Abstract
IL-10 produced by dendritic cells (DC) can limit or terminate ongoing inflammatory responses by inhibiting the proinflammatory cytokine production. Currently, the molecular mechanism by which IL-10 suppresses cytokine production is still ill-defined. In this study, we showed that IL-10 produced by DC dampens myeloid differentiation factor (MyD)88-dependent, but not MyD88-independent signaling. At the molecular level, IL-10 induces ubiquitination and subsequent protein degradation of MyD88-dependent signaling molecules, including IL-1 receptor-associated kinase 4 and TNF-receptor associated factor 6. Protein degradation by IL-10 was associated with decreased phosphorylation of p38, JNK, and IKK. All of these events were prevented by either blocking IL-10 receptor signaling or inhibiting proteasome degradation. IL-10 induced LPS hyporesponsiveness using the same mechanisms, i.e., ubiquitination and protein degradation. Thus, a previously undescribed regulatory mechanism by which IL-10-mediated protein degradation contributes to the inhibition of inflammatory cytokine production and endotoxin tolerance in DC.
Keywords: cytokine, ubiquitination, endotoxin tolerance
The proinflammatory response, essential to protect the host from infection, must be tightly regulated. Uncontrolled or excessive inflammation can have detrimental effects on hosts and result in inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and septic shock. Dendritic cells (DC) have a key role in keeping the immune response in check. On infection, DC can produce many first-line defensive cytokines, including IL-1, IL-6, IL-12, and TNF-α. Cytokines produced by DC can shape a type of an adaptive immune response that is tailored to the pathogen. DC can also produce anti-inflammatory cytokines such as IL-10 to limit or terminate the inflammatory responses.
IL-10 was first identified as cytokine synthesis inhibitory factor produced by Th2 cells (1). Further studies showed that an inhibitory effect of IL-10 on T cells was indirect and mediated mainly through antigen-presenting cells like macrophages and DC (2). IL-10 was shown to inhibit antigen presentation, expression of costimulatory molecules, and proliferation of DC (3). Cytokine production by DC was also suppressed by IL-10. The antiinflammatory role of IL-10 is supported by genetic evidence and unequivocally established in various models of infection. However, it has been a matter of debate how IL-10 mediates its immunosuppressive activities at the molecular level (2, 4, 5). JAK1 and STAT3 were shown to be essential proximal mediators for IL-10 signaling (5, 6). However, it is not yet clear what downstream targets of STAT3 execute IL-10-mediated immunosuppression. As suggested by the term cytokine inhibitory factor, IL-10 controls the transcription of cytokine genes (7–9). Bcl-3, one of IL-10-induced gene, exerts an inhibitory effect on TNF-α, but not IL-6 transcription in macrophages (10). Posttranscriptional regulation such as mRNA stability also has a role in inhibition of cytokine production by IL-10, but the exact mechanism remains elusive.
After activation by Toll-like receptor (TLR) ligands, DC produce multiple cytokines. However, the same DC lose this capability when they are reexposed to the TLR ligand, phenomenon known as endotoxin tolerance (11). IL-10 is required for the induction of endotoxin tolerance (2). Neutralizing IL-10 and TGF-β during LPS priming prevented the induction of endotoxin tolerance as measured by TNF-α production, whereas pretreatment of IL-10 renders monocytes/macrophages refractory to subsequent LPS challenge (12). However, IL-10-deficient mice still showed hyporesponsiveness to LPS (13), suggesting additional mechanisms contribute to endotoxin tolerance. The underlying mechanism of endotoxin tolerance remains unclear.
In the current study, we investigated the molecular mechanism by which IL-10 regulates both cytokine production and endotoxin tolerance in DC. The results demonstrate that IL-10 produced by DC induces ubiquitination and subsequent protein degradation of myeloid differentiation factor (MyD)88-dependent signaling molecules, such as IL-1 receptor-associated kinase (IRAK)4 and TNF-receptor associated factor (TRAF)6, to dampen MyD88-dependent, but not MyD88-independent signaling pathway. Also, IL-10-mediated signaling uses ubiquitination and protein degradation to induce LPS hyporesponsiveness. Together, our present study revealed a previously uncharacterized mechanism for IL-10-mediated immune regulation in DC.
Results
IL-10 Inhibits MyD88-Dependent, But Not MyD88-Independent Signaling Pathway.
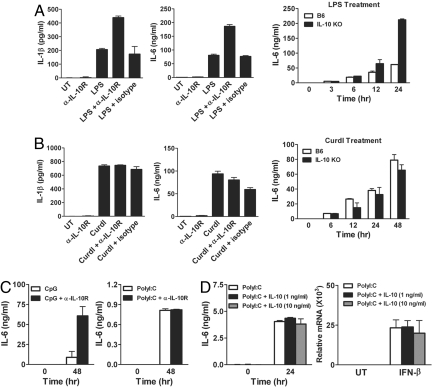
To investigate the antiinflammatory function of IL-10, we first measured cytokine production by DC after treating them with LPS. As expected, DC produced the proinflammatory cytokines such as IL-1β and IL-6 on LPS stimulation (Fig. 1A Left and Center). This induction was further enhanced when endogenous IL-10 was neutralized by adding the anti-IL-10 receptor antibody (α-IL-10R), but not the control antibody, suggesting an inhibitory role of IL-10 (Fig. 1A). Consistent with the antibody blocking data, DC from IL-10-deficient (Il10−/−) mice also showed a greater amount of IL-6 when they were stimulated with LPS (Fig. 1A Right). We next examined whether IL-10 exerts the same inhibitory function on DC when they are treated with different stimuli. To test this question, we used curdlan to stimulate DC, and found that the neutralizing α-IL-10R did not show the same effect (Fig. 1B). Also, the level of IL-6 in Il10−/− DC was comparable with that of WT even after 48 h of treatment (Fig. 1B). This observation was intriguing, because curdlan activates STAT3 and produces comparable amounts of IL-10 with LPS stimulation (Fig. S1A). Curdlan-inducible gene expression of TNF-α, IL-12p35, and IL-12p40 was also similar between B6 and Il10−/− DC (Fig. S1B). These data indicate that IL-10 inhibits LPS-mediated, but not curdlan-mediated signaling pathway.
Fig. 1.
IL-10 inhibits the cytokine production in DC on stimulation with LPS and CpG, but not curdlan or PolyI:C. (A and B) DC prepared from C57BL/6 or Il10−/− mice were treated with 1 μg/mL LPS (A) or 100 μg/mL curdlan (B) alone or together with α-IL-10R or rat IgG1 (both at 15 μg/mL) for 24 h or as indicated. IL-1β and IL-6 levels were quantified by ELISA in culture supernatants. (C) DC prepared from C57BL/6 mice were treated with 1 μg/mL CpG or 100 μg/mL PolyI:C alone or together with α-IL-10R for the indicated time. IL-6 level was quantified by ELISA. (D) DC from C57BL/6 mice were treated with 100 μg/mL PolyI:C alone or together with IL-10 for the indicated time. IL-6 was measured by ELISA and IFN-β was quantified by qRT-PCR. Values are presented as means ± SD.
LPS and curdlan use a different set of receptors and signaling pathways (14). Therefore, we next investigated whether the inhibitory effect of IL-10 in LPS, but not curdlan treated DC could be mediated through TLR signaling pathway. LPS binds to TLR4 and activates both MyD88- and TRIF-dependent pathways. To dissect which signaling pathway is responsible for the inhibition by IL-10, we examined TLR9 and TLR3, which stimulates MyD88-dependent and MyD88-independent signaling pathway, respectively. When cells were treated with CpG, a TLR9 ligand, IL-6 production was increased in the presence of α-IL-10R (Fig. 1C Left). Messenger RNA expression of IL-12p35, IL-23p19, TNF-α, and IFN-β after CpG treatment was also enhanced in Il10−/− DC (Fig. S2). In contrast, the same antibody treatment did not increase the IL-6 level when cells were treated with PolyI:C that activates Myd88-independent signaling (Fig. 1C Right). However, DC produce limited amounts of IL-10 with PolyI:C treatment (Fig. S3). Therefore, it is possible that the lack of the neutralizing α-IL-10R effect was due to an insignificant amount of IL-10 in the culture. To address this question, we added IL-10 to the culture. As shown in Fig. 1D, adding IL-10 as high as 10 ng/mL had little effect on IL-6 production after PolyI:C stimulation. We also compared the expression level of IFN-β, because PolyI:C is known to induce type I IFNs (15). INF-β mRNA levels were comparable with or without IL-10 (Fig. 1D Right). Together, these data suggest that IL-10 inhibits the production of cytokines induced by MyD88-dependent signaling.
IL-10 Degrades MyD88-Dependent Signaling Molecules.
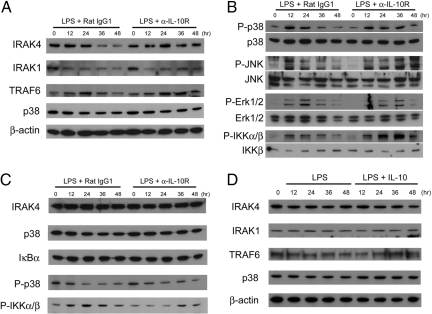
Having observed that IL-10 appears to inhibit MyD88-dependent signaling, we next examined signaling molecules involved in this pathway, including IRAK4, IRAK1, and TRAF6. To assess the status of these MyD88-dependent signaling molecules, we compared the protein levels during the course of the LPS treatment. We found that the amount of IRAK4 protein, but not mRNA, was gradually decreased on LPS stimulation (Fig. 2A; Figs. S4 and S5). Protein levels of TRAF6 were also reduced to some extend at later time points. When IL-10 signaling was blocked, IRAK4 and TRAF6 were maintained at a level comparable with unstimulated cells (Fig. 2A). As previously shown (16, 17), IRAK1 was diminished quickly after adding LPS, and the change of IRAK1 level was less prominent with or without α-IL-10R. We next investigated the phosphorylation status of downstream signaling molecules, including p38, JNK, Erk1/2, and IKK. We observed that each of them was phosphorylated in response to LPS, and that the degree of phosphorylation declined over time. When IL-10 signaling was blocked, phosphorylation of p38, JNK, and IKK was sustained (Fig. 2B). However, phosphorylation of Erk1/2 was not affected in the presence of α-IL-10R. Interestingly, there was little protein degradation or the loss of phosphorylation in the absence of MyD88, even though MyD88-deficient cells still can respond to LPS via MyD88-independent pathways (Fig. 2C; Fig. S4). MyD88-deficient cells are known to produce little IL-10 in response to LPS (18). To further test whether MyD88 is required for IL-10-mediated protein degradation, DC from MyD88-deficient mice were treated with LPS or LPS together with IL-10. Protein levels of IRAK4, IRAK1, and TRAF6 were not affected by exogenous IL-10 (Fig. 2D), indicating MyD88-dependent degradation of proteins. Also, exogenously added IL-10 alone could not induce protein degradation even in the presence of MyD88 unless cells were treated with LPS (Fig. S6). Thus, our data suggest that the inhibition mediated by IL-10 specifically target the pathway involving MyD88, and that concurrent LPS stimulation is necessary for IL-10-mediated protein degradation.
Fig. 2.
IL-10 induces degradation of MyD88-dependent signaling molecules and down-regulation of phosphorylation of p38, JNK, and IKK. DC prepared from C57BL/6 mice were treated with 1 μg/mL LPS + 15 μg/mL α-IL-10R or 1 μg/mL LPS + 15 μg/mL rat IgG1. At the indicated time, whole-cell lysates were prepared. (A) IRAK4, IRAK1, TRAF6, p38, and β-actin expression was analyzed by immunoblotting. (B) P-p38, p38, P-JNK, JNK, P-Erk1/2, Erk1/2, P-IKKα/β, and IKKβ were detected by immunoblotting. (C) DC from Myd88−/− mice were treated the same way as in A. IRAK4, p38, IκBα, P-p38, and P-IKKα/β were detected by immunoblotting. (D) DC from Myd88−/− mice were treated with 1 μg/mL of LPS or 1 μg/mL of LPS together with 10 ng/mL of IL-10. At the indicated time, whole-cell lysates were prepared and used to examine IRAK4, IRAK1, TRAF6, p38, and β-actin by immunoblotting.
IL-10 Signaling Leads to Ubiquitination of MyD88-Dependent Signaling Molecules.
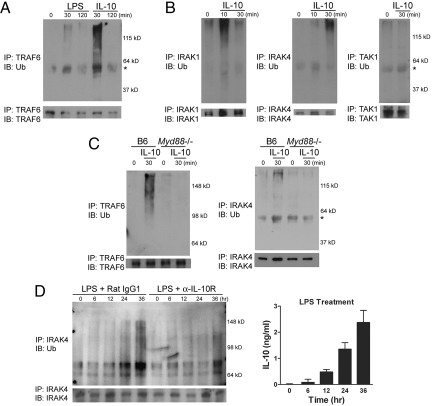
We next examined whether the reduction in protein levels may result from protein modification such as ubiquitination. When cells were treated with IL-10, a substantial ubiquitination of TRAF6 was detected (Fig. 3A). Ubiquitination of IRAK1 and IRAK4, but not TGF-β-activated kinase (TAK)1, was also observed as early as 10 min after stimulation (Fig. 3B). Other signaling molecules, including IKKγ, IκBα, and TANK-binding kinase (TBK)1, did not show a detectable level of ubiquitination. Interestingly, MyD88 was required for IL-10-mediated ubiquitination, because both TRAF6 and IRAK4 were not ubiquitinated by IL-10 in the absence of MyD88 (Fig. 3C). To directly test whether MyD88-dependent signaling molecules can be ubiquitinated by endogenously produced IL-10, we compared ubiquitination levels of IRAK4 during the course of LPS treatment. We found that the ubiquitination of IRAK4 was gradually increased over time, which was strongly correlated with the increase in the IL-10 level in the culture supernatant (Fig. 3D Right). Also, ubiquitination of IRAK4 was greatly reduced when IL-10 signaling was inhibited (Fig. 3D Left).
Fig. 3.
IL-10 signaling leads to ubiquitination of IRAK1, IRAK4, and TRAF6. (A and B) DC from C57BL/6 mice were treated with 1 μg/mL LPS or 10 ng/mL IL-10 as indicated. Cells were lysed and TRAF6, IRAK1, IRAK4, or TAK1 was immunoprecipitated as described in Materials and Methods. Ubiquitinated proteins and corresponding total proteins were determined by immunoblotting using the anti-ubiquitin (Ub) antibody (Upper) and the antibody recognizing TRAF6, IRAK1, IRAK4, or TAK1 (Lower), respectively. (C) DC prepared from C57BL/6 or Myd88−/− mice were treated with IL-10 and subjected to immunoprecipitation followed by blotting as described in A. The asterisk indicates heavy chain. Of note, the molecular weights shown are different due to use of a different percentage of gels (12 or 8%). (D) DC from C57BL/6 mice were stimulated as indicated in Fig. 2. Immunoprecipitates were immunoblotted using the anti-Ub or the anti-IRAK4 antibody (Left). Cells were treated with LPS (1 μg/mL) for the indicated time and the IL-10 level was quantified by ELISA (Right).
Blocking Proteasomal Degradation Restores Cytokine Production.
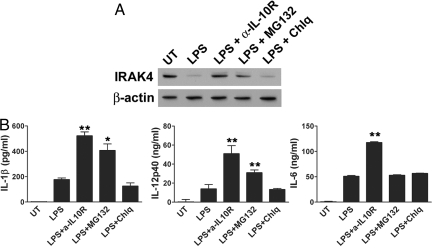
If protein degradation by IL-10-mediated ubiquitination is responsible for the decrease in cytokine levels, blocking protein degradation could sustain the cytokine production. To test this question, we treated DC with LPS in the presence of proteasome inhibitors (MG132 and lactacystin) or lysosome inhibitors (chloroquine and NH4Cl). As shown in Fig. 4A, cells treated with MG132, similar to α-IL-10R treatment, showed less protein degradation of IRAK4 than the control. Another proteasome inhibitor lactacystin was also effective for inhibiting protein degradation. However, protein degradation was not inhibited with lysosome inhibitors. These data indicate that protein degradation by IL-10 is primarily mediated through proteasomal degradation. We then tested whether cytokine production can also be rescued if protein degradation is prevented. We added MG132 at 4.5 h after LPS treatment, which allows the initial TLR activation by LPS. As shown in Fig. 4B, IL-1β and IL-12p40 production was significantly restored by the proteasome inhibitor, but not the lysosome inhibitor. MG132 alone did not enhance cytokine production (Fig. S7). This result suggests that IL-10-mediated proteasomal degradation contributes to the inhibition of cytokine production. However, cytokine rescue by the proteasome inhibitor was selective, because IL-6 production was not changed (Fig. 4B Right).
Fig. 4.
Cytokine production was partially rescued by preventing IL-10-mediated protein degradation. DC were treated with LPS (1 μg/mL) alone or together with α-IL-10R (15 μg/mL), MG132 (0.5 μM), chloroquine (10 μM), or left untreated for 48 h. The antibody and inhibitors were added 4.5 h after LPS treatment. IRAK4 and β-actin expression was analyzed at by immunoblotting (A); and IL-1β, IL-12p40, and IL-6 levels were quantified by ELISA (B). *, P < 0.05; **, P < 0.01, LPS versus LPS with inhibitor treatment.
IL-10-Mediated Protein Degradation Has a Role in Endotoxin Tolerance.
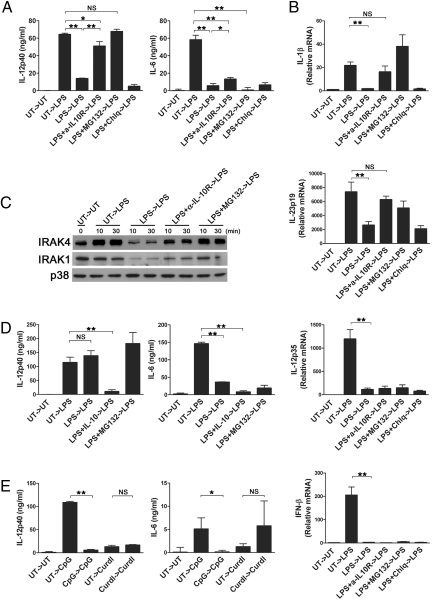
It has been speculated that IL-10 would exploit the same mechanism to suppress the proinflammatory cytokine production as observed in endotoxin tolerance (2). To test whether IL-10-mediated protein degradation also has a role in endotoxin tolerance of DC, cells were treated with 100 ng/mL LPS for 48 h followed by restimulation with 1 μg/mL LPS. Similar to the IL-10 effect on the primary response of DC, IL-12p40, but not IL-6 production on restimulation was substantially increased by neutralizing IL-10 during initial LPS stimulation (Fig. 5A). Also, blocking proteasome degradation during primary stimulation restored IL-12p40 production. We also compared the amount of mRNA of IL-1β and IL-23p19 after primary and secondary stimulation. As shown in Fig. 5B, the transcript levels of IL-1β and IL-23p19 were increased by blocking IL-10 signaling, as well as by inhibiting proteasomal degradation during primary stimulation. In contrast, mRNA expression of IL-12p35 and IFN-β was not recovered in the presence of α-IL-10R or the protein degradation inhibitor. Consistent with these data, the immunoblot data showed greatly reduced IRAK4 and IRAK1 in endotoxin-tolerant DC, which was recovered in DC treated with α-IL-10R or MG132 (Fig. 5C).
Fig. 5.
IL-10-mediated protein degradation contributes to endotoxin tolerance. (A) DC prepared from C57BL/6 mice were treated with LPS (0.1 μg/mL) alone or together with α-IL-10R (15 μg/mL), MG132 (0.5 μM), or chloroquine (10 μM), or left untreated for 48 h. After extensive washing, cells were restimulated with 1 μg/mL LPS for 24 h, and cytokines in the culture supernatants were determined by ELISA. (B) DC were treated as in A, except that cells were harvested 6 h after restimulation. RNA was prepared, and qRT-PCR was performed to assess the amount of IL-23p19, IL-12p35 and IL-1β, and IFN-β mRNA. (C) DC were prepared and restimulated with LPS for the indicated time. Total cell lysates were prepared and subjected to immunoblot analysis to determine IRAK4, IRAK1, and p38 expression. (D) DC from Il10−/− mice were stimulated with LPS (0.1 μg/mL) alone or together with IL-10 (10 ng/mL), MG132 (0.5 μM), or left untreated for 48 h. After extensive washing, cells were restimulated with 1 μg/mL LPS for 24 h, and IL-12p40 and IL-6 levels were quantified by ELISA. (E) DC from C57BL/6 mice were treated with CpG (1 μg/mL), curdlan (100 μg/mL), or left untreated for 48 h. Cells were then restimulated for 24 h with CpG (1 μg/mL) or curdlan (100 μg/mL). IL-12p40 and IL-6 in the culture supernatants were assayed by ELISA. *, P < 0.05; **, P < 0.01.
To confirm the role of IL-10 in endotoxin tolerance, we tested Il10−/− DC. As shown in Fig. 5D, IL-12p40 production was no longer inhibited by subsequent LPS challenge in Il10−/− DC. On the contrary, IL-6 production was still inhibited in Il10−/− DC. We showed that CpG, but not curdlan signaling was negatively regulated by IL-10 (Fig. 1 B and C). Similar to this observation, CpG caused tolerance of DC evidenced by reduced IL-12p40 and IL-6 production, whereas restimulation with curdlan did not induce the tolerance response (Fig. 5E). Together, these data indicate that the degradation of MyD88 signaling molecules caused by IL-10 contributes to endotoxin tolerance.
Discussion
Our study showed that IL-10 negatively regulates TLR, specifically MyD88-dependent signaling, but not Dectin-1 signaling pathway. TLR signaling is shown to be subjected to multiple levels of negative regulation, and most of negative regulators seem to target MyD88-dependent pathway (19, 20). The reason for this observation is still unclear, but might be related to the toxicity and/or the strength of the MyD88-dependent pathway (19). Likewise, IL-10 may use several mechanisms to regulate proinflammatory cytokine production. IL-10 is known to regulate the expression of proinflammatory cytokines at the level of transcription (5). However, cytokine production can be controlled not only by transcriptional regulation, but also by posttranscriptional or posttranslational regulation steps. Earlier studies support our data demonstrating that blocking MyD88 signaling via posttranscriptional regulation is also crucial for the inhibitory effect of IL-10. Prolonged TLR stimulation leads to down-regulation of IRAK4 protein (17). Stimulation of TLR2, TLR4, or TLR9, but not TLR3, caused the decrease in the IRAK4 protein level without affecting its mRNA level in a macrophage cell line RAW264.7 (17). Consistent with these observations, on LPS stimulation, IRAK4 mRNA levels in DC were not affected by IL-10 (Fig. S5), suggesting that the decrease is at the protein level. Based on our study, it is likely that the observed down-regulation of IRAK4 in RAW264.7 was due to IL-10 produced by RAW264.7 on prolonged TLR stimulation. Our data showed that RAW264.7 produced comparable amounts of IL-10 at 24 h after LPS treatment, and that IL-6 production was enhanced by blocking IL-10 signaling with α-IL-10R (Fig. S8). Recently, it was shown that an addition of IL-10 for 6 days during the generation of human monocyte-derived antigen-presenting cells down-regulated various proteins along the TLR signaling cascade (21). However, no significant down-regulation of MyD88, IRAK1, and TRAF6 was seen on mRNA levels, suggesting posttranscriptional regulation of protein levels by IL-10 treatment (21).
IL-10-deficient (Il10−/−) mice develop a spontaneous enterocolitis (22), and are hypersensitive toward endotoxic shock (13). Interestingly, it has been demonstrated that Il10−/− mice fail to develop intestinal pathology if they also lack MyD88 (23). Mice lacking both IL-10 and MyD88 were shown to be free from any signs of colitis or aberrant immune responses. This result unequivocally demonstrated that IL-10 negatively regulates MyD88-dependent signaling pathway in vivo.
MyD88 is required for IL-10-induced ubiquitination and protein degradation in DC, although the underlying mechanisms for these processes regulated by MyD88 are not yet clear. It is tempting to speculate that IL-10 signaling might disengage MyD88 from TLR and expedite ubiquitination of multiple targets such as IRAK1, IRAK4, and TRAF6 by recruiting them together followed by proteosome-mediated degradation. However, ubiquitination by IL-10 was not sufficient to have ubiquitinated proteins degraded unless cells were stimulated by LPS. Therefore, it is possible that LPS stimulation triggers activation of the degradation machinery. Also, E3 ligases known to regulate TLR signaling may be responsible for ubiquitination and degradation by IL-10. Isoforms of Pellino were recently reported to be E3 ubiquitin ligases of IRAK1 and IRAK4 (24). It has been shown that Pellino isoforms only became competent to form polyubiquitination chains after phosphorylation by IRAK1 or IRAK4 (25). Thus, concurrent LPS stimulation may be necessary for activation of E3 ligases such as Pellino.
We demonstrated that neutralization of IL-10 can prevent endotoxin tolerance and the addition of IL-10 can induce LPS hyporesponsiveness in DC. By either blocking IL-10 signaling or proteasome degradation, we showed that cytokines such as IL-12p40 and IL-1β, but not IL-6, could be rescued for the cytokine production and their LPS hyporesponsiveness. These results suggest that IL-10-mediated degradation of MyD88-dependent signaling molecules is one of molecular events for both cytokine inhibition and endotoxin tolerance in DC. A posttranscriptional down-regulation of IRAK1 is associated with endotoxin tolerance of intestinal epithelial cells (IECs) (26). IECs acquire TLR tolerance immediately after birth by exposure to exogenous endotoxin. Postnatal depletion of IRAK1 was shown to significantly contribute to the LPS nonresponsive phenotype of IECs. These data are in agreement with our data that IRAK4 and IRAK1 depletion has been observed in endotoxin tolerant DC. However, the fact that blocking IL-10 signaling or proteasomal degradation did not completely rescue all cytokines suggests an involvement of other regulatory mechanisms. Cytokine regulation by IL-10 or endotoxin tolerance is very complex processes, and our current study revealed one of many mechanisms used by DC.
Based on its potent immunosuppressive activities, IL-10 has been considered as an attractive therapeutic candidate for inflammatory diseases but efforts have yielded little success (2). Therefore, it is undoubtedly important to have a better understanding of the molecular mechanisms of the signaling pathway and the immune regulation mediated by IL-10. Future studies on IL-10 and IL-10-mediated immune responses would help us to develop a way to resolve an inflammatory response and to provide information that can be used to design a therapy for inflammatory diseases.
Materials and Methods
Mice and Cells.
IL-10-deficient (Il10−/−) mice were purchased from the Jackson Laboratory. Myd88−/− and C57BL/6 mice were bred and maintained under specific pathogen-free conditions at the University of Michigan animal facility. All animal procedures were approved by the University of Michigan Committee on the Use and Care of Animals. Bone marrow-derived DC were prepared as previously described (27).
Reagents.
The following reagents were used to treat DC: 1 μg/mL LPS (or as indicated in the figure legends; Sigma–Aldrich), 100 μg/mL curdlan (Wako), 1 μg/mL CpG ODN 1668 (InvivoGen), 100 μg/mL PolyI:C (InvivoGen), and 10 ng/mL recombinant mouse IL-10 (or as indicated in the figure legends; Peprotech). Other reagents included 10 ng/mL recombinant GM-CSF (Peprotech), 0.5 μM MG132 (or as indicated in the figure legends; Calbiochem), 3 μM lactacystin (Calbiochem), 10 μM chloroquine (Sigma–Aldrich), and 20 mM NH4Cl (Sigma–Aldrich). The 15 μg/mL of the neutralizing α-IL-10R antibody and isotype control rat IgG1 were purchased from BD PharMingen. Antibodies used for immunoblot analysis included p38 MAPK, phospho-p38 MAPK (Tyr-180/Tyr-182), p44/42 MAPK, phospho-p44/42 MAPK (Thr-202/Tyr-204), IKKβ, phospho-IKKα (Ser-180)/IKKβ (Ser-181), SAPK/JNK, phospho-SAPK/JNK (Thr-183/Tyr-185), Stat3, phospho-Stat3 (Tyr-705), IRAK4, IκBα (Cell Signaling Technology), Tak1 (M-579), TRAF6 (D-10), Ub (P4D1), IRAK1 (H-273) (Santa Cruz Biotechnology), IRAK4, TBK1 (Imgenex), and β-actin (Sigma–Aldrich).
ELISA and Quantitative (q)RT-PCR.
Cytokine concentrations in supernatants were detected by ELISA, and mRNA accumulation of cytokines was analyzed by qRT-PCR as previously described (27). The primers used for GAPDH, IL-12p35, IL-12p40, IL-10 and IL-6 were described previously (27). The primers used for TNF-α were 5′-CCCCAAAGGGATGAGAAGTT-3′ and 5′-CACT TGGTGGTTTGCTACGA-3′, for IL-23p19 were 5′-CCAGCGGGACATATGAATCT-3′ and 5′-AGGCTCCCCTTTGAAGATGT-3′, for IFN-β were 5′-TCCAAGAAAGGACGAACATTC G-3′ and 5′-TGAGGACATCTCCCACGTCAA-3′ and for IL-1β were 5′-CAACCAACAAGTG ATATTCTCCATG-3′ and 5′-GATCCACACTCTCCAGCTGCA-3′.
Immunoprecipitation and Immunoblot Analysis.
DC were lysed in lysis buffer [20 mM Tris·HCl, pH 7.4/150 mM NaCl/1 mM EDTA/1 mM EGTA/2 mM DTT/1% Triton X-100/1 mM PMSF/1% protease inhibitor mixture (Sigma–Aldrich)/25 μM MG132]. Lysates were precleared for 1 h by incubation with Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) and incubated overnight with specific primary antibody at 4 °C. One microgram of an antibody was added to 2.5 mg of cell lysate to pull down an equivalent amount of immunoprecipitated protein of an interest. Agarose beads were added for the last 8 h. Immune complexes were collected and washed three times with ice-cold lysis buffer and once with lysis buffer without Triton X-100. Immunoprecipitates were resuspended and boiled in sample buffer (60 mM Tris·HCl, pH 6.8/25% glycerol/2% SDS/5% β-mercaptoethanol/0.1% bromophenol blue) and resolved by SDS/PAGE. After transfer to a PVDF membrane (PerkinElmer), proteins were probed with the indicated antibodies and visualized by enhanced chemiluminescence (Thermo Fisher Scientific). The same antibodies were used for both immunoprecipitation and blotting.
Statistical Analysis.
Results are given as the mean ± SD. Data were analyzed by a two-tailed t test. Differences with a P value of 0.05 or less were regarded as statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Philip King (University of Michigan, Ann Arbor, MI) and Dr. Mark Kaplan (Indiana University, Indianapolis, IN) for critical reading of the manuscript. This work was partly supported by National Institutes of Health (NIH) Grants HL031237, HL089216, and HL031963 (to S.K.), and NIH Grant AI070448 (to C.-H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905815106/DCSupplemental.
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 3.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 4.Williams LM, et al. Interleukin-10 suppression of myeloid cell activation–a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song S, et al. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood. 1997;89:4461–4469. [PubMed] [Google Scholar]
- 9.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwata H, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 11.Mengozzi M, Ghezzi P. Cytokine down-regulation in endotoxin tolerance. Eur Cytokine Netw. 1993;4:89–98. [PubMed] [Google Scholar]
- 12.Randow F, et al. Mechanism of endotoxin desensitization: Involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg DJ, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatao F, et al. Prolonged Toll-like receptor stimulation leads to down-regulation of IRAK-4 protein. J Leukoc Biol. 2004;76:904–908. doi: 10.1189/jlb.0504277. [DOI] [PubMed] [Google Scholar]
- 18.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474–482. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 19.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 20.Lang T, Mansell A. The negative regulation of Toll-like receptor and associated pathways. Immunol Cell Biol. 2007;85:425–434. doi: 10.1038/sj.icb.7100094. [DOI] [PubMed] [Google Scholar]
- 21.Knodler A, et al. Post-transcriptional regulation of adapter molecules by IL-10 inhibits TLR-mediated activation of antigen-presenting cells. Leukemia. 2008;23:535–544. doi: 10.1038/leu.2008.301. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 23.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Smith H, et al. Identification of the phosphorylation sites on the E3 ubiquitin ligase Pellino that are critical for activation by IRAK1 and IRAK4. Proc Natl Acad Sci USA. 2009;106:4584–4590. doi: 10.1073/pnas.0900774106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordureau A, et al. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem J. 2008;409:43–52. doi: 10.1042/BJ20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotz M, et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.