Abstract
Ubiquitination by the anaphase-promoting complex (APC/C) is essential for proliferation in all eukaryotes. The human APC/C promotes the degradation of mitotic regulators by assembling K11-linked ubiquitin chains, the formation of which is initiated by its E2 UbcH10. Here, we identify the conserved Ube2S as a K11-specific chain elongating E2 for human and Drosophila APC/C. Ube2S depends on the cell cycle-dependent association with the APC/C activators Cdc20 and Cdh1 for its activity. While depletion of Ube2S already inhibits APC/C in cells, the loss of the complete UbcH10/Ube2S-module leads to dramatic stabilization of APC/C substrates, severe spindle defects, and a strong mitotic delay. Ube2S and UbcH10 are tightly co-regulated in the cell cycle by APC/C-dependent degradation. We conclude that UbcH10 and Ube2S constitute a physiological E2-module for APC/C, the activity of which is required for spindle assembly and cell division.
Keywords: K11-linked chain, proteasome, ubiquitin
The proteasomal degradation of proteins is essential for cell division in all eukaryotes. Proteins are targeted to the proteasome by modification with ubiquitin chains, whose assembly depends on a cascade of E1, E2, and E3 enzymes (1, 2). E3s containing a RING-domain recruit substrates and ubiquitin-charged E2s, and promote the transfer of ubiquitin from the E2 active site to a substrate lysine. The E2-E3 pair then switches to modifying one of the seven Lys residues in ubiquitin, which results in chain elongation. E2s with dedicated roles in elongating K48- (UBE2K/E2–25K) or K63-linked chains (UBE2N-UEV1A) have been described (3, 4).
The essential RING-E3 anaphase-promoting complex (APC/C) is a key regulator of cell division in eukaryotes (5). Loss of APC/C activity arrests cells at metaphase and results in severe aberrations in the structure of the mitotic spindle (6–8). Human APC/C regulates progression through mitosis by modifying a large family of substrates with K11-linked ubiquitin chains, which triggers their degradation by the proteasome (10–12). Its specific E2, UBE2C/UbcH10, initiates chain formation by recognizing a substrate motif, the TEK-box, which is homologous to residues around K11 in ubiquitin (11). However, depletion of UbcH10 stabilizes APC/C substrates to a lesser extent than inhibition of APC/C or mutation of K11 in ubiquitin (11, 12), suggesting that additional K11-specific E2s operate with human APC/C.
As expected from its central role in proliferation, the capacity of the APC/C to assemble ubiquitin chains is tightly regulated. When cells enter mitosis, the APC/C is partially activated through phosphorylation of core subunits and binding of the WD40-repeat protein Cdc20 (9, 13, 14). The APC/C becomes fully active after all chromosomes have achieved bipolar attachment to the mitotic spindle, which results in silencing of the spindle checkpoint and dissociation of Cdc20 from its inhibitors Mad2 and BubR1 (13). Soon after anaphase onset, Cdc20 is degraded and replaced by Cdh1, which maintains APC/C activity during late mitosis and early G1 (15, 16). Accordingly, inactivation of the APC/C before S phase involves phosphorylation and degradation of Cdh1 and binding of the Cdh1-inhibitor Emi1 (5). Cdc20 and Cdh1 recruit substrates to the APC/C (17, 18), but also increase the catalytic activity of the APC/C by a poorly understood mechanism.
Here, we identify the highly conserved Ube2S as a regulator of human and Drosophila APC/C. Ube2S functions as a K11-specific chain elongating E2 of APC/C, which depends on chain initiation by UbcH10. Together, UbcH10 and Ube2S are required for the degradation of all APC/C substrates tested so far, spindle formation, and progression of cells through mitosis. Ube2S and UbcH10 are tightly co-regulated during the cell cycle, and APC/C itself promotes their ubiquitination and degradation. Our data suggest that UbcH10 and Ube2S constitute the physiological E2-module of human APC/C during mitosis.
Results
Ube2S Promotes the Assembly of K11-Linked Ubiquitin Chains by the APC/C.
The human APC/C triggers the proteasomal degradation of its substrates by modifying them with K11-linked ubiquitin chains (10, 11). Its specific E2, UbcH10, initiates chain formation by recognizing TEK-boxes in substrates and ubiquitin (11). UbcH10 is the only known APC/C-E2 with K11-specificity, but its depletion from extracts fails to completely stabilize the APC/C substrate securin (21). Thus, an unidentified E2 must be able to catalyze the formation of K11-linked ubiquitin chains by the APC/C.
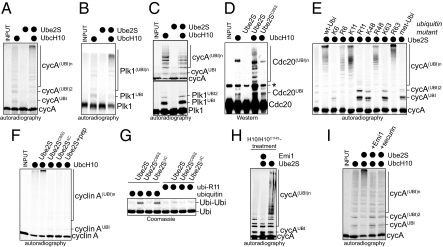
To isolate the unknown E2, we purified human E2 enzymes and measured their activity in catalyzing ubiquitination by the APC/C. Using this approach we identified the conserved E2 Ube2S, which dramatically promotes the formation of ubiquitin chains on several APC/C substrates, when added together with the APC/C-specific E2 UbcH10 (Fig. 1 A and B and Fig. S1 A–C). Albeit less efficiently, Ube2S also cooperates with the non-specific E2 UbcH5 (Fig. S1E). Ube2S functions with both APC/CCdc20 and APC/CCdh1 (Fig. 1D), while it is inactive with other E3s tested (Fig. S1F).
Fig. 1.
Ube2S catalyzes the formation of K11-linked ubiquitin chains on APC/C-substrates. (A) Ube2S promotes chain formation on cyclin A. 35S-cyclin A was incubated with APC/CCdh1, E1, ubiquitin, and UbcH10, and/or Ube2S. Reaction products were analyzed by autoradiography. (B) Ube2S promotes chain formation on Plk1. The ubiquitination of 35S-Plk1 was analyzed as described for cyclin A. (C) Ube2S does not promote chain initiation. 35S-cyclin A and 35S-Plk1 were incubated with APC/CCdh1 and methyl-ubiquitin, as described above. (D) Ube2S cooperates with APC/CCdc20. APC/CCdc20 was activated as described (23), and used to ubiquitinate endogenous Cdc20, as detected by Western blot. (E) Ube2S assembles K11-linked chains on APC/C substrates. The ubiquitination of 35S-cyclin A by APC/CCdh1 and Ube2S was analyzed in the presence of ubiquitin mutants, as indicated. “K6” describes ubiquitin containing Lys-6, “R6” is ubiquitin, in which K6 has been exchanged to Arg. (F) Ube2S requires its catalytic Cys and its C terminus for APC/C-dependent chain formation. Ubiquitination of 35S-cyclin A was analyzed in the presence of APC/CCdh1, Ube2S, Ube2SC95S, or Ube2S1–196/Ube2SΔC. In addition, the activity of Ube2S was competed by a C-terminal peptide encompassing the last 26 amino acids of Ube2S (Ube2S+pep). (G) Ube2S catalyzes formation of K11-linked ubiquitin dimers independently of an E3. Ube2S and indicated mutants were incubated with E1 and ubiquitin or ubi-R11, and the formation of ubiquitin dimers (ubi-ubi) was detected by Coomassie staining. (H) Ube2S does not require UbcH10 during chain elongation. 35S-cyclin A was briefly incubated with APC/CCdh1 and UbcH10, before UbcH10 was inactivated by adding a 100-fold excess of UbcH10C114S. Then, Ube2S was added alone or together with the APC/C inhibitor Emi1. (I) Ube2S depends on the APC/C for activity. 35S-cyclin A was briefly incubated with APC/CCdh1 and UbcH10, and then Ube2S alone or Ube2S together with the APC/C inhibitor Emi1 or an excess of the APC/C substrate securin were added.
Ube2S does not modify substrate Lys residues itself (Fig. 1 A and B), and as seen in reactions with methyl-ubiquitin, it does not promote additional chain initiation by UbcH10 (Fig. 1C). These findings indicate that Ube2S elongates ubiquitin chains previously initiated by UbcH10.
The addition of recombinant ubiquitin mutants revealed that chains assembled by Ube2S and APC/C are strictly linked through K11 of ubiquitin, independently of whether UbcH10 or UbcH5c are used to initiate chain formation (Fig. 1E and Fig. S1 A–C and G–I). In promoting chain elongation, Ube2S is reminiscent of E2–25K and Ube2N-Uev1A, which extend K48- or K63-linked ubiquitin chains, respectively (2). However, E2–25K and Ube2N-Uev1A do not cooperate with UbcH10 in catalyzing chain formation on APC/C substrates (Fig. S1I).
Ube2S contains a UBC-domain and a conserved C-terminal extension. Mutation of the catalytic Cys of Ube2S (Ube2SC95S) or deletion of the C terminus (Ube2SΔC) abrogates its activity toward APC/C substrates (Fig. 1F). In a similar manner, addition of a peptide comprising the C terminus of Ube2S (CTP) blocks the activity of Ube2S on APC/C. Ube2S catalyzes the formation of K11-linked ubiquitin-dimers independently of APC/C (22). This activity depends on the active-site Cys of Ube2S, but is not affected by deletion of its C terminus (Fig. 1G), indicating that the C terminus of Ube2S is specifically required for APC/C-dependent chain formation.
To determine whether Ube2S depends on UbcH10 or APC/C during chain elongation, we separated chain initiation from elongation. We briefly incubated the APC/C substrate cyclin A with APC/C and UbcH10, which results in short ubiquitin chains on cyclin A (Fig. 1 H and I). In one experiment, we then added Ube2S together with an excess of inactive UbcH10C114S to block further UbcH10-activity. Under these conditions, Ube2S still elongates ubiquitin chains on cyclin A, indicating that it does not require UbcH10 for chain extension (Fig. 1H). In a parallel experiment, we added Ube2S and the APC/C inhibitor Emi1 or an excess of a competitor APC/C substrate to displace preubiquitinated cyclin A from APC/C. This treatment interferes with chain-extension (Fig. 1I), demonstrating that Ube2S depends on APC/C to elongate chains on APC/C substrates. Together, these experiments point to Ube2S as a K11-specific chain-elongating E2 of human APC/C.
Ube2S Binds APC/C and APC/C Activators.
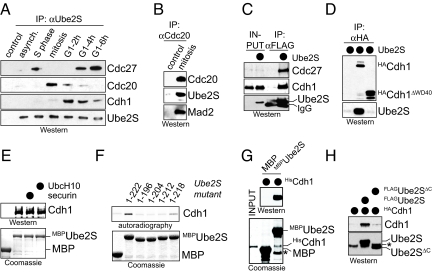
To test whether Ube2S cooperates with APC/C in cells, we precipitated endogenous Ube2S from synchronized HeLa cells and detected co-purifying proteins by Western analysis. We find a strong association between Ube2S and the APC/C activator Cdc20, which occurs specifically during mitosis (Fig. 2A). This interaction is also observed when FLAGUbe2S or endogenous Cdc20 are immunoprecipitated from mitotic cells (Fig. 2B and Fig. S2A). During late mitosis and early G1, APC/C is activated by Cdh1, and at this cell cycle stage, Ube2S-precipitates only contain Cdh1, and no Cdc20. FLAGUbe2S also associates with Cdh1 (Fig. 2C), and the reverse affinity-purification of Cdh1 recovers high levels of Ube2S (Fig. 2D). Thus, Ube2S interacts with both APC/C activators Cdc20 and Cdh1 in a cell cycle-dependent manner.
Fig. 2.
Ube2S interacts with APC/C. (A) Ube2S binds Cdc20 and Cdh1 in a cell cycle-dependent manner. Ube2S was precipitated from extracts of synchronized HeLa cells, and co-purifying proteins were detected by Western blot. (B) Cdc20 binds Ube2S in mitosis. Cdc20 was precipitated from mitotic HeLa S3 cells. Co-purifying Ube2S and Mad2 were detected by Western blot. (C) Ube2S binds Cdh1. FLAGUbe2S was precipitated from 293T cells, and co-purifying HACdh1 was detected by Western blot. (D) Cdh1 binds Ube2S. HACdh1 or HACdh1ΔWD40 were precipitated from 293T cells, and co-purifying Ube2S was detected by Western blot. (E) Ube2S binds Cdh1 from G1 extracts. Immobilized MBP or MBPUbe2S were incubated with HeLa S3 extracts. Recombinant securin or UbcH10 (≈100× excess over Cdh1) were added as indicated. Bound Cdh1 was detected by Western blot. (F) Ube2S binds Cdh1. 35S-Cdh1 was incubated with immobilized MBPUbe2S or the indicated truncation mutants. Bound Cdh1 was detected by autoradiography. (G) Ube2S directly binds Cdh1. HisCdh1 purified to homogeneity was incubated with MBP and MBPUbe2S. Bound Cdh1 was detected by Western blot. (H) The C terminus of Ube2S is required for its interaction with Cdh1. FLAGUbe2S or FLAGUbe2SΔC were precipitated from 293T cells, and bound HACdh1 was analyzed by Western blot.
To determine whether Ube2S directly binds Cdc20 or Cdh1, we performed pulldown-assays using MBPUbe2S. In these experiments, we focused on Cdh1, which does not require mitotic phosphorylations for activity. As expected for a direct interaction, MBPUbe2S efficiently binds Cdh1 from G1 extracts (Fig. 2E), Cdh1 synthesized by IVT/T (Fig. 2F), and HisCdh1 purified to homogeneity from insect cells (Fig. 2G). The interaction between Ube2S and Cdh1 in vitro as well as in 293T cells requires the WD40-domain of Cdh1 (Fig. 2D and Fig. S2B). Although the WD40-domain of Cdh1 recognizes APC/C substrates (17, 18), an excess of APC/C substrates does not block the Cdh1/Ube2S-interaction (Fig. 2E and Fig. S2C), and binding studies show that Cdh1 binds substrates and Ube2S at the same time (Fig. S2D). The association of Ube2S with Cdh1 depends on the C terminus of Ube2S and is blocked by the C-terminal Ube2S-peptide (Fig. 2F and Fig. S2E). Accordingly, Ube2SΔC fails to interact with Cdh1 in cells (Fig. 2H). We conclude that Ube2S directly binds Cdh1 using its C-terminal tail, which is also required for the activity of Ube2S toward APC/C.
In addition to its interaction with Cdc20 and Cdh1, Ube2S binds core APC/C, as Ube2S-precipitates from late G1 and S phase contain the APC/C subunit Cdc27, but little Cdc20 or Cdh1 (Fig. 2A). Indeed, FLAGUbe2S affinity-purified from stably transfected 293T cells efficiently precipitates core APC/C subunits, as determined by mass spectrometry (Table S1). Surprisingly, the levels of Cdc27 co-purifying with Ube2S are reduced in mitosis and G1, which we attribute to a low efficiency of our antibody to precipitate Ube2S*APC/C*Cdc20/Cdh1-complexes. This hypothesis is supported by the reciprocal purification of APC/C using Cdc27-antibodies, which leads to co-purification of Ube2S during mitosis and G1 (Fig. S3A); by purification of FLAGUbe2S from stably transfected, mitotic 293T cells, which co-precipitates Cdc27 (Fig. S3B); and by co-fractionation of Ube2S with core APC/C in sucrose gradient centrifugations of mitotic and G1-extracts (Fig. S3 C and D). The regulated interaction between Ube2S and APC/C and its activators demonstrates that Ube2S is a component of the APC/C machinery in cells.
Ube2S Is Crucial for APC/C Activity In Vivo.
To determine whether Ube2S functions with APC/C in vivo, we depleted Ube2S and/or UbcH10 in human HeLa and U2OS and in Drosophila S2 and Kc cells. The fly Ube2S cooperates with the UbcH10 homolog Vihar in catalyzing chain formation on APC/C substrates (Fig. S4A). If loss of Ube2S and/or UbcH10 abrogates APC/C activity, cells should be delayed in mitosis due to defects in spindle formation, which activate the spindle checkpoint (6–8); failure to disassemble spindle checkpoint complexes (23–25); and stabilization of APC/C substrates, which interferes with mitotic exit (5, 6).
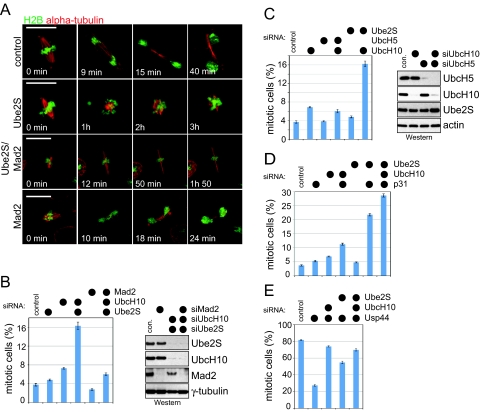
We depleted Ube2S in Drosophila S2 cells stably expressing GFP-tagged histone 2B and mCherry-tagged α-tubulin and analyzed progression through mitosis by time-lapse microscopy. As a control, we depleted Drosophila UbcH10/Vihar, which is known to activate APC/C in flies (26). Importantly, the depletion of either Ube2S or UbcH10 results in a strong delay in a metaphase-like state (Fig. 3A and Fig. S4B; t >3 h, compared to ≈10 min in control cells). This suggests that like UbcH10/Vihar, Drosophila Ube2S is important for progression through mitosis.
Fig. 3.
Ube2S regulates progression of cells through mitosis. (A) Drosophila Ube2S is required for mitosis. S2 cells stably expressing histone H2B-GFP and α-tubulin-mCherry were transfected with RNAi and filmed by time-lapse microscopy. (B) Ube2S and UbcH10 cooperate to promote progression through mitosis. HeLa cells were transfected with the indicated siRNAs, and scored for mitotic cells. Error bars, SEM derived from at least seven independent frames of different experiments, counting at least 1,000 cells in each frame. The right panel shows the depletion efficiency of Western blot. (C) UbcH10 shows genetic interactions with Ube2S, but not UbcH5. UbcH10 was depleted by siRNA alone, together with all four UbcH5-homologs, or with Ube2S, and the mitotic index of the cell population was determined. The right panel shows the depletion efficiency, as detected by Western blot. (D) Ube2S contributes to APC/C-dependent spindle checkpoint silencing. HeLa cells were transfected with the indicated siRNA and scored for cells arresting in mitosis. (E) Ube2S counteracts the DUB Usp44. HeLa cells were transfected with indicated siRNA, and treated with taxol to activate the spindle checkpoint. The number of cells arrested in mitosis were counted.
We then depleted Ube2S and/or UbcH10 in asynchronous cells and determined the number of mitotic cells in the population. We used five siRNAs against human Ube2S, and three siRNAs against human UbcH10, all of which effectively knock down the respective protein and result in identical phenotypes (compare Fig. 3B and Fig. S4C). In both human and fly cells, reducing Ube2S levels does not strongly affect the mitotic distribution (Fig. 3B and Fig. S4 C and D), and depletion of UbcH10 results in a weak increase in the mitotic index (Fig. 3B), as reported previously (21, 26). This is consistent with experiments showing that siRNA-dependent depletion of Cdc20, Cdh1, or single APC/C subunits triggers negligible increases in the mitotic index (27–29). Importantly, the co-depletion of Ube2S and UbcH10 strongly increases the mitotic index of human and Drosophila cells (Fig. 3B and Fig. S4 C and D). By contrast, co-depleting UbcH10 and all UbcH5 homologs does not strongly impact progression through mitosis (Fig. 3C). The effects on mitosis caused by UbcH10/Ube2S depletion are among the most dramatic seen by siRNA against APC/C, underscoring the importance of UbcH10 and Ube2S in APC/C-dependent chain formation.
We next determined whether co-depletion of Ube2S and UbcH10 results in aberrations in spindle structure and function (Fig. S5). Using immunofluorescence and time-lapse microscopy, we observed detachment of spindle poles (Fig. S5 A and D) and spindle elongation (Fig. S5F). Chromosome missegregation (Fig. S5C), defects in chromosome congression in early mitosis, and an inability to maintain a metaphase plate were evident in experiments performed by time-lapse microscopy. All of these phenotypes had been reported after APC/C inactivation (7, 8, 30, 31). To test whether this leads to checkpoint activation, we concurrently depleted Mad2, which abrogates spindle checkpoint function even in the presence of nocodazole or taxol. Switching off the checkpoint partially rescues the mitotic delay caused by Ube2S/UbcH10 depletion (Fig. 3B and Fig. S4D), indicating that the checkpoint had been activated. However, cells co-depleted of Ube2S, UbcH10, and Mad2 are still delayed in progression through mitosis, as seen by time-lapse microscopy in Drosophila S2 cells (Fig. 3A), and by accumulation of human cells in prometaphase and metaphase (Fig. S5E). Thus, as expected for inhibition of APC/C, loss of Ube2S and UbcH10 results in spindle defects, activation of the spindle checkpoint, and in mitotic delay independent of the checkpoint.
Next, we tested for a role of Ube2S in APC/C-dependent spindle checkpoint silencing by analyzing genetic interactions with p31comet and Usp44. p31comet cooperates with UbcH10 and APC/C to promote checkpoint silencing (23, 25), which is counteracted by the deubiquitinating enzyme Usp44 (24, 32). Accordingly, the depletion of both UbcH10 and p31comet results in synthetic mitotic arrest, and depletion of UbcH10 inhibits spindle checkpoint bypass caused by siRNA against Usp44 (Fig. 3 D and E). In a similar manner, Ube2S- and p31comet-siRNA strongly synergize in increasing the mitotic index (Fig. 3D), and loss of Ube2S rescues the defective checkpoint response observed after Usp44 depletion (Fig. 3E), showing that Ube2S contributes to APC/C-dependent spindle checkpoint silencing.
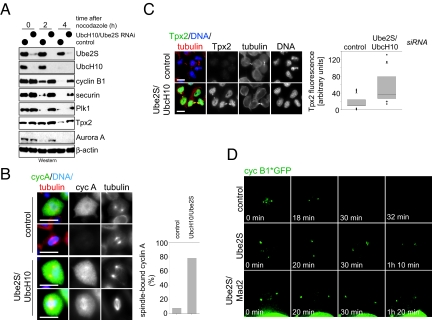
Finally, we tested whether Ube2S triggers the degradation of APC/C substrates in vivo. When Ube2S/UbcH10-depleted human cells are released from mitotic arrest, the degradation of several APC/C substrates is strongly delayed (Fig. 4A). When analyzed by fluorescence microscopy, multiple APC/C substrates are stabilized in siRNA-treated cells, including cyclin A, cyclin B, and Tpx2 (Fig. 4 B and C and Fig. S6A). Tpx2-levels remain high in siRNA-treated cells even in G1, with Tpx2 accumulating at remnants of the spindle midzone (Fig. 4C). Tpx2 is also stabilized in G1 if only Ube2S is depleted (Fig. S6C). As observed by time-lapse microscopy, the depletion of in Drosophila S2 cells stably expressing GFP-tagged cyclin B leads to strong stabilization of cyclin B on the spindle pole (Fig. 4D). Thus, Ube2S plays a critical role in driving the degradation of APC/C substrates in cells.
Fig. 4.
Ube2S and UbcH10 promote the degradation of APC/C substrates in cells. (A) Depletion of Ube2S and UbcH10 strongly stabilizes APC/C substrates. Mitotic HeLa cells transfected with siRNA against UbcH10 and Ube2S were released into G1, and the levels of several APC/C-substrates were measured by Western blot. (B) The UbcH10/Ube2S module drives degradation of cyclin A. HeLa cells were transfected with control or UbcH10/Ube2S siRNA, and cyclin A was detected by immunofluorescence (green). The spindle was visualized by antibodies against α-tubulin (red), and DNA was stained with DAPI (blue). (Scale bar, 10 μm.) The right panel shows the quantification of mitotic HeLa cells before anaphase with detectable cyclin A staining on the spindle. (C) Accumulation of Tpx2 in postmitotic HeLa cells after depletion of Ube2S and UbcH10. Tpx2 was detected in HeLa cells transfected with the indicated siRNAs by immunofluorescence. Cells in early G1 were identified, and representative images are shown. (Scale bar, 10 μm.) The right panel shows a quantification of Tpx2 levels in HeLa cells in G1. (D) Ube2S is required for degradation of cyclin B in Drosophila. S2 cells stably expressing cyclin B-GFP were transfected with the RNAi, and imaged by time-lapse microsopy. Depletion of Ube2S stabilizes cyclin B-GFP on the spindle pole (arrow) independently of spindle checkpoint activation.
Several lines of evidence indicate that depletion of Ube2S and UbcH10 directly affect APC/C activity, and not only indirectly through activation of the spindle checkpoint: first, cyclin A is stabilized by UbcH10/Ube2S depletion (Fig. 4B), although it is not regulated by the checkpoint (33); second, Tpx2 is stabilized at mitotic stages in which the checkpoint is inactive (Fig. 4C); third, fly GFP-cyclin B1 is stable in Ube2S/Mad2-depleted S2 cells as monitored by time-lapse microscopy (Fig. 4D); and fourth, depletion of Ube2S also stabilizes APC/C substrates during interphase (Fig. S6B). Together, our experiments demonstrate a key role for Ube2S in activating APC/C. The phenotypes of co-depleting Ube2S and UbcH10 are very similar to complete APC/C inhibition: mitotic arrest, spindle defects, prolonged activation of the checkpoint, and stabilization of all tested APC/C substrates.
Ube2S Is Regulated by APC/C-Dependent Degradation.
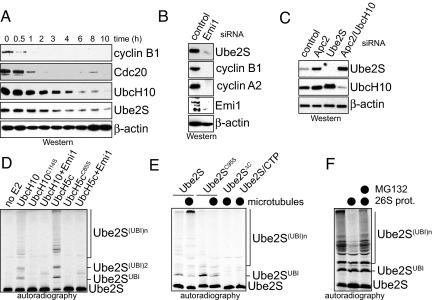
Overexpression of Ube2S transforms cells in culture and promotes tumor growth in mice (19, 20). This suggests that Ube2S levels have to be regulated, and indeed, Ube2S is degraded during G1 (Fig. 5A). The proteolysis of Ube2S occurs at similar times as the degradation of UbcH10, but is delayed compared to APC/C substrates. Ube2S is undetectable in cells arrested in quiescence (Fig. S7A) and synthesized in parallel with UbcH10 when cells re-enter the cell cycle upon stimulation with serum.
Fig. 5.
Ube2S is regulated by the APC/C. (A) Ube2S is degraded during G1. Mitotic HeLa cells were released into fresh medium to allow mitotic exit, and the indicated proteins were detected by Western blot. (B) Ube2S levels decrease upon activation of the APC/C. HeLa cells were treated with siRNA against the APC/C inhibitor Emi1, and the indicated proteins were detected by Western blot. (C) Ube2S-levels increase after depletion of APC/C subunits. HeLa cells were treated with the indicated siRNAs, and the levels of Ube2S were determined by Western blotting. (D) Ube2S is ubiquitinated by APC/CCdh1. Affinity-purified APC/CCdh1 was incubated with 35S-Ube2S in the absence or presence of UbcH5c, UbcH10, or Emi1. Reaction products were detected by autoradiography. (E) Microtubules promote the ubiquitination of Ube2S. APC/CCdh1 was incubated with taxol-stabilized microtubules, UbcH5c, and 35S-Ube2S, 35S-Ube2SC95S, 35S-Ube2SΔC, or 35S-Ube2S, and the C-terminal Ube2S-peptide. Reaction products were detected by autoradiography. (F) Ubiquitinated Ube2S is degraded by the 26S proteasome. 35S-Ube2S was ubiquitinated by APC/CCdh1 in the presence of microtubules. Subsequent to the ubiquitination, purified 26S proteasomes were added. MG132 was added as indicated. The reaction products were visualized by autoradiography.
Because Ube2S is co-regulated with UbcH10, its levels might be controlled by APC/C itself, as reported for UbcH10 (21). Indeed, APC/C activation, accomplished by depleting its inhibitor Emi1, leads to a dramatic decrease in the concentration of Ube2S, which is also observed for APC/C substrates (Fig. 5B). Conversely, inhibition of the APC/C by depleting its activator Cdh1 or by co-depletion of Apc2 and UbcH10 results in a strong increase in Ube2S (Fig. 5C and Fig. S7B).
Consistent with Ube2S being an APC/C substrate, APC/CCdh1 catalyzes the ubiquitination of Ube2S in vitro, which can be blocked by the APC/C inhibitor Emi1 (Fig. 5D). This reaction requires UbcH5 or UbcH10 to promote the attachment of the first ubiquitin to Ube2S. Although substrates do not compete with Ube2S binding to Cdh1 or APC/C, they interfere with the APC/C-dependent ubiquitination of Ube2S (Fig. S7C). Interestingly, ubiquitination of Ube2S by the APC/C is strongly increased by microtubules (Fig. 5E and Fig. S7 D and E), which is consistent with the known spindle binding of APC/C (5). This efficient ubiquitination of Ube2S depends on its active-site Cys and on its C-terminal tail (Fig. 5E), and the ubiquitin chains attached to Ube2S are specifically linked through K11 (Fig. S7E). 26S proteasomes trigger the degradation of ubiquitinated Ube2S, which is blocked by addition of the proteasome inhibitor MG132 (Fig. 5F). These findings indicate the APC/C promotes the ubiquitination and degradation of both Ube2S and UbcH10 (21), resulting in the tight co-regulation of these E2 enzymes.
Discussion
The APC/C is essential for cell cycle progression in all eukaryotes (5). The human APC/C modifies substrates with K11-linked ubiquitin chains to trigger their proteasomal degradation (11). The formation of K11-linked chains is initiated by its E2 UbcH10, which recognizes TEK boxes in substrates and ubiquitin (11). Here, we identify the conserved Ube2S as a K11-specific chain elongating E2 for human and Drosophila APC/C. Together, UbcH10 and Ube2S promote the efficient degradation of APC/C substrates, spindle assembly, and progression through mitosis. We propose that UbcH10 and Ube2S constitute the physiological E2 module of human APC/C during mitosis.
The cooperation between Ube2S and UbcH10 is reminiscent of yeast APC/C, which uses a chain-initiating E2, Ubc4, and a chain-elongating E2, Ubc1 (34). However, Ubc4 and Ubc1 function sequentially to assemble K48-linked ubiquitin chains, whereas human UbcH10 and Ube2S most likely bind APC/C at the same time. This hypothesis is supported by competition studies, in which a large excess of UbcH10 does not block the binding of Ube2S to Cdh1 or APC/C, and by ubiquitination assays, in which Ube2S and UbcH10 promote the APC/C-dependent ubiquitination of each other. By binding to different sites on the APC/C, UbcH10 and Ube2S can truly cooperate in the assembly of K11-linked ubiquitin chains, allowing them to promote the degradation of the extended family of human APC/C substrates.
UbcH10 binds the RING subunit Apc11 and the cullin subunit Apc2 (25, 34). Consistent with a different mode of APC/C interaction, Ube2S directly binds the APC/C activators Cdc20 and Cdh1. The deletion of C-terminal amino acids of Ube2S ablates its binding to Cdh1 and abrogates its activity toward APC/C, which implies that the association between Ube2S and Cdh1 is required for Ube2S activity. The interaction between Ube2S and Cdc20/Cdh1 is cell-cycle regulated and occurs only at times when Cdc20 and Cdh1 activate APC/C. We propose that recruitment of Ube2S in part explains the capability of Cdc20 and Cdh1 to increase the catalytic activity of the APC/C.
The strongest effects on cell cycle progression are observed, if Ube2S and UbcH10 are co-depleted from cells. This indicates that APC/C ubiquitinates at least some substrates, if one E2 is present at low concentrations. It is possible that other E2s partially compensate for loss of either UbcH10 or Ube2S. Such E2s are unlikely to include UBE2D/UbcH5 or UBE2K/E2–25K, which neither assemble specific K11-linked chains (11), nor target APC/C substrates for degradation in extracts (11), nor show genetic interactions with UbcH10. We believe it is more likely that even low levels of UbcH10 support initiation of short chains, which could then be extended by Ube2S. In support of this notion, mutation of UbcH10 in flies or its efficient depletion in several human cell lines lead to cyclin B stabilization and a delay in mitosis (21, 23, 26, 35–37), while incomplete depletion of UbcH10 does not produce these phenotypes (38).
Consistent with their close cooperation in modifying APC/C substrates, UbcH10 and Ube2S are tightly co-regulated in cells. Both E2s are degraded during G1, which is promoted by APC/C-dependent ubiquitination. The APC/C turns against its own E2s only after most, if not all, APC/C substrates have been degraded. As co-depletion of UbcH10 and Ube2S inactivates APC/C during mitosis, it can be assumed that the degradation of both E2s during G1 also shuts off most of APC/C, as previously proposed (21).
The identification of Ube2S as a K11-specific E2 for the APC/C underscores the importance of K11-linked ubiquitin chains for cell cycle control (11). The phenotypes observed after co-depletion of Ube2S and UbcH10 are reminiscent of the strong mitotic delay caused by injection of a K11-deficient ubiquitin mutant into Xenopus embryos (11). A proteomic analysis revealed that p97/CDC48, whose depletion causes mitotic arrest in HeLa cells (39), efficiently binds K11-linked ubiquitin chains (40). K11-linked ubiquitin chains also accumulate in diseases with impaired proteasome activity, such as Huntington's or Alzheimer's (41), and they are abundant in normally dividing cells (12). Together, these findings point to K11-linked chains as critical, if not essential, regulators of proteasomal degradation and cell cycle progression in higher eukaryotes.
In conclusion, we have identified the highly conserved Ube2S as a chain-elongating E2 of human and Drosophila APC/C. UbcH10 and Ube2S likely constitute the physiological E2 module for the APC/C during mitosis, in which UbcH10 initiates and Ube2S elongates K11-linked ubiquitin chains. The inhibition of mitotic APC/C is an attractive goal for chemotherapy, which, as shown here, could be accomplished by small molecules targeting both UbcH10 and Ube2S.
Materials and Methods
Detailed materials and methods can be found in the SI Methods. Ube2S was purified as an N-terminal MBP fusion protein, and the MBP-tagged was cleaved off by TEV protease before Ube2S was used in assays. UbcH10 and UbcH5c were purified from E. coli, while E1 and HisCdh1 were purified from Sf9 cells. In vitro ubiquitinations were performed as described, with APC/C being obtained from human HeLa S3 cells (11). Immunofluorescence analysis was performed in HeLa cells as described (21). siRNAs were obtained from Dharmacon (for sequences see SI Methods). Ube2S-binding partners were identified by MudPIT-mass spectrometry performed the UCB Proteomics/Mass Spectrometry Laboratory (P/MSL).
Supplementary Material
Acknowledgments.
We thank Deborah Zajchowski and Rick Feldman for providing antibodies against Ube2S, Eric Griffis and Ron Vale for S2 cells expressing cyclin B1*GFP, Claudio Sunkel (IBMC, Portugal) for Mad2 antibody, Chris Fromme for help with sucrose gradients, Isaac Oderberg for technical help, the Drubin/Barnes laboratory for help with microscopy, and Julia Schaletzky for discussions and critically reading the manuscript. This work was supported by National Institutes of Health Grants RO1 GM066272 (to G.H.K.) and RO1 GM083064 (to M.R.) and a National Institutes of Health Director New Innovator Award (to M.R.). M.R. is a Pew fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907887106/DCSupplemental.
References
- 1.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 2.Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–150. doi: 10.1146/annurev.biophys.36.040306.132820. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Eddins MJ, Carlile CM, Gomez KM, Pickart CM, Wolberger C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat Struct Mol Biol. 2006;13:915–920. doi: 10.1038/nsmb1148. [DOI] [PubMed] [Google Scholar]
- 5.Peters JM. The anaphase-promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 6.Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 7.Goshima G, et al. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 2007;316:417–421. doi: 10.1126/science.1141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somma MP, et al. Identification of Drosophila mitotic genes by combining co-expression analysis and RNA interference. PLoS Genet. 2008;4:e1000126. doi: 10.1371/journal.pgen.1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft C, et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkpatrick DS, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H. Cdc20: A WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 16.Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 17.Burton JL, Solomon MJ. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 2001;15:2381–2395. doi: 10.1101/gad.917901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Jung CR, et al. E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis. Nature Med. 2006;12:809–816. doi: 10.1038/nm1440. [DOI] [PubMed] [Google Scholar]
- 20.Tedesco D, et al. The ubiquitin-conjugating enzyme E2-EPF is overexpressed in primary breast cancer and modulates sensitivity to topoisomerase II inhibition. Neoplasia. 2007;9:601–613. doi: 10.1593/neo.07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 2004;432:588–595. doi: 10.1038/nature03023. [DOI] [PubMed] [Google Scholar]
- 22.Baboshina OV, Haas AL. Novel multiubiquitin chain linkages catalyzed by the conjugating enzymes E2EPF and RAD6 are recognized by 26 S proteasome subunit 5. J Biol Chem. 1996;271:2823–2831. doi: 10.1074/jbc.271.5.2823. [DOI] [PubMed] [Google Scholar]
- 23.Reddy SK, Rape M, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 24.Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 25.Summers MK, Pan B, Mukhyala K, Jackson PK. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell. 2008;31:544–556. doi: 10.1016/j.molcel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Máthé E, et al. The E2-C vihar is required for the correct spatiotemporal proteolysis of cyclin B and itself undergoes cyclical degradation. Curr Biol. 2004;14:1723–1733. doi: 10.1016/j.cub.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarten AJ, Felthaus J, Wäsch R. Strong inducible knockdown of APC/CCdc20 does not cause mitotic arrest in human somatic cells. Cell Cycle. 2009;8:643–646. doi: 10.4161/cc.8.4.7810. [DOI] [PubMed] [Google Scholar]
- 28.Kittler R, et al. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature. 2004;432:1036–1040. doi: 10.1038/nature03159. [DOI] [PubMed] [Google Scholar]
- 29.Wei W, et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 30.Giménez-Abián JF, et al. Regulated separation of sister centromeres depends on the spindle assembly checkpoint but not on the anaphase promoting complex/cyclosome. Cell Cycle. 2005;4:1561–1575. doi: 10.4161/cc.4.11.2146. [DOI] [PubMed] [Google Scholar]
- 31.Ban KH, et al. The END network couples spindle pole assembly to inhibition of the anaphase-promoting complex/cyclosome in early mitosis. Dev Cell. 2007;13:29–42. doi: 10.1016/j.devcel.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Kim AH, et al. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136:322–336. doi: 10.1016/j.cell.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geley S, et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Z, et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell. 2001;12:3839–3851. doi: 10.1091/mbc.12.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner KW, et al. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene. 2004;23:6621–6629. doi: 10.1038/sj.onc.1207861. [DOI] [PubMed] [Google Scholar]
- 36.Fujita T, et al. Clinicopathological relevance of UbcH10 in breast cancer. Cancer Sci. 2008 doi: 10.1111/j.1349–7006.2008.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berlingieri MT, et al. UbcH10 is overexpressed in malignant breast carcinomas. Eur J Cancer. 2007;43:2729–2735. doi: 10.1016/j.ejca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Walker A, Acquaviva C, Matsusaka T, Koop L, Pines J. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator. J Cell Sci. 2008;121:2319–2326. doi: 10.1242/jcs.031591. [DOI] [PubMed] [Google Scholar]
- 39.Wójcik C, Yano M, DeMartino GN. RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J Cell Sci. 2004;117:281–292. doi: 10.1242/jcs.00841. [DOI] [PubMed] [Google Scholar]
- 40.Alexandru G, et al. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134:804–816. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett EJ, et al. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.