Abstract
The importance of proper ion channel trafficking is underpinned by a number of channel-linked genetic diseases whose defect is associated with failure to reach the cell surface. Conceptually, it is reasonable to suggest that the function of ion channels depends critically on the precise subcellular localization and the number of channel proteins on the cell surface membrane, which is determined jointly by the secretory and endocytic pathways. Yet the precise mechanisms of the entire ion channel trafficking pathway remain unknown. Here, we directly demonstrate that proper membrane localization of a small-conductance Ca2+-activated K+ channel (SK2 or KCa2.2) is dependent on its interacting protein, α-actinin2, a major F-actin crosslinking protein. SK2 channel localization on the cell-surface membrane is dynamically regulated, and one of the critical steps includes the process of cytoskeletal anchoring of SK2 channel by its interacting protein, α-actinin2, as well as endocytic recycling via early endosome back to the cell membrane. Consequently, alteration of these components of SK2 channel recycling results in profound changes in channel surface expression. The importance of our findings may transcend the area of K+ channels, given that similar cytoskeletal interaction and anchoring may be critical for the membrane localization of other ion channels in neurons and other excitable cells.
Keywords: ion channel trafficking, early endosome, cardiac myocytes, small conductance Ca2+-activated K+ channel, calmodulin binding domain
The function of ion channels depends critically on the precise number and subcellular localization of the channel proteins on the cell-surface membrane (1, 2). The steady-state cell-surface expression of ion channels is intricately and dynamically governed by the anterograde (forward) and retrograde trafficking (2, 3). Ion channel molecules are first synthesized in the endoplasmic reticulum (ER), assembled and processed, then trafficked to the membrane where they function. Trafficking of ion channel proteins to the surface membrane involves a series of tightly regulated events coordinated by ER resident proteins, microtubules, transport vesicle and Golgi apparatus, the actin cytoskeleton, myosins, and anchoring proteins (2). The importance of correct ion channel trafficking is highlighted by a number of channel-linked genetic diseases whose defect is associated with failure to reach the cell surface (4–8).
Small-conductance Ca2+-activated K+ (SK or KCa2) channels belong to a family of Ca2+-activated K+ channels (KCa) that have been reported from a wide variety of cells (9–11). SK channels represent a highly unique family of K+ channels, in that they are directly gated by changes in intracellular Ca2+ concentration and hence function to integrate changes in Ca2+ concentration with changes in K+ conductance and membrane potentials. SK channels have been shown to mediate afterhyperpolarizations in neurons (9, 12, 13) and action potential repolarization in cardiac tissues (14, 15). Previous studies have provided strong support that ion channels are components of macromolecular complexes containing the main pore-forming and auxiliary subunits interacting with scaffolding and regulatory proteins that are linked to the cytoskeleton (16). We have previously shown that SK2 channel interacts with α-actinin2, a cytoskeletal protein that localizes the channel to L-type Ca2+ channel in cardiac myocytes (17) to mediate the entry of external Ca2+. In this study, we directly demonstrate that cell-surface expression of SK2 channel is dynamically regulated by its interaction with α-actinin2 cytoskeletal protein and involves early endosome–mediated endocytic recycling. The interaction sites have been localized to the EF-hand motifs in the C terminus of α-actinin2 protein and the helical core region of the calmodulin-binding domain (CaMBD) in SK2 channel.
Results
α-Actinin2 Cytoskeletal Protein Is Required for the Proper Surface Membrane Localization of SK2 Channel.
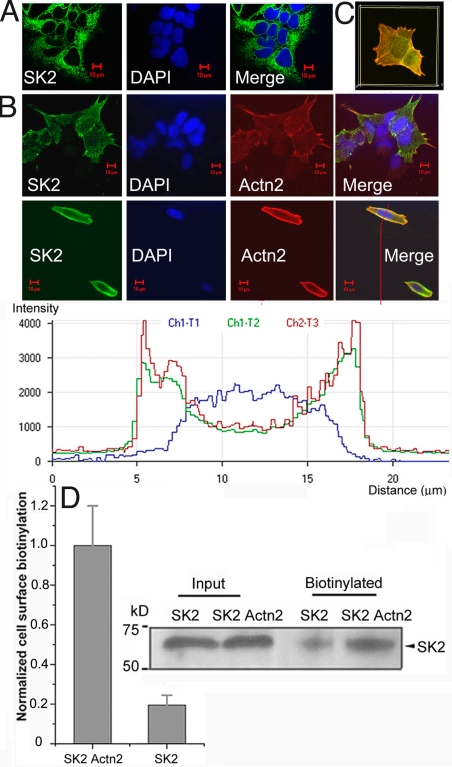
We have previously documented the interaction of SK2 channel with α-actinin2 cytoskeletal protein, which localizes SK2 channel to Ca2+ channel to regulate the channel function (17). To directly probe the functional roles of SK2 channel interacting protein, α-actinin2, we used immunofluorescence confocal microscopy and human embryonic kidney cells (HEK 293) as an expression system. Surprisingly, when SK2 channels were expressed alone, instead of being found on the plasma membrane, the channels were localized mostly in the cytosol of the cells (Fig. 1A). It is possible that the cytosolic localization may be the result of an overloading of the cell machinery by excessive amounts of protein from the transfection. However, there were no significant differences in SK2 channel localization in the stable cell line compared with the transient transfection [supporting information (SI) Fig. S1], suggesting that the cytosolic localization of SK2 channel did not result from the overexpression. Consequently, when SK2 channel and α-actinin2 were coexpressed in HEK 293 cells, SK2 channels displayed proper membrane localization pattern (Fig. 1B). SK2 channel and α-actinin2 were found to be colocalized, as illustrated by intensity profile analyses (Fig. 1B, Inset). Three-dimensional reconstructions of confocal Z-stack images further illustrated that SK2 channel and α-actinin2 were mostly colocalized in the plasma membrane (yellow) (Fig. 1C). Furthermore, using cell-surface biotinylation assay, we demonstrated that HEK 293 cells transfected by SK2 plasmid alone exhibited diminished cell-surface expression when compared with cells cotransfected with SK2 and α-actinin2 plasmids, supporting the notion that α-actinin2 could aid in the proper membrane localization of SK2 channels (Fig. 1D).
Fig. 1.
α-Actinin2 cytoskeletal protein aids in the proper localization of SK2 channel to the plasma membrane in HEK 293 cells. (A) Confocal images from HEK 293 cells transfected with SK2 channel alone and treated with anti-SK2 antibody (green, Left) and DAPI stain for nuclei (blue, Middle). The merged image (Right) shows the localization pattern of SK2 channel protein mainly in the cytosol. (B) Confocal images from HEK 293 cells cotransfected with SK2 channel and α-actinin2 and stained using anti-SK2 and anti–α-actinin2 antibodies in stably transfected cells (Top) and transiently tranfected cells (Bottom). Inset: Colocalization of SK2 channels and α-actinin2 proteins at the plasma membrane as illustrated by intensity profile analyses. (C) Three-dimensional reconstructions of confocal Z-stack images of HEK 293 cells doubly labeled by anti-SK2 and anti–α-actinin2 antibodies. (D) Cell-surface biotinylation of SK2 channel from HEK 293 cells transfected with SK2 channel alone (SK2) compared with cells cotransfected with SK2 channel and α-actinin2 (SK2 Actn2), using NHS-LC-Biotin, pulled down with streptavidin beads, and probed with anti-SK2 antibody. Signals were corrected to channel protein input and normalized (n = 3; *, P < 0.05). A representative cell-surface biotinylation immunoblot of SK2 channel is shown (Right).
SK2 Channel Interacts with α-Actinin2 Cytoskeletal Protein via the EF-Hand Motifs in α-Actinin2 Protein and the Helical Core Region of CaMBD in the C Terminus of SK2 Channel.
To define the binding site on α-actinin2, we dissected the shortest positive α-actinin2 clone containing roughly the last 2 spectrin repeats and the EF hand, which we have previously uncovered in a human heart library screen using C terminus of SK2 channel as the bait (17). Three different constructs containing the last spectrin repeat, first and second EF hand (EF-1.2) and third and fourth EF hand (EF-3.4), were made separately to detect the interaction with SK2 channel using yeast two-hybrid (YTH) assay. However, none of the constructs showed a positive interaction. We suspected that short fragments of α-actinin2 might lead to conformation changes of the protein. Hence, site-directed mutagenesis was used instead to map the interaction sites. From our previous library screen, all positive clones of α-actinin2 contained EF-hand motifs, suggesting that the motifs may be required for the interaction. Hydrophobicity analysis revealed 2 separate hydrophobic regions in EF-3.4 of α-actinin2 (Fig. S2A, Right). Indeed, previous reports have demonstrated that EF-3.4 of α-actinin2 displays hydrophobic interaction with its partner and that hydrophobic interactions are the most important noncovalent forces that drive the folding of the linear polypeptide into a compact structure (18). From the above reasoning 2 different mutants were generated, in which amino acids with high hydrophobicity were mutated to glycine, which is ambivalent, nonpolar, and the smallest of amino acids. Wild-type α-actinin2 displayed a strong interaction with SK2 channel. In contrast, point mutations of phenylalanine (F835, Actn2mut2) or tyrosine (Y881) and phenylalanine (F884) residues to glycine (Actn2mut3) completely suppressed the interaction between SK2 channel and α-actinin2 protein (Fig. 2B). Intriguingly, these 2 mutants still retained a strong interaction with Ca2+ channel Cav1.3 (Fig. S2B), suggesting that the binding sites were different between SK2 channel and Cav1.3. It also suggested that the 2 point mutations are unlikely to disrupt the protein conformation. In contrast, point mutation of a glutamic acid residue, which is located in the area with low hydrophobicity (E829, Actn2mut1), to glycine (Fig. S2A, Right), did not significantly change the interaction strength compared with wild-type α-actinin2 (Fig. 2A).
Fig. 2.
Mapping of the interaction sites between SK2 channel and α-actinin2 protein using YTH assays. (A) Different mutant and wild-type constructs of SK2 channel and α-actinin2 display different strengths of interaction using YTH assays on the screening media with high (SD/-Ade/-His/-Leu/-Trp/X-α-gal) and low (SD/-Leu/-Trp) stringency. pGADT7-T with p53 was used as a positive control. The interaction of SK2 channel with α-actinin2 was further quantified using β-galactosidase activity (Right) (n = 6; *, P < 0.05). (B) Immunofluorescence confocal microscopic images. A marked overlap in the distribution of SK2 channels and α-actinin2 proteins was found at the plasma membrane when HEK 293cells were cotransfected by pIRES-SK2 with pcDNA3.1-Actn2-wild-type (top row). The second and third rows show a low level of plasma membrane localization of SK2 channels when cells were cotransfected by pIRES-SK2 with pcDNA3.1-Actn2mut2 or pcDNA3.1-Actn2mut3. Bottom rows show HEK 293 cells cotransfected with SK2 channel and truncated Actn2-N (aa 1–251) containing only the 2 calponin homology domains or SK2 with truncated Actn2-Rod (aa 344–745) containing only the rod domains. Of note, the negative immunolabeling for the truncated forms of α-actinin2 in B indicated that the epitopes for the antibody (Sigma monoclonal anti–α-actinin sarcomeric clone EA-53) may be localized outside the truncated portion of the α-actinin2 protein. (C) Top row shows immunofluorescence confocal microscopic images labeled with trans-Golgi marker (P230) and anti-SK2 antibodies. Second, third, and fourth rows show images labeled with anti-SK2 and anti-Rab8, anti-Rab5, or anti-EEA1 antibodies, respectively, showing differences in the colocalization among different proteins with SK2 channels. Scatterplots (right column) show a poor correlation between a trans-Golgi marker (P230) with SK2 channels and high correlation between EEA1 with SK2 channels. All pixels in the images have been assigned a position on the scatterplots and are placed according to the intensity of color (red or green). Bottom row shows immunofluorescence confocal microscopic images from HEK 293 cells after treatment with 120 μM primaquine for 4 h labeled with anti-SK2 and anti-EEA1 antibodies.
Because the EF hand of α-actinin2 belonged to the calmodulin (CaM)-like protein family, and because our previous YTH data showed that the proximal C-terminal tail of SK2 interacts physically with α-actinin2 (17), we hypothesize that the binding site on the SK2 channel may be localized within the CaMBD. Previous published data have demonstrated that the CaMBD exhibits a helical core region between residues 423 and 437 and that disruption of the helical domain abolishes constitutive association of CaMBD with apo-CaM (19) (Fig. S2A, Inset). On the basis of these published data, we generated mutant SK2 channels containing mutations of threonine (T431) and tryptophan (W432), located within the helical core region, to alanine (SK2Mut1), and threonine (T438) and leucine (L440), located outside the helical core region, to alanine (SK2Mut2). SK2Mut1 sharply blocked the interaction between SK2 and α-actinin2. In contrast, SK2Mut2 showed similar interaction strength compared with the wild-type SK2 (Fig. 2A). Similarly, SK2Mut1 also significantly abolished the interaction between SK2 and CaM but not SK2Mut2. β-Galactosidase activity quantification data further supported the results of YTH assays (Fig. 2A, Right), suggesting that the binding site of α-actinin2 on SK2 channel was located within the helical core region of CaMBD.
Direct Interactions Between SK2 Channel and α-Actinin2 Are Required for the Cell-Surface Localization of SK2 Channel.
We directly assessed the effect of binding of α-actinin2 with SK2 channel on membrane localization (Fig. 2B). Confocal microscopic analysis showed a marked overlap in the distribution of SK2 channel and α-actinin2 at the plasma membrane when HEK 293 cells were cotransfected with plasmids containing SK2 and wild-type α-actinin2 (Fig. 2C, top row). In contrast, when SK2 channels were coexpressed with Actn2mut2 or Actn2mut3, the channels showed a low level of plasma membrane localization (Fig. 2B, 2 middle rows), suggesting that the 2 mutant α-actinin2 proteins failed to aid in SK2 channel membrane localization. Similarly, truncated α-actinin2-N (aa 1–251) containing the 2 calponin homology domain, important for the binding to actin filaments, or α-actinin2-Rod (aa 344–745) containing only the spectrin domains, also failed to aid in proper SK2 membrane localization (Fig. 2B, 2 bottom rows). Taken together, these data suggest that interaction between SK2 channel and α-actinin2 protein is required for the proper SK2 channel membrane localization.
Critical Involvement of Early Endosome–Mediated Endocytic Recycling in the Retrograde Trafficking of SK2 Channels.
To test whether SK2 channel protein was retained in the cytosol during trafficking through ER to Golgi or Golgi to the plasma membrane when SK2 channel was expressed alone in HEK 293 cells, a set of Golgi marker antibodies that included antibodies against the cis- to the medial- and the trans-Golgi network were used (Fig. 2C). None of the Golgi markers demonstrated colocalization with SK2 channel (Fig. 2C, Top, and Fig. S3A). We further investigated the involvement of specific Rab GTPases and tested whether SK2 channels were retained in the endocytic recycling pathway by using an early endosome marker [early endosomal antigen 1 (EEA1)] and Rab5 antibodies. EEA1 marker and Rab5 showed a higher correlation with SK2 channel compared with the Golgi marker or Rab8, which plays a role in vesicular transport from the trans-Golgi network (Fig. 2C). To further test whether the regulation involves SK2 channel trafficking from recycling endosomes, we examined SK2 channel surface expression in the presence of primaquine, which acutely and selectively inhibits recycling but not endocytosis (20, 21). Pretreatment with primaquine dramatically abolished surface expression of SK2 channel, even in the presence of α-actinin2 (Fig. 2C, Bottom). Taken together, our data suggest the critical involvement of early endosome–mediated endocytic recycling in the retrograde trafficking of SK2 channels.
SK2 Channel Interacts with α-Actinin2 in a Ca2+-Independent Manner.
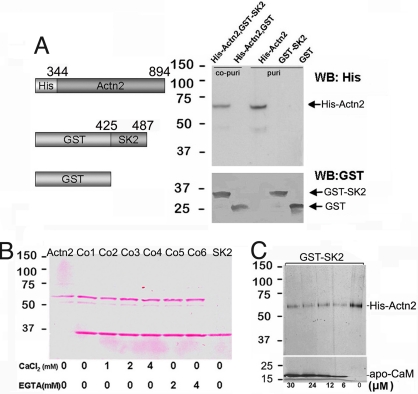
Copurification assay of recombinant fusion proteins of GST-SK2 (encoding residues 425–487 of SK2) and His-α-actinin2 (containing the EF-hand fragment of α-actinin2) was performed (Fig. 3A). When GST-SK2 was immobilized on glutathione column and then incubated with 6xHis-tagged α-actinin2, both GST-SK2 and His-α-actinin2 fusion proteins could be eluted and detected in the immunoblot (Fig. 3A, lane 1), suggesting that His-α-actinin2 fusion protein can be copurified via interaction with GST-SK2. In contrast, His-α-actinin2 protein could not be copurified with GST alone (Fig. 3A, lane 2). Moreover, as shown in Fig. 3B, His-α-actinin2 could be copurified with GST-SK2 in the presence of different concentrations of Ca2+ or EGTA, suggesting that SK2 interacted with α-actinin2 in a Ca2+-independent manner.
Fig. 3.
SK2 channels interact with α-actinin2 in a Ca2+-independent manner; in addition, the interaction was decreased in the presence of apo-CaM. (A Left) Schematic diagrams of recombinant fusion proteins for His-Actn2 and GST-SK2. The numbers refer to the amino acids of these 2 proteins. Both proteins could be induced by isopropyl-β-D-thiogalactopyranoside (IPTG) and expressed in Escherichia coli. (Right) Expressions of 6xHis-tagged α-actinin2 purified with Ni-NTA agarose and GST-tagged recombinant C terminus of SK2 channel, purified over glutathione Sepharose were detected at the predicted size by Western blot using anti-His and anti-GST antibodies, respectively (lanes 3 and 4, labeled as puri). Lanes 1 and 2 (labeled as co-puri) are the Western blot results probed by anti-His and anti-GST antibodies separately after copurification of GST-SK2 and His-Actn2 proteins. (B) Purified and copurified proteins stained by Ponceau S showing that His-Actn2 can be copurified with GST-SK2 in the presence of different concentrations of Ca2+ and EGTA. (C) In the presence of different concentrations of apo-CaM, His-Actn2 could still be copurified with GST-SK2, but the binding between GST-SK2 and His-Actn2 was decreased in the presence of apo-CaM.
We next tested whether the presence of apo-CaM could affect the interaction between SK2 and α-actinin2 (Fig. 3C). When GST-SK2 fusion protein was incubated with His-α-actinin2 in the presence of apo-CaM, weaker binding was observed compared with the interaction in the absence of apo-CaM. However, with increasing amounts of apo-CaM, there was no detectable change in the α-actinin2 binding. These data suggest possible competitive binding between α-actinin2 and apo-CaM to the SK2 channel protein.
Requirement of α-Actinin2 in SK2 Channel Surface Membrane Expression in Native Cells.
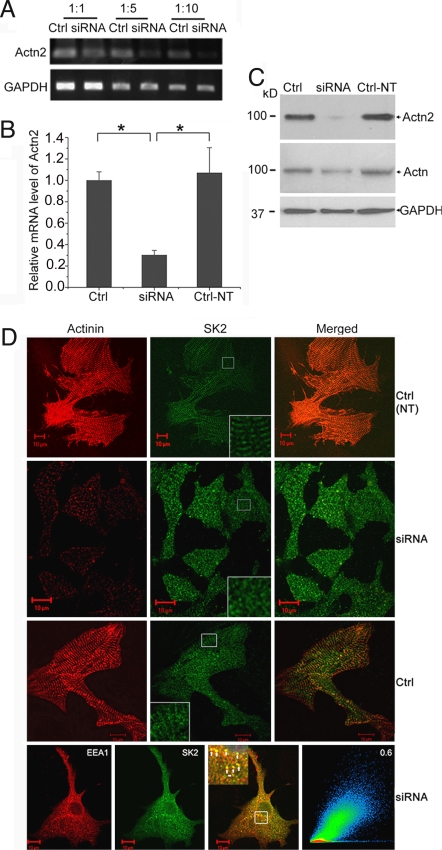
Most studies on ion channel trafficking to date have used mainly expression systems or cell lines (2, 22, 23). However, ion channel trafficking may be distinct in native systems, owing to differences in intracellular milieu and interacting proteins. Here, we directly tested whether α-actinin2 was required for the trafficking of SK2 channel in cardiomyocytes using siRNA-mediated knockdown of α-actinin2 protein. To demonstrate the efficacy of the specific siRNA targeted against α-actinin2, we measured mRNA and protein levels of α-actinin2 by RT-PCR and Western blot analysis (Fig. 4 A–C). Nontransfected cardiomyocytes (Ctrl-NT) or control cells transfected with scramble siRNA (Ctrl) showed intense staining of α-actinin2 along the Z-lines with a striated pattern (Fig. 4D). Moreover, SK2 channels were localized not only at the sarcolemma but also along the Z-line (Fig. 4D). In contrast, when cardiomyocytes were transfected by siRNA specific to α-actinin2, the distribution of SK2 channels displayed an abnormal pattern, with random staining localized in the cytosol associated with loss of striation (Fig. 4D). Moreover, these abnormally localized SK2 channels showed a high correlation with EEA1, suggesting that the channels were retained within the recycling pathway (Fig. 4D, Bottom). Taken together, these data suggest that α-actinin2 was required for proper SK2 channel localization in cardiomyocytes, possibly by decreasing the internalization and/or increasing the recycling of the channels from recycling endosomes.
Fig. 4.
α-Actinin2 cytoskeletal protein is required for normal SK2 channels localization in neonatal cardiomyocytes. (A and B) mRNA level of α-actinin2 was analyzed by semiquantitative RT-PCR using different dilutions of cDNA (A) and real-time RT-PCR (B) in neonatal cardiomyocytes treated with specific siRNA targeted against α-actinin2 or scrambled siRNA (Ctrl) and nontransfected cells (Ctrl-NT). There was a significant reduction in α-actinin2 mRNA level by specific siRNA compared with scrambled siRNA according to semiquantitative RT-PCR (A) as well as real-time RT-PCR (B). GAPDH mRNA level was used as control. Values are means ± SEM (n = 6; *, P < 0.05). (C) Western blot analysis showing α-actinin2 protein level detected by monoclonal rabbit anti-actinin2 antibody (Top) and monoclonal anti–α-actinin (sarcomeric) antibody (Middle) and GAPDH (Bottom) for loading control in cultured neonatal mouse cardiomyocytes with different treatments. Consequently, α-actinin2 protein level detected using rabbit monoclonal anti–α-actinin2 antibody was reduced significantly after transfection by specific siRNA. (D) Confocal microscopic images of immunostaining using anti-SK2 and anti–α-actinin antibodies for siRNA-treated or control groups. Cardiomyocytes transduced by siRNA specific to α-actinin2 show a significant decrease in the α-actinin2 staining; the SK2 channels were expressed but displayed abnormal pattern with localization mainly in the cytosol. Insets: Higher magnifications of the original marked area. Bottom row shows a high correlation between the abnormally localized SK2 channels with EEA1 in cardiomyocytes treated with siRNA.
In mouse ventricular myocytes, the sarcolemmal membrane forms invaginations called transverse tubules (T-tubules), which extend into the cell interior and are oriented in register with Z bands of the myofibrils. Similarly, atrial cells have prominent sarcoplasmic reticulum elements, which have been described as “Z-tubules.” Fig. S3B shows distinct T-tubule-like structures, also termed “Z-tubules,” in mouse atrial myocytes (K and L in Fig. S3B). Moreover, electron microscopic postembedding immunogold labeling demonstrates that SK2 channels and α-actinin2 are clustered together at the sarcomeric Z-discs in adult mouse cardiomyocytes (H and I in Fig. S3B).
SK2 Channel Cell-Surface Expression Is Dynamically Regulated by Its Interaction with α-Actinin2 Cytoskeletal Protein.
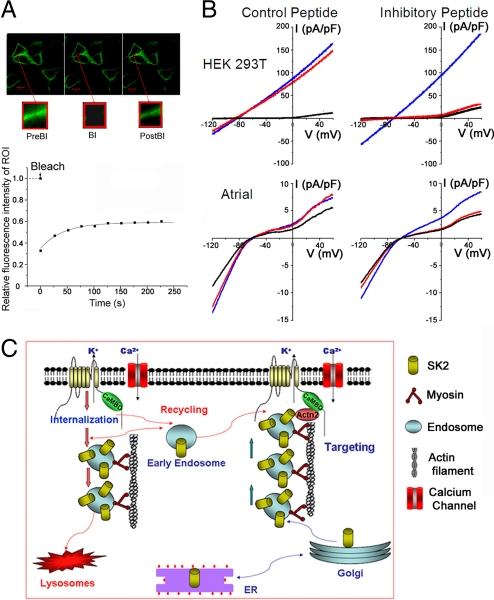
The dynamic behavior of SK2 channels in living cells was directly investigated using a construct encoding CFP-SK2 fusion protein in HEK 293 cells. We first determined that the CFP-SK2 fusion protein remained functional by recording whole-cell Ca2+-activated K+ current (IK,Ca) (Fig. S4A). Fluorescence recovery after photobleaching (FRAP) experiments in HEK 293 cells transfected with CFP-SK2 and α-actinin2 were performed (Fig. 5A). After photobleaching, the bleached region of interest (ROI) recovered within 2 min and reached approximately half of the original intensity. The observations support the dynamic process of SK2 channel recruitment to the cell membrane.
Fig. 5.
Dynamic regulation of SK2 channel cell-surface expression probed using live-cell imaging and patch-clamp recording with inhibitory peptide. (A) FRAP experiments were performed in HEK 293T cells transfected with CFP-SK2 and α-actinin2 plasmids. Insets: Sequential images of an ROI. PreBl, Prebleach; Bl, Bleach; PostBl, Postbleach. Bottom: Relative fluorescence intensity of the ROI pre- and postbleach. Time constant of recovery (τ) was 42.5 ± 4.3 s (n = 5). (B) Whole-cell IK,Ca was recorded immediately after establishment of whole-cell configuration (blue line), 20 min thereafter (red line), and after application of apamin (100 pM; black line). A voltage-ramp protocol was applied from −120 to +60 mV, with a slope of 360 mV/s from a holding potential of −55 mV. SK2-mediated whole-cell IK,Ca from HEK 293T cells cotransfected with pIRES2-EGFP-SK2 and pcDNA3-α-actinin2 plasmids (Top) and IK,Ca recorded from mouse atrial myocytes (Bottom), using control peptide and inhibitory peptide. (C) A working model for the anterograde and retrograde trafficking of SK2 channel. SK2 channels directly interact with α-actinin2 cytoskeletal proteins, which help to anchor the channels at the surface membrane and along the T-tubular structure in cardiac myocytes (pathway on the right). Absence of α-actinin2 may lead to an increase in the rate of internalization and/or decrease in the targeting of the early endosomes to the surface membrane (pathway on the left).
To functionally assess the effects of α-actinin2 binding on SK2 channel in native atrial myocytes (Fig. 5B, Bottom) as well as HEK 293T cells transfected with SK2 and α-actinin2 (Fig. 5B, Top), we designed an inhibitory peptide (IP) derived from the α-actinin2 binding site within the helical core region of CaMBD in the SK2 channel (Fig. S2A). A scrambled peptide (CP) was used as control. Inclusion of IP in the pipette solution resulted in nearly complete inhibition of IK,Ca after 20 min of recordings compared with the initial traces (Fig. 5B, Right), with no further reduction after application of apamin. In contrast, parallel experiments using the same concentrations of CP (Fig. 5B, Left) showed stable current recordings after 20 min. Summary data in Fig. S4B show a significant inhibition of IK,Ca quantified at a test potential of −120 mV by IP. Our data showed that inclusion of IP in the pipette solution resulted in nearly complete inhibition of IK,Ca after 20 min of recordings compared with the initial traces, with no further reduction after application of apamin. Therefore, it is difficult to assess whether the Ca2+ or apamin sensitivity and channel kinetics were altered after inclusion of IP in the pipette solution.
Discussion
CaM-Like Domain in the C Terminus of α-Actinin2 Protein.
α-Actinin, a major F-actin crosslinking protein present in both muscle and non-muscle cells, is a typical example of an EF-hand protein. EF hands are helix–loop–helix binding motifs involved in the regulation of many cellular functions. In general, EF hands exist as side-by-side pairs, forming a globular domain capable of coordinating 2 Ca2+ ions. Skeletal, cardiac, and smooth-muscle isoforms, α-actinin2 and -3, are localized within the Z-disk, which forms a complex network of interactions in the assembly and maintenance of the sarcomere (24). The C-terminal CaM-like domain of α-actinin is composed of 4 EF-hand motifs. However, muscle isoforms have lost their ability to bind Ca2+ owing to several mutations of residues engaged in Ca2+ coordination.
α-Actinin2 Interacts with the CaMBD in the C Terminus of SK2 Channels.
SK channels are voltage-independent and gated solely by intracellular Ca2+ in the submicromolar range. This high affinity for Ca2+ results from Ca2+-independent association of the SK2 subunit with CaM (19, 25, 26). Previous work has demonstrated that the CaMBD in the C terminus of SK2 exhibits a helical region between residues 423 and 437 (Fig. S2A Inset), and disruption of the helical domain abolishes constitutive association of CaMBD with Ca2+-free CaM but did not interfere with interactions between the CaMBD and Ca2+-loaded CaM, indicating a high degree of molecular specificity (19, 27). Our study demonstrates that α-actinin2 interacts with SK2 channel via the helical region of the channel in a Ca2+-independent manner (Figs. 2 and 3). Moreover, α-actinin2 could coexist or compete with apo-CaM at the CaMBD of SK2 channel. In contrast to the localization pattern of CaM, our findings indicate that the majority of α-actinin2 proteins are highly localized at the plasma membrane in the HEK 293 cells or at the Z-disc in the native cardiac myocytes. Moreover, we found that EF hand 3, 4 of α-actinin2 was essential for the localization of SK2 channel on the plasma membrane (Figs. 1 and 4). CaM is not only essential for gating but is also required for channel assembly and trafficking (28). Our data demonstrate that targeting or anchoring of SK2 channel at the plasma membrane requires the binding of α-actinin2, which crosslinks actin filaments to stabilize the muscle contractile apparatus and signal transduction. Furthermore, our FRAP analyses indicated that the localization of SK2 channel at the plasma membrane was dynamic, and IP derived from the core region of CaMBD in the SK2 channel could sharply block IK,Ca. Because both binding sites of apoCaM/CaMBD complex and α-actinin2/CaMBD were located within the same region, SK2 trafficking aided by CaM or SK2 targeting anchored by α-actinin2 might be blocked simultaneously during the IP incubation.
Surface Expression of SK2 Channel Is Dependent on Early Endosome–Mediated Endocytic Recycling.
The steady-state cell surface expression of ion channels is dynamically governed by the anterograde (forward) and retrograde trafficking (Fig. 5C) (2, 3). Endocytosis represents the initial step in the recycling process whereby different fates for the channel proteins take place, including targeting of the internalized proteins for lysosomal degradation. Alternatively, the endosomes may be recycled back to the surface membrane. Indeed, the pathways taken by internalized channel proteins that eventually recycle to the cell surface are highly complex and tightly regulated (2, 3). Recent studies have provided evidence for the critical roles of different isoforms of Rab-proteins in sorting the internalized protein in early endosomes to Rab-GTPase-specific compartments (22, 23, 29). Rab proteins represent the largest branch of the small G protein superfamily and regulate each of the major steps in vesicular transport (22, 23, 29). Rab GTPases define the various intracellular trafficking vesicles by recruiting the effectors required for their function, for example, Rab5 is required for clathrin-mediated endocytosis and early endosome formation (30) and recruits EEA1 to these vesicles.
Our data demonstrate that the surface expression of SK2 channel is dependent on early endosome–mediated endocytic recycling because expression of SK2 channel alone in a heterologous system or knockdown of the α-actinin2 proteins in native cells, as well as pretreatment with primaquine, result in SK2 channel localization mainly in the early endosomes. Moreover, α-actinin2 cytoskeletal protein is crucial for SK2 channel anchoring at the surface membrane and along the T-tubular structure in cardiac myocytes. Lack or knockdown of α-actinin2 may lead to an increase in the rate of internalization and/or a decrease in the targeting of the early endosomes to the surface membrane (Fig. 5C), supporting the notion that α-actinin2 anchoring is required for the proper retrograde trafficking of SK2 channels. Additional studies are required to further differentiate between these possibilities. New understanding into ion channel trafficking may provide insights into unique therapeutic opportunities to modify channel-trafficking diseases.
Methods
Additional details can be found in SI Materials and Methods.
Site-Directed Mutagenesis.
Point mutations were generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (catalog no. 210518; Stratagene).
Cell-Surface Biotinylation and Isolation of Cell-Surface Proteins.
Biotinylation of surface proteins was carried out using cell-surface protein isolation kit (catalog no. 89881; Pierce) as per the manufacturer's instructions.
YTH Assays.
YTH assays were performed with the GAL4 system using the Matchmaker GAL4 Two-Hybrid System 3 (Clontech).
siRNA Gene Silencing.
For the siRNA experiments, 80 pmol per 35-mm Petri dish of fully annealed, 4 different α-actinin2 siRNA were transfected into cultured neonatal mouse cardiomyocytes 2 days after isolation using GeneSilencer siRNA Transfection Reagent (Gene Therapy Systems), according to the recommendations of the manufacturer.
Immunofluorescence Confocal Microscopy and FRAP Experiments.
Immunofluorescent labeling was performed as described in ref. 17. FRAP experiments were performed on the temperature-controlled stage at 37 °C with a Zeiss LSM 510 META confocal microscope and MultiTime software.
Immunogold-Labeled Transmission Electron Microscope Experiment.
Mouse hearts were fixed in 4% formaldehyde [Electron Microscopy Sciences (EMS)] in 0.1 M Sorenson phosphate buffer (pH 7.2; EMS) for >1 h. Other procedures were described in ref. 31.
Functional Study Using IP and Patch-Clamp Recordings.
Whole-cell IK,Ca was recorded from freshly isolated atrial myocytes and transfected HEK 293T cells using voltage-ramp protocol and patch-clamp techniques, as previously described (14, 32).
Supplementary Material
Acknowledgments.
We thank Dr. E. N. Yamoah for helpful suggestions and comments. This work was funded by National Institutes of Health Grants HL85727 and HL85844 (to N.C.) and a Department of Veterans Affairs Merit Review Grant (to N.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908207106/DCSupplemental.
References
- 1.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 2.Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. J Physiol. 2007;582(Pt 1):17–26. doi: 10.1113/jphysiol.2007.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele DF, Zadeh AD, Loewen ME, Fedida D. Localization and trafficking of cardiac voltage-gated potassium channels. Biochem Soc Trans. 2007;35(Pt 5):1069–1073. doi: 10.1042/BST0351069. [DOI] [PubMed] [Google Scholar]
- 4.Le Scouarnec S, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivaprasadarao A, Taneja TK, Mankouri J, Smith AJ. Trafficking of ATP-sensitive potassium channels in health and disease. Biochem Soc Trans. 2007;35(Pt 5):1055–1059. doi: 10.1042/BST0351055. [DOI] [PubMed] [Google Scholar]
- 6.Ficker E, Dennis A, Kuryshev Y, Wible BA, Brown AM. HERG channel trafficking. Novartis Found Symp. 2005;266:57–69. discussion 70–74, 95–99. [PubMed] [Google Scholar]
- 7.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb Exp Pharmacol. 2006;2006(171):349–355. doi: 10.1007/3-540-29715-4_14. [DOI] [PubMed] [Google Scholar]
- 8.Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: Ion channel protein trafficking. Circ Res. 2004;94:1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea. [DOI] [PubMed] [Google Scholar]
- 9.Kohler M, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 10.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 11.Stocker M. Ca2+-activated K+ channels: Molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 12.Pennefather P, Lancaster B, Adams PR, Nicoll RA. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci USA. 1985;82:3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedarzani P, et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current IAHP and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 15.Tuteja D, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 16.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, et al. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via α-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson RA, et al. Ca2+-independent binding of an EF-hand domain to a novel motif in the alpha-actinin-titin complex. Nat Struct Biol. 2001;8:853–857. doi: 10.1038/nsb1001-853. [DOI] [PubMed] [Google Scholar]
- 19.Wissmann R, et al. A helical region in the C terminus of small-conductance Ca2+-activated K+ channels controls assembly with apo-calmodulin. J Biol Chem. 2002;277:4558–4564. doi: 10.1074/jbc.M109240200. [DOI] [PubMed] [Google Scholar]
- 20.van Weert AW, Geuze HJ, Groothuis B, Stoorvogel W. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur J Cell Biol. 2000;79:394–399. doi: 10.1078/0171-9335-00062. [DOI] [PubMed] [Google Scholar]
- 21.Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen DP, et al. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282(40):29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 23.Zadeh AD, et al. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol. 2008;586(Pt 20):4793–4813. doi: 10.1113/jphysiol.2008.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjoblom B, Salmazo A, Djinovic-Carugo K. Alpha-actinin structure and regulation. Cell Mol Life Sci. 2008;65(17):2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554(Pt 2):255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumacher MA, Crum M, Miller MC. Crystal structures of apocalmodulin and an apocalmodulin/SK potassium channel gating domain complex. Structure. 2004;12:849–860. doi: 10.1016/j.str.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin: Cell surface expression and gating. J Biol Chem. 2003;278:25940–25946. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- 29.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 30.Bucci C, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 31.Erickson AH, Ginns EI, Barranger JA. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985;260:14319–14324. [PubMed] [Google Scholar]
- 32.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.