Abstract
A critical step in synapse formation is the clustering of neurotransmitter receptors in the postsynaptic membrane, directly opposite the nerve terminal. At the neuromuscular junction, a widely studied model synapse, acetylcholine receptors (AChRs) initially aggregate to form an ovoid postsynaptic plaque. As the synapse matures, the plaque becomes perforated and is eventually transformed into a complex, branched structure. We found that this transformation also occurs in myotubes cultured in the absence of neurons, and used this system to seek machinery that orchestrates postsynaptic maturation. We show that perforations in the AChR aggregate bear structures resembling podosomes, dynamic actin-rich adhesive organelles involved in matrix remodeling in non-neuronal cells but not described in neural structures. The location and dynamics of synaptic podosomes are spatiotemporally correlated with changes in AChR aggregate topology, and pharmacological disruption of podosomes leads to rapid alterations in AChR organization. Our results indicate that synaptic podosomes play critical roles in maturation of the postsynaptic membrane.
Keywords: acetylcholine receptor, endocytosis, neuromuscular junction, synapse formation
An early step in the formation of chemical synapses is the aggregation of neurotransmitter receptors in the postsynaptic membrane. Subsequently, as the synapse matures, receptor aggregates change in size, shape, receptor density, and the composition of associated signaling and cytoskeletal proteins (1, 2). Whereas the initial aggregation of receptors renders the synapse functional, the subsequent alterations are critical for synaptic growth and adaptation to changing conditions.
A dramatic example of postsynaptic maturation is in evidence at the skeletal neuromuscular junction (NMJ), a synapse that has been studied extensively because it is more accessible and some 100 times larger than typical synapses in the brain (1). Newly formed myotubes synthesize and assemble acetylcholine receptors (AChRs) and cluster them in small, ovoid plaques. In some cases, axons preferentially form synapse in apposition to the plaques, while at other synapses, axons organize AChR clustering (2, 3). Then, during a brief postnatal period, the plaque is transformed into a complex pretzel-shaped array of AChR-rich branches.
Over the past years, several key molecular players in AChR aggregation have been identified. They include the tyrosine kinase MuSK, a low density lipoprotein-related protein, LRP4, a MuSK-associated adapter, DOK7, an AChR-binding cytoskeletal protein, rapsyn, and a nerve-derived proteoglycan, z-agrin (1, 3–6). In contrast, little is known about the cellular or molecular mechanisms that regulate postsynaptic maturation. Proteins implicated in this transformation including members of the dystrophin-glycoprotein complex, such as dystroglycan and α-dystrobrevin, and laminin isoforms that are associated with AChR aggregates and bind dystroglycan (7–10). The cellular mechanisms by which these proteins act remain obscure.
Recently, we found that AChR-rich ovoid plaques on myotubes cultured aneurally spontaneously mature into branched, pretzel-shaped arrays of AChR-rich branches. These arrays resemble those in mature NMJs in many respects: their size and topology are similar, they arise through a series of transitional stages similar to those in vivo, and their formation, like that of the NMJ, requires MuSK and rapsyn (11). These results are significant in two respects: first, they show that although postsynaptic maturation is clearly regulated by the nerve, the muscle has a cell autonomous capacity to form topologically complex postsynaptic structures. Second, they provide a simple system in which postsynaptic maturation can be monitored and manipulated.
Here, we used cultured myotubes to explore muscle-intrinsic mechanisms that might account for patterning of the postsynaptic membrane. We were surprised to find that AChR aggregates contain dynamic actin-rich, adhesive organelles called podosomes. Podosomes were described originally in monocyte-derived cells such as macrophages, dendritic cells, and osteoclasts but recently were found in many other cell types (12–16). They are capable of remodeling the extracellular matrix and are believed to play a role in cell migration (12–15, 17, 18). To date, they have not been described in neurons or synapses. We show that myotubes can use these organelles to remodel AChR aggregates. Our results suggest that they are critical mediators of the “plaque-to-pretzel” transition central to synaptic maturation.
Results
Actin-Rich Aggregates at AChR Clusters in Myotubes.
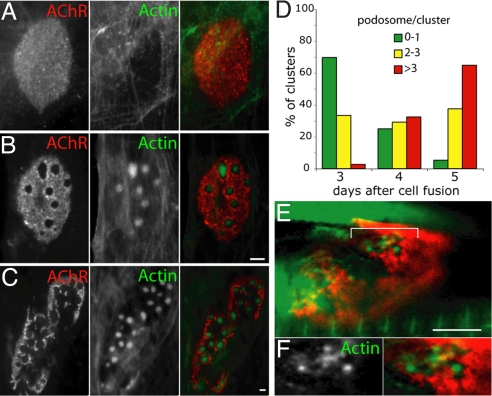
As the NMJ matures, an initially plaque-shaped postsynaptic apparatus is remodeled, via perforated transitional stages, to form an elaborate, branched “pretzel-like” structure (1). Mechanisms that underlie this transformation are unknown, but previous studies have suggested that regulated polymerization of cytosekeletal actin leads to the movement of AChRs during initial cluster formation (19, 20). We therefore examined the organization of actin as AChR clusters mature. To this end, we plated mononucleated cells of the C2 myogenic cell line on laminin-coated surfaces under conditions that promote fusion of myoblasts into myotubes, formation of plaque-shaped AChR clusters, and maturation of plaques on the substrate-facing surface of the myotubes into complex arrays (2, 11). We stained the myotubes with phalloidin, which binds to F-actin, and α-bungarotoxin (BTX), which binds to AChRs. Fine actin cables were present beneath plaque-shaped clusters (Fig. 1A). In contrast, complex clusters bore larger actin-rich puncta, which were localized precisely beneath perforations in the plaques (Fig. 1B), and between branches in the “pretzel-shaped” arrays (Fig. 1C). Puncta were generally circular and ranged in diameter from <0.5 μm to approximately 5 μm, but some were ovoid and exceeded 10 μm in length. In some cases, puncta were composed of closely-packed subunits and clouds of actin (Fig. S1). The number of puncta per AChR aggregate increased several-fold as clusters matured (Fig. 1D). Similar puncta were associated with AChR clusters formed on myotubes obtained from dissociated neonatal mouse myoblasts (Fig. S2).
Fig. 1.
Actin–rich puncta lie within the perforations of AChR aggregates. C2C12 myotubes cultured on laminin were incubated with BTX (red) and phalloidin (green) to label AChR and F-actin, respectively. (A) In immature AChR plaques (day 3 after cell fusion) fine actin cables lie below aggregates. (B) As plaques become perforated (day 4 after cell fusion), actin-rich puncta appear within perforations. (C) As aggregates become branched (day 5 after cell fusion), they bear multiple actin-rich puncta. (D) The number of actin-rich puncta increases during maturation of the AChR clusters in C2C12 myotubes (n = >45 per time point). (E) Similar actin-rich structures were found in perforations at the developing NMJ (postnatal day 8). Tibialis anterior muscle was infected with adenovirus expressing β–actin-GFP (green) and counterstained with BTX (red). Area bracketed in (E) is shown in higher magnification in (F). (Scale bars, 5 μm.)
To seek actin-rich puncta at NMJs in vivo we infected muscles in neonatal mice with adenovirus expressing a β-actin-GFP fusion protein. Although some GFP was associated with sarcomeres, confocal microscopy revealed that the postsynaptic apparatus contained actin-rich structures during the first postnatal week, as NMJs mature (Fig. 1E). As in cultured myotubes, the puncta were associated with perforations that form in AChR-rich plaques as they transform into branched arrays.
Podosomes at AChR Clusters in Myotubes.
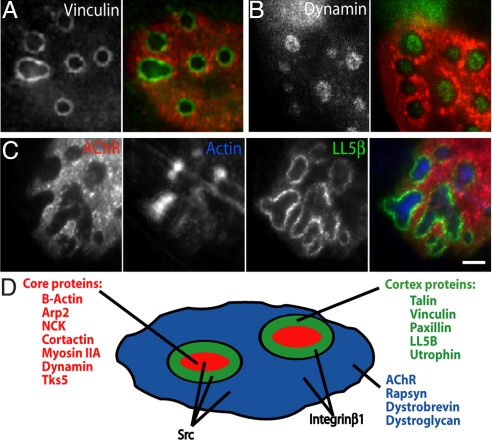
We next asked whether proteins known to associate with actin cytoskeleton were co-localized with actin puncta in the perforated regions of AChR clusters. We assessed two known components of actin-rich focal adhesions, vinculin and talin, both of which have been reported to be present at NMJs in vivo (21, 22). Surprisingly, immunoreactivity was most concentrated in narrow ring-like structures that lined the internal edge of perforations in the AChR clusters (Fig. 2A and Fig. S3). Triple-labeling confirmed that these cytoskeletal proteins were precisely positioned between areas of highest actin and AChR density (Fig. S3).
Fig. 2.
Components of synaptic podosomes. C2C12 myotubes were labeled with BTX (red) and antibodies to the indicated components (green): vinculin (A), dynamin (B), LL5β and actin (C). (Scale bar, 5 μm.) (D) Schematic localization of proteins in synaptic podosomes. AChR-rich region (blue) also contains dystroglycan, α-dystrobrevin, rapsyn, integrin β1, and Src. Perforations in the receptor clusters are occupied by cores (red) containing β-actin, Arp2, NCK, cortactin, myosin IIA, dynamin, Tks5, and Src. The core is surrounded by a cortex (green) rich in talin, vinculin, paxillin, LL5β, integrin β1, Src, and utrophin.
The presence of a cortex of focal adhesion components ensheathing an actin-rich core is a characteristic of podosomes, adhesive organelles that are believed to play roles in cell migration and matrix remodeling, but have not previously been observed in the nervous system (see Introduction). To ask whether the AChR-associated structures were indeed podosomes, we stained C2C12 cells with antibodies to proteins known to be components of this organelle. These included Arp2, NCK, cortactin, myosin IIa, dynamin, Tks5, paxillin, src kinase, integrin β1, and utrophin (12, 14, 23–28). All of these proteins were present at the podosome-like structures, concentrated in core or cortex as shown in other cells, although src and integrin β1 were also present at substantial levels in the AChR-rich domain (Fig. 2 B and D and Fig. S3). LL5β, a phosphatidylinositol-binding protein previously shown to be associated with focal adhesions (29) and AChR clusters (30), was also concentrated in the cortex domain (Fig. 2C). Together, these results provide strong evidence that podosomes are associated with maturing AChR aggregates in myotubes, and identify LL5β as a novel podosomal component.
Functional Attributes of Synaptic Podosomes.
Previous studies have shown that podosomes are dynamic structures that mediate strong adhesion to and remodeling of the underlying substratum (13, 14, 31). We asked whether synaptic podosomes share these attributes.
Substrate Adhesion.
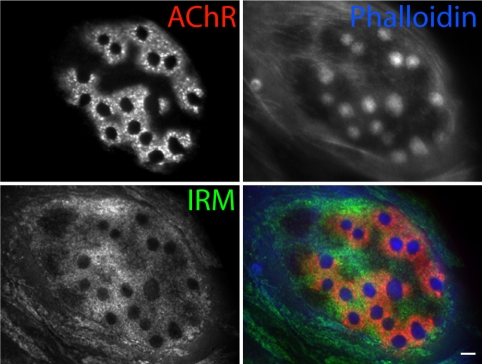
We used internal reflection microscopy to visualize localization of adherent sites in myotubes. In this technique, the amount of reflected light depends on the distance between the cell membrane and substratum, so closer appositions appear darker. Immature AChR plaques were generally present within broad regions of close cell-substratum adhesion (Fig. S4) as shown previously (32). In branched arrays of AChRs, in contrast, adhesivity was markedly non-uniform, with sites of highest adhesion regularly associated with podosomes (Fig. 3). Thus, as in other cells (17, 18), synaptic podosomes are sites of attachment to the substratum.
Fig. 3.
Synaptic podosomes are sites of adhesion. Adhesion of C2C12 myotubes to substratum revealed by internal reflection microscopy (IRM). In mature clusters, podosomes are sites of strong adhesion. Dark spots in IRM image reveal close apposition to substratum. (Scale bar, 5 μm.)
Remodeling of Extracellular Matrix.
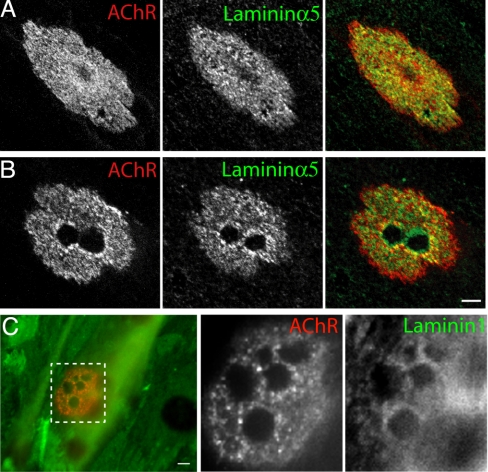
Laminins containing the α5 and β2 chains are secreted by myotubes and concentrated in the matrix apposed to AChR clusters (10, 33). Here, we asked whether the distribution of these laminins within clusters corresponded to that of podosomes. Both chains were co-distributed with AChRs in plaques (Fig. 4A and Fig. S5A). As plaques remodeled to form complex arrays, however, the laminins were lost from beneath podosomes (Fig. 4B and Fig. S5 A and B), as were the matrix receptors dystroglycan and integrin β1 (Fig. S5 E–H), raising the possibility that proteases associated with podosomes (13) had selectively degraded the matrix in these areas. To test this idea, we examined the distribution of the exogenous laminin 111 (α1/β1/γ1) used to coat the substratum. Much of the laminin persisted throughout the culture period but levels were selectively reduced beneath podosomes (Fig. 4C). To rule out the possibility that the reduced immunoreactivity reflected inaccessibility of the substratum to antibodies, we treated cells with the detergent saponin at 37 °C before fixation and with Triton X-100 after fixation, then stained with anti-laminin antibody. Residual patches of AChR allowed identification of synaptic sites; laminins were absent from the perforations in the AChR clusters (Fig. S5 C and D). Together, these results indicate that synaptic podosomes remodel extracellular matrix at the AChR clusters.
Fig. 4.
Synaptic podosomes remodel extracellular matrix. (A and B) C2C12 myotubes were stained for AChR and laminin α5. In immature clusters, laminin α5 (green) and β2 (Fig. S5A) are distributed underneath the AChRs (red) (A). After formation of podosomes, laminin α5 (B) is lost from sites of perforations in the AChR. A thin ring of laminins often remains at the edge of the perforation, corresponding to sites of increased concentrations of integrin β1 (Fig. S3). (C) Exogenous laminin 1 (green) that had been used to coat slide surface before plating cells was also removed from beneath AChR perforations. (Scale bar, 5 μm.)
Localized Endocytosis.
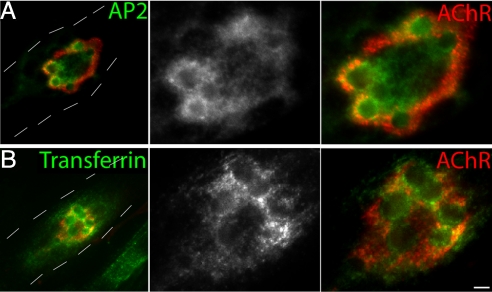
Previous studies have shown that clathrin is associated with podosomes in osteoclasts (31, 34) suggesting that they may be sites of endocytosis. Using fluorescent protein fusions, we found that the clathrin light chain and AP2, another component of the endocytic machinery, were enriched around a subset of synaptic podosomes. Both proteins were concentrated in regions of AChR clusters and, within these clusters, were preferentially associated with podosomes (Fig. 5A and Fig. S6).
Fig. 5.
Enhanced endocytosis associated with synaptic podosomes. (A) C2C12 myotubes expressing AP2 sigma subunit. AP2 was concentrated at the AChR clusters (Left; lower magnification; dotted lines outline the location of myotube) and within the clusters, they were enriched around podosomes in the AChRs (Center and Right; higher magnification). (B) Internalization of endocytic tracers, transferrin, occurs at similar locations. (Scale bar, 5 μm.)
As a direct test of the idea that endocytosis is enhanced at or near podosomes, we incubated cells for 5–10 min with three fluorescently-tagged endocytic tracers: dextran (molecular weight, 3,000), a marker of fluid phase uptake; transferrin, which is internalized by a receptor-mediated process; and FM4–64, a lipophilic dye taken up as membranes are internalized (35). All three markers accumulated at AChR clusters on myotubes (Fig. 5B and Fig. S6 B and C Left). Within AChR-rich regions, all three tracers were present at highest concentrations near the edges of podosomes (Fig. 5B and Fig. S6 B and C Center and Right). The accumulation represented internalization rather than binding to the surface, as indicated by lack of labeling when incubation was performed on ice (Fig. S6 E–G). Thus, synaptic podosomes are sites of intense endocytotic activity.
Dynamism.
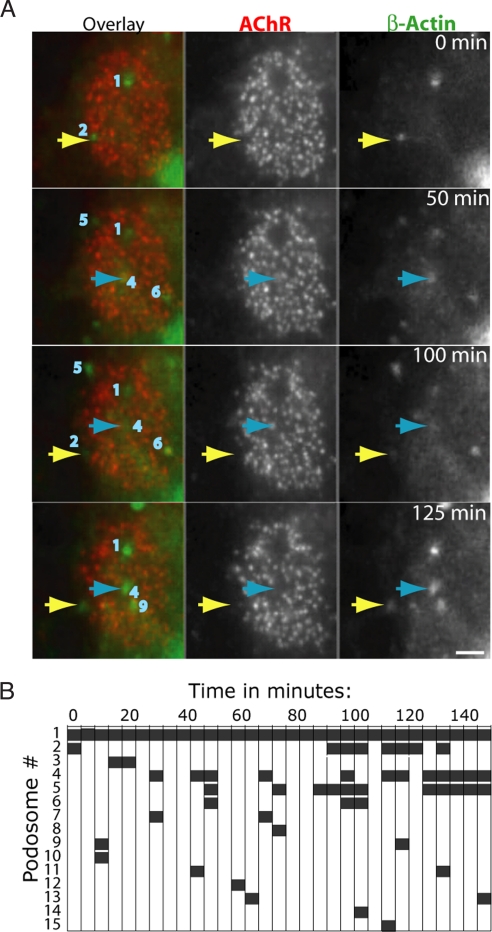
Time-lapse imaging of macrophages, osteoclasts, and fibroblasts has shown that podosomes can appear, disappear, grow, shrink, change shape, fuse or split within minutes (14, 17, 36, 37). To ask whether synaptic podosomes exhibit similar dynamism, we infected myotubes with an adenoviral vector encoding a β-actin-GFP fusion, waited 2 days, labeled the cells with α-bungarotoxin, and imaged AChR clusters at intervals of 5 min for 2–24 h. Fig. 6A shows images from a movie. At the first view, two podosomes were associated with the cluster. During the subsequent 2 h, one (no. 1) remained unchanged while the other (no. 2, yellow arrows) disappeared and then reappeared at the same site. Of 15 podosomes that appeared within this cluster during the period of 2.5 h, six disappeared rapidly (nos. 3, 8, 10, 12, 14, and 15), eight disappeared and reappeared (nos. 2, 4–7, 9, 11, and 13) and four were present at the end of this imaging session (nos. 1, 4, 5, and 13) (Fig. 6B). Fig. S7, which diagrams data from another culture, illustrates the lateral dynamics of synaptic podosomes: their movement, growth, shrinkage, fusion, and fission. Thus, although some synaptic podosomes are stable for periods of hours, many appear, disappear, and remodel over periods of minutes.
Fig. 6.
Dynamic behavior of podosomes revealed by time-lapse imaging. (A) Selected frames from a 2.5-h imaging session. (B) Each podosome was numbered and its presence through the session is plotted. While some podosomes are stable, others are dynamic. Podosomes often disappear then reappear at the same location, as shown for podosome 2 (yellow arrow in A) or 4 (blue arrow in A). (Scale bar, 5 μm.)
Correlation of Podosome Dynamism with AChR Cluster Remodeling.
AChR aggregates on cultured myotubes remodel by formation, fusion and fission of perforations within the plaque (11). The association of dynamic podosomes with these perforations raised the possibility that podosomes could drive the remodeling. To investigate this possibility we analyzed the relationship between podosomes and AChRs at high spatial and temporal resolution. The appearance, disappearance and remodeling of podosomes were frequently associated with changes in the shape and size of AChR aggregates.
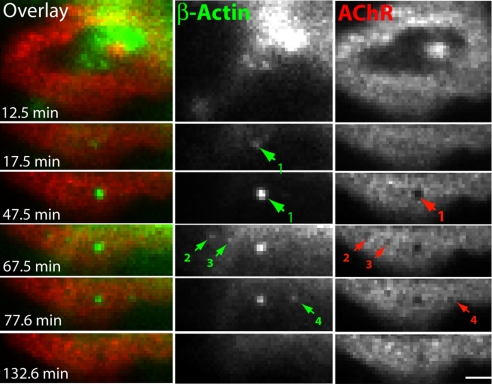
Several examples are evident in Fig. 7. Four podosomes (nos. 1–4) appeared within 1 h, each accompanied by formation of a perforation in the AChR aggregate. In one case (no. 1) formation of the perforation clearly follows the appearance of the podosome. In two cases (nos. 2 and 3), the disappearance of the podosome is followed by the collapse of the perforation. Another example of podosome-associated perforation collapse is shown in Fig. S8. Thus, formation of podosomes correlates well in time and space with changes in assembly of AChRs.
Fig. 7.
Appearance of podosomes correlates with maturation of AChR clusters. Time-lapse analysis revealed that podosomes (green arrow) appear underneath AChR clusters at sites where perforations subsequently form (red arrow no. 1). Each perforation formed during analyzed period was marked with red arrow and numbered. While perforation 1 was long lived, other collapsed soon after podosomes disappeared. (Scale bar, 5 μm.)
Inhibition of Podosome Formation Affects AChR Localization.
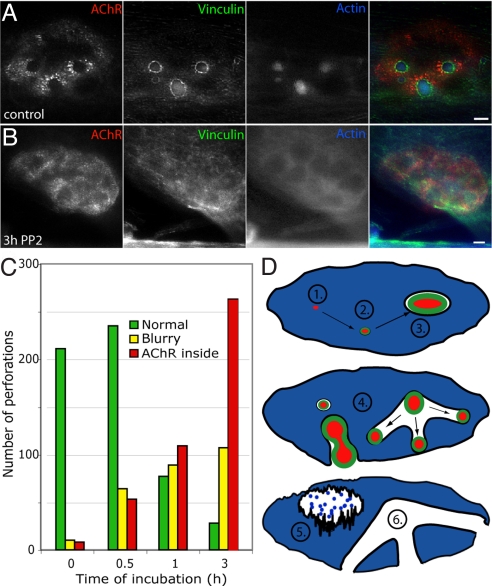
The observation that podosomes often appear or disappear before formation or collapse of perforations in AChR clusters (Fig. 7 and Fig. S8), suggest a causal role for podosomes in cluster remodeling. To test this idea, we sought agents that would rapidly block podosome formation or provoke their disassembly without detectably affecting myotube shape or viability. The Src kinase inhibitor, PP2, which is known to block podosome formation in other cell types (38, 39), led to loss of most podosomes in myotubes within 0.5 h of addition, and nearly complete loss by 3 h (Fig. 8 A–C and Fig. S9A). As podosomes disassembled, perforations lost their sharp boundaries and AChRs appeared within them (Fig. S9), just as seen following spontaneous podosome disassembly (Fig. S8). Importantly, significant changes in AChR distribution were visible by 0.5 h after addition of inhibitor (Fig. 8C and Fig. S9A), but no significant alterations in cellular morphology were observed for at least 2.5 additional hours. PP2 also blocked selective endocytosis at perforations within AChR clusters as assayed by dextran uptake (Fig. S10). Dynasore, an inhibitor of dynamin function, which is known to be essential for podosome formation (39), had effects similar to, although weaker than those of PP2 on podosomes and AChR clusters (Fig. S9).
Fig. 8.
Loss of podosomes leads to altered distribution of AChRs. (A and B) Incubation with Src family kinase inhibitor PP2 led to disassembly of podosomes as shown by loss of actin puncta and dispersal of vinculin. This disruption was associated with redistribution of AChRs into the perforation. (C) Quantification of PP2-depemdent alteration in AChRs distribution. Graph shows measurements from a total of three cultures. (D) Schematic summary of results providing a model for podosomes dependent remodeling of AChR clusters. Muscle cells deposit synaptic basal lamina components underneath AChR plaques (blue). Small actin complexes associated with AChRs give rise to podosomes that grow and form perforations in the cluster (2). Podosomes secrete proteases that remodel basal lamina; along with local endocytosis, this leads to remodeling of the postsynaptic membrane (3). Growth of podosomes leads to formation of branches (4) whereas disassembly of podosomes leads to entry of AChR into perforations (5). With time, however perforations become stable and no longer require podosomes for their maintenance (6). (Scale bar, 5 μm.)
Discussion
Podosomes have been studied in many cell types (12–17, 23–28, 31, 36–44), but they have not previously been described in neurons or neural structures. Moreover, their functions remain obscure. We report here that the developing postsynaptic membrane in skeletal myotubes contains structures that resemble podosomes in many respects. We provide evidence that one function of synaptic podosomes is to direct the maturation of the postsynaptic membrane.
Synaptic Podosomes.
The presence of organelles resembling podosomes in synaptic structures was unexpected. We therefore compared their composition and bioactivities carefully with those of “canonical” podosomes. In fact, synaptic podosomes share many structural and functional features with podosmes described in other cells. First, their size, shape, and occurrence in groups are within reported ranges (12, 14, 36, 40, 41). Second, they contain numerous proteins found in other podosomes, including actin, Arp2/3, cortactin, dynamin, myosin IIA, Tks5/FISH, talin, vinculin, paxillin, integrin β1, utrophin, and src (Fig. 2 and Fig. S3) (12, 14, 16, 39, 42, 43). Third, the components are arragned in two domains, an actin-rich core surrounded by an annular cortex (Fig. 2) (12, 14). Fourth, they are sites of attachment to the substratum (Fig. 3) (18, 24, 44). Fifth, they remodel extracellular matrix components (Fig. 4 and Fig. S5) (13). Sixth, they are sites of active endocytosis (Fig. 5 and Fig. S6), a feature shared with some (31, 34, 40, 45–48) but perhaps not all (49) other podosomes. Seventh, they are highly dynamic: they can appear, disassemble, change their size or shape, and undergo fission and fusion within minutes (Fig. 6 and Fig. S7) (36, 37, 41). Finally, they are disassembled by inhibitors known to disrupt other podosomes (Fig. 8 and Fig. S9) (49–51). In view of these many similarities, we feel justified in calling the structures we have studied podosomes.
Podosomes Shape the Postsynaptic Membrane.
Several observations indicate that synaptic podosomes are involved in remodeling the postsynaptic membrane as it matures. First, podosomes are precisely localized to perforations and indentations in AChR clusters (Fig. 1). Second, podosomes appear underneath AChR clusters that subsequently become perforated at this location (Fig. 7). Third, AChRs are removed locally at sites where podosomes form, and AChRs diffuse into sites from which podosomes have recently disappeared (Fig. 7 and Fig. S8). Finally, pharmacological disruption of podosomes leads to similar disaggregation of AChRs and collapse of perforations. (Fig. 8 and Fig. S9).
Our observations suggest a scenario by which podosomes might lead to transformation of an AChR plaque into a pretzel (Fig. 8D). Newly formed mytobes synthesize and assemble AChRs, aggregate them into plaques, and secrete specialized basal lamina components that are closely apposed to the plaques (10, 52). The plaques initially overlie small adhesion complexes (Fig. 1A) that mature to form podosomes (Fig. 8D, nos. 1 and 2). As the podosomes grow, AChRs are displaced and proteases locally degrade components of the synaptic basal lamina. Some podosomes grow, divide and move laterally, forming indentations and elongated branches in the AChR aggregate, thereby transforming the plaque into a complex, pretzel-like shaped structure (Fig. 8D, no. 4). When podsomes disassemble during this period, AChRs diffuse into the perforation, but eventually the AChR-rich region becomes stabilized, and therefore remains intact even after podosomes are lost (Fig. 8D, nos. 5 and 6).
Although the molecular mechanisms that link podosomal dynamics to synaptic remodeling remain to be discovered, our observations suggest several possibilities. First, localized endocytosis could remove AChRs and associated components from sites of podosomes, to generate perforations (1, 11). Second, components of the cortex could serve as a diffusion barrier, maintaining the separation between AChR-rich and poor regions (30). The entry of AChRs into perforations following podosome disassembly supports this idea. Third, podosomes may generate lateral force to displace AChRs and other postsynaptic components through actin-myosin interactions, aggregation of membrane-distorting proteins such as dynamin and cortactin, and engagement of the AChR-associated cytoskeleton. Fourth, focal removal of matrix components from beneath podosomes could lead to localized disassembly of postsynaptic structures. Indeed, myotubes deposit specialized laminins in the basal lamina directly abutting AChR aggregates (52) and these laminins act through receptors such as dystroglycan, to promote AChR stabilization and postsynaptic maturation (8, 10). Local removal of laminins at podosomes is likely to provoke destabilization of AChRs in these regions, increasing their susceptibility to disassembly by endocytosis or lateral displacement. Finally, proteases associated with podosomes may directly degrade dystroglycan (53, 54) and potentially other components of the postsynaptic apparatus.
It is tempting to speculate that similar processes may be involved in the formation or maturation of neuron-neuron synapses. Although these synapses are too small to contain conventional podosomes, their postsynaptic receptors are often organized into plaques that contain scattered perforations and are enriched in several components of podosomes, such as actin, Arp2/3, and cortactin.
Materials and Methods
Plasmids and Adenoviral Constructs.
Adenoviral plasmid pAd-β-actin-GFP, encoding β-actin-GFP was a kind gift from Bernard Hoflack (Biotechnologisches Zantrum). Plasmids pAP2-Tom, encoding rat adaptor protein complex subunit sigma 1(AP2S1) fused to Tomato, and pCLA-GFP enocding rat clathrin light chain (CLA1) fused to GFP, were kind gifts from Tom Kirchhausen (Harvard University, MA) (55, 56). An adenoviral plasmid encoding rapsyn-cherry was constructed from rapsyn and Cherry DNAs, using the Gateway system. Adenoviral particles were produced in our lab using the AdEasy System (Stratagene) or by Welgen, Inc.
Cell Culture.
C2C12 cells (American Type Culture Collection, CRL-1772) and primary muscle cells were cultured as described in (11). Detailed protocols are provided in SI Text. Fluorescently labeled endocytic tracers, 1 mg/mL FITC-dextran (3000 MW, Invitrogen), 80 μg/mL transferrin labeled with Alexa Fluor 568 (Invitrogen) and 10 μM FM4–64 (Molecular Probes) were added to the cultured myotubes for 5–7 min. Cells were then washed three times with fusion media and fixed with PFA as above. Transient transfection was performed with Metafectene PRO (Biontex) according to the product manual. To inhibit formation of podosomes, cells were incubated with 25 μM PP2 (Alexis Biochemicals) or 80 μM Dynasore (Tocris).
Histology and Imaging.
Protocols for epifluoresence, confocal, time-lapse, and interference contrast imaging of the stained samples is provided in SI Text.
Supplementary Material
Acknowledgments.
We thank B. Hoflack and T. Kirchhausen for plasmids and Corey McCann for help in initial time-lapse imaging. This work was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (to J.R.S.) and a fellowship from the International Human Frontier Science Program Organization (to T.J.P.).
Note Added in Proof.
Finally, while this article was under review, Lee et al. (57) published results on Xenopus muscle consistent with our conclusion that actin-based structures participate in the remodeling of AChR clusters.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910391106/DCSupplemental.
References
- 1.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 2.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: Paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- 4.Kim N, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, et al. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada K, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- 7.Grady RM, et al. Maturation and maintenance of the neuromuscular synapse: Genetic evidence for roles of the dystrophin-glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 8.Grady RM, et al. Tyrosine-phosphorylated and nonphosphorylated isoforms of alpha-dystrobrevin: Roles in skeletal muscle and its neuromuscular and myotendinous junctions. J Cell Biol. 2003;160:741–752. doi: 10.1083/jcb.200209045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J Cell Biol. 2001;152:435–450. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimune H, et al. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol. 2008;182:1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kummer TT, Misgeld T, Lichtman JW, Sanes JR. Nerve-independent formation of a topologically complex postsynaptic apparatus. J Cell Biol. 2004;164:1077–1087. doi: 10.1083/jcb.200401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Linder S. The matrix corroded: Podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Linder S, Aepfelbacher M. Podosomes: Adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 15.Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol. 2006;85:191–194. doi: 10.1016/j.ejcb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Thompson O, et al. Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts. PLoS ONE. 2008;3:e3638. doi: 10.1371/journal.pone.0003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JG, Correia I, Krasavina O, Watson N, Matsudaira P. Macrophage podosomes assemble at the leading lamella by growth and fragmentation. J Cell Biol. 2003;161:697–705. doi: 10.1083/jcb.200212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchisio PC, Capasso O, Nitsch L, Cancedda R, Gionti E. Cytoskeleton and adhesion patterns of cultured chick embryo chondrocytes during cell spreading and Rous sarcoma virus transformation. Exp Cell Res. 1984;151:332–343. doi: 10.1016/0014-4827(84)90384-7. [DOI] [PubMed] [Google Scholar]
- 19.Dai Z, Luo X, Xie H, Peng HB. The actin-driven movement and formation of acetylcholine receptor clusters. J Cell Biol. 2000;150:1321–1334. doi: 10.1083/jcb.150.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbins GC, Zhang B, Xiong WC, Mei L. The role of the cytoskeleton in neuromuscular junction formation. J Mol Neurosci. 2006;30:115–118. doi: 10.1385/JMN:30:1:115. [DOI] [PubMed] [Google Scholar]
- 21.Bloch RJ, Hall ZW. Cytoskeletal components of the vertebrate neuromuscular junction: Vinculin, alpha-actinin, and filamin. J Cell Biol. 1983;97:217–223. doi: 10.1083/jcb.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sealock R, Paschal B, Beckerle M, Burridge K. Talin is a post-synaptic component of the rat neuromuscular junction. Exp Cell Res. 1986;163:143–150. doi: 10.1016/0014-4827(86)90566-5. [DOI] [PubMed] [Google Scholar]
- 23.Block MR, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Kaverina I, Stradal TE, Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–4924. doi: 10.1242/jcs.00818. [DOI] [PubMed] [Google Scholar]
- 25.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 26.Tehrani S, Faccio R, Chandrasekar I, Ross FP, Cooper JA. Cortactin has an essential and specific role in osteoclast actin assembly. Mol Biol Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: Roles of the protein interaction domains. Exp Cell Res. 2006;312(6):760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Webb BA, Eves R, Mak AS. Effects of tyrosine phosphorylation of cortactin on podosome formation in A7r5 vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C463–471. doi: 10.1152/ajpcell.00350.2005. [DOI] [PubMed] [Google Scholar]
- 29.Lansbergen G, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006;11:21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Kishi M, Kummer TT, Eglen SJ, Sanes JR. LL5beta: A regulator of postsynaptic differentiation identified in a screen for synaptically enriched transcripts at the neuromuscular junction. J Cell Biol. 2005;169:355–366. doi: 10.1083/jcb.200411012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akisaka T, Yoshida H, Suzuki R, Takama K. Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res. 2008;331:625–641. doi: 10.1007/s00441-007-0552-x. [DOI] [PubMed] [Google Scholar]
- 32.Bloch RJ, Geiger B. The localization of acetylcholine receptor clusters in areas of cell-substrate contact in cultures of rat myotubes. Cell. 1980;21:25–35. doi: 10.1016/0092-8674(80)90111-7. [DOI] [PubMed] [Google Scholar]
- 33.Martin PT, Ettinger AJ, Sanes JR. A synaptic localization domain in the synaptic cleft protein laminin beta 2 (s-laminin) Science. 1995;269:413–416. doi: 10.1126/science.7618109. [DOI] [PubMed] [Google Scholar]
- 34.Akisaka T, Yoshida H, Suzuki R, Shimizu K, Takama K. Clathrin sheets on the protoplasmic surface of ventral membranes of osteoclasts in culture. J Electron Microsc. 2003;52:535–543. doi: 10.1093/jmicro/52.6.535. [DOI] [PubMed] [Google Scholar]
- 35.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinardi L, et al. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Evans JG, Matsudaira P. Structure and dynamics of macrophage podosomes. Eur J Cell Biol. 2006;85:145–149. doi: 10.1016/j.ejcb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Destaing O, et al. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruzzaniti A, et al. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol Biol Cell. 2005;16:3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buccione R, Orth JD, McNiven MA. Foot and mouth: Podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 41.Luxenburg C, Addadi L, Geiger B. The molecular dynamics of osteoclast adhesions. Eur J Cell Biol. 2006;85:203–211. doi: 10.1016/j.ejcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seals DF, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 45.Basbaum CB, Werb Z. Focalized proteolysis: Spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- 46.Boyd ND, Chan BM, Petersen NO. Adaptor protein-2 exhibits alpha 1 beta 1 or alpha 6 beta 1 integrin-dependent redistribution in rhabdomyosarcoma cells. Biochemistry. 2002;41:7232–7240. doi: 10.1021/bi011501f. [DOI] [PubMed] [Google Scholar]
- 47.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heckel T, et al. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proc Natl Acad Sci USA. 2009;106:1451–1456. doi: 10.1073/pnas.0804464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochoa GC, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopp P, et al. The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol Biol Cell. 2006;17:2811–2823. doi: 10.1091/mbc.E05-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 52.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada H, et al. Processing of beta-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genet. 2001;10:1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- 54.Michaluk P, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- 55.Ehrlich M, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Massol RH, Boll W, Griffin AM, Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci USA. 2006;103:10265–10270. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee CW, et al. Regulation of acetylcholine receptor clustering by ADF/cofolin-directed vesicular trafficking. Nat Neurosci. 2006;12:848–856. doi: 10.1038/nn.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.