Abstract
Weimberg, Ralph (Northern Regional Research Laboratory, Peoria, Ill.), and William L. Orton. Synthesis and breakdown of the polyphosphate fraction and acid phosphomonoesterase of Saccharomyces mellis and their locations in the cell. J. Bacteriol. 89:740–747. 1965.—The conditions for accumulation of polyphosphate in cells of Saccharomyces mellis differ in several respects from those for acid phosphomonoesterase biosynthesis and maintenance. Polyphosphate can be synthesized or degraded in vivo by resting cells, provided an energy source is present. Experiments with growing cells indicate that the enzyme systems involved in the metabolism of the polyphosphate fraction are constitutive, since cells respond immediately to changes in the level of inorganic phosphate in the external medium. There is no change in the acid phosphatase level in either resting cells or in cells in the lag phase of growth. Enzyme formation or breakdown occurs only in cells that are exponentially dividing. Enzyme is lost rapidly from derepressed cells when they are transferred to a phosphate-rich medium, falling to a very low value by the time the cell mass had doubled. Protoplasts of repressed cells were prepared to determine the location of ortho- and polyphosphates in the cell. Previous studies have shown that phosphomonoesterase is released as a soluble enzyme when derepressed cells become protoplasts. Unlike phosphomonoesterase in derepressed cells, the two phosphate fractions in repressed cells are still attached to the protoplast after the cell wall has been digested and are eluted only when the protoplast structure is lysed in cold water. However, it is also possible to extract a part of the two phosphate fractions from intact cells in the absence of snail gut extract by osmotic shock if the cells are first suspended in a solution of high salt concentration. This treatment with salt does not affect viability. These results do not permit a definite conclusion concerning the location of ortho- and polyphosphates in the cell, other than that they are associated with the protoplast and thus occupy a position different from that of the phosphomonoesterase.
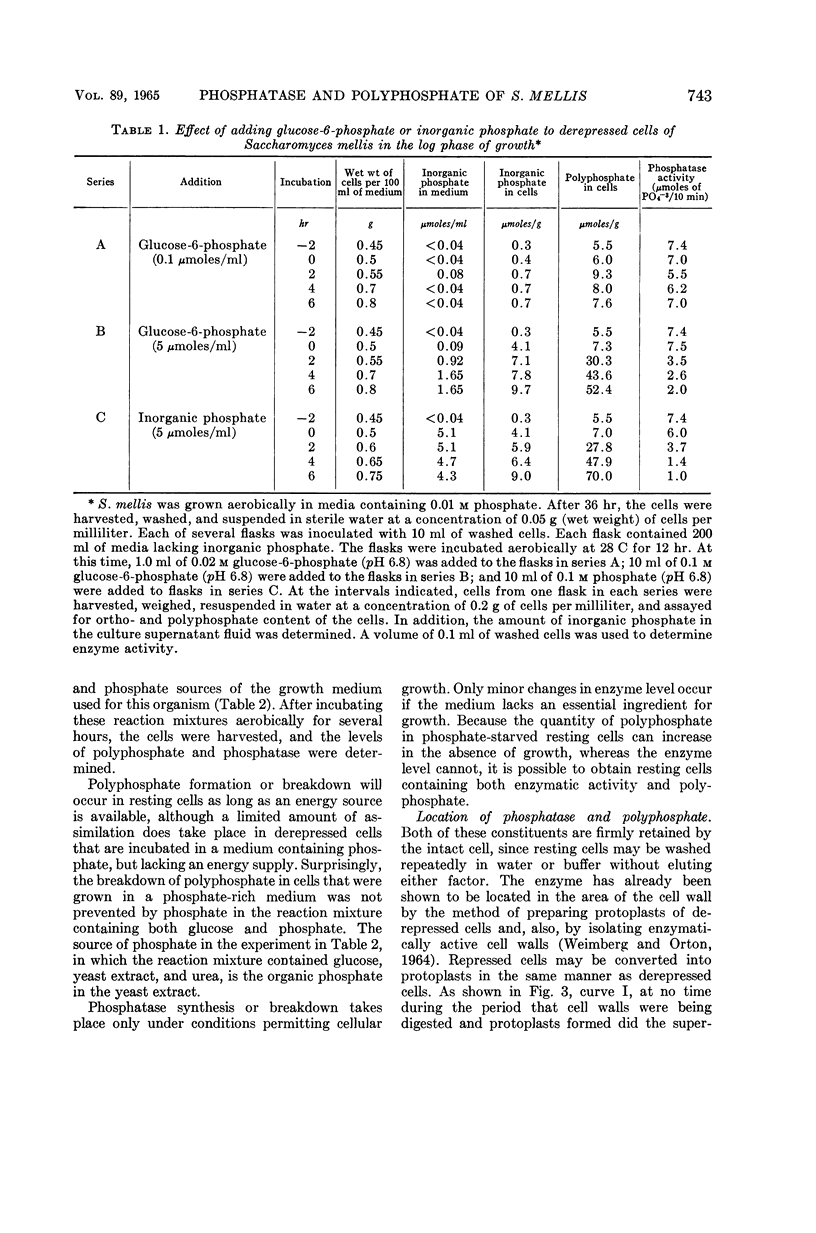
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- EHRENBERG M. [Phosphorus metabolism by Saccharomyces cerevisiae in relation to intracellular and extracellular phosphate concentration]. Arch Mikrobiol. 1961;40:126–152. [PubMed] [Google Scholar]
- HEREDIA C. F., YEN F., SOLS A. Role and formation of the acid phosphatase in yeast. Biochem Biophys Res Commun. 1963 Jan 18;10:14–18. doi: 10.1016/0006-291x(63)90259-6. [DOI] [PubMed] [Google Scholar]
- KATCHMAN B. J., FETTY W. O. Phosphorus metabolism in growing cultures of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):607–615. doi: 10.1128/jb.69.6.607-615.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGEN P., LISS E. Uber Bildung und Umsatz der Polyphosphate der Hefe. Biochem Z. 1958;330(6):455–466. [PubMed] [Google Scholar]
- LISS E., LANGEN P. [Experiments on polyphosphate overcompensation in yeast cells after phosphate deficiency]. Arch Mikrobiol. 1962;41:383–392. [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- PRICE C. A. Repression of acid phosphatase synthesis in Euglena gracilis. Science. 1962 Jan 5;135(3497):46–46. doi: 10.1126/science.135.3497.46. [DOI] [PubMed] [Google Scholar]
- SCHMIDT G., BARTSCH G., LAUMONT M. C., HERMAN T., LISS M. Acid phosphatase of bakers' yeast: an enzyme of the external cell surface. Biochemistry. 1963 Jan-Feb;2:126–131. doi: 10.1021/bi00901a022. [DOI] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. REPRESSIBLE ACID PHOSPHOMONOESTERASE AND CONSTITUTIVE PYROPHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1963 Oct;86:805–813. doi: 10.1128/jb.86.4.805-813.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDISCH F., HINKELMANN S., STIERAND D. Histochemischer Nachweis der im Zytoplasma regenerativ nach P-Mangelzüchtung auftretenden phosphatischen Uberkompensation. Experientia. 1955 Aug 15;11(8):307–309. doi: 10.1007/BF02158389. [DOI] [PubMed] [Google Scholar]


