Abstract
Tail resorption during amphibian metamorphosis has been thought to be controlled mainly by a cell-autonomous mechanism of programmed cell death triggered by thyroid hormone. However, we have proposed a role for the immune response in metamorphosis, based on the finding that syngeneic grafts of tadpole tail skin into adult Xenopus animals are rejected by T cells. To test this, we identified two tail antigen genes called ouro1 and ouro2 that encode keratin-related proteins. Recombinant Ouro1 and Ouro2 proteins generated proliferative responses in vitro in T cells isolated from naive adult Xenopus animals. These genes were expressed specifically in the tail skin at the climax of metamorphosis. Overexpression of ouro1 and ouro2 induced T-cell accumulation and precocious tail degeneration after full differentiation of adult-type T cells when overexpressed in the tail region. When the expression of ouro1 and ouro2 were knocked down, tail skin tissue remained even after metamorphosis was complete. Our findings indicate that Ouro proteins participate in the process of tail regression as immune antigens and highlight the possibility that the acquired immune system contributes not only to self-defense but also to remodeling processes in vertebrate morphogenesis.
Keywords: amphibian, skin, cell death, T cell, remodeling
During amphibian metamorphosis, most tissues in the tadpole undergo a complete transformation from the larva to the adult. However, the tail tissue is unique in that it retains its larval form until it is resorbed (1, 2). It has been hypothesized as early as 1916 that amphibian metamorphosis is regulated by the thyroid gland (3). Indeed, recent studies have shown that thyroid hormone (TH) plays a crucial role in tail regression and may regulate programmed cell death in a variety of tissues in a cell-autonomous manner (4, 5). However, it is also possible that neighboring cells induce nonautonomous tissue destruction (6).
We have suggested a possible role for the immune system in degeneration of larval tail tissues during metamorphosis, based on several lines of evidence (7–13). For instance, syngeneic grafts of larval tail skin into adult frogs are rejected and exhibit an accelerated secondary immune response (7). Moreover, T cells from adults and larvae at the metamorphic climax stage—but not earlier—show a prominent proliferative response against larval tails in vitro, and tail explants undergo apoptosis in the presence of adult Xenopus serum (8). Based on these observations, we proposed that newly differentiated adult-type, nonthymic T cells recognize and eliminate larval cells as “non-self” targets during metamorphosis. This model leads to the prediction that larval-specific antigens recognized by adult T cells are expressed in the larval skin (9). Recently, we isolated 59- and 53-kDa proteins as candidate target antigens using alloantiserum produced by larval skin grafts in adult frogs (10). The spatiotemporal localization of these two proteins in larval tail skin (11) is compatible with their predicted role as immune antigens involved in metamorphic tail regression (13). However, it is unresolved whether these proteins mediate an immune-based mechanism of tail regression.
In this study, we isolated genes encoding 59- and 53-kDa proteins, named Ouro1 and Ouro2, respectively, and carried out gain- and loss-of-function analyses. We show that ouro1 and ouro2 are specifically expressed in the regressing tail skin at the climax of metamorphosis and that recombinant Ouro proteins are recognized in vitro by adult T cells as foreign antigens. By analyzing the phenotypes of single- and double-transgenic (DT) tadpoles, we demonstrate that overexpression of ouro genes results in a significant acceleration of tail regression, whereas knockdown causes delayed tail regression. Together, these data provide the evidence for an unprecedented immune-based mechanism regulating the process of tissue reorganization in Xenopus metamorphosis.
Results
Ouro1 and Ouro2 Proteins Induce Adult T-Cell Proliferation.
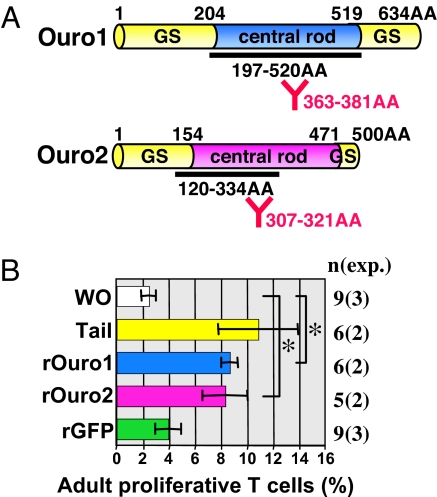
The isolated 59- and 53-kDa larval skin proteins (10) were sufficiently pure to determine their partial amino acid sequences [Fig. 1A, red Y marks for amino acids (AA) nos. 363–381 in Ouro1 and AA nos. 307–321 in Ouro2]. Rat antibodies raised against synthetic peptides containing these amino acid sequences specifically recognized the 59-kDa (10, 11) and 53-kDa proteins (see Fig. 2C), and these peptides elicited a T-cell response in vitro with cells isolated from syngeneic adult frogs immunized by grafting the larval skin (10). Oligonucleotide primers designed for the partial amino acid sequences of the 59- and 53-kDa proteins were used to amplify corresponding cDNA fragments, which were subsequently used to clone 2,009- and 1,764-bp cDNAs (accession nos. AB299972 and AB299973, respectively). These cDNAs contained coding sequences (CDS) for 59-kDa (634 residues) and 53-kDa (500 residues) proteins, both of which included a central rod domain flanked by glycine-serine-rich domains with no apparent strong homology to one another (Fig. 1A). We named these genes ouro1 for the 59-kDa CDS and ouro2 for the 53-kDa one, derived from the Greek word ouroboros, which means “one who devours his own tail.” These genes are putative orthologs of the hagfish thread keratin genes α and γ, respectively, which have unknown function and belong to different subgroups of the keratin superfamily (14).
Fig. 1.
Recombinant Ouro1 and Ouro2 proteins induce adult T-cell proliferation. (A) Schematic presentation of Ouro1 and Ouro2. Both proteins are predicted to contain central rod domains flanked by glycine-serine rich domains (GS). Bold lines with amino acid (AA) nos. represent sequences used for His-tagged recombinants. Y marks with AA nos. (red) show sequences used for raising specific antisera (see Results). (B) T-cell proliferation assay. Columns indicate the percentage of proliferating cells (mean ± SD from two to three independent experiments) cultured without a stimulus (WO) or with syngeneic larval tail tissues (Tail), Ouro1 recombinant protein (rOuro1), Ouro2 recombinant protein (rOuro2), or GFP recombinant protein (rGFP). The ANOVA test was used to assess statistical significance among values. *, P < 0.01. Significant differences are indicated by the Tukey's HSD test. n, number of assays; exp., number of experiments.
Fig. 2.
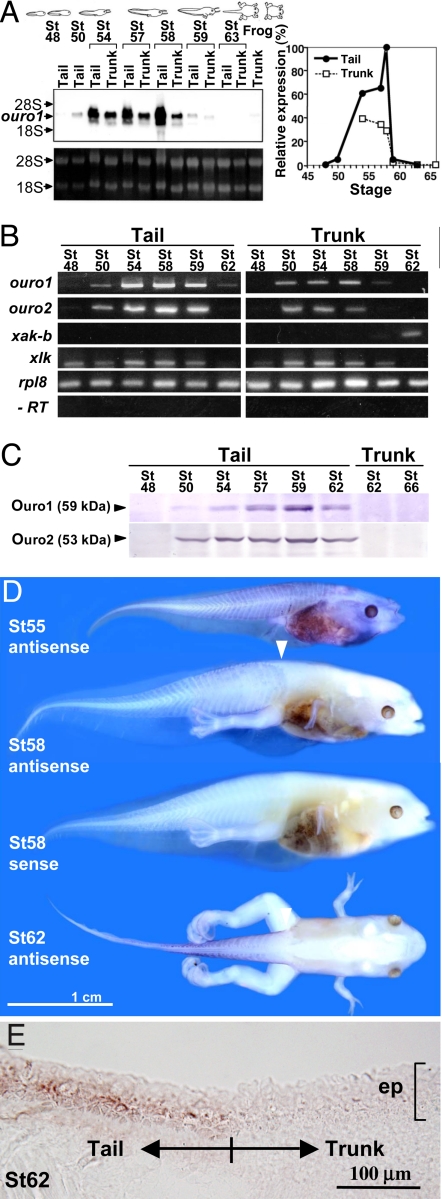
ouro1 and ouro2 are expressed in the skin during metamorphosis. (A) Northern blot analysis for ouro1 expression in J strain tadpoles. Tail and trunk skin tissues were isolated from various stages of tadpoles as indicated. A representative blot is shown (Upper Left), because five independent sets of experiments showed basically the same results. Ribosomal RNA visualized by ethidium bromide as a loading control (Lower Left). Relative expression levels were calculated using the image J software (Right). (B) RT-PCR with J strain tadpoles. Tail and trunk skin tissues as indicated were analyzed for ouro1, ouro2, Xenopus adult keratin (xak-b), Xenopus larval keratin (xlk), and Xenopus rpl8 (rpl8) as an internal control. -RT, rpl8 without RT. (C) Western blot analysis for Ouro1 and Ouro2 with J strain tadpoles. Tail and trunk skin cell lysates were used. (D) WISH with albino (non-J strain) X. laevis tadpoles. ouro1 antisense probe was used for tadpoles at stage 55 (n = 7), 58 (n = 7), and 62 (n = 3). ouro1 sense probe was used as a negative control for tadpoles at stage 58 (n = 5). Positive signals in blue were reproducibly detected in the tail and trunk (stage 55) or in the tail (stages 58 and 62). Arrowheads show the boundary between the tail and trunk region. (E) The vertical section of the tadpole at stage 62 after WISH using ouro1 antisense probe. The section includes the boundary between the tail and trunk skin as indicated. Purple signals are specifically seen in the tail epidermis (n = 2). ep, epidermis.
To characterize the Ouro1 and Ouro2 proteins, we produced recombinant proteins in Escherichia coli using their partial cDNA sequences (Fig. 1A, bold lines; AA nos. 197–520 in Ouro1, AA nos. 120–334 in Ouro2). Both recombinant proteins stimulated adult T cells as strongly as larval tail tissues from stage 57 tadpoles (Fig. 1B; see Figs. 2 A and B for the expression of ouro genes at stage 57), indicating that Ouro proteins function as antigens for adult immune cells.
The ouro1 and ouro2 Transcripts and Proteins Are Expressed in the Metamorphosing Tadpole Skin.
To examine whether ouro1 and ouro2 are expressed in the appropriate spatiotemporal pattern to be involved in tail regression, we performed Northern blot analysis and RT-PCR amplification for tadpole tissues. The transcripts for both genes were detected in the tail skin in a restricted period from stages 50–62 during metamorphosis (Fig. 2 A and B). Although weak expression levels of ouro1 and ouro2 were also observed in the trunk, such a sharp peak in expression in the tail skin appears to be unique to the ouro genes, compared with other types of keratin, adult (xak-b) and larval (xlk) keratin (Fig. 2B).
Western blotting analyses showed that both Ouro proteins could be detected at stage 50, and they peaked at stage 59 in the tail (Fig. 2C). Substantial expression of both proteins was observed at stages 54–62. Although the tail regresses dramatically during the metamorphic climax stage (stages 62–65), immunostaining of the tail with the Xenopus serum against the larval antigens including Ouro1 and Ouro2 showed that expressions of Ouro proteins were still detected at stage 64 at a high level (Fig. S1), similar to stage 59 (11). Thus, even though ouro transcripts were not detected after stage 62 (see Fig. 2 A and B), the Ouro proteins are likely to be present in the tail at a certain level from the prometamorphic stage (stages 54–57) to the climax stage (see Fig. S2).
Whole mount in situ hybridization (WISH) on albino tadpoles (non-J strain) demonstrated that the expression of ouro1 began throughout the entire body but diminished in the trunk at stage 58, with a clear boundary between the tail and the trunk (Fig. 2D, white arrowheads). The expression in the tail persisted until stage 62 (Fig. 2D and Fig. S3 for enlargement) and was strongly reduced by stage 63 (data not shown). No signal was detected with the sense probe control (Fig. 2D). Apparent inconsistency in expression levels at stage 62 between RT-PCR (Fig. 2B) and WISH (Fig. 2D) may have been caused by interindividual variability (Fig. S4) or differences between the J strain and the non-J albino strain. Cross-sections of stained tadpoles at stage 62 revealed that the expression of ouro1 was mainly confined to the epidermis of the skin (Fig. 2E). Notably, T-cell accumulation was observed in the tail epidermis (Fig. S1). Taken together, the results indicate that ouro genes are expressed in the tail epidermis specifically during metamorphosis, suggesting the possibility that Ouro proteins function to recruit T cells to the tail skin for regression.
Overexpression of ouro1 and ouro2 Enhances Tail Degeneration and T-Cell Accumulation.
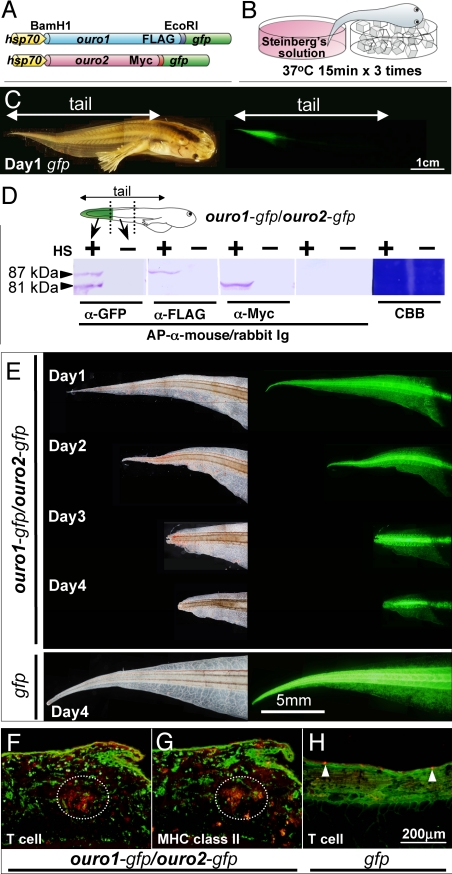
The potent antigenicity and expression pattern of the ouro transcripts and proteins, as well as T-cell accumulation in the tail at the metamorphic climax as described above, support our hypothesis that the Ouro proteins functioning as tail antigens mediate an immune-based mechanism of tail degeneration. To analyze the function of the ouro genes in vivo, transgenic animals generated by nuclear transplantation (15) were used to express FLAG- or Myc-tagged GFP-Ouro fusion proteins under the control of the Xenopus heat shock (HS) protein promoter (Fig. 3A). When the tail tip of transgenic tadpole was subjected to HS at stages 57–59 (Fig. 3B and Fig. S5), GFP expression was successfully induced with a clear boundary between HS-treated and nontreated areas (Fig. 3C). Western blot analysis confirmed that the tagged Ouro-GFP fusion proteins (87 kDa for Ouro1-FLAG-GFP, 81 kDa for Ouro2-Myc-GFP) were induced by HS in transgenic tadpoles as expected (Fig. 3D). In the ouro1-gfp/ouro2-gfp DT tadpoles, both Ouro proteins were detected in the same cells as assayed by confocal microscopy of immunostaining with anti-FLAG and anti-Myc tag antibodies (data not shown). Subsequently, F1 and F2 lines 1–9 were generated with the F0 transgenic frogs (Fig. S6).
Fig. 3.
Precocious tail degeneration by overexpression of ouro1 and ouro2 genes. (A) DNA constructs used to generate transgenic animals. Ouro proteins were fused to the FLAG- or Myc-tag and the GFP protein. The expression constructs are under the control of the HS promoter hsp70. (B) HS treatment. The distal part of the tadpole tail was heat-treated by immersion in Steinberg's solution at 37 °C. (C) Induction of GFP expression by HS. GFP was only detected in a HS-treated region of the tail. The panel shows a typical case, which is the gfp F2 transgenic line (see Fig. S6, line 9) tadpole on day 1 after HS treatment. (D) Western blot analysis of induced Ouro fusion proteins. ouro1-gfp/ouro2-gfp DT F2 tadpoles (see Fig. S6, line 2) were used. Expression of both introduced genes was detected in the HS-treated area (+), but not in nontreated area (−) on day 1 after HS. Arrowheads indicate the Ouro fusion proteins. Blotted proteins were stained with Coomassie Brilliant Blue (CBB). A representative blot from two independent experiments is shown. (E) Induction of precocious tail regeneration by HS. Tails of ouro1-gfp/ouro2-gfp DT (line 2) on days 1–4 after HS at stage 58/59 showed precocious degeneration (Upper). HS-induced gfp-transgenic tadpoles (line 9) showed a normal tail (Lower). Bright field (Left) and GFP fluorescence image (Right) are paired. (F–H) Accumulation of T cells in the HS-treated tails. Vertical frozen sections of HS-treated tails of ouro1-gfp/ouro2-gfp DT tadpole (line 1) (n = 8) (F and G) and gfp transgenic tadpole (line 8) (n = 8) (H) F1 tadpoles on day 3 after HS were stained with anti-GFP antibody (green) (F–H) and anti-Xenopus T cells (red) (F and H) or with anti-Xenopus MHC class II antibody (red) (G). Dotted circles in serial sections (F and G) indicate an assembly of T cells (F) expressing MHC class II (G). Arrowheads, a few T cells seen in the tail epidermis of the gfp control (H).
To observe the effect of ouro1 and ouro2 overexpression on the tail before tail regression normally starts at stage 62, HS was administered at stages 57–59. Notably, precocious degeneration of tail tissue was observed in a GFP-positive region on days 1–4 after HS when both Ouro1-GFP and Ouro2-GFP were induced (Fig. 3E). This phenomenon was observed in F0 tadpoles (Table S1) in F1 and F2 lines 1–3 with a high incidence of 72% (31/43) (Table 1, and see Table S1 for reproducibility in each line). By contrast, no degeneration was observed in the single transgenic ouro1-gfp (0/37), ouro2-gfp (0/20), and gfp alone (0/72) tadpoles (Table 1, lines 4–9). These data suggest that a combination of Ouro1 and Ouro2 overproduction can initiate tail degeneration.
Table 1.
Effects of overexpression of ouro genes on tail regression in transgenic tadpoles
| Transgene | Stage of HS | Line (F) | No. of HS tadpoles (nHS) | No. of GFP-expressing tadpoles (nG) | nG/nHS, % | Degenerated tails (nD) | nD/nG, % | P |
|---|---|---|---|---|---|---|---|---|
| Line 1 (F1) | ||||||||
| ouro1-gfp/ouro2-gfp | 57–59 | Line 2 (F2) | 55 | 43 | 78 | 31 | 72 | — |
| Line 3 (F2) | ||||||||
| Line 1 (F1) | ||||||||
| 50–52 | Line 2 (F2) | 38 | 27 | 71 | 0 | 0 | <0.01 | |
| Line 3 (F2) | ||||||||
| Line 4 (F1) | ||||||||
| ouro1-gfp | 58/59 | Line 5 (F1) | 74 | 37 | 50 | 0 | 0 | <0.01 |
| Line 6 (F2) | ||||||||
| ouro2-gfp | 58/59 | Line 7 (F2) | 36 | 20 | 56 | 0 | 0 | <0.01 |
| gfp | 57–59 | Line 8 (F1) | 97 | 72 | 74 | 0 | 0 | <0.01 |
| Line 9 (F2) |
F1 and F2 lines 1–9 were generated by mating their transgenic founders as described in Table S1 and Fig S6. GFP-expressing animals (nG) were selected from HS-treated specimens (nHS) on day 1 after HS as described in Materials and Methods. The phenotype ″Degenerated tails″ (nD) indicates that >50% of the GFP-expressing area disappeared by day 4 (around stages 61) as shown in Fig. 3E. The number of samples and phenotypes in the same experiment groups were combined (see Table S1 for details of each line). Note: HS treatment at the earlier stages (stages 50–52) for ouro1-gfp/ouro2-gfp double transgenic lines 1–3 did not initiate premature tail degeneration (see Results). P, one-way ANOVA, followed by Duncan's multiple range test against ouro1-gfp/ouro2-gfp DT tadpoles treated with HS at stage 57–59.
We next examined the possibility that Ouro-initiated premature degeneration is mediated by a T-cell immune response. For this purpose, HS treatment was done at the premetamorphic stages 50–52 before differentiation of adult-type T cells at stage 54 (13, 16). As expected, precocious tail degeneration was not observed in the HS-treated transgenic tadpoles (0/27; Table 1, lines 1–3). Furthermore, a second administration of HS at stages 57–59 to pretreated tadpoles (Table S1, line 1) resulted in severe tail degeneration (7/9) with a high incidence (8/9) than the single HS treatment at stages 57–59 (see Table S1). This enhanced response by the two rounds of HS treatment resembles a boosted immune response.
The precocious degenerating tails of ouro1-gfp/ouro2-gfp DT tadpoles were bloody as a result of hemolysis and/or dilation of peripheral capillaries, whereas the tails of gfp control tadpoles appeared transparent (Fig. 3E), like the normal metamorphosing tadpoles. Therefore, we next analyzed T-cell accumulation in degenerating tails. Immunohistochemistry of tail tissues for a Xenopus pan-T-cell marker showed an accumulation of a large number of T cells in the GFP-positive degenerating area (Fig. 3F). The accumulated T cells did not express GFP, indicating that they migrated from outside the HS-treated region. No such accumulation was seen in gfp (Fig. 3H) or single ouro transgenic tadpoles (data not shown). In addition, T cells in the HS-treated tail of ouro1-gfp/ouro2-gfp DT tadpoles expressed MHC class II (Fig. 3G), a marker of adult-type T cells (13, 16). Thus, tail degeneration was observed at stages after adult-type T-cell differentiation (see Fig. S2). These data suggest that T cells are primed by Ouro antigens during metamorphosis. These data are all consistent with the idea that tail degeneration by Ouro1 and Ouro2 involves T-cell-mediated immune responses.
Knockdown of ouro1 and ouro2 Gene Expression Results in Retention of the Tadpole Tail.
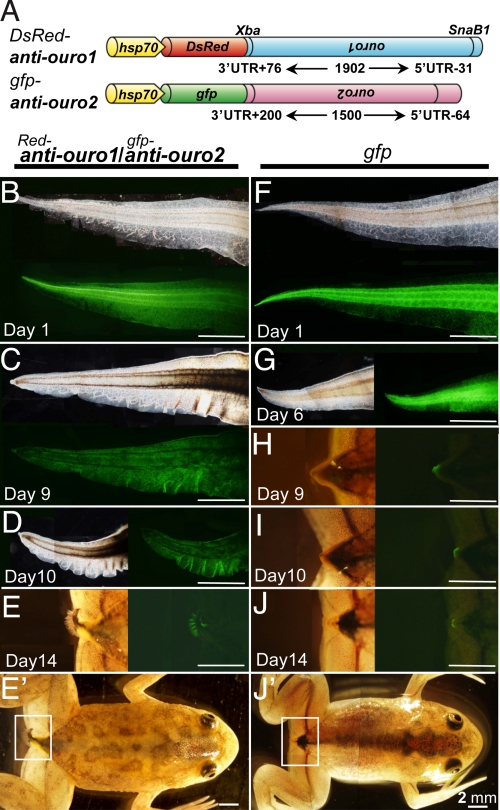
Next, loss-of-function analysis was performed by expressing antisense RNA in vivo in transgenic animals using DsRed-anti-ouro1/gfp-anti-ouro2 constructs (Fig. 4A). In this analysis, we were unable to generate F1 antisense-ouro tadpoles, and it was even difficult to raise their F0, possibly because of some toxicity from the leaky expression of antisense RNAs (see Table S2). The distal part of the transgenic tadpole tail was heat-treated in the same manner as shown in Fig. 3B and Fig. S5. On day 1 after HS, expressions of GFP (Fig. 4B) and DsRed (data not shown) were observed in the HS-treated area in the antisense-DT tadpole, similar to the GFP expression as shown in Fig. 3C. RT-PCR analysis confirmed that the antisense RNAs had been induced correctly by HS treatment (Fig. S7). Repression of the endogenous ouro2 gene upon HS was clearly shown at both the RNA and protein levels by comparing HS and non-HS regions in the same tail of antisense-DT or single-trasngenic tadpoles (Fig. S8).
Fig. 4.
Knockdown of ouro1 and ouro2 gene expression results in retention of tail skin. (A) Antisense constructs for ouro1 and ouro2. Reverse-oriented ouro1 or ouro2 cDNA were placed after DsRed or gfp, respectively, which were driven by the hsp70 promoter. (B–E and E′) Suppression of tail regression in DsRed-anti-ouro1/gfp-anti-ouro2 DT tadpoles. Tails of DsRed-anti-ouro1/gfp-anti-ouro2 DT tadpoles (F0) on days 1–14 after HS at stage 58/59 are shown by bright field and fluorescence microscopy (only GFP is shown). Note: DsRed-anti-ouro1/gfp-anti-ouro2 DT animals exhibit a pronounced delay in tail regression with a folded epidermis. (F–J and J′) gfp transgenic control. HS-induced gfp transgenic tadpole (line 9, F2) show normal tail degeneration. Boxed areas in E′ and J′ are magnified for E and J, respectively.
Remarkably, tail degeneration in DsRed-anti-ouro1/gfp-anti-ouro2 DT tadpoles was delayed in the HS-treated region, compared with that of the gfp control tadpoles (Fig. 4, compare C and H on day 9). Furthermore, some of HS-treated tails were retained even after day 14 (Fig. 4E), even though control animals had completed tail degeneration by stage 66. We defined the criterion for a “retained tail” in that the tail fails to regress; mainly the epidermis remains resulting in a “folded tail” phenotype (see Fig. 4 C–E). As summarized in Table 2, this retained tail phenotype was observed in 58% (7/12) of the DsRed-anti-ouro1/gfp-anti-ouro2 DT tadpoles. Furthermore, this phenotype was also observed in 20% (2/10) of the gfp-anti-ouro2 single-transgenic tadpoles (Table 2), suggesting that both Ouro1 and Ouro2 are required for tail degeneration.
Table 2.
Effects of knockdown of ouro genes on tail metamorphosis in transgenic tadpoles
| Transgene | Stage of HS | F0 or line (F) | No. of HS tadpoles (nHS) | No. of GFP/DsRed-expressing tadpoles (nG) | nG/nHS, % | Retained tails (nR) | nR/nG, % | P |
|---|---|---|---|---|---|---|---|---|
| DsRed-anti-ouro1/gfp-anti-ouro2 | 58/59 | F0 | 144 | 12 | 8 | 7 | 58 | <0.01 |
| gfp-anti-ouro2 | 58/59 | F0 | 115 | 10 | 9 | 2 | 20 | <0.05 |
| gfp | F0 | 19 | 6 | 32 | ||||
| 57–59 | Line 8 (F1) | 73 | 56 | 77 | 0 | 0 | — | |
| Line 9 (F2) |
The number of samples and phenotypes in the same experiment groups were combined (see Table S2 for details of each F0 experiment or line). F1 and F2 gfp lines were generated as described in Fig. S6D. GFP-expressing animals (nG) were selected on day 1 from HS-treated tadpoles (nHS). Retained tails (nR) were counted on days 7–14 (stages 64–66). The same specimens of gfp lines 8 and 9 as in Table 1 were analyzed for tail retention. Note: the number of HS-tadpoles was reduced because some of tadpoles were killed for histochemical examination or died before being counted. P, one-way ANOVA, followed by Duncan's multiple range test against gfp.
Some of the folded tails in antisense tadpoles were retained for over 2 weeks after metamorphosis had been completed. This retention was not caused by a transformation from larva- to adult-type skin, because the expression of adult-type keratin xak-b was not detected in the tail (Fig. S8A). In normal metamorphosing tadpoles, the endogenous ouro1 is expressed specifically in the epidermis as shown above (see Fig. 2E). This expression pattern can explain our observation that the epidermis persisted even though the other tail tissue components completely degenerated when ouro gene expression was knocked down.
Discussion
In this paper, we have shown that the Ouro1 and Ouro2 proteins are necessary and sufficient for the regression of the tail skin during Xenopus metamorphosis. Our data substantiate that the Ouro1 and Ouro2 proteins represent the long-postulated 59- and 53-kDa molecules, respectively (9, 10), based on their antigenicity, calculated molecular mass, and specific expression in the skin epidermis peaking at, and limited to, the metamorphic periods.
Immunocompetent cells undergo profound reorganization during metamorphosis, including the large-scale death of larval lymphocytes and the emergence of adult-type T cells (16). Precocious tail regression by overexpressed Ouro proteins depends on the period when adult T cells are fully differentiated, being consistent with our previous finding that the T cells from climax-stage, but not prometamorphic-stage animals, display a prominent proliferative response against larval tail tissues (8). Taken together, we propose an immune-based mechanism, in which the Ouro proteins actively function in tail regression as targets for newly differentiated adult-type immune cells during metamorphosis. This is in contrast to the role of innate immunity to clean up larval cells during metamorphosis, which had been generally believed since Weber's original observations (17).
Almost a century has passed since scientific argument began in 1916 concerning the mechanism underlying amphibian metamorphosis, in which removal of the thyroid from tadpoles inhibited their metamorphosis (3). To date, two mechanisms underlying induction of apoptosis in amphibian metamorphosis have been proposed (6). The first is the “suicide model,” in which a cell-autonomous apoptosis pathway is triggered by TH. The second is the “murder model,” in which death factors secreted from the neighboring cells kill larval cells. Our data demonstrate a third mechanism, as detailed above.
We have shown that, individually, neither the Ouro1 nor Ouro2 protein is sufficient for inducing precocious tail degeneration in overexpression (Table 1) that suppression of Ouro2 alone is necessary for tail retention in knockdown experiments (Table 2). These phenomena could be explained by the formation of a complex, because it has been reported that the hagfish counterparts, thread keratin α and γ, form a stable complex in vitro (18, 19).
ouro1 and ouro2 expressions are initiated during early metamorphosis in both the tail and trunk epidermis, but become restricted to the tail region at the climax of metamorphosis. Although the mechanisms regulating ouro gene expression remain elusive, the down-regulation of ouro expression in the trunk may be caused by TH, because the Ouro1 protein is down-regulated in the trunk of TH-treated tadpoles more rapidly than in the tail (11), and because TH is present in higher concentrations in the trunk than in the tail cells (20). Whatever the mechanism, this tail-specific expression of ouro genes is likely to function as a prepattern for tail regression, which is targeted by an immune response.
Our results demonstrate not only the role of the acquired immune system in self/non-self recognition, but also how adult/larva recognition contributes to the tissue remodeling process. This report shows how this discovered mechanism is involved in amphibian metamorphosis and suggests that the immune system may participate more generally than suspected in tissue remodeling during vertebrate morphogenesis. Although here we reported on Ouro proteins in Xenopus, we have also isolated some putative orthologs from other amphibians and fish (unpublished data), which lead to the idea that a similar mechanism may be involved in the development of species other than Xenopus.
Materials and Methods
Animals.
An MHC-homozygous inbred J strain of Xenopus laevis was used for most experiments and was staged according to Nieuwkoop and Faber (21). Outbred X. laevis animals were used for preparing transgenic animals. A partially inbred albino strain (non-J strain) of X. laevis was used for WISH. All larvae and adults were reared at 23 ± 1 °C in dechlorinated tap water.
Cloning of ouro Genes.
Degenerate primers were designed against partial peptide sequences of 59- and 53-kDa proteins that were immunopurified from tail skin lysates of stage 57 J strain tadpoles using a frog alloantiserum (10). PCR products were used as probes to clone full-length ouro1 and ouro2 cDNAs by screening a ZAP cDNA library of whole tails at stage 62 (a kind gift from Dr. Yoshio Yaoita). 5′ RACE was performed to identify the 5′UTR of ouro1.
T-Cell Proliferation Assay with His-Tagged Recombinant Proteins.
cDNA fragments encoding AA197–520 of ouro1 and AA120–334 of ouro2 were cloned into the pQE70 vector with a 6× His-tag at the C-terminal end. gfp was a gift from Qiagen GmbH. IPTG-induced recombinant proteins were obtained using the E. coli M15 strain, the QIA expressionist kit (Qiagen), a Ni-NTA column, dialyzed against PBS, and sterilized through a 0.22-μm filter after concentration in Centricon columns (Millipore). The T-cell proliferation assay was performed as described (8, 9). Briefly, 5 × 107 leukocytes, including antigen presenting cells (APCs) from spleens of 1- to 2-year-old J strain adult frogs, were cocultured in 70% L15 medium with 2 × 2 mm2 square cut larval tail fins from the same strain of tadpoles at stage 57 or with each His-tagged recombinant at a concentration of 35 μM in 10% resin-treated FCS, from which hormones (including TH) had been excluded. Proliferation was evaluated by BrdU incorporation.
Northern Blot Analysis.
Total RNA was extracted from the tail or trunk skin from one or five pooled J strain tadpoles at stages 48–66. The tail skin was carefully separated from the muscle and notochord. The trunk skin was excised from the dorsal region of the trunk and head, including the forelimb skin. RNA (10 μg) was run on a 1% agarose formaldehyde gel, transferred onto a membrane, and hybridized at 50 °C with 32P-labeled ouro1 cDNA probe (nucleotide nos. 1090–1497).
RT-PCR Analysis.
Total RNA was extracted from the tail or trunk skin as described above. RNA (2 μg) was reverse-transcribed using random primers (Invitrogen). Specific primers were used for ouro1 (sense: 5′-TTT-GAT-AAC-ACG-CCC-AAA-CTG-G-3′, antisense 5′-CAT-CTT-CAC-TGC-CAA-GAG-GTC-3′) and ouro2 (sense: 5′-GGC-ATT-TTC-TTT-GGG-GCG-TTC-TTT-GAC-TGC-3′, antisense: 5′-GCT-CTC-AGT-TTG-TTT-AAT-GCA-GTG-GTG-AGG-3′). PCR was performed for 22–32 cycles as described for xlk, xak (22), and rpl8 (23). The amplified products were run on 2% agarose gels and visualized by ethidium bromide.
Western Blot Analysis.
Western blot analysis was performed essentially as described in ref. 11. Briefly, lysates obtained from J strain tadpole tail and trunk skins, or from whole tissues of transgenic tadpoles, were electrophoresed on 10% SDS-polyacrylamide gel (50 μg total protein per lane). Immunoblots were performed with rat antiserum against synthetic peptides for Ouro1 (10) or Ouro2 (see Fig. 1), mouse anti-GFP monoclonal antibody (Santa Cruz Biotechnology), rabbit anti-FLAG polyclonal antibodies (Sigma), and mouse anti-c-Myc monoclonal antibody (Roche Molecular Biochemicals). AP-conjugated rat, mouse, or rabbit IgG antibodies were used as secondary antibodies for color detection.
Whole Mount in Situ Hybridization.
WISH was performed on albino (non-J strain) X. laevis tadpoles with digoxigenin-labeled probes as described in ref. 24 with a few modifications. Antisense and sense probes were synthesized from the CDS of ouro1 in the pBSIISK+ plasmid vector (Stratagene) and hybridized at 60 °C for 25 h.
Immunohistochemistry on Sections.
Nonfixed frozen skin tissues were embedded in OCT-Compound, and 4-μm sections were cut (11) and incubated with mouse anti-Xenopus T-cell monoclonal antibody (XT-1) (25), mouse anti-Xenopus MHC class II monoclonal antibody (AM20) (26), or rabbit anti-GFP polyclonal antibody (Medical Biological Lab). Secondary antibody detection was performed using Cy3 or Alexa488-conjugated mouse or rabbit IgG antibodies.
Construction of Plasmids for Transgenesis.
The Xenopus HS promoter-driven expression plasmids pHsS1/EGFP (27) and pHsS1/DsRed were used as vectors. CDS fragments of ouro1 or ouro2 with FLAG- or Myc-tag, respectively, were subcloned into the BamHI and EcoRI sites of pHsS1/EGFP. pHsS1/EGFP was used as the gfp control. Antisense constructs were made by inserting the ouro1 or ouro2 cDNA (the ouro1 cDNA including 31-bp 5′UTR and 76-bp 3′UTR or the ouro2 cDNA including 64-bp 5′UTR and 200-bp 3′UTR) in the reverse orientation.
Nuclear Transplantation Transgenic Technique.
Transgenesis was performed as described in ref. 15. Briefly, NotI-linearized plasmid was mixed with sperm nuclei, and this mixture was then microinjected into unfertilized eggs. Transgenic embryos were raised to feeding stage in 6 days in Steinberg's solution with 50 μg/mL gentamicin.
HS Treatment and Screening Animals.
The tip of tail was heat-shocked for 15 min at 37 °C three times, keeping them at room temperature in Steinberg's solution for 15 min in between (see Fig. S5). To identify transgenic animals, expression of GFP or DsRed in the HS area was analyzed by fluorescence microscopy on day 1 after HS. Animals with high expression of GFP or DsRed were selected, and negative-, weak-, moderate-, or mosaic-expressing animals were subsequently excluded. The ouro1-gfp/ouro2-gfp DT tadpoles were confirmed by double-staining for FLAG- and Myc-tags using immunohistochemistry as described above. The selected animals were carefully reared individually in 200-mL glass beakers with autoclaved Steinberg's solution to avoid undesirable effects. Tails were observed without anesthetizing the animals, using a fluorescent microscope. A picture of the tail was constructed from partially overlapped serial images captured using a digital camera (at least 6 to 15 images for a tadpole tail).
Supplementary Material
Acknowledgments.
We thank M. Asashima and T. Michiue (University of Tokyo, Tokyo, Japan) for providing the transgenic vector, and Y. Yaoita (Hiroshima University, Higashi-Hiroshima, Japan) for the Xenopus cDNA library. We also thank Yu-ichi G. Watanabe, K. Fujiwara, K. Yamazaki, M. Yashima, N. Sudou, and Y. Ito for technical assistance, K. Igarashi, T. Ohshima, and A. Sasaki for technical support producing transgenic tadpoles, M. Yamamoto, M. Ito, M. Ato, Y. Maeda, and M. Yamaguchi for comments, N. Funayama, I. Hamano, and Y. Okubo for their many kindnesses, and C. Katagiri and C. Hannah for critical reading.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Ouroboros1, Accession no. AB299972; Ouroboros2, Accession no. AB299973 have been deposited in the DNA Data Bank of Japan (DDBJ).
This article contains supporting information online at www.pnas.org/cgi/content/full/0708837106/DCSupplemental.
References
- 1.Izutsu Y, Kaiho M, Yoshizato K. Different distribution of epidermal basal cells in the anuran larval skin correlates with the skin's region-specific fate at metamorphosis. J Exp Zool. 1993;267:605–615. [Google Scholar]
- 2.Izutsu Y, Tochinai S, Onoe K. Loss of reactivity to pan-cadherin antibody in epidermal cells as a marker for metamorphic alteration of Xenopus skin. Dev Growth Differ. 2000;42:377–383. doi: 10.1046/j.1440-169x.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen BM. The results of extirpation of the anterior lobe of the hypophysis and of the thyroid of Rana pipiens larvae. Science. 1916;44:755–758. doi: 10.1126/science.44.1143.755. [DOI] [PubMed] [Google Scholar]
- 4.Das B, et al. Gene expression changes at metamorphosis induced by thyroid hormone in Xenopus laevis tadpoles. Dev Biol. 2006;291:342–355. doi: 10.1016/j.ydbio.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz DR, Heimeier RA, Das B, Washington T, Shi YB. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2006;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima K, Yaoita Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn. 2003;227:246–255. doi: 10.1002/dvdy.10300. [DOI] [PubMed] [Google Scholar]
- 7.Izutsu Y, Yoshizato K. Metamorphosis-dependent recognition of larval skin as non-self by inbred adult frogs (Xenopus laevis) J Exp Zool. 1993;266:163–167. doi: 10.1002/jez.1402660211. [DOI] [PubMed] [Google Scholar]
- 8.Izutsu Y, Yoshizato K, Tochinai S. Adult-type splenocytes of Xenopus induce apoptosis of histocompatible larval tail cells in vitro. Differentiation. 1996;60:277–286. doi: 10.1046/j.1432-0436.1996.6050277.x. [DOI] [PubMed] [Google Scholar]
- 9.Izutsu Y, Tochinai S, Iwabuchi K, Onoe K. Larval antigen molecules recognized by adult immune cells of inbred Xenopus laevis: Two pathways for recognition by adult splenic T cells. Dev Biol. 2000;221:365–374. doi: 10.1006/dbio.2000.9681. [DOI] [PubMed] [Google Scholar]
- 10.Izutsu Y, Tochinai S, Maeno M, Iwabuchi K, Onoe K. Larval antigen molecules recognized by adult immune cells of inbred Xenopus laevis: Partial characterization and implication in metamorphosis. Dev Growth Differ. 2002;44:477–488. doi: 10.1046/j.1440-169x.2002.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Ohshima M, Morohashi M, Maeno M, Izutsu Y. Ontogenic emergence and localization of larval skin antigen molecule recognized by adult T cells of Xenopus laevis: Regulation by thyroid hormone during metamorphosis. Dev Growth Differ. 2003;45:77–84. doi: 10.1046/j.1440-169x.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Izutsu Y, Maeno M. Analyses of immune responses to ontogeny-specific antigens using an inbred strain of Xenopus laevis (J strain) Methods Mol Med. 2005;105:149–158. doi: 10.1385/1-59259-826-9:149. [DOI] [PubMed] [Google Scholar]
- 13.Izutsu Y. The immune system is involved in Xenopus metamorphosis. Front Biosci. 2009;14:141–149. doi: 10.2741/3235. [DOI] [PubMed] [Google Scholar]
- 14.Schaffeld M, Schultess J. Genes coding for intermediate filament proteins closely related to the hagfish “thread keratins (TK)” alpha and gamma also exist in lamprey, teleosts and amphibians. Exp Cell Res. 2006;312:1447–1462. doi: 10.1016/j.yexcr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 16.Rollins-Smith LA, Needham DA, Davis AT, Blair PJ. Late thymectomy in Xenopus tadpoles reveals a population of T cells that persists through metamorphosis. Dev Comp Immunol. 1996;20:165–174. doi: 10.1016/0145-305x(96)00018-3. [DOI] [PubMed] [Google Scholar]
- 17.Weber R. Ultrastructural changes in regressing tail muscles of Xenopus larvae at metamorphosis. J Cell Biol. 1964;22:481–487. doi: 10.1083/jcb.22.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RH, Downing SW, Koch EA, Salo WL, Saidel LJ. Hagfish slime gland thread cells. II. Isolation and characterization of intermediate filament components associated with the thread. J Cell Biol. 1984;98:670–677. doi: 10.1083/jcb.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch EA, Spitzer RH, Pithawalla RB, Castillos FA, 3rd, Parry DA. Hagfish biopolymer: A type I/type II homologue of epidermal keratin intermediate filaments. Int J Biol Macromol. 1995;17:283–292. doi: 10.1016/0141-8130(95)98156-s. [DOI] [PubMed] [Google Scholar]
- 20.Brown DD. The role of deiodinases in amphibian metamorphosis. Thyroid. 2005;15:815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis Daudin. The Netherlands: North-Holland, Amsterdam; 1956. [Google Scholar]
- 22.Watanabe Y, et al. Metamorphosis-dependent transcriptional regulation of xak-c, a novel Xenopus type I keratin gene. Dev Dyn. 2002;225:561–570. doi: 10.1002/dvdy.10196. [DOI] [PubMed] [Google Scholar]
- 23.Sachs LM, Shi YB. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc Natl Acad Sci USA. 2000;97:13138–13143. doi: 10.1073/pnas.260141297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashiro S, Sedohara A, Asashima M, Izutsu Y, Maeno M. Characterization of myeloid cells derived from the anterior ventral mesoderm in the Xenopus laevis embryo. Dev Growth Differ. 2006;48:499–512. doi: 10.1111/j.1440-169X.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 25.Nagata S. Development of T lymphocytes in Xenopus laevis: Appearance of the antigen recognized by an anti-thymocyte mouse monoclonal antibody. Dev Biol. 1986;114:389–394. doi: 10.1016/0012-1606(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 26.Flajnik MF, Ferrone S, Cohen N, Du Pasquier L. Evolution of the MHC: Antigenicity and unusual tissue distribution of Xenopus (frog) class II molecules. Mol Immunol. 1990;27:451–462. doi: 10.1016/0161-5890(90)90170-5. [DOI] [PubMed] [Google Scholar]
- 27.Michiue T, Asashima M. Temporal and spatial manipulation of gene expression in Xenopus embryos by injection of heat shock promoter-containing plasmids. Dev Dyn. 2005;232:369–376. doi: 10.1002/dvdy.20233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.