores likely play a key role in the generation and maintenance of the conspicuously high plant diversity in the tropics.
Keywords: plant defenses, community assembly, phylogenetic signal, herbivory, tropical diversity
Because plants and herbivores constitute more than half of the macroscopic diversity on Earth, their interactions play a fundamental role in biodiversity and ecosystem function. Two central issues are how defenses against herbivores have evolved and how such variation in defenses among species might regulate plant community composition (1–6). Here, we use a phylogenetic approach to investigate the diversification of antiherbivore defenses and their role in community assembly.
Several paradigms dominate our understanding of plant/herbivore macroevolution. Ehrlich and Raven (7) observed that related plants host similar herbivores and suggested that plant–herbivore coevolution is driven by changes in plant secondary metabolites. Their hypothesis predicts that more closely related species should have more similar chemistry (8, 9). An alternative hypothesis is that natural selection caused by herbivores may result in rapid trait evolution such that closely related species have divergent defenses. Another distinct, and widely accepted, proposition suggests that species coexisting at a single site are likely to differ in key ecological niche dimensions (10, 11).
Here, we examine the diversity of antiherbivores defenses, their phylogenetic signal, and their contribution to species coexistence in the tree genus Inga (Fabaceae: Mimosoideae). Inga diversified rapidly during the last 2 million to 10 million years and now has >300 species distributed throughout the Neotropics (12, 13). Furthermore, Inga is one of the most species-rich and abundant genera in local communities (14) with 43 species comprising 6.0% of the stems recorded from 25 ha in Ecuador (15). In 50 ha in Panama, Inga comprises 7% of all tree species (16).
We measured antiherbivore defenses in Inga for 12 species in Panama and 31 species in Peru (six species are shared). We focused our study on the defenses of young, expanding leaves because their rate of damage is ≈100-fold higher than for mature leaves and they receive >80% of the damage accrued throughout the lifetime of a leaf (17, 18). During the very short period (1–3 weeks) of leaf expansion in the Inga study species, 25% of the leaf area was eaten in Panama and 37% was eaten in Peru (Fig. 1A). Therefore, we argue that the defense traits under the strongest natural selection are those of young leaves. Chemical defenses are likely important for young leaves as Inga species invest up to 50% dry weight (DW) of young leaves in secondary metabolites (19). Young leaves are also defended against herbivory through developmental defenses such as rapid leaf expansion or delayed chloroplast development (20–23). Finally, Inga leaves have extrafloral nectaries that produce nectar and attract leaf-defending ants only during leaf expansion (Fig. 1B and refs. 24–26). Thus, we measured the following defensive traits on young leaves: the presence or absence of chemical compounds from classes that are relevant to antiherbivore defense, the rate of leaf expansion, chloroplast development (chlorophyll content), and the average number of ants per extrafloral nectary.
Fig. 1.
Young leaves of I. poeppigiana being damaged by a hemipteran (Left; photo by Robyn Burnham) and young leaves of I. thibaudiana with extrafloral nectaries being visited by the ant Ectatomma (Right; photo by Tania Brenes-Arguedes).
The primary goal of this study is to determine how interspecific variation in defenses may contribute to our understanding of the origin and maintenance of tropical diversity. Therefore, we also constructed a phylogenetic hypothesis for all of the focal species by using plastid DNA sequence data. We also assessed coexistence in the field by using data on the abundance and richness of Inga from the 50-ha forest dynamics plot in Panama and a network of tree plots in southern Peru. These data allowed us to test the above-mentioned hypotheses that pertain to defense evolution (7–9) and community assembly (10, 11) by asking whether or not (i) defense traits are phylogenetically conserved and (ii) coexisting species differ more in defense strategies than would be expected by chance.
Results and Discussion
Chemical defenses vary considerably among Inga species and include compounds belonging to several distinct classes that are known for their antiherbivore effects (27). Secondary metabolites include phenolics, saponins and nonprotein amino/imino acids, and an overexpressed protein amino acid, tyrosine (28–30). Twelve types of metabolites were identified that arise from branch points in the shikimic acid, phenylpropanoid and flavonoid pathways (Fig. S1; referred to as “phenolics”). These include quinic, gallic, and cinnamic acid derivatives and tyrosine and tyramine, either in pure or conjugated form. The most common and widespread phenolics were the flavan-3-ols, the polymeric forms of which are called condensed tannins.
Thirty-two species contained one or more forms of phenolic metabolite; 12 species contained saponins. In all, the 37 study species were distributed among 13 distinct chemotypes, each a unique combination of phenolics and saponins (Table S1). The most common chemotype, polymers of the flavan-3-ol, gallocatechin-epigallocatechin gallate, was shared by 11 species. In addition to Inga, this class of metabolites is known from at least two genera of mimosoid legumes Stryphnodendron (31) and Cojoba (Table S2), which suggests that it may be the ancestral chemical defense in the genus. All other chemotypes were considerably less common: nine of the 13 chemotypes were represented by only one or two species (Table S1).
Eleven species from Panama were also examined for protein/nonprotein amino/imino acid content, a class characteristic of legumes (32). There were 17 different compounds (Table S1), 11 of which are derived from five different amino acid precursors (33), with the remainder being uncharacterized. No two species had the same suite of compounds.
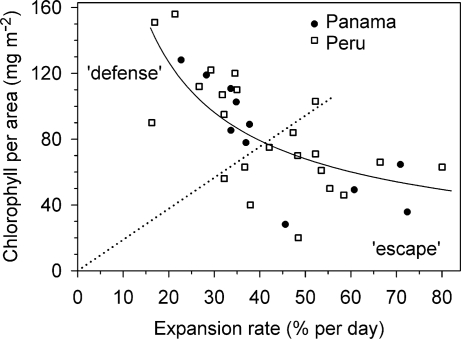
The rate of leaf expansion, a key developmental defense, determines how long a leaf is tender and vulnerable to herbivores. This rate varied widely among species, ranging from 16% to 80% per day (Fig. 2), equivalent to the variation among all non-Inga species sampled (34, 35). The chlorophyll content of young leaves also varied (Fig. 2), with some species having almost white young leaves (20 mg·m−2) and others with normal greening (156 mg·m−2). There was a significant negative relationship between expansion and chlorophyll within Inga (Fig. 2), which was true for each site independently (Panama, r2 = 0.71, P < 0.001; Peru, r2 = 0.41, P < 0.001) and all species together (r2 = 0.50, P < 0.001).
Fig. 2.
The rate of expansion of young leaves expressed as the percentage increase in area per day (% per day) versus the chlorophyll content (mg·m−2) for Inga species from Panama (n = 11) and Peru (n = 23). For all species combined, there is a significant negative relationship [exp = 971 × (chl−0.68); r2 = 0.50, P < 0.0001]. The dotted diagonal line separates species into two equal-sized groups designated as defense and escape.
To test whether the correlations between expansion and chlorophyll could be caused by convergence, we examined their phylogenetically independent contrasts (PICs) (36). There was a significant negative correlation between PICs for the two traits (across 200 randomly selected Bayesian trees: r̄2 = 0.37, P̄ = 0.0001, P < 0.05 for 99% of trees). We have argued that this widespread tradeoff may be caused by an unavoidable physiological constraint that restricts simultaneous allocation of resources to both rapid expansion and the costly photosynthetic system (21–23). A second, although not mutually exclusive, explanation is that this association could arise because of pleiotropic effects.
Ant abundance, a biotic defense, also varied among Inga species. Ant visitation to the extrafloral nectaries of young leaves differed 20-fold among species and on average was 2.3 times higher in Peru compared with Panama (t test, P < 0.001; Fig. S2). We tested the hypothesis that ant visitation could be associated with developmental defenses. Using nonphylogenetic regressions and PICs, we found no significant correlation between expansion rates and ant visitation (r2 = 0.01, P = 0.72, Fig. S2; PICs: r̄2 = 0.01, P̄ = 0.48) or between chlorophyll and ant visitation (r2 = 0.01, P = 0.51; PICs: r̄2 = 0.01, P̄ = 0.45).
The chemical, nectary, and leaf-development traits discussed above vary considerably among species. The broad distribution of traits seen in Inga could result from two quite distinct evolutionary trajectories. The species with dissimilar defenses could be the most phylogenetically distant (high phylogenetic signal), a pattern consistent with Ehrlich and Raven's (7) view of plant–herbivore macroevolution. Alternatively, close relatives may have divergent defenses (low phylogenetic signal), an unexpected pattern that would suggest distinct macroevolutionary processes.
Are More Closely Related Species More Similar in Their Defenses?
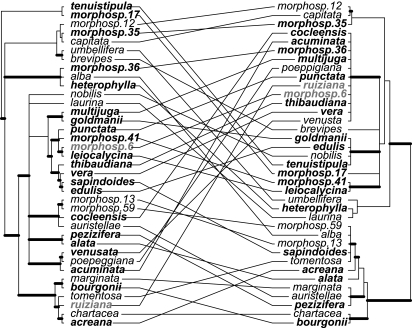
The assumption that more closely related species have similar defenses has dominated coevolutionary theory (7). However, within the genus Inga, we inferred that defense strategy shows little phylogenetic signal. Our phylogenetic hypothesis for Inga species, based on ≈6,000 bp of plastid DNA sequence and inferred by using Bayesian approaches, resolves several clades with strong support (posterior probabilities ≥95%; Fig. 3). This phylogenetic tree shows more resolution than that of Richardson et al. (13), who sampled fewer Inga species and used only ≈1,500 bp of DNA sequence.
Fig. 3.
Phylogenetic tree (Left; 50% majority rule consensus tree from Bayesian analysis of 6,000 bps of plastid DNA) and chemistry dendrogram (Right) for Inga species. Thickened branches on the phylogeny represent >0.95 posterior probability for the adjacent node. The chemistry dendrogram was generated by using hierarchical clustering of presence/absence data for 13 defense chemicals (phenolics and saponins, weighted equally). Thickened branches are adjacent to nodes with P < 0.1 according to multiscale bootstrapping analysis (approximately >90% bootstrap support; see SI Text). Species in bold are classified as defense (see Fig. 2), and other species are classified as escape. Gray species are unclassified because of lack of data.
We quantified the chemical distances between species based on the number of compounds for which species differed (see Materials and Methods). We found a weak correlation between phylogenetic distance and chemical distance between species, based on combining data on saponin and phenolic compounds and weighting them equally (Mantel Tests across 200 Bayesian trees, r̄ = 0.13, P̄ = 0.041, P < 0.05 for 70% of trees; Fig. S3). The weak phylogenetic signal for total chemical distance derives from moderate similarity in phenolic chemistry of closely related species (Mantel Tests, r̄ = 0.23, P̄ = 0.021, P < 0.05 for 95% of trees). There was no relationship between phylogenetic distance and saponin chemical distance (Mantel Tests, r̄ = 0.03, P̄ = 0.31), and for Panamanian species, there was no relationship between phylogenetic and amino/imino acid chemical distances (Mantel Tests, r̄ = −0.04, P̄ = 0.51).
We also compared the phylogeny with a dendrogram of the chemical similarities among species of Inga (Fig. 3). Inspection of Fig. 3 showed weak congruence. Some closely related species (I. capitata, I. morphosp.12 and I. morphosp.35) shared similar chemistry, but more frequently, close relatives were chemically dissimilar. For example, the clade containing I. umbellifera, I. brevipes, I. morphosp.36, and I. heterophylla exhibited chemical profiles that spanned the entire chemical space. For the five species having only saponins, two, I. morphosp.59 and I. morphosp.13, are sister in the phylogeny, whereas the other three species, I. alba, I. sapindoides, and I. tomentosa, are placed in three other clades. We conclude that, for Inga, related species are more divergent in their secondary metabolite composition than expected by chance.
To test for a phylogenetic signal for developmental and ant defenses, we first conducted a principal component analysis (PCA) to determine the orthogonality of these two proposed defense strategies. The first axis was highly correlated with expansion rate and chlorophyll content (development), whereas the second axis was highly correlated with ant visitation rates. The first axis showed significant phylogenetic signal (Blomberg's K across 200 Bayesian trees: K̄ = 0.69, P̄ = 0.04, P < 0.05 for 73% of trees) (37), although still lower than the expected value of one under pure Brownian evolution. The second axis showed no detectable phylogenetic signal (K̄ = 0.42, P̄ = 0.87). To further visualize the evolution of these characters, we classified species into the conventional categories of “escape” vs. “defense” (35) based on species' coordinates in Fig. 2 and as having high vs. low ant visitation. These binary characters showed no detectable phylogenetic signal when mapped onto 100 Bayesian trees sampled at stationarity (Fig. S4) (ref. 38 and http://mesquiteproject.org). We also show in Fig. 3 how species were classified with respect to escape vs. defense. Hence, we found a significant phylogenetic signal only for developmental traits and only in the case when we treated these defenses as quantitative traits and analyzed them by PCA.
We also assessed Mantel correlations of the chemical distances between species with the differences between species in the values along each of the two principal component axes representing developmental and ant defense. Neither was correlated with chemistry (first axis: Mantel r = −0.03, P = 0.49; second axis: Mantel r = −0.01, P = 0.85). These results and the PCAs suggest that developmental, ant, and chemical profiles are independent defensive strategies that also have evolved independently of one another. Species in the cotton clade also showed independent evolution of ant and chemical defenses (39).
We have sampled only 37 species of Inga, and further sampling could result in subclades showing more uniformity in defensive strategies. However, that is unlikely for several reasons. First, our sampling spans the taxonomic diversity of the genus, representing 9 of 14 sections recognized by Pennington (12). Second, some of the species included are definitively shown to be closely related but chemically divergent. For example, two species pairs show a profound lack of sequence divergence in the rapidly evolving plastid regions we have used. These are I. goldmanii and I. multijuga, identical for ≈6,000 bp of plastid DNA, and I. leiocalycina and I. morphosp.6, differing by two substitutions in 6,000 bp; and they nevertheless show contrasting chemical defenses (Fig. 3).
Although there is a significant association of developmental defense strategy with phylogeny, we find little evidence for phylogenetic signal in the other, independent defense traits, ants, and chemistry. Our data show both extensive variation in defense traits and considerable differences among close relatives, a pattern that is consistent with defense strategies in Inga being evolutionarily labile (40). This finding contrasts with the prevailing hypothesis [Ehrlich and Raven (7)], which implies that closely related plant species should be similar in defense strategies. Few studies have examined defenses across many related species, although terpenes in the genus Bursera (Burseraceae) showed low congruence between a chemical dendrogram and a phylogeny (3).
Coexistence and Defense Trait Similarity.
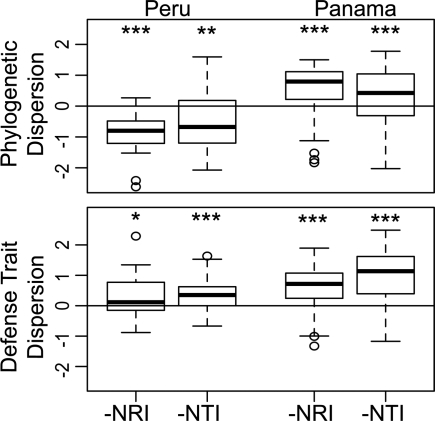
Could antiherbivore defenses be an important mechanism of niche partitioning in community assembly? To address this question, we assessed phylogenetic and defense trait dispersion in Inga communities. We used the inverse of the metrics of Webb (41) so that positive values represent overdispersion and negative values represent underdispersion. In the 50-ha forest dynamics plot in Panama, we found that co-occurring Inga species are more distantly related than expected by chance (overdispersion; Fig. 4; across 200 Bayesian trees, two-tailed t tests: N̄RI = 0.68, t̄ = 15.83, P̄ < 0.00001, P < 0.05 for 100% of trees; N̄TI = 0.50, t̄ = 7.83, P̄ = 0.005, P < 0.05 for 99% trees). In contrast, in Peru we found phylogenetic underdispersion of Inga communities (Fig. 4; two-tailed t tests: N̄RI = −0.66, t̄ = −5.08, P̄ < 0.0002, P < 0.05 for 100% of trees; N̄TI = −0.50, t̄ = −3.15, P̄ = 0.021, P < 0.05 for 91% trees). This site difference likely reflects the different spatial and environmental scales that were sampled. In Panama, we assessed co-occurrence within the homogenous habitat of the 50-ha plot (local spatial scale). In Peru, we sampled across a 150-×-200-km area (regional spatial scale) and in two different habitat types, terra firme and floodplain forest (42). We suggest that a phylogenetic signal for traits associated with habitat preference (cf. ref. 41) may underlie the contrasting phylogenetic structures.
Fig. 4.
Phylogenetic and defense trait dispersion for Inga communities in Peru and Panama. Phylogenetic results are for one, randomly selected Bayesian tree. Values given are the inverse of the net relatedness index (NRI) and nearest taxon index (NTI) following Webb (41). Values > 0 indicate overdispersion, and values < 0 indicate underdispersion. The departure of communities from the null expectation (zero) of communities being randomly assembled was evaluated by using two-sided t tests for phylogenetic dispersion and one-sided t tests for defense trait dispersion (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
At both spatial scales, including Peru where neighbors were close relatives, the co-occurring species were more dissimilar than expected at random for a defense index combining the developmental, ant, and chemical traits (see Materials and Methods; Fig. 4; Peru: one-tailed t test: N̄RI = 0.32, t = 2.26, P = 0.016; N̄TI = 0.40, t = 3.56, P = 0.0007; Panama: one-tailed t test: N̄RI = 0.60, t = 13.75, P < 0.00001; N̄TI = 1.06, t = 17.35, P < 0.00001). We also assessed the dispersion of two leaf traits that are likely unrelated to herbivore defense, the presence vs. absence of wings on the rachis and the number of leaflets per leaf. We found no evidence for overdispersion of leaf traits in Panama (one-tailed t test: N̄RI = −0.68, t = −8.96, P < 0.00001; N̄TI = −0.05, t = −0.74, P = 0.46) or Peru (N̄RI = 0.29, t = 1.33, P = 0.196; N̄TI = −0.04, t = −0.16, P = 0.87).
These results illustrate that, although nondefense traits such as leaflet number and presence of wings are randomly or underdispersed, defensive traits at both sites are overdispersed. Divergence in chemical defenses at single sites has also been shown for another speciose tree genus, Bursera (4). The co-occurrence of species having divergent defense strategies could be caused by herbivores preferentially foraging on individuals with common defense phenotypes. By such a mechanism, herbivores may structure the assembly of rainforest tree communities.
Conclusions
Our analysis of defenses is consistent with the idea that, in Inga, the arms race between plants and their herbivores has led to rapid and divergent trait evolution. The young leaves of Inga make substantial investments in chemical defenses [up to 50% of DW (19)] and developmental defenses and extrafloral nectar, suggesting that herbivory is a strong selective agent. The chemical, biotic, and developmental defenses of young leaves showed considerable variation among species. The observations of Ehrlich and Raven (7) suggest that defenses evolve by small changes (8, 9) and low trait divergence also might be predicted from the recent radiation of Inga (13). Instead we found a weak or no correlation of phylogeny with defensive traits. These results suggest divergent selection on, and rapid evolution of, antiherbivore defenses in Inga (40) and thus are consistent with the recognized importance of the interactions between hosts and natural enemies in driving diversifying evolution in both plants and animals (43).
We also argue that divergent defenses could be a principal mechanism structuring community assembly. Most species of Inga are restricted to similar habitats and may vary little in resource use; they also have similar flower and fruit morphologies (12), thus suggesting similar pollinator and dispersal syndromes. Therefore, the high number of species of Inga, up to 43, that coexist at a single site (15) presents an enigma. We found that, regardless of whether the co-occurring species of Inga are less related (Panama) or more closely related (Peru) than expected by chance, the assemblage of Inga at a single site differs more in defense strategy than random. In addition, most lepidopteran herbivores specialize on a subset of the species of Inga present at a given site (44). Therefore, for Inga species, high local abundance and diversity may be caused by antagonistic, density-dependent interactions with these specialized natural enemies. Thus, niche differentiation may occur via differences in antiherbivore defenses, rather than differences in resource use, pollination, or dispersal.
Are there parallels with other genera? A striking pattern in the tropics is the disproportionate contribution to total diversity made by genera having 100 or more species, such as Eugenia, Miconia, Piper, Pouteria, and Psychotria. Many such genera also have high local abundance and diversity (14–16). For example, in 25 ha in Ecuador 10.7% of the species, 17% of the basal area, and 17% of the stems are contributed by only three genera (15). In most speciose genera, as with Inga, the congeners appear to have similar functional morphologies, reproductive syndromes and habitat preferences, raising yet again the enigmatic issue of coexistence. Perhaps interactions with natural enemies will be key to understanding the maintenance of diversity in the tropics.
It also is unclear what ecological processes have driven speciation in the large genera typical of tropical rainforests. We argue that Inga's radiation seems unlikely to result from adapting to differences in the abiotic environment, pollinators, or seed dispersers. Instead, we suggest that the Inga radiation was driven by interactions with natural enemies (6) through the diversification of antiherbivore defenses. Thus, although links between herbivore pressure and host speciation in tropical forests are tentative, our hypothesis for rapid, divergent selection on antiherbivore defenses also may explain the radiations in Inga and other large genera in the tropics (6).
Materials and Methods
Study Sites.
Data were collected in Panama and Peru. Barro Colorado Island (BCI) is a field station administered by the Smithsonian Tropical Research Institute in the Republic of Panama (9° N, 79° W). The average total yearly rainfall is 2,600 mm, 90% of which falls during the rainy season from May through December, and the average daily temperature is 27 °C. The vegetation is moist-lowland forest (45). We used 0.25-ha subplots of the 50-ha forest dynamics plot on BCI to provide Inga community composition data for Panama (46). The second field site was at Los Amigos Biological Station in Peru and is operated by the Amazon Conservation Association (13° S, 70° W). The site is ≈250 m above mean sea level, receives 2,600 mm of rainfall annually, and averages 24 °C. The forest is lowland rainforest and encompasses terra firme (upland) forest and seasonally flooded forest. We used a network of 0.25-ha Inga plots across the department of Madre de Dios (in which Los Amigos is located and where two surveys were located) to provide community composition data for Peru (42). We worked on the most common Inga species present at each site. We collected data on 12 species from Panama and 31 from Peru. Six species occurred in both Panama and Peru, based on similar morphology and DNA sequences. I. laurina, I. nobilis, I. ruiziana, and I. sapindoides had similar secondary metabolite profiles, whereas the secondary metabolites of I. marginata and I. umbellifera, although similar, were not identical between sites.
Sample Collection.
Ecological and defense trait data were taken for young leaves on understory saplings. Rates of young-leaf expansion were calculated as the percentage increase in area per day for leaves between 20% and 80% of full size. The number of ants visiting extrafloral nectaries of young leaves was counted (number of ants per nectary) during censuses along trails between 10 AM and 3 PM. Chlorophyll content (mg·m−2) was determined for young leaves estimated to be between 60% and 80% of full size. See SI Text for detailed methods and Table S3 for species values.
Chemical Analyses.
Leaves collected for chemical analysis were processed and stored on site in Peru and Panama before shipment to Utah (see SI Text for details). An Inga-specific fractionation protocol (Fig. S5) developed in the lab at the University of Utah produced seven chemically distinct (19, 47) fractions: lipids, phenolics, saponins, amino acids, organic acids, proteins, and the insoluble parts of the cell walls (marc). Two of these, phenolics and saponins, either have been shown to account for the great majority of the deterrent activity in Inga extracts (19, 47) or are presumed to be deterrent based on their mass abundance and the literature (27). The phenolic fraction, when present, was analyzed in detail for chemical content. Analysis was by HPLC with detection by photo-diode array (for UV-absorbing metabolites) and evaporative light-scattering (ELS) or electrospray ionization (ESI) MS. Based on earlier work with Inga defense chemistry (19, 28–30, 47), we were in many cases able to identify whole classes of compounds (e.g., catechin/epicatechin polymers) solely by their UV absorption and mass spectra. Where this was not possible, the structures of unknowns were solved explicitly by NMR and high-resolution MS (28–30, 47). For a complete description of phenolic chemical structures, see Fig. S1. Each species was classified according to chemotypes that were based on the class of phenolic metabolite and the presence/absence of saponins when either one or both were present. If either phenolics or saponins was 5% or less of the total active fraction (as estimated by ELS detection), it was omitted from the analysis. Ten species from Panama were analyzed by HPLC-ESI-MS for protein/nonprotein amino/imino acid content. Metabolites having primary or secondary amines were first derivatized (48). Tertiary amines were analyzed directly. One other Panama species, I. laurina, was analyzed by gas chromatography after derivatization (49). Several individuals from each species were analyzed separately and, in all cases, chemical profiles based on presence/absence of compounds were identical within a species.
Phylogenetic Reconstruction.
Samples for DNA were dried in silica gel, and sterile and fertile herbarium vouchers were identified by using the taxonomic monograph of Inga (12) and verified by its author T.D. Pennington (Royal Botanic Gardens, Kew; Table S4). Total genomic DNA extraction followed Richardson et al. (13). Six plastid DNA regions were sequenced: trnLF (50), trnD-T (47, 51) and additional internal sequencing primers designed for this study: psbA-trnH (52, 53), rps16 (54), rpoC1 (55), and ndhF-rpl32 (56). All primer sequences plus PCR and sequencing protocols are in SI Text.
Bayesian analysis was performed by using MrBayes 3.1.1 (57) with 5,000,000 generations of four simultaneous MCMC chains, sampling one tree every 10,000 generations. MrModelTest 3.7 (58) was used to select the best-fitting substitution model for each plastid region. Phylogenetic trees were rooted by using outgroup sequences from Zygia, which is shown to be most closely related to Inga in phylogenetic analyses using multiple genera from tribe Ingeae.
We assessed correlations between expansion rate, chlorophyll content, and ant visitation by using linear regressions and PICs (36). We evaluated chemical distances between species as the number of compounds for which species have a different state (presence vs. absence), standardized by the maximum observed distance. We assessed the correlation between chemical and phylogenetic distance by using Mantel tests. The chemistry dendrogram (Fig. 3) was constructed by using hierarchical clustering (59). We conducted a PCA on continuous trait data to derive independent axes of defense trait variation. We evaluated phylogenetic signal for the first two axes (the two axes that showed eigenvalues >1) by using analyses of Blomberg's K (37). We evaluated a possible evolutionary relationship between chemical defense strategy and these axes by using a Mantel test relating chemical distance to differences between species along the first two axes.
We evaluated the distance between species in defense traits as the average of distances for the different chemical classes and for distances between species in escape/defense (developmental) strategy and ant visitation rates. We standardized each chemical or other defense class or axis to vary from 0 and 1, such that each was weighted equally in the total defense trait analyses. We evaluated the distance between species in leaf traits (wings and leaflet number) as the average distance between species along two axes derived from a principal component analysis of the leaf traits. All analyses were conducted in the R statistical environment (R Core Development Team 2009), and details can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank E. Leigh for stimulating discussions and O. Acevedo and B. Jimenez for moral and logistical support at the Smithsonian Tropical Research Institute; K. Rudolph, K. Bromberg, D. Dvorett, S. Ring, B. Wolfe, and M.-J. Epps for field assistance in Panama; N. Pitman for intellectual input and J. Saldaña, C. Lazo, and F. Para for field work in Peru; officials from Autoridad Nacional del Ambiente of Panama and Instituto Nacional de Recursos Naturales of Peru for permission to conduct field work; S. Lee and D. Grapov for assistance with the chemical characterizations at the University of Utah; officials at the Center for Tropical Forest Science of the Smithsonian Tropical Research Institute for use of the data from the 50-ha plot (http://ctfs.si.edu/datasets/bci); and M. Lavin, M. Lemes, M. Hollingsworth, and A. Clark for phylogenetic research. K.G.D. was supported by National Science Foundation Grant DDIG-0608368 and grants from the Amazon Conservation Association, American Philosophical Society, Duke University, Explorer's Club, Society for Systematic Biology, and Sigma Xi. T.A.K. and P.D.C. were supported by National Science Foundation Grants DEB-0234936 and DEB-0640630 and Research Experience for Undergraduate supplements. R.T.P. was supported by Leverhulme Trust Study Abroad Fellowship RF/2/2006/0142.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.A.A. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. FJ974145, FJ974151, FJ974164, FJ974180, FJ974241, FJ974279, FJ974351, FJ974409, FJ974438, FJ974449, FJ974509, FJ974628, FJ974673, FJ974683, FJ974713, FJ974870, FJ974883, FJ974910, FJ974975, FJ974988, FJ975003, FJ975011, FJ975039, GQ118709–GQ118890, GQ871263–GQ871278, and GQ892055).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904786106/DCSupplemental.
References
- 1.Agrawal AA. Macroevolution of plant defense strategies. Trends Ecol Evol. 2007;22:103–109. doi: 10.1016/j.tree.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S149. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Becerra JX. Insects on plants: Macroevolutionary chemical trends in host use. Science. 1997;276:253–256. doi: 10.1126/science.276.5310.253. [DOI] [PubMed] [Google Scholar]
- 4.Becerra JX. The impact of herbivore–plant coevolution on plant community structure. Proc Natl Acad Sci USA. 2007;104:7483–7488. doi: 10.1073/pnas.0608253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell BD, Dussourd DE, Mitter C. Escalation of plant defense: Do latex and resin canals spur plant diversification? Am Nat. 1991;138:881–900. [Google Scholar]
- 6.Schemske DW. In: Foundations of Tropical Forest Biology: Classic Papers with Commentaries. Chazdon RL, Whitmore TC, editors. Chicago: Univ Chicago Press; 2002. pp. 163–173. [Google Scholar]
- 7.Ehrlich PR, Raven PH. Butterflies and plants: A study in plant coevolution. Evolution (Lawrence, Kans) 1964;18:586–608. [Google Scholar]
- 8.Berenbaum MR, Feeny P. Toxicity of angular furanocoumarins to swallowtail butterflies: Escalation in a coevolutionary arms race? Science. 1981;212:927–929. doi: 10.1126/science.212.4497.927. [DOI] [PubMed] [Google Scholar]
- 9.Berenbaum MR, Zangerl AR. Chemical phenotype matching between a plant and its insect herbivore. Proc Natl Acad Sci USA. 1998;95:13743–13748. doi: 10.1073/pnas.95.23.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. Am Nat. 2004;163:823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- 11.Webb CO, Gilbert GS, Donoghue MJ. Phylodiversity-dependent seed mortality, size structure, and disease in a Bornean rain forest. Ecology. 2006;87:S123–S131. doi: 10.1890/0012-9658(2006)87[123:psmssa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Pennington TD. The Genus Inga. London: Royal Botanic Gardens; 1997. [Google Scholar]
- 13.Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science. 2001;293:2242–2245. doi: 10.1126/science.1061421. [DOI] [PubMed] [Google Scholar]
- 14.Valencia R, Balslev H, Miño GP. High tree α diversity in Amazonian Ecuador. Biodiver Conserv. 1994;3:21–28. [Google Scholar]
- 15.Valencia R, et al. In: Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network. Losos EC, Leigh EG Jr, editors. Chicago: Univ Chicago Press; 2004. pp. 609–628. [Google Scholar]
- 16.Hubbell SP, Foster RB, Condit R, Lao S, Perez R. Demographic Tree Data from the 50-ha Barro Colorado Island Forest Dynamics Plot, 1982–1995. Washington, DC: Smithsonian Institution; 1995. CTFS Forest Dynamic Plot Data Series, CD-ROM. [Google Scholar]
- 17.Brenes-Arguedas T, et al. Contrasting mechanisms of secondary metabolite accumulation during leaf development in two tropical tree species with different leaf expansion strategies. Oecologia. 2006;149:91–100. doi: 10.1007/s00442-006-0423-2. [DOI] [PubMed] [Google Scholar]
- 18.Coley PD, Aide TM. In: Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Price PW, Lewinsohn TM, Fernandes WW, Benson WW, editors. New York: Wiley; 1991. pp. 25–49. [Google Scholar]
- 19.Lokvam J, Kursar TA. Divergence in structure and function of young leaf chemical defenses in two co-occurring Inga species. J Chem Ecol. 2005;31:2563–2580. doi: 10.1007/s10886-005-7614-x. [DOI] [PubMed] [Google Scholar]
- 20.Kursar TA, Coley PD. Nitrogen content and expansion rate of young leaves of rainforest species: Implications for herbivory. Biotropica. 1991;123:141–150. [Google Scholar]
- 21.Kursar TA, Coley PD. Delayed development of the photosynthetic apparatus in tropical rainforest species. Func Ecol. 1992;6:411–422. [Google Scholar]
- 22.Kursar TA, Coley PD. The consequences of delayed greening during leaf development for light absorption and ligh use efficiency. Plant Cell Environ. 1992;15:901–909. [Google Scholar]
- 23.Kursar TA, Coley PD. Delayed greening in tropical leaves: An antiherbivore defense? Biotropica. 1992;24:256–262. [Google Scholar]
- 24.Brenes-Arguedas T, Coley PD, Kursar TA. Divergence in the chemical ecology of Inga between two neotropical sites. J Ecol. 2008;96:127–135. [Google Scholar]
- 25.Koptur S. Experimental evidence for defense of Inga (Mimosoideae) saplings by ants. Ecology. 1984;65:1787–1793. [Google Scholar]
- 26.Koptur S. Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology. 1985;66:1639–1650. [Google Scholar]
- 27.Seigler DS. Plant Secondary Metabolism. Boston: Kluwer; 1998. [Google Scholar]
- 28.Lokvam J, Brenes-Arguedas T, Lee JS, Coley PD, Kursar TA. Allelochemic function for a primary metabolite: The case of l-tyrosine hyper-production in Inga umbellifera (Fabaceae) Am J Bot. 2006;93:1109–1115. doi: 10.3732/ajb.93.8.1109. [DOI] [PubMed] [Google Scholar]
- 29.Lokvam J, Clausen TP, Grapov D, Coley PD, Kursar TA. Galloyl depsides of tyrosine from young leaves of Inga laurina. J Nat Prod. 2007;70:134–136. doi: 10.1021/np060491m. [DOI] [PubMed] [Google Scholar]
- 30.Lokvam J, Coley PD, Kursar TA. Cinnamoyl glucosides of catechin and dimeric procyanidins from young leaves of Inga umbellifera (Fabaceae) Phytochemistry. 2004;65:351–358. doi: 10.1016/j.phytochem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Palazzo de Mello J, Petereit F, Nahrstedt A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry. 1996;41:807–813. [Google Scholar]
- 32.Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64:3–19. doi: 10.1016/s0031-9422(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 33.Romeo JT. Functional multiplicity among nonprotein amino acids in Mimosoid legumes: A case against redundancy. Ecoscience. 1998;5:287–294. [Google Scholar]
- 34.Coley PD, Kursar TA. In: Tropical Forest Plant Ecophysiology. Mulkey SS, Chazdon R, Smith AP, editors. New York: Chapman & Hall; 1996. pp. 305–336. [Google Scholar]
- 35.Kursar TA, Coley PD. Convergence in defense syndromes of young leaves in tropical rainforests. Biochem Syst Ecol. 2003;21:929–949. [Google Scholar]
- 36.Garland T, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
- 37.Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution (Lawrence, Kans) 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 38.Maddison W, Maddison D. Mesquite: A Modular System for Evolutionary Analysis. Austin, TX: Mesquite Software; 2007. version 2.01. [Google Scholar]
- 39.Rudgers JA, Strauss SY, Wendel JE. Tradeoffs among antiherbivore resistance traits: Insights from Gossypieae (Malvaceae) Am J Bot. 2004;91:871–880. doi: 10.3732/ajb.91.6.871. [DOI] [PubMed] [Google Scholar]
- 40.Revell LJ, Harmon LJ, Collar DC. Phylogenetic signal, evolutionary process, and rate. Syst Biol. 2008;57:591–601. doi: 10.1080/10635150802302427. [DOI] [PubMed] [Google Scholar]
- 41.Webb CO. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am Nat. 2000;156:145–155. doi: 10.1086/303378. [DOI] [PubMed] [Google Scholar]
- 42.Dexter KG. Durham, NC: Duke University; 2008. The effects of dispersal on macroecological patterns. PhD Thesis. [Google Scholar]
- 43.Brockhurst MA. Ecology: Death and destruction determine diversity. Curr Biol. 2007;17:R512–R514. doi: 10.1016/j.cub.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Kursar TA, Wolfe BT, Epps MJ, Coley PD. Food quality, competition, and parasitism influence feeding preference in a neotropical lepidopteran. Ecology. 2006;87:3058–3069. doi: 10.1890/0012-9658(2006)87[3058:fqcapi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Leigh EG. Tropical Forest Ecology: A View from Barro Colorado Island. New York: Oxford Univ Press; 1999. [Google Scholar]
- 46.Hubbell SP, Condit R, Foster RB. Barro Colorado Forest Census Plot Data. Panama: Smithsonian Tropical Research Institute; 2005. [Google Scholar]
- 47.Coley PD, et al. Divergent defensive strategies of young leaves in two neotropical species of Inga. Ecology. 2004;86:2633–2643. [Google Scholar]
- 48.Tapuhi Y, Schmidt DE, Lindner W, Karger BL. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem. 1981;115:123–129. doi: 10.1016/0003-2697(81)90534-0. [DOI] [PubMed] [Google Scholar]
- 49.Stalling DL, Gehrke CW, Zumwalt RW. A new silylation reagent for amino acids bis(trimethylsilyl)trifluoroacetamide (BSTFA) Biochem Biophys Res Commun. 1968;31:616–622. doi: 10.1016/0006-291x(68)90523-8. [DOI] [PubMed] [Google Scholar]
- 50.Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three noncoding regions of chloroplast DNA. Plant Mol Biol. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 51.Grivet D, Heinze B, Vendramin GG, Petit RJ. Genome walking with consensus primers: Application to the large single copy region of chloroplast DNA. Mol Ecol Notes. 2001;1:345–349. [Google Scholar]
- 52.Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae) Am J Bot. 1997;84:1120–1136. [PubMed] [Google Scholar]
- 53.Tate JA, Simpson BB. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst Bot. 2003;28:723–737. [Google Scholar]
- 54.Oxelman B, Liden M, Berglund D. Chloroplast rps16 intron phylogeny of the tribe Sileneae (Caryophyllaceae) Plant Syst Evol. 1997;206:393–410. [Google Scholar]
- 55.Hollingsworth ML, et al. Selecting barcoding loci for plants: Evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Res. 2009;9:439–457. doi: 10.1111/j.1755-0998.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- 56.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am J Bot. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- 57.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 58.Posada D, Crandall KA. Selecting the best-fit model of nucleotide substitution. Syst Biol. 2001;50:580–601. [PubMed] [Google Scholar]
- 59.Everitt BS, Landau S, Leese M. Cluster Analysis. 4th Ed. Florence, KY: Taylor & Francis; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.