Abstract
Mobile group II introns retrohome by an RNP-based mechanism in which the excised intron lariat RNA fully reverse splices into a DNA site via 2 sequential transesterification reactions and is reverse transcribed by the associated intron-encoded protein. However, linear group II intron RNAs, which can arise by either hydrolytic splicing or debranching of lariat RNA, cannot carry out both reverse-splicing steps and were thus expected to be immobile. Here, we used facile microinjection assays in 2 eukaryotic systems, Xenopus laevis oocyte nuclei and Drosophila melanogaster embryos, to show that group II intron RNPs containing linear intron RNA can retrohome by carrying out the first step of reverse splicing into a DNA site, thereby ligating the 3′ end of the intron RNA to the 5′ end of the downstream exon DNA. The attached linear intron RNA is then reverse transcribed, yielding an intron cDNA whose free end is linked to the upstream exon DNA. Some of these retrohoming events result in the precise insertion of full-length intron. Most, however, yield aberrant 5′ junctions with 5′ exon resections, 5′ intron truncations, and/or extra nucleotide residues, hallmarks of nonhomologous end-joining. Our findings reveal a mobility mechanism for linear group II intron RNAs, show how group II introns can co-opt different DNA repair pathways for retrohoming, and suggest that linear group II intron RNAs might be used for site-specific DNA integration in gene targeting.
Keywords: DNA repair, gene targeting, retrotransposition, reverse transcriptase, ribozyme
Mobile group II introns found in bacterial and organellar genomes are retroelements that consist of an autocatalytic intron RNA and an intron-encoded protein (IEP) with reverse transcriptase (RT) activity (1, 2). The intron RNA and IEP function together in a ribonucleoprotein particle (RNP) to promote the integration of the intron into specific DNA sites by a mechanism in which the intron RNA reverse splices directly into a DNA strand and is then reverse transcribed by the IEP (1). Group II introns use this mechanism both to retrohome to the ligated-exon junction (homing site) in intronless alleles at high frequency and to retrotranspose to ectopic sites that resemble the normal homing site at low frequency. These processes enabled the dispersal of mobile group II introns to diverse bacteria and may have been used for the invasion and proliferation of group II introns in the nuclear genomes of early eukaryotes, where they evolved into spliceosomal introns (1, 3, 4).
Like spliceosomal introns, most group II introns splice via 2 sequential transesterification reactions that result in the formation of an intron lariat RNA (2). For mobile group II introns, the splicing reactions are assisted by the IEP, which binds specifically to the intron RNA to stabilize the catalytically active RNA structure (5). The IEP then remains bound to the excised intron lariat RNA in an RNP that promotes intron mobility (6). RNPs initiate intron mobility by recognizing DNA target sites using both the IEP and base pairing of the intron RNA to bind to 5′ and 3′ exon sequences flanking the intron insertion site (7, 8). The excised intron lariat RNA in the RNPs then inserts directly into the DNA strand by reverse splicing in 2 steps: ligation of the 3′ end of the intron RNA to the 5′ end of the 3′ exon DNA, followed by ligation of the 5′ end of the intron RNA to the 3′ end of the 5′ exon DNA. After reverse splicing, the inserted intron RNA is reverse transcribed by the IEP, using either the opposite DNA strand cleaved by the IEP or a nascent strand at a DNA replication fork to prime cDNA synthesis (1). Finally, the resulting intron cDNA is integrated into the recipient DNA by host cell DNA recombination or repair mechanisms, which have been only partially characterized (9, 10).
Some group II introns use a variation of the splicing mechanism in which 5′ splice site cleavage occurs by hydrolysis rather than branching, leading to the excision of a linear rather than lariat intron RNA. Such hydrolytic splicing has been observed in group II intron self-splicing reactions under nonphysiological conditions (high monovalent salt and/or Mg2+ concentrations) (2) and was shown to occur in vivo by using branchpoint mutants of the yeast mitochondrial (mt) group II intron aI5γ (11). A subset of naturally occurring group II introns lack a bulged branchpoint nucleotide in DVI, and some of these splice in vivo by a mechanism that yields linear intron RNA without detectable lariat RNA (12). In addition to hydrolytic splicing, linear group II intron RNAs can be generated from excised lariat RNAs by the action of debranching enzyme, which is used in eukaryotes for the debranching and turnover of excised spliceosomal introns (13).
Linear group II intron RNAs cannot carry out both steps of reverse splicing and were thus thought to be immobile. They can, however, carry out the first step of reverse splicing into either RNA or DNA sites, thereby ligating their 3′ end to the 5′ end of the 3′ exon (14–16). This partial reverse splicing reaction could potentially be used for intron mobility. If partial reverse splicing occurs into an RNA site, then the recombined RNA would need to be reverse transcribed, and the resulting cDNA integrated into the host genome by recombination, whereas if partial reverse splicing occurs into a DNA site, the attached intron RNA could be reverse transcribed and the intron cDNA integrated by DNA repair. The latter pathway is more likely for group II introns that encode RTs, because association with the IEP in RNPs strongly biases the intron RNA to reverse splice into DNA rather than RNA sites (17).
The Lactococcus lactis Ll.LtrB group II intron used in this work retrohomes by a mechanism involving the full reverse splicing of intron lariat RNA into a DNA site combined with second-strand cleavage by its IEP (denoted LtrA protein) to generate the primer for reverse transcription (18). Ll.LtrB RNPs recognize a DNA target site that extends over ≈35 bp, using both LtrA and base pairing of intron RNA sequences denoted exon-binding sites 1 and 2 (EBS1 and 2) and δ to bind 5′ and 3′ exon sequences flanking the intron insertion site (7, 8).
Recently, we investigated whether Ll.LtrB RNPs reconstituted with linear intron RNA could use their reverse splicing and second-strand cleavage activities to introduce a double-strand break at a DNA target site (15). The introduction of a recombinogenic double-strand break by a protein endonucleases, such as a Zn-finger nuclease or meganuclease, is a favored mode of gene targeting in higher organisms (19), and we reasoned that group II introns might be advantageous for this approach, as they can be retargeted readily to introduce the double-strand break at desired sites simply by modifying the RNA sequences that base pair to the DNA target site (8). Biochemical assays showed that Ll.LtrB RNPs containing linear intron RNA could efficiently carry out the first step of reverse splicing into a DNA target site, after which the IEP uses its En domain to cleave the bottom strand and reverse transcribes the attached linear intron RNA (15). Further, we found that these linear RNPs microinjected into Xenopus laevis oocyte nuclei could use these reactions in vivo to introduce a site-specific double-strand break that stimulates gene targeting by homologous recombination with a coinjected DNA (15). In addition to showing the potential utility of group II introns for gene targeting by double-strand-break-stimulated homologous recombination, these experiments raised the possibility that linear group II intron RNAs might be able to retrohome in vivo by a mechanism involving partial reverse splicing into the DNA target site followed by reverse transcription of the attached RNA, provided the resulting intron cDNA could be integrated into the recipient DNA by DNA repair (15).
Here, we used microinjection assays in 2 eukaryotic systems, X. laevis oocyte nuclei and D. melanogaster embryos, to show that RNPs containing linear group II intron RNA can retrohome by this mechanism in vivo and may use nonhomologous end joining (NHEJ) to ligate the free end of the intron cDNA to the 5′ exon of the target DNA.
Results
Retrohoming of RNPs Containing Linear Intron RNA in X. laevis Oocyte Nuclei.
To test the retrohoming of linear group II intron RNA, we used a plasmid-based assay developed for bacteria (20) and later adapted to test retrohoming of Ll.LtrB lariat RNPs (Lar RNPs) microinjected into X. laevis oocyte nuclei (15) (Fig. 1). In this assay, an Ll.LtrB intron with a phage T7 promoter inserted near its 3′ end integrates into a target site cloned in a recipient plasmid upstream of a promoterless tetR gene, thereby activating that gene. For assays in X. laevis oocytes, we use the recipient plasmid pBRR3-ltrB, which carries the Ll.LtrB target site/tetR gene cassette and a separate ampR marker, and RNPs reconstituted with purified IEP and in vitro-synthesized 0.9-kb Ll.LtrB-ΔORF intron RNA carrying a T7 promoter. The recipient plasmid was injected first into the nucleus of stage VI oocytes, followed within 1 min by Ll.LtrB RNPs containing lariat or linear intron RNA. After incubation for 2 h at 25 °C, nucleic acids were isolated and transformed into Escherichia coli HMS174(DE3), which expresses T7 RNA polymerase. The cells were then plated on LB medium containing ampicillin plus tetracycline or ampicillin alone, and the integration efficiency was quantified as the ratio of (TetR + AmpR)/AmpR colonies.
Fig. 1.
Plasmid assay for group II intron retrohoming in X. laevis oocyte nuclei and D. melanogaster embryos. The assay uses the recipient plasmid pBRR3-ltrB, which contains the Ll.LtrB intron target site (ligated ltrB exon 1 and 2 sequences; E1 and E2) cloned upstream of a promoterless tetR gene in a pBR322-based vector carrying an ampR marker (20). The plasmid is microinjected into X. laevis oocyte nuclei or D. melanogaster embryos, followed by Ll.LtrB RNPs containing lariat or linear intron RNA with a phage T7 promoter inserted in intron RNA domain IV. Site-specific integration of the intron into the target site introduces the T7 promoter upstream of the promoterless tetR gene. After extraction, nucleic acids are electroporated into E. coli HMS174(DE3), which expresses T7 RNA polymerase, and colonies containing retrohoming products are selected by plating on LB medium containing tetracycline and ampicillin. Integration efficiencies are determined as the ratio of (TetR + AmpR)/AmpR colonies. T1 and T2 are E. coli rrnB transcription terminators, and Tφ is a phage T7 transcription terminator.
First, we used this assay to compare the retrohoming efficiencies of Ll.LtrB RNPs containing lariat or linear intron RNA. In initial experiments, we tested 2 forms of the linear intron RNA, one whose 5′ end corresponds precisely to that of the Ll.LtrB intron (Lin) and the other with 2 extra 5′ G residues (GG-Lin), enabling more efficient transcription by phage T3 RNA polymerase (21). These extra 5′ residues are not expected to affect the ability of the GG-Lin RNA to carry out the first step of reverse splicing (14), nor to serve as a 5′ exon for RNA splicing to produce lariat RNA. The latter was confirmed by in vitro splicing assays (supporting information (SI) Fig. S1) (22).
Table 1 summarizes the findings of 4 separate experiments, with 10 oocytes injected and pooled for each condition in each experiment. The retrohoming efficiency of Ll.LtrB Lar RNPs in these experiments was 8.3%−22%, in agreement with previous results (15). For Lin and GG-Lin RNPs, retrohoming efficiencies were 0.004%−0.04%, readily detectable in these assays, but more than 500- to 2,000-fold lower than for the Lar RNPs. Controls showed no retrohoming for GG-Lin RNPs reconstituted with an RT-deficient mutant LtrA protein (DD−; Table 1), confirming dependence upon the RT activity of the IEP, as shown previously for retrohoming of Lar RNPs in X. laevis oocyte nuclei (15).
Table 1.
Retrohoming efficiencies of lariat and linear group II intron RNPs in X. laevis oocyte nuclei
| RNPs | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 |
|---|---|---|---|---|
| WT Lar | 22% | 20% | 8.3% | 9.0% |
| WT Lin | 0.04% | 0.018% | ||
| WT GG-Lin | 0.016% | 0.008% | 0.004% | 0.004% |
| WT GG-Lin (2′-OMe) | 0.002% | 0.005% | ||
| WT GG-Lin-ΔA2486 | 0.002% | 0.002% | 0.001% | |
| DD− GG-Lin | <4 × 10−5% |
Retrohoming assays were done by injecting recipient plasmid pBRR3-ltrB and the indicated Ll.LtrB RNPs into X. laevis oocyte nuclei, as described in Fig. 1 and Materials and Methods. Different RNPs contained lariat intron RNA (Lar); linear intron RNA (Lin); linear intron RNA transcribed from a DNA template with 2 extra 5′ G residues (GG-Lin); GG-Lin transcribed from a DNA template with 2′ -OMe nucleotide residues at the 2 terminal positions; GG-Lin RNA with the branchpoint A residue deleted (GG-Lin-ΔA2486); and GG-Lin RNA + RT-deficient LtrA protein (DD− ; YADD→YAAA). RNP concentrations: Exps. 1 and 2, 2.6 mg/mL; exp. 3, 1.8 mg/mL; exp. 4, 3.4 mg/mL. After incubating the injected oocytes for 2 h at 25°C, nucleic acids were transformed into E. coli HMS174(DE3). Retrohoming efficiencies were calculated as the ratio of (TetR + AmpR)/AmpR colonies.
The reverse-splicing activity of linear group II intron RNA depends critically upon the RNA having the correct 3′ terminal nucleotide residues. This dependence is problematic for RNAs synthesized with T7 RNA polymerase, which adds extra nontemplated nucleotide residues to the 3′ ends of runoff transcripts (23). For our experiments, we synthesized linear Ll.LtrB intron RNAs with phage T3 RNA polymerase, which differs from T7 RNA polymerase in giving runoff transcripts with more homogenous 3′ ends (24). The use of DNA templates ending with C2′-methoxy (2′-OMe) nucleotide residues, which reduces the 3′-end heterogeneity of T7 transcripts (16, 25), had no significant effect on the retrohoming efficiency of GG-Lin RNAs synthesized with T3 RNA polymerase and thus appears unnecessary (Table 1).
Finally, we confirmed that GG-Lin RNPs with the branchpoint A residue deleted (GG-Lin-ΔA2486) still retained substantial retrohoming activity, albeit 2- to 4-fold lower than that for wild-type GG-Lin RNPs assayed in parallel (0.001%–0.002% and 0.004%–0.008%, respectively; Table 1). The insertion of the GG-Lin-ΔA2486 intron was confirmed by sequencing through the branchpoint region. These findings indicate that retrohoming of GG-Lin RNPs is not dependent upon branching at the normal site. It is unclear whether the somewhat decreased retrohoming efficiencies of the ΔA2486 RNA are significant and if so, whether they reflect differences in the quality of the RNP preparations or somewhat decreased reverse splicing activity due to the effect of deleting the branchpoint nucleotide on intron RNA structure.
PCR and Sequence Analysis of Integration Junctions for Retrohoming of Lin RNPs in X. laevis Oocyte Nuclei.
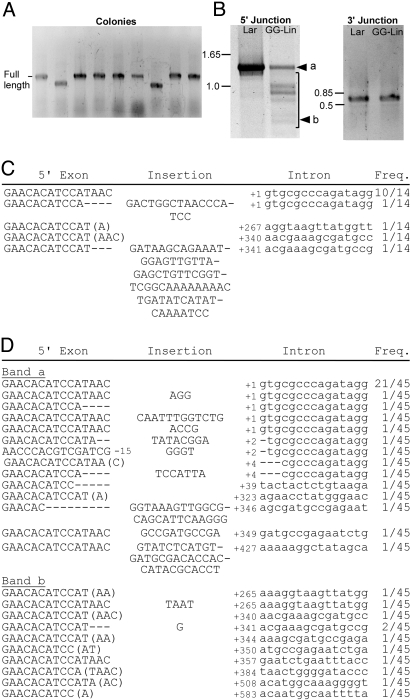
To confirm retrohoming of the linear intron RNA, we carried out PCR of randomly selected TetR + AmpR colonies, using primers flanking the intron insertion site in the recipient plasmid. For Lar RNPs, the colony PCR gave products of the size expected for insertion of the full-length Ll.LtrB-ΔORF intron for all 23 retrohoming events analyzed (one example is shown in Fig. 2A), whereas for Lin RNPs, the colony PCR gave some products of the size expected for insertion of the full-length intron, along with smaller products (Fig. 2A).
Fig. 2.
Retrohoming of Ll.LtrB Lin RNPs in X. laevis oocyte nuclei. Retrohoming of group II intron RNPs into recipient plasmid pBRR3-ltrB in X. laevis oocyte nuclei was assayed as described in Fig. 1 and Materials and Methods. After incubating the injected oocytes for 2 h at 25 °C, nucleic acids were extracted and electroporated into E. coli HMS174(DE3), followed by selection for TetR + AmpR colonies. (A) E. coli colony PCR. TetR + AmpR colonies resulting from retrohoming of Lin RNPs (Lin) were analyzed by colony PCR using the primers P1 and P4, which flank the Ll.LtrB insertion site in the recipient plasmid (Materials and Methods). The PCR products were analyzed in a 1% agarose gel, which was stained with ethidium bromide. Colony PCR for a retrohoming product obtained in a parallel assay with Lar RNPs (Lar) is shown for comparison. Lane M, 1-kb plus DNA ladder (Invitrogen). (B) Sequences of 5′ integration junctions resulting from retrohoming of Lin RNPs. The sequences were determined by sequencing the colony PCR products using the primer Rseq. 5′ exon sequences and extra nucleotide residues inserted at the junction are in uppercase letters, and intron sequences are in lowercase letters. The position numbers at the beginning of the intron sequences are indicated. Microhomologies at the ligation junction in the 5′ exon are shown in parentheses. The frequency of occurrence of a sequence (Freq.) is indicated to the right.
The retrohoming events were further characterized by sequencing the 5′ and 3′ integration junctions. For Lar RNPs, the sequencing showed precise insertion of the full-length intron at the target site, as expected for full reverse splicing, for all 23 retrohoming products analyzed here and others in previous work (15). For Lin RNPs, all 24 retrohoming products analyzed had the 3′ junction sequence expected for the first step of reverse splicing into the DNA target site. However, only one event analyzed for Lin RNPs gave insertion of full-length intron with an accurate 5′ junction sequence (Fig. 2B). The remainder had 5′ junctions with some combination of: (i) resection of 5′ exon sequences; (ii) insertion of 5′ truncated introns; and/or (iii) insertion of extra nucleotide residues at the integration junction (Fig. 2B). These anomalies presumably reflect errors during the process used to link the intron cDNA to the 5′ exon. Similar colony PCR results and 5′ and 3′ junction sequences were obtained for retrohoming products of GG-Lin RNA (Fig. S2 A and B). Some of the junction sequences, particularly those with extensive 5′ intron truncations, showed microhomologies of 1–5 nucleotide residues between the 5′ end of the intron and the ligated 5′ exon sequences (nucleotides in parentheses in Fig. 2B). The characteristics of the 5′ junctions for retrohoming of Lin and GG-Lin RNPs are similar to those of ligation junctions formed during DNA repair by NHEJ (26–28).
To more fully analyze the spectrum of retrohoming products for linear RNPs and confirm that cDNA ligation occurred in the eukaryotic host, we carried out PCR of 5′ and 3′ integration junctions in the nucleic acids extracted from X. laevis oocyte nuclei without transformation into E. coli (Fig. 3). As expected from the colony PCR results, the 5′ junction PCR for Lin and GG-Lin RNPs showed some products of the same size as those for Lar RNPs, along with smaller products (Fig. 3A Left). Sequencing of cloned PCR products confirmed that the larger products (band a) had insertions of full-length or almost full-length introns, whereas PCR products of decreasing sizes (bands b and c) had insertions with progressively longer 5′ intron truncations (Fig. 3B and Fig. S2C). Some of the 5′ junction sequences also showed variably sized 5′ exon resections, insertion of extra nucleotide residues, and microhomologies, similar to the junction sequences analyzed by colony PCR. We note that although the transcription of GG-Lin RNA was confirmed by single-nucleotide omission experiments to initiate preferentially at the upstream G residues, only a few PCR products retained one or both of the extra 5′ G residues at the ligation junction (Fig. S2 B and C), possibly reflecting their preferential removal or exclusion at some step during retrohoming. One possibility is that because these extra nucleotide residues lie outside the folded RNA structure in RNPs, they are prone to removal by RNase trimming.
Fig. 3.
PCR analysis of integration junctions for retrohoming of Ll.LtrB Lin RNPs in X. laevis oocyte nuclei. Retrohoming assays with Lin, GG-Lin, and Lar RNPs were done in X. laevis oocyte nuclei as in Fig. 2, except that the extracted nucleic acids were analyzed directly by PCR without transformation into E. coli. (A) PCR of the 5′ junction using primers P1 and P2, and PCR of the 3′ junction using primers P3 and P4 (Materials and Methods). The control lanes show parallel PCR of X. laevis genomic DNA and a water blank. (B) Sequences of 5′ integration junctions for retrohoming of Lin RNPs. PCR products corresponding to regions a, b, and c of the gel were cloned into pCRII-TOPO and sequenced (Materials and Methods). Intron and exon sequences are depicted as in Fig. 2. Mutations in the intron are highlighted in gray.
By contrast to the 5′ junction, the 3′ junction PCR for retrohoming products of Lin and GG-Lin RNPs gave a single strong band of the same size as that for Lar RNPs (Fig. 3A Right), and sequencing showed the expected junction corresponding to the 3′ end of the intron inserted precisely at the target site by partial reverse splicing (Fig. S3A).
Together, the above findings suggest that retrohoming of linear intron RNA in X. laevis oocyte nuclei occurs by a mechanism involving partial reverse splicing of the intron RNA into the DNA target site, reverse transcription of the attached intron RNA, and attachment of the free end of the intron cDNA to the upstream exon by a mechanism that gives 5′ junctions characteristic of NHEJ.
Retrohoming of Ll.LtrB Lin RNPs in D. melanogaster Embryos.
Finally, to test the generality of our findings, we carried out similar retrohoming assays for RNPs containing lariat or linear intron RNA microinjected into early (precellular blastoderm) stage D. melanogaster embryos (Fig. 4). Because group II intron integration into double-stranded DNA is more efficient at higher temperatures (7, 29), the injected embryos were incubated at 37 °C for a short time (30 min) before analysis of the retrohoming products, as done previously for chromosomal gene targeting (15). Initial experiments in which retrohoming products were recovered by transforming extracted nucleic acids into E. coli showed that as in X. laevis, the retrohoming efficiency measured by the ratio of (TetR + AmpR)/AmpR colonies was much lower for GG-Lin RNPs than for Lar RNPs (0.002% and 5%, respectively; 3.4 mg/mL RNPs injected in both cases).
Fig. 4.
Retrohoming of Ll.LtrB Lin RNPs in D. melanogaster embryos. Retrohoming assays using Lar and GG-Lin RNPs were done in D. melanogaster precellular blastoderm embryos, as described in Fig. 1 and Materials and Methods. After incubating the embryos for 30 min at 37 °C, nucleic acids were extracted and either transformed into E. coli HMS174(DE3) for plating assays and colony PCR or used directly for PCR analysis of integration products, as described in Figs. 2 and 3 for X. laevis oocyte assays. (A) E. coli colony PCR of 5′ integration junctions in randomly selected TetR + AmpR colonies obtained after retrohoming of GG-Lin RNPs. (B) Direct PCR analysis of 5′ and 3′ integration junctions using primers P1 and P2 for the 5′ junction and primers P3 and P4 for the 3′ junction (Materials and Methods). (C) Sequences of 5′ junctions from colony PCR products. (D) Sequences of 5′ junctions in PCR products amplified from extracted DNA without transformation into E. coli. Intron and exon sequences are depicted as in Fig. 2.
Analysis of retrohoming products, both by E. coli colony PCR and by direct PCR of nucleic acids extracted from D. melanogaster embryos, gave results similar to those for X. laevis oocyte nuclei (Fig. 4 A and B). The 3′ junction sequences for retrohoming of GG-Lin RNPs were again homogenous, with all 24 products analyzed showing the 3′ end of the intron integrated precisely at the target site, as did sequencing of the 3′ junction direct PCR product (Fig. S3B). By contrast, the 5′ junction sequences were heterogeneous, with some showing full-length intron insertions, but most showing some combination of 5′ exon resections, insertion of truncated introns, and/or as many as 70 extra nucleotide residues at the integration junction (Fig. 4 C and D). As in X. laevis oocyte nuclei, 5′ junctions with microhomologies between the 5′ end of the intron and 5′ exon (nucleotides in parentheses in Fig. 4 C and D) were more prevalent for events with long 5′ intron truncations. Similar sequence data were obtained for retrohoming of GG-Lin RNPs in D. melanogaster embryos at lower temperature (25 °C for 1 h; Fig. S4). Considered together, these findings indicate that retrohoming of linear RNPs in D. melanogaster embryos occurs by a mechanism similar to that in X. leavis oocyte nuclei.
Discussion
Our findings suggest that RNPs containing linear group II intron RNA can retrohome in vivo by the mechanism proposed in Fig. 5. The pathway begins with the linear intron RNA catalyzing the first step of reverse splicing into a DNA target site, resulting in the ligation of the 3′ end of the intron to the 5′ end of the 3′ exon DNA. The associated IEP then uses its En domain to cleave the opposite strand and synthesizes a cDNA copy of the linear intron RNA. These initial reactions were demonstrated previously for GG-Lin RNPs in vitro (15), and their occurrence in vivo is indicated here by the expected 3′ junction sequences for retrohoming of Lin and GG-Lin RNPs. A key step found here to complete retrohoming in vivo is the attachment of the free end of the intron cDNA to the upstream exon DNA. This step occasionally leads to the precise insertion of the full-length intron, just as for retrohoming of lariat RNA, but more frequently occurs with loss of 5′ exon or 5′ intron sequences and insertion of additional nucleotide residues at the junction. As we observed no duplication of target site sequences resulting from the initial staggered double-strand break made by the RNPs, we infer that the single-stranded 5′ overhang attached to the 5′ exon DNA bottom strand is resected before cDNA ligation. Like retrohoming of lariat RNA, the retrohoming of linear intron RNA is presumably completed by the degradation or displacement of the intron RNA template strand followed by second-strand DNA synthesis and the sealing of nicks by host DNA polymerases and ligases (10).
Fig. 5.
Model for retrohoming of linear group II intron RNA. RNPs containing linear group II intron RNA recognize DNA target sites and carry out the first step of reverse splicing into the intron insertion site (IS) in the recipient DNA, resulting in ligation of the 3′ end of the intron RNA to the 5′ end of the 3′ exon DNA. The IEP then uses its En domain to cleave the bottom strand between positions +9 and +10 of the 3′ exon (CS) and reverse transcribes the attached linear intron RNA. After resection of the 5′ overhang resulting from the initial staggered double-strand break at the target site, the intron cDNA is linked to the 5′ exon. This attachment step is inaccurate and often occurs with loss of 5′ exon sequences due to excessive resection, insertion of 5′ truncated introns due to incomplete cDNA synthesis or degradation, and/or insertion of extra nucleotide residues at the ligation junction, which is done by a repair DNA polymerase during NHEJ (26–28). As for lariat RNA, retrohoming of the linear intron RNA is likely completed by degradation or displacement of the intron RNA template strand, second-strand DNA synthesis, and sealing of nicks using host enzymes. Intron RNA, red; IEP, gray; recipient DNA, black; cDNA, green; second-strand DNA, blue. 5′SS and 3′SS, 5′ and 3′ splice sites, respectively.
The characteristics of the 5′ junction sequences for retrohoming of linear RNPs, particularly 5′ exon resection and insertion of extra nucleotide residues, suggest that cDNA attachment occurs by a mechanism akin to NHEJ. These characteristics cannot be explained by cDNA extension into the 5′ exon without ad hoc assumptions about the biochemical activities of the group II intron RT, which does not efficiently use DNA templates (10). In classical NHEJ, the free DNA ends are tethered by the Ku protein complex (27, 28). In retrohoming of linear group II intron RNA, however, the group II intron RNP could itself contribute to such tethering, as it binds to both 5′ and 3′ exon sequences for DNA target site recognition (7, 8), and the RNP contacts with both exons appear to be maintained at least through the initiation of cDNA synthesis (30). A bridging role for the RNPs could explain why microhomologies between the ligated ends are infrequent for long intron cDNAs, which extend up to the 5′ end of the intron, but more frequent for truncated cDNAs, whose free ends are farther from the eventual site of attachment. It will be of interest to investigate to what extent host proteins involved in classical Ku-dependent or alternative NHEJ function in group II intron cDNA integration, and how they may be recruited to the site by group II intron insertion, which makes an unconventional double-strand break with the intron RNA and cDNA attached on one side.
In both X. laevis oocyte nuclei and D. melanogaster embryos, the insertion of 5′ truncated introns most likely results either from degradation of the linear intron RNA or resection of unattached cDNA ends, rather than abortive cDNA synthesis, as such 5′ truncations are not observed for retrohoming of lariat RNA. The latter is identical to the linear intron RNA, except that its 5′ end is protected by the 2′–5′ linkage, and then ligated directly to the 5′ exon by the second step of reverse splicing. As a result, DNA synthesis can extend continuously into the upstream exon without leaving an unattached cDNA end, as is the case for linear intron RNA. Additionally, in both X. laevis oocyte nuclei and D. melanogaster embryos, most of the truncated introns (62% and 67%, respectively) have 5′ ends at positions consistent with termination of cDNAs synthesis within single-stranded RNA regions, which may be prone to RNase cleavage (Fig. S5).
Previous biochemical assays showed that linear and lariat Ll.LtrB-ΔORF RNAs can carry out the first step of reverse splicing into DNA target sites with similar efficiency in vitro (15), and this step also appears to be efficient for linear intron RNA in vivo, as judged by PCR analysis of 3′ integration junctions (Figs. 3 and 4 and additional PCRs under semiquantitative conditions). Thus, the lower retrohoming efficiency of linear intron RNA is likely due mainly to its greater nuclease sensitivity and/or low NHEJ activity in the systems tested thus far (see ref. 31). If so, the retrohoming efficiency of linear intron RNA may be increased by RNA modifications that confer nuclease resistance and could be higher in other hosts with higher NHEJ activity. We also note that the retrohoming efficiency of the linear intron RNA is likely underestimated by our genetic assay, which requires integration of sufficiently long cDNA to introduce the T7 promoter upstream of the promoterless tetR gene (Fig. 1).
NHEJ is ubiquitous in eukaryotes, and a related NHEJ pathway with a Ku homolog and ATP-dependent DNA ligase exists in many prokaryotes, albeit not in E. coli K12 (32). Thus, the mechanism elucidated here could be used in both prokaryotes and eukaryotes for the retrohoming of linear group II intron RNAs generated by hydrolytic splicing or by the action of host debranching enzymes on lariat intron RNAs. Hydrolytic splicing is a side pathway for group II introns that splice via branching, and is the exclusive splicing pathway for group II introns that cannot branch (see the Introduction). It is possible that ancestral group II introns spliced exclusively via the hydrolytic pathway and were mobile by the type of mechanism elucidated here, and intron branching was a later evolutionary adaptation that ultimately predominated because it increased the efficiency and fidelity of retromobility.
Finally, our findings suggest that RNPs containing linear group II intron RNAs could be used for site-specific insertion in gene targeting. The lower retrohoming efficiency of linear intron RNA might be offset both by its greater ease of preparation, which does not require the self-splicing and purification steps needed for lariat RNA, and by the facility of incorporating selectable or screenable genetic markers, which can substantially decrease the splicing and reverse splicing efficiency of lariat RNA. Indeed, we previously reported targeted integration of linear Ll.LtrB intron RNA into the D. melangogaster yellow gene (15), which likely occurred by the mechanism elucidated here.
Materials and Methods
Synthesis of Group II Intron RNAs.
Ll.LtrB-ΔORF intron RNAs were transcribed from DNA templates generated by PCR of plasmid pACD5C, a derivative of pACD4C (8) with a T7 promoter sequence inserted at the SalI site in DIV. The PCRs used to generate DNA templates for lariat precursor RNA and linear intron RNA are described in SI Materials and Methods. In vitro transcription and the preparation of lariat and linear intron RNAs were as described (15).
Preparation of LtrA Protein and Ll.LtrB RNPs.
Wild-type and RT-deficient mutant LtrA protein (DD-; YADD→YAAA at the RT active site) were expressed in E. coli BL21(DE3) from the plasmids pImp-1P and pImp-10P, respectively (6). These plasmids express LtrA proteins with a C-terminal tag containing an intein-linked chitin-binding domain. The proteins were purified via a chitin-affinity column and intein cleavage, as described (6), except that the column buffer contained 50 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, and 0.1% Nonidet P40. Ll.LtrB RNPs were reconstituted with the purified LtrA protein and in vitro-synthesized lariat or linear Ll.LtrB-ΔORF intron RNA, as described (15), except that the RNPs were resuspended in 10 mM KCl, 5 mM MgCl2, and 40 mM Hepes (pH 8.0).
X. laevis Methods.
X. laevis oocyte microinjection and retrohoming assays were as described (15). Briefly, 18 nL of recipient plasmid pBRR3-ltrB (0.25 μg/μL with 500 mM MgCl2 and 17 mM dATP, dCTP, dGTP and dTTP; Invitrogen) (20) was injected into the oocyte nucleus, followed within 1 min by 18 nL of group II intron RNPs (1.5–8.7 mg/mL based on A260), using a different needle to avoid prior mixing. To obtain sufficient material for analysis, 10 oocytes were injected for each condition in each experiment. After incubation for 2 h at 25°C, the oocytes were pooled, and nucleic acids were extracted as described (15). To select retrohoming products, the extracted nucleic acids were electroporated into E. coli HMS174(DE3), which was then grown in LB medium for 1 h at 37 °C and plated at different dilutions on LB medium containing ampicillin (100 μg/mL) with or without tetracycline (25 μg/mL). Retrohoming efficiencies were calculated as the ratio of (TetR + AmpR)/AmpR colonies (15). Colony PCR was done using the primers P1 (5′-CTGATCGATAGCTGAAACGC) and P4 (5′-AATGGACGATATCCCGCA) flanking the intron-insertion site in the recipient plasmid, and the PCR products were sequenced by using the primers Rseq (5′-CCATGCGAGAGTAGGGAAC) and P3 (5′-CAGTGAATTTTTACGAACGAACAATAAC) for the 5′ and 3′ integration junctions, respectively. Direct PCR of the extracted nucleic acids was done using the primers P1 (see above) and P2 (5′-CGGCTCTGTTATTGTTCGTTCG) for the 5′ integration junction, and the primers P3 and P4 (see above) for the 3′ integration junction. Gel-purified PCR products were cloned into a TOPO TA cloning vector (pCRII-TOPO; Invitrogen) and sequenced using primer M13For-40 (5′-GTTTTCCCAGTCACGAC) and M13Rev-48 (5′-AGCGGATAACAATTTCACACAGGA).
D. melanogaster Methods.
Fly stock OR-R was grown in standard fly media at 22 °C, and precellular blastoderm stage embryos were microinjected as described [(15) and SI Materials and Methods]. The posterior of the embryo was injected first with ∼0.3 nL of recipient plasmid (1.4 mg/mL DNA, 500 mM MgCl2, and 17 mM dATP, dCTP, dGTP, and dTTP), followed within 5 min by ∼0.3 nL of RNPs (3.4 mg/mL based on A260) using a different needle. For each experimental condition, 80 embryos were injected and incubated in a humidified chamber for 30 min at 37° C. The embryos were pooled, and nucleic acids were extracted as described (15), then either transformed into E. coli for selection of retrohoming products, or used directly for PCR analysis, as described above for X. laevis assays.
Supplementary Material
Acknowledgments.
We thank Tanya Paull, John Moran, and Tom Eickbush for comments on the manuscript, and Paul Macdonald for use of equipment for D. melanogaster growth and microinjection experiments. This work was supported by National Institutes of Health Grants GM037949 and HD054349 and Welch Foundation Grant F-1607.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910277106/DCSupplemental.
References
- 1.Lambowitz AM, Zimmerly S. Mobile group II introns. Annu Rev Genet. 2004;38:1–35. doi: 10.1146/annurev.genet.38.072902.091600. [DOI] [PubMed] [Google Scholar]
- 2.Pyle A, Lambowitz AM. In: The RNA World. 3rd Ed. Gesteland RF, Cech TR, Atkins JF, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2006. pp. 469–505. [Google Scholar]
- 3.Martin W, Koonin EV. Introns and the origin of nucleus-cytosol compartmentalization. Nature. 2006;440:41–45. doi: 10.1038/nature04531. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Trelles F, Tarrío R, Ayala FJ. Origins and evolution of spliceosomal introns. Annu Rev Genet. 2006;40:47–76. doi: 10.1146/annurev.genet.40.110405.090625. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura M, Noah JW, Lambowitz AM. Mechanism of maturase-promoted group II intron splicing. EMBO J. 2001;20:7259–7270. doi: 10.1093/emboj/20.24.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saldanha R, et al. RNA and protein catalysis in group II intron splicing and mobility reactions using purified components. Biochemistry. 1999;38:9069–9083. doi: 10.1021/bi982799l. [DOI] [PubMed] [Google Scholar]
- 7.Singh NN, Lambowitz AM. Interaction of a group II intron ribonucleoprotein endonuclease with its DNA target site investigated by DNA footprinting and modification interference. J Mol Biol. 2001;309:361–386. doi: 10.1006/jmbi.2001.4658. [DOI] [PubMed] [Google Scholar]
- 8.Perutka J, Wang W, Goerlitz D, Lambowitz AM. Use of computer-designed group II introns to disrupt Escherichia coli DExH/D-box protein and DNA helicase genes. J Mol Biol. 2004;336:421–439. doi: 10.1016/j.jmb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Eskes R, Yang J, Lambowitz AM, Perlman PS. Mobility of yeast mitochondrial group II introns: Engineering a new site specificity and retrohoming via full reverse splicing. Cell. 1997;88:865–874. doi: 10.1016/s0092-8674(00)81932-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith D, et al. Recruitment of host functions suggests a repair pathway for late steps in group II intron retrohoming. Genes Dev. 2005;19:2477–2487. doi: 10.1101/gad.1345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podar M, Chu VT, Pyle AM, Perlman PS. Group II intron splicing in vivo by first-step hydrolysis. Nature. 1998;391:915–918. doi: 10.1038/36142. [DOI] [PubMed] [Google Scholar]
- 12.Vogel J, Börner T. Lariat formation and a hydrolytic pathway in plant chloroplast group II intron splicing. EMBO J. 2002;21:3794–3803. doi: 10.1093/emboj/cdf359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green MR. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- 14.Mörl M, Schmelzer C. Integration of group II intron bI1 into a foreign RNA by reversal of the self-splicing reaction in vitro. Cell. 1990;60:629–636. doi: 10.1016/0092-8674(90)90666-3. [DOI] [PubMed] [Google Scholar]
- 15.Mastroianni M, et al. Group II intron-based gene targeting reactions in eukaryotes. PLoS One. 2008;3:e3121. doi: 10.1371/journal.pone.0003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roitzsch M, Pyle AM. The linear form of a group II intron catalyzes efficient autocatalytic reverse splicing, establishing a potential for mobility. RNA. 2009;15:473–482. doi: 10.1261/rna.1392009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerly S, et al. A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell. 1995;83:529–538. doi: 10.1016/0092-8674(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 18.Cousineau B, et al. Retrohoming of a bacterial group II intron: Mobility via complete reverse splicing, independent of homologous DNA recombination. Cell. 1998;94:451–462. doi: 10.1016/s0092-8674(00)81586-x. [DOI] [PubMed] [Google Scholar]
- 19.Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat Biotech. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, et al. Group II introns designed to insert into therapeutically relevant DNA target sites in human cells. Science. 2000;289:452–457. doi: 10.1126/science.289.5478.452. [DOI] [PubMed] [Google Scholar]
- 21.Bailey JN, Klement JF, McAllister WT. Relationship between promoter structure and template specificities exhibited by the bacteriophage T3 and T7 RNA polymerases. Proc Natl Acad Sci USA. 1983;80:2814–2818. doi: 10.1073/pnas.80.10.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquier A, Rosbash M. Efficient trans-splicing of a yeast mitochondrial RNA group II intron implicates a strong 5′ exon-intron interaction. Science. 1986;234:1099–1104. doi: 10.1126/science.2430332. [DOI] [PubMed] [Google Scholar]
- 23.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumder HK, Maitra U, Rosenberg M. Termination of transcription by bacteriophage T3 RNA polymerase: Homogeneous 3′-terminal oligonucleotide sequence of in vitro T3 RNA polymerase transcripts. Proc Natl Acad Sci USA. 1979;76:5110–5113. doi: 10.1073/pnas.76.10.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao C, Rudisser S, Zheng M. A simple and efficient method to transcribe RNAs with reduced 3′ heterogeneity. Methods. 2001;23:201–205. doi: 10.1006/meth.2000.1131. [DOI] [PubMed] [Google Scholar]
- 26.Hagmann M, et al. Homologous recombination and DNA-end joining reactions in zygotes and early embryos of zebrafish (Danio rerio) and Drosophila melanogaster. Biol Chem. 1998;379:673–681. doi: 10.1515/bchm.1998.379.6.673. [DOI] [PubMed] [Google Scholar]
- 27.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 28.Weterings E, van Gent DC. The mechanism of non-homologous end-joining: a synopsis of synapsis. DNA Repair. 2004;3:1425–1435. doi: 10.1016/j.dnarep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerly S, Guo H, Perlman PS, Lambowitz AM. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell. 1995;82:545–554. doi: 10.1016/0092-8674(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 30.Noah JW, et al. Atomic force microscopy reveals DNA bending during group II intron ribonucleoprotein particle integration into double-stranded DNA. Biochemistry. 2006;45:12424–12435. doi: 10.1021/bi060612h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagmann M, et al. Dramatic changes in the ratio of homologous recombination to nonhomologous DNA-end joining in oocytes and early embryos of Xenopus laevis. Biol Chem Hoppe Seyler. 1996;377:239–250. doi: 10.1515/bchm3.1996.377.4.239. [DOI] [PubMed] [Google Scholar]
- 32.Bowater R, Doherty AJ. Making ends meet: Repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2006;2:e8. doi: 10.1371/journal.pgen.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.