Abstract
In functional brain imaging there is controversy over which hemodynamic signal best represents neural activity. Intrinsic signal optical imaging (ISOI) suggests that the best signal is the early darkening observed at wavelengths absorbed preferentially by deoxyhemoglobin (HbR). It is assumed that this darkening or “initial dip” reports local conversion of oxyhemoglobin (HbO) to HbR, i.e., oxygen consumption caused by local neural activity, thus giving the most specific measure of such activity. The blood volume signal, by contrast, is believed to be more delayed and less specific. Here, we used multiwavelength ISOI to simultaneously map oxygenation and blood volume [i.e., total hemoglobin (HbT)] in primary visual cortex (V1) of the alert macaque. We found that the hemodynamic “point spread,” i.e., impulse response to a minimal visual stimulus, was as rapid and retinotopically specific when imaged by using blood volume as when using the initial dip. Quantitative separation of the imaged signal into HbR, HbO, and HbT showed, moreover, that the initial dip was dominated by a fast local increase in HbT, with no increase in HbR. We found only a delayed HbR decrease that was broader in retinotopic spread than HbO or HbT. Further, we show that the multiphasic time course of typical ISOI signals and the strength of the initial dip may reflect the temporal interplay of monophasic HbO, HbR, and HbT signals. Characterizing the hemodynamic response is important for understanding neurovascular coupling and elucidating the physiological basis of imaging techniques such as fMRI.
Keywords: fMRI, imaging, macaque, visual, neurovascular coupling
Cerebral hemodynamics respond quickly and specifically to local neural activity (1, 2). Hemodynamic signals are thus used extensively as proxies for such activity in functional neuroimaging techniques like fMRI and intrinsic signal optical imaging (ISOI). There has been considerable debate, however, as to which of the possible hemodynamic signals, e.g., changes in local blood oxygenation, volume, or flow, with their distinct response properties, constitutes the “best” signal for inferring neural activity (1, 3–5).
The debate regarding the best signal sharpened with reports of initial dips in both ISOI and fMRI signals. ISOI studies consistently found a brief stimulus-evoked darkening followed by a strong brightening at imaging wavelengths preferentially absorbed in deoxyhemoglobin (HbR) [e.g., at 605 nm (6)]. The darkening, later termed the initial dip*, was interpreted as a local conversion of oxyhemoglobin (HbO) to HbR caused by increased oxygen consumption by local neurons before any active vascular response (1, 7) The subsequent brightening was taken to measure the “rebound” in [HbO]† caused by a delayed, stimulus-triggered increase in cerebral blood flow (7).
In parallel, some fMRI studies reported finding an initial dip before the rise in the blood oxygen level-dependent (BOLD) signal (8). Because the BOLD signal measures changes in [HbR] alone‡, the fMRI initial dip was seen as clear evidence for an initial increase in [HbR]. It was equated to the ISOI dip (8) and was argued to be the best marker of neural activity, free of the venous artifacts seen in positive BOLD (8). By extension, positive BOLD was equated to the ISOI rebound (9). A key implication of these studies was the presence of a significant delay between the start of neural activity-triggered oxygen consumption, believed to underlie the initial dip, and the subsequent active vascular response generating the rebound in fMRI or ISOI (4, 7, 9, 10). The ISOI studies also suggested that the rebound was more spatially diffuse and less stimulus-specific than the initial dip (4, 7, 10). The initial dip thus became the signal of choice in a number of functional imaging studies, not only in ISOI (11–15) but also in fMRI (16).
There are a number of unresolved issues, however, questioning the interpretation of the ISOI initial dip as local deoxygenation preceding the active vascular response. First, the initial dip, while robust and reliable in ISOI, has proved to be elusive in fMRI (3, 9). Next, oxygen consumption preceding increased blood flow should reduce [HbO] in addition to increasing [HbR]. However, even studies proposing an initial deoxygenation failed to see the expected [HbO] decrease (7). Finally, a number of ISOI studies in the rodent that quantitatively separated the imaging signal into [HbR] and [HbO] either failed to see any transient [HbR] increase (17–20) or found a simultaneous rise in [HbO] consistent with a stimulus-evoked increase in total hemoglobin [HbT] (21, 22).
The ISOI dip has been successfully used to map cortical columns (e.g., orientation or ocular dominance) where activated columns darken more than inactive ones. However, despite only a transient initial dip in the global signal, the mapping signal (i.e., the additional darkening in active columns) remains throughout the entire duration of the hemodynamic response despite the rebound observed in the global signal. The mapping signal is typically interpreted as a maintained increase in [HbR] in the activated columns, persisting through the presumed spatially diffuse rebound of oxygenated blood (7, 23). Such a maintained [HbR] increase demands a sustained elevation of local oxygen consumption for many seconds (>10 s) after cessation of neural activity (23). This interpretation is not supported by fMRI. The BOLD mapping signal shows a tuned increase in activated columns, indicating a spatially specific decrease of [HbR], during the rebound (24). The observed orientation-tuned decrease in [HbR] suggests that the prolonged mapping signal is not caused by deoxygenation and, by extension, also raises doubts about the standard interpretation of the initial dip.
These conundrums might be resolved by considering alternate explanations, other than deoxygenation, for the darkening seen at typical imaging wavelengths (e.g., 605 nm). Darkening at these wavelengths may result from a rise in local blood volume, increasing absorption by both HbO and HbR, or even an increase in [HbO] alone. Most hemoglobin in cerebral blood is highly oxygenated [saturation between 95% and 60% (25)] and HbO is a significant absorber at all imaging wavelengths. Even small fractional increases in [HbO] could yield increased absorption. The current study was designed to test these distinct interpretations of the ISOI signals with a focus on the initial dip.
Results
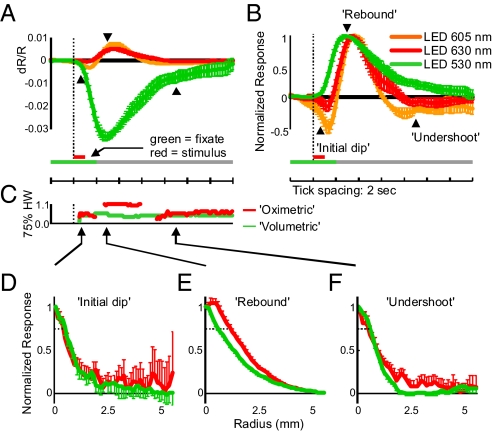
We analyzed stimulus-evoked hemodynamic signals in the brain by using dual-wavelength ISOI in primary visual cortex (V1) of the alert behaving macaque (ref. 26; two animals V and S). With this technique we imaged the hemodynamic point spread, i.e., the impulse response to a minimal visual stimulus (a ¼ deg line) flashed (1 s) while the animal held its gaze steady in a fixation task (4-s fixation periods, trial length 10 s). Stimulated trials alternated with nonstimulated trials (giving 20 s between stimuli for hemodynamic response to return to baseline). The imaging wavelengths were chosen pairwise from three light-emitting diode (LED) sources (Fig. 1A): a “volumetric” LED (green, centered at 530 nm) that was absorbed equally in HbO and HbR giving a measure of [HbT] or blood volume§; and “oximetric” LEDs (orange: center, 605 nm; red, center 630 nm), absorbed more strongly in HbR than HbO, and thus being sensitive to blood oxygenation changes. Consistent with earlier findings, the signals at oximetric wavelengths showed a robust initial dip followed by a rebound and then a delayed undershoot (secondary darkening). Notably, however, volumetric signals started at the same time as the initial dip and showed similar spatial specificity.
Fig. 1.
Optical imaging wavelengths and corresponding pointspreads. (A) In vitro absorbance spectra for HbR and HbO (molar extinction coefficients) along with normalized emission spectra for the three LEDs used. The full LED spectra were used to compute effective absorptions and path-lengths for the three imaging wavelengths (see Methods). (B) Imaged point spreads in one representative session. (Upper) Red: 630-nm (oximetric) LED. (Lower) Green: 530-nm (volumetric) LED. Image frames were averaged over the times in parentheses to capture the peaks of the initial dip, rebound, etc. of the oximetric signal. Note different scale bars to the right of each image.
To get a quantitative measure of the responses as blood oxygenation and volume we used the absorption spectra of HbR and HbO to spectrally decompose the measured imaging signals into [HbO], [HbR], and [HbT]. Notably, we used the Beer-Lambert Law modified to account for the wavelength-dependent optical path lengths in cortical tissue at different imaging wavelengths (see Methods and refs. 17 and 27). Path length correction, although missing from prior work in monkeys and cats, is necessary to avoid errors in the spectral decomposition and interpretation of ISOI signals (17, 27) This quantitative analysis showed that the earliest stimulus-evoked response was driven by a rapid increase in [HbT] with no change in [HbR]. The full multiphasic time course of typical imaging signals, including the initial dip, rebound, and undershoot, could be well explained by a fast increase in [HbT] preceding a more transient decrease in [HbR].
Spatial and Temporal Properties of Absorption Point Spreads Measured at Different Wavelengths.
The point spread at oximetric wavelengths (605 and 630 nm) had a triphasic time course with an initial darkening [i.e., increased absorption; Figs. 1B and 2A: initial dip; 605 nm: peak at 1.5 s, dR/R = 0.31 (0.05)%; 630 nm: peak at 1.3 s, dR/R = 0.05 (0.03)%¶], a subsequent larger decrease in absorption [rebound; 605 nm: peak at 3.7 s, dR/R = 0.69 (0.09)%; 630 nm: peak at 3.6 s, dR/R = 0.49 (0.05)%], and finally a small secondary increase in absorption [undershoot; 605 nm: peak at 8.0 s, dR/R = 0.15 (0.04)%; 630 nm: peak at 8.8 s, dR/R = 0.04 (0.02)%]. Volumetric responses at 530 nm, by contrast, were large and monophasic [peak at 2.9 s, dR/R = 3.3 (0.2) %]. The spatial properties of these signals are summarized in Table 1. For comparison, the 75% half-width of the point spread measured in V1 using voltage-sensitive dyes was ≈0.6 mm (estimated from ref. 28).
Fig. 2.
Imaged point spreads at oximetric and volumetric wavelengths suggest a blood volume contribution to the initial dip. (A) Temporal profiles of signal changes measured at the point spread center, for the three LEDs used. Note the distinct triphasic signal at 605 and 630 nm vs. the monophasic signal at 530 nm averaged over all experiments. Error bars show the SEM. Dotted vertical line indicates stimulus onset. Arrowheads indicate time points used in the analysis of spatial profiles in C–E for the initial dip, rebound, and undershoot. (B) Same data as in A, but scaled to maximum for each session before averaging. Note the much larger initial dip and undershoot observed with 605 nm than with 630 nm. Also note that scaling the image makes it appear artifactually as if the initial dip at 605 and 630 nm starts earlier than changes at 530 nm. (C) Time courses of 75% half-widths for oximetric (red) and volumetric (green) point spreads. Time axis is aligned to A. (D–F) Radial profiles normalized to the center point, for oximetric (red, combining 605 and 630 nm) and volumetric (green, 530 nm) point spreads during the three response phases calculated by averaging the signal on radial annuli after masking off the vasculature. (D) Profiles early into the initial dip (t = 0.8 s) are shown. Note the similar spatial extent of both signals. Dashed lines indicate 75% response level. (E) Same as D, but for the rebound (t = 3 s). Note the much wider oximetric signal with an extended and rounded peak (red) vs. the sharp and narrow profile of the volumetric image (green). (F) Same as D, but for the undershoot (t = 9 s). Note the similarity of point spreads across wavelengths during the initial dip and undershoot.
Table 1.
Half-widths of the hemodynamic response point-spreads over V1
| Time | Oximetric | Volumetric | HbR | HbO | HbT |
|---|---|---|---|---|---|
| 75% half-width (mm) | |||||
| 0.8 s (dip) | 0.48 (0.08) | 0.5 (0.2) | – | 0.5 (0.2) | 0.5 (0.1) |
| 3.0 s (rebound) | 1.15 (0.08) | 0.60 (0.05) | 0.80 (0.03) | 0.66 (0.04) | 0.61 (0.05) |
| 9.0 s (undershoot) | 0.6 (0.2) | 0.57 (0.05) | 0.59 (0.03) | 0.58 (0.04) | 0.58 (0.04) |
| 50% half-width (mm) | |||||
| 0.8 s (dip) | 1.0 (0.4) | 0.73 (0.08) | – | 0.7 (0.1) | 0.74 (0.09) |
| 3.0 s (rebound) | 1.86 (0.09) | 1.35 (0.08) | 1.53 (0.07) | 1.41 (0.07) | 1.37 (0.07) |
| 9.0 s (undershoot) | 0.7 (0.2) | 0.88 (0.05) | 0.94 (0.05) | 0.91 (0.04) | 0.90 (0.04) |
The interpretation of the initial dip as a focal deoxygenation preceding a delayed and spatially diffuse active vascular response leads to specific predictions when comparing oximetric and volumetric signals. (i) The oximetric point spread should appear first. Any volumetric response should appear considerably later, well after the onset of the initial dip. (ii) This volumetric response should be distributed more diffusely over cortex than the initial dip. (iii) During the oximetric rebound (which is presumably dominated by the blood volume response), the oximetric and volumetric responses should have comparable spatial extents. To test these predictions, we compared the volumetric and oximetric point spreads at the three phases of the oximetry signal. Our results failed to bear out any of the above predictions.
Darkening at the Initial Dip Is Unlikely to Measure a Selective Focal Increase of [HbR].
Although the initial dip at both 630 and 605 nm was reliable, it started no earlier than the volumetric (530 nm) response. Quantifying response onsets for each wavelength as the first time point where the response showed a statistically significant inflection in slope (Fig. S1) revealed that onset times were essentially identical for all three wavelengths (0.4 s at 530 nm; 0.4 s at 605 nm; 0.5 s at 630 nm). Thus, there was no delay in the blood volume increase relative to the initial dip. We noted that the ratio of the initial dip to the subsequent rebound was reliably higher for 605 than for 630 nm (Fig. 2B), a point that we return to later.
We found no difference in the spatial extent of signals at oximetric and volumetric wavelengths during the initial dip. Even at its earliest clearly measurable time point (0.8 s), the initial dip point spread was identical to that of the volumetric point spread [Fig. 2 C and D; 75% half-width = 0.5 mm (SEM = 0.2 mm)‖ (volumetric); 0.48 (0.08) mm (oximetric), P = 0.8, n = 11]. This surprising result shows that the initial dip is no more focal than blood volume and suggests that the two may share a common underlying mechanism.
Volumetric Signal Remains Spatially Focused, Unlike the Diffuse and Delayed Oximetric Rebound.
Both the volumetric and oximetric responses broadened during the rebound. However, the spatial spread at the center of the volumetric response remained largely unchanged, maintaining a sharp profile. At its peak (3 s after stimulus onset), the 75% half-width of the volumetric response grew by only 13% [half-width: from 0.5 (0.2) to 0.60 (0.05) mm] despite a 13-fold increase in amplitude [from 0.26 (0.09) % to 3.36 (0.16) %] (Table 1 and Fig. 2 A, C, and E). By contrast, the spatial spread at the center of the oximetric response broadened significantly during the rebound, taking on a distinctly rounded profile [75% half-width grew from 0.48 (0.08) to 1.15 (0.08) mm, an increase of a factor of 2.4; P = 9e-6, n = 11]. It is noteworthy that the cross-sectional shape and spatial extent of the volumetric point spread closely matches those observed with voltage sensitive dyes (28, 29). This finding suggests that the volumetric response, is not only distinct from the oximetric rebound, but is likely a better neuroimaging signal tightly coupled to underlying neural activity.
Late Undershoot at the Oximetric Wavelengths Matches the Spatial Extent of the Volumetric Signal.
At oximetric wavelengths we often detected a small increase in absorption after the rebound that was spatially localized [half-width at 75% = 0.6 (0.2) mm; Table 1 and Fig. 2F]. Other investigators have shown this “late undershoot” emerging many seconds after the cessation of neural activity and have suggested that it may indicate a secondary increase in [HbR] (30). This late oximetric signal matched spatially the long-lived volumetric signal [volumetric half-width = 0.57 (0.05) mm], suggesting that both could reflect lingering blood volume.
Spectral Decomposition.
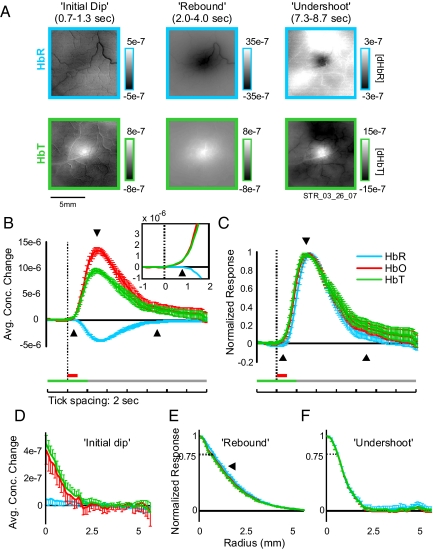
The spectrally decomposed [HbO], [HbR], and [HbT] signals showed similar spatially localized responses, each monophasic in time (Table 1 and Fig. 3). To relate absorption changes at oximetric wavelengths during the initial dip, rebound, and undershoot to these analyzed hemodynamic components we examined the pointspreads in HbR and HbT separately during these phases.
Fig. 3.
Spectral decomposition of imaging signal shows no increase in HbR during the initial dip period as defined for the oximetric signal. (A) Spectrally decomposed point spreads: same experiment as in Fig. 1B. Conventions are as in Figs. 1 and 2. Only the images for HbR and HbT are shown. (B) The average temporal profile of concentration changes at response center after visual stimulation. Spectral decomposition was done separately for each experiment and then averaged. Conventions are as in Fig. 2A. Note the lack of any detectable increase in HbR despite clear initial dip signals in Fig. 2. (Inset) Magnified view of concentration changes during early portion of response, 1–2 s after stimulus. Note the early increase in HbT and the lack of any increase (only a late decrease) in HbR. (C) Same as B, but normalized to maximal response (HbR signal is thus flipped in sign). Note HbR signal lagging behind HbT at onset but decaying faster toward baseline. (D–F) The radial profiles of concentration changes for HbR, HbO, and HbT during the three oximetric response phases (as in Fig. 2). (D) Concentration changes early into the initial dip (t = 0.8 s). Unnormalized because there was no reliable change in HbR at this time point (see A and B). Compare with Fig. 2D. (E) Same as D, but for the rebound phase and scaled to maximum (t = 3 s). Note HbR signal is slightly broader than HbT. (F) Same as E, but for the undershoot phase (t = 9 s). Note similar point spreads in all components.
Initial Dip Is Dominated by HbT.
Surprisingly, we saw no significant change in [HbR] during the initial dip (0.8 s after stimulus, P = 0.9, n = 11; Fig. 3 A and B and Fig. S2). Unlike the darkening at oximetric wavelengths, [HbR] at this time point had no reliable spatial structure. Instead, the response was entirely dominated by an increase in [HbT] (P = 0.02; Fig. 3 B–D), whereas [HbR] showed only a delayed monophasic decrease. The [HbT] point spread was identical to the volumetric point spread (Figs. 1 and 2 and Table 1). Our data suggest that the initial dip at oximetric wavelengths is indeed a result of an early and spatially localized increase in [HbT] and not an increase in [HbR]. These results were independent of the wavelength pairs used for imaging (Figs. S2 and S3) or the model parameters used for decomposition (see Methods and Fig. S4).
Observed Oximetric Rebound Is Likely Shaped by an Interaction Between HbT and HbR.
We found that the [HbT] signal during the rebound had a spatial spread that was consistently, albeit slightly narrower than the concomitant decrease in [HbR] {Table 1 and Fig. 3E arrowhead: [HbR] width = 0.80 (0.03); [HbT]: 0.61 (0.05); P = 0.0017} (31, 32). Notably, both the [HbR] and [HbT] profiles were sharply peaked at the center and much narrower than the raw oximetric rebound [half-width = 1.15 (0.08) mm; P = 0.0007; Table 1 and Fig. 2E]. Thus, neither the [HbT] nor the [HbR] signal by itself can account for the width and the diffuse peak of the oximetric rebound.
We hypothesize that the oximetric rebound reflects the difference between the absorption increase from increasing [HbT] and the somewhat broader absorption decrease from decreasing [HbR]. A simple model of such a difference image, using computed profiles for [HbR] and [HbT], plausibly reproduces the shape of the rebound (Fig. S5).
Late Undershoot Is a Result of Residual HbT.
Our analysis also confirmed that the late darkening is dominated by slowly decaying [HbT], persisting for 15 s, rather than a late increase in [HbR].
Predicting the Imaging Signal Time Course Across Wavelengths.
Our calculated hemodynamic time courses, although obtained with three imaging wavelengths, correctly predict ISOI time courses observed across the full spectrum (Fig. 4). These predictions were made by using the calculated early increase in [HbT] (increased darkening) and delayed decrease in [HbR] (decreased darkening). Signals at isosbestic wavelengths result from a rapid, monophasic darkening matching the [HbT]. For wavelengths away from the isosbestic point, with progressively stronger absorption by HbR vs. HbO, the delayed [HbR] decrease (Fig. 3B) becomes progressively more prominent. This delayed [HbR] decrease leads to a delayed reduction (e.g., at 592 nm) or reversal of the initial HbT-linked darkening (e.g., at ≈600–720 nm where the predicted signal displays the familiar initial dip and rebound). These predicted time courses match published results (e.g., figure 2 of ref. 19). In particular, wavelengths with stronger absorption in HbR vs. HbO show stronger and more prolonged rebounds after weaker and more transient initial dips (Fig. 4 B and C) matching our own (Fig. 2B) and previous observations (30) of stronger dips closer to the isosbestic point. By contrast, a model of the initial dip as deoxygenation failed to account for these observations, yielding similarly sized dips across wavelengths, and delayed darkening near isosbestic points (Fig. S6).
Fig. 4.
Changes in blood volume predict size of initial dip. (A) Molar extinction coefficient for HbR and HbO. Vertical dashed lines indicate isosbestic points. Shaded regions indicate: ranges where HbR absorbs more strongly than HbO. (B) Predicted response time courses at wavelengths moving from isosbestic to oxymetric marked in A, showing progression from a monophasic signal at the isosbestic 584 nm to a biphasic signal at 600 nm. (Note: The predicted signal time courses here are for the pure spectral wavelengths indicated; while qualitatively similar to our measured imaging signals they are quantitatively different because our LED sources had finite bandwidths giving the corresponding admixture of responses. All quantitative calculations using our LED sources accounted for this finite bandwidth; see Methods). (C) The predicted ratio of the size of the initial dip relative to the rebound across wavelength. Note an explosive increase in this ratio toward isosbestic points. (D) Predicted spatial profiles at 3 s poststimulus onset normalized to maximum response amplitude. Wavelengths same as in B. Note broader spreads at wavelengths showing a rebound. (Inset) Not normalized for amplitude.
Our findings suggest that the multiphasic temporal profile of the ISOI signals at typical oximetric wavelengths, including the magnitude and duration of the initial dip, rebound, and undershoot, reflect the interplay of absorptions by HbT and HbR and are problematic as estimates of neural oxygen metabolism.
Discussion
With ISOI in alert macaque we observed the expected, robust initial dip. Our interpretation of this dip is very different, however, from earlier interpretations in macaque or cat. The likely reason for this difference is that we separated our imaging signal into [HbR] and [HbO] by using a model that incorporates changes in optical path length caused by the large changes in absorptivity across wavelengths (27). The earlier results (7, 23) were obtained with fixed path lengths, which, as now widely acknowledged (4, 10), can lead to significant errors in spectroscopic analysis (17, 27). Our results are robust over a broad range of model parameters when using wavelength-dependent path lengths (Fig. S4). However, control analyses of our imaging data assuming fixed path lengths resulted in transient initial increases in [HbR] similar to the earlier published results.
Although we applied these analysis techniques to ISOI in the macaque, our results are consistent with a large body of work pioneering similar analysis in rodents [including alert rodents (22)] and alert humans (33). Those studies arrived at conclusions similar to ours: that the primary response after stimulus onset is a rapid and robust increase in [HbT]. Those groups either failed to see any initial increase in [HbR] (e.g., refs. 18–20) or found only a modest increase accompanied by a large rise in [HbO], consistent with a net increase in [HbT] (e.g., refs. 21, 22, and 34). Discrepancies between rodent and earlier monkey results had been attributed to species differences (35, 36). By contrast, our findings add to a growing body of evidence suggesting that neurovascular coupling mechanisms may be conserved across species.
Independent of modeling, we see that the volumetric (530 nm) signal is as fast as the oximetric (605 or 630 nm) initial dip, indicating no delay in the rise of [HbT]. We identified signal onset times by using a quantitative statistical measure. Our findings are consistent with those from fMRI (35) and rat ISOI (18, 19). Prior work in cats and monkeys suggested that changes in HbT are delayed, but that delay could be an artifact of signal normalization and appears to be contradicted by other data from the same group (e.g., figure 3 a and b in ref. 7). Our results argue against any significant delays between the start of oxygen extraction from blood and the initiation of the active vascular response (4, 7).
It is important to note that the lack of increased [HbR] does not preclude increased oxygen consumption or decreased tissue pO2 caused by neural activity (19). Stimulation leads to a rapid (<400 ms) increase in local cerebral blood flow (18, 19, 37), increasing [HbO] in local blood vessels (38). This increase in flow could support increased oxygen consumption and a decrease of tissue pO2 even as the local blood supply gets enriched in [HbO] and depleted of [HbR] (19). Our findings highlight the complexity of the relationship between measures of blood volume, flow, and oxygenation with local metabolic demand or neural activity.
Our observations could shed light on the elusive nature of the fMRI initial dip compared with the ISOI. Unlike in ISOI, a dip in BOLD equals increased [HbR]. However, a large number of careful fMRI investigations have either failed to see any initial dip (e.g., refs. 24 and 35), found it unreliable (39), or obtained it only after unnaturally intense stimulation (40). Our work together with the large body of work in rodents suggests an explanation: the hemodynamic response during the ISOI dip (unlike in fMRI) is driven by a rapid active vascular response, dominated by increases in [HbT], and only occasionally accompanied by small increases in [HbR].
Our results suggest that blood volume ([HbT]) is a more reliable signal to use for neuroimaging than the initial dip. The initial dip likely carries no privileged information about neural activity or metabolic demand. The [HbT] signal is as rapid and spatially focused and more than an order of magnitude stronger and longer-lived. For ISOI maps, e.g., of orientation, the initial dip has been reported to give cleaner maps than the [HbT] signal, i.e., with fewer vascular artifacts (1, 10). However, these cleaner maps may result from spatial filtering and the fortuitous cancellation of common vascular patterns by the difference signal that comprises the initial dip (increasing [HbT] with decreasing [HbR]).
An important open question is the relation of these various hemodynamic signals to underlying neural activity. The [HbT] signal was earlier believed to be diffuse, nonspecific, and dominated by vascular artifacts (10). Our point spreads imaged using the volumetric 530 nm (or the computed [HbT], [HbO], or [HbR]) are sharply peaked and remarkably similar in shape and extent to those obtained with voltage sensitive dyes (28, 29). The [HbT] response is thus likely a tight neurovascular signal that faithfully reports the spatial pattern of spiking plus subthreshold neural activity. This likely close link between [HbT] signals and neural activity is also consistent with work in anesthetized rodent barrel cortex showing that neural activity correlates well with [HbT] or [HbO] signals but not with the initial dip (5).
The correct interpretation of stimulus-driven hemodynamic signals is critical to modern neuroimaging techniques such as fMRI. The proper separation of the imaging signal into its hemodynamic components is a prerequisite for linking it to underlying neural activity.
Methods
Results were obtained by using continuous, multiwavelength ISOI in alert macaque V1. Two animals (V and S) were trained on a periodic visual fixation task. Visual stimuli, consisting of 0.25° light bars against a gray background, were flashed on passively for 1 s while the animal held fixation. Standard alert-monkey optical imaging techniques (23) were used to record the intrinsic cortical signal, continuously, through a clear artificial dura and glass-fronted recording chamber mounted over the animals' V1. The brain surface was illuminated by using two LED arrays chosen from three sets with wavelengths centered at 530, 605, and 630 nm. The LED arrays were switched on and off alternately in synchrony with the camera (15 Hz) giving, in effect, simultaneous optical imaging at 7.5 Hz at the separate LED wavelengths.
Importantly, the imaging signals were spectrally decomposed by using modified Beer-Lambert equations with wavelength-dependent optical path lengths: the cortical tissue was modeled as a scattering medium with embedded absorbers (HbO, HbR). The path lengths of light traveling into and out of cortex were estimated separately for each LED wavelength by using Monte Carlo simulations. These wavelength-dependent path lengths were then used to decompose the measured imaged signal into the underlying Δ[HbR] and Δ[HbO].
Details of animal training, visual stimuli, optical imaging, and data analysis are in SI Appendix. All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
Supplementary Material
Acknowledgments.
We thank M. E. Goldberg for supporting a dedicated machine shop at the Columbia University Medical Center; P. P. Mitra (Cold Spring Harbor Labs, Cold Spring, NY) for providing Chronux analysis software; K. Korinek for designing and fabricating the optical imaging hardware (with Y.B.S.); and G. Cantone and C. Ma for helping with recordings. Monte Carlo code was developed by C.B. and E.M.C.H., based on the previous works of Drs. Lihong Wang (Washington University, St. Louis) and Andrew Dunn (University of Texas, Austin). This work was supported by National Institutes of Health Grants F31 NS056834 and T32 MH015174 (to Y.B.S.), R01 EY013759 and R01 EY019500 (to A.D.), R21 NS053684 and R01 NS063226 (to E.M.C.H.), and R24 EY015634 (to Dr. M. E. Goldberg), the Human Frontier Science Program (E.H.), the Columbia Research Initiatives in Science and Engineering, the Gatsby Initiative in Brain Circuitry, The Dana Foundation Program in Brain and Immuno Imaging, and the Esther and Joseph Klingenstein Foundation Fellowship in the Neurosciences (to A.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The term initial dip has been used in different senses in the literature. Here, we use it to refer to the observed phenomenon of a dip, i.e. the observed initial darkening in ISOI (e.g., ref. 30) or transiently reduced BOLD signal in fMRI (e.g., ref. 8). We are not using the term to refer to the calculated increase in [HbR] (e.g., ref. 3).
The square brackets [] indicate concentration per volume of imaged cortical tissue. This measure combines the concentration of a species in blood with the fraction of the cortical pixel occupied by blood vessels. This measure of concentration is similar to the measure of concentration per voxel in fMRI.
Unlike in fMRI BOLD, both [HbR] and [HbO] contribute to ISOI absorption at all wavelengths.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905509106/DCSupplemental.
HbT is equivalent to cerebral blood volume (CBV) as measured in fMRI for fixed hematocrit. Because CBV in MR studies often measures plasma volume (e.g. via a contrast agent) the two differ if the hematocrit changes.
All data cited are averaged across 11 experiments in two animals.
Half-widths measured at the 75% assess the focal spatial resolution of the point spread.
References
- 1.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, et al. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxton RB. The elusive initial dip. NeuroImage. 2001;13:953–958. doi: 10.1006/nimg.2001.0814. [DOI] [PubMed] [Google Scholar]
- 4.Vanzetta I, Grinvald A. Evidence and lack of evidence for the initial dip in the anesthetized rat: Implications for human functional brain imaging. NeuroImage. 2001;13:959–967. doi: 10.1006/nimg.2001.0843. [DOI] [PubMed] [Google Scholar]
- 5.Nemoto M, et al. Functional signal- and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J Neurosci. 2004;24:3850–3861. doi: 10.1523/JNEUROSCI.4870-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- 7.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: Implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 8.Menon RS, et al. BOLD-based functional MRI at 4 Tesla includes a capillary bed contribution: Echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- 9.Ances BM. Coupling of changes in cerebral blood flow with neural activity: What must initially dip must come back up. J Cereb Blood Flow Metab. 2004;24:1–6. doi: 10.1097/01.WCB.0000103920.96801.12. [DOI] [PubMed] [Google Scholar]
- 10.Vanzetta I, Hildesheim R, Grinvald A. Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry. J Neurosci. 2005;25:2233–2244. doi: 10.1523/JNEUROSCI.3032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chisum HJ, Mooser F, Fitzpatrick D. Emergent properties of layer 2/3 neurons reflect the collinear arrangement of horizontal connections in tree shrew visual cortex. J Neurosci. 2003;23:2947–2960. doi: 10.1523/JNEUROSCI.23-07-02947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995;375:780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- 13.Basole A, White LE, Fitzpatrick D. Mapping multiple features in the population response of visual cortex. Nature. 2003;423:986–990. doi: 10.1038/nature01721. [DOI] [PubMed] [Google Scholar]
- 14.Vnek N, Ramsden BM, Hung CP, Goldman-Rakic PS, Roe AW. Optical imaging of functional domains in the cortex of the awake and behaving monkey. Proc Natl Acad Sci USA. 1999;96:4057–4060. doi: 10.1073/pnas.96.7.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RM, Raffi M, Phinney RE, Turner JA, Jando G. Functional architecture of eye position gain fields in visual association cortex of behaving monkey. J Neurophysiol. 2003;90:1279–1294. doi: 10.1152/jn.01179.2002. [DOI] [PubMed] [Google Scholar]
- 16.Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci. 2000;3:164–169. doi: 10.1038/72109. [DOI] [PubMed] [Google Scholar]
- 17.Lindauer U, et al. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. NeuroImage. 2001;13:988–1001. doi: 10.1006/nimg.2000.0709. [DOI] [PubMed] [Google Scholar]
- 18.Sheth SA, et al. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. NeuroImage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Hillman EM, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. NeuroImage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayhew J, et al. Spectroscopic analysis of neural activity in brain: Increased oxygen consumption following activation of barrel cortex. NeuroImage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- 22.Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. NeuroImage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J Neurosci. 2000;20:8111–8121. doi: 10.1523/JNEUROSCI.20-21-08111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon CH, Fukuda M, Park SH, Kim SG. Neural interpretation of blood oxygenation level-dependent fMRI maps at submillimeter columnar resolution. J Neurosci. 2007;27:6892–6902. doi: 10.1523/JNEUROSCI.0445-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries, and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 1999;437:617–623. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- 26.Sirotin YB, Das A. Anticipatory hemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl M, et al. Physical model for the spectroscopic analysis of cortical intrinsic optical signals. Phys Med Biol. 2000;45:3749–3764. doi: 10.1088/0031-9155/45/12/317. [DOI] [PubMed] [Google Scholar]
- 28.Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Geisler WS, Seidemann E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat Neurosci. 2006;9:1412–1420. doi: 10.1038/nn1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: Implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culver JP, Siegel AM, Franceschini MA, Mandeville JB, Boas DA. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxygenated hemoglobin. NeuroImage. 2005;27:947–959. doi: 10.1016/j.neuroimage.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 32.Berwick J, et al. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J Neurophysiol. 2008;99:787–798. doi: 10.1152/jn.00658.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller CJ, et al. Intracranial microprobe for evaluating neuro-hemodynamic coupling in unanesthetized human neocortex. J Neurosci Methods. 2009;179:208–218. doi: 10.1016/j.jneumeth.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: The relationship between blood flow, oxygenation, and volume in rodent barrel cortex. NeuroImage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- 35.Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Vanzetta I, Grinvald A. Coupling between neuronal activity and microcirculation: Implications for functional brain imaging. HFSP J. 2008;2:79–98. doi: 10.2976/1.2889618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masamoto K, Vazquez A, Wang P, Kim SG. Trial-by-trial relationship between neural activity, oxygen consumption, and blood flow responses. NeuroImage. 2008;40:442–450. doi: 10.1016/j.neuroimage.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoge RD, et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magn Reson Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Logothetis N. Can current fMRI techniques reveal the micro-architecture of cortex? Nat Neurosci. 2000;3:413–414. doi: 10.1038/74768. [DOI] [PubMed] [Google Scholar]
- 40.Yesilyurt B, Ugurbil K, Uludag K. Dynamics and nonlinearities of the BOLD response at very short stimulus durations. Magn Reson Imaging. 2008;26:853–862. doi: 10.1016/j.mri.2008.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.