Abstract
A central paradigm in the field of plant–herbivore interactions is that the diversity and complexity of secondary compounds in plants have intensified over evolutionary time, resulting in the great variety of secondary products that currently exists. Unfortunately, testing of this proposal has been very limited. We analyzed the volatile chemistry of 70 species of the tropical plant genus Bursera and used a molecular phylogeny to test whether the species' chemical diversity or complexity have escalated. The results confirm that as new species diverged over time they tended to be armed not only with more compounds/species, but also with compounds that could potentially be more difficult for herbivores to adapt to because they belong to an increasing variety of chemical pathways. Overall chemical diversity in the genus also increased, but not as fast as species diversity, possibly because of allopatric species gaining improved defense with compounds that are new locally, but already in existence elsewhere.
Keywords: Bursera, chemical complexity, chemical diversity, evolution of secondary compounds, coevolution
Herbivore–plant coevolution has been proposed to be a major factor promoting the escalation of secondary chemistry in plants (1, 2). Progressive steps of herbivore attack and plant defense are thought to be largely responsible for the incremental elaboration, proliferation, and intricacy of plant compounds (3, 4). It has been further argued that the evolution of plant chemical defenses and herbivore counterdefenses has allowed plants and herbivores to colonize new adaptive zones, within which further evolutionary divergence could occur in the relative absence of previous competition and predation (1). These reciprocal selective pressures could thus be of paramount importance in the generation of biological diversity (5, 6).
Despite the prominent conceptual role of this arms-race paradigm, very few studies have reported chemical escalation in plants. In the Apiaceae, for example, it has been postulated that possession of hydroxycoumarins is an ancestral trait relative to the more toxic linear furanocoumarins, and that the more complex angular furanocoumarins are the most derived of the three conditions (3, 7). In Asclepias, complex cardenolides appear to be phylogenetically novel (8). Nevertheless, one of the few studies to use explicit phylogenetic models of character evolution actually reported a pattern of phyletic decline of defensive cardenolides in Asclepias (9, 10).
Another important question is whether this ostensible chemical escalation may have resulted in an overall increase in plant chemical diversity over time (4). The use of modern analytical techniques has already unveiled the existence of thousands of secondary chemical structures in plants and this number will only increase as chemical surveys continue (11, 12). However, it has not been possible to determine whether this diversity has been the result of the purported plant–herbivore arms race, as predicted by coevolutionary theory. For this it is necessary to confirm that the accumulation of new compounds in plant groups has increased over time, as coevolutionary interactions have taken place. Alternatively, it is possible that individual species have increased their diversity and/or complexity using compounds that are already produced by other closely related species and, therefore, the average pool of compounds produced by a group may have remained constant or may have even decreased over time.
Here, we test whether the secondary chemistry of a group of plants, the genus Bursera, has intensified over evolutionary time. We also examine whether this trend is affected by plants having additional mechanical defenses. Last, we try to determine whether chemical escalation in the species has resulted in an increase in the total number of chemical secondary structures in the genus over time.
Bursera includes ≈100 species divided approximately equal into two subgenera, Bullockia and Bursera. The genus is distributed from the southwestern United States to Peru and it is most diverse and abundant in the tropical dry forests of Mexico, where ≈85 species occur (13). Bursera produces resin-containing terpenes (mostly monoterpenes and sesquiterpenes) and other compounds stored in reticulating networks of canals that run throughout the leaves. These compounds are toxic or repellent to insect herbivores, and in Bursera they decrease the survival and growth of their specialized herbivores in the chrysomelid genus Blepharida (14, 15). Previous screening of the species indicates great variation in the number, identity, and relative abundance of individual compounds (16, 17). Some of the species produce one dominant compound, whereas others may produce complex mixtures containing up to 35 individual compounds (17, 18). In some Bursera species, a mechanical defense is added to the chemical protection (15, 19). Resin canals are pressurized, and when a leaf is damaged, an abundant release of resin is triggered, often as a squirt that shoots up to 2 m (Fig. 1). Besides being repellent and toxic, resins solidify when exposed to air and may entomb small insects completely. Thus, insects feeding on these burseras confront both chemical and squirt challenges. Blepharida species feeding on these hosts have to overcome the plant's chemical defenses but they also have responded evolutionarily by cutting the leaf veins where canals are to stop the flow of resin before feeding on the leaves. Interestingly, the chemistry of these squirting burseras tends to be very simple. They produce very few compounds, with one or two highly dominant compounds (15).
Fig. 1.
Squirt defense in Bursera. In some species resins are stored under pressure in a grid of canals that run throughout the cortex of the stems and in the leaves. When these canals are severed or punctured by an insect, a high-pressure flow of resins is released, soaking the attacker.
Bursera's main herbivores, the genus Blepharida, includes ≈45 species that all feed on Bursera. Many of them are monophagous, feeding on a single Bursera species, whereas others feed on many Bursera hosts (20). Fossil and biogeographic information were used to time-calibrate the DNA phylogenies of both genera, and calibrations suggest that their association has coevolved for the last 100 million years (My) (21). These phylogenies also indicate that the plant's defensive chemical and mechanical traits and the beetles' counterdefensive strategies have synchronous times of origin, consistent with the suggestion that these traits evolved in response to concurrent coadaptation.
Although Bursera is an old genus, it only reached its peak rate of diversification ≈13.5 Mya. Rigorous testing using log-likelihood models suggests that its extinction rate has been low and that diversity in the group is mostly driven by speciation (22, 23). This is an important piece of information because incomplete taxon sampling may yield unreliable reconstruction of ancestral characters and distort results of evolutionary models based on maximum likelihood that are now the standard methods to infer evolutionary trends (24–26). The availability of information on diversification and coevolutionary information on its specialized herbivores make Bursera an exceptional system to test for potential patterns of chemical escalation.
Our approach uses a robust molecular phylogeny available for Bursera (Fig. S1). It was reconstructed by using sequences from the internal transcribed spacer (ITS) region, the external transcribed spacer (ETS) region, and the 5S nontranscribed region of the nuclear ribosomal DNA (21, 27, 28).
Our study is based on the analysis of the volatile chemistry of 70 Bursera species by using gas chromatography and mass spectrometry. This number represents ≈82% of the Mexican species and ≈70% of the whole genus. Analyses focused on volatile terpenes, alkanes, and aromatic compounds, which are the most abundant secondary compounds in Bursera and with known negative effects on chrysomelid beetles. For each species we measured the number of compounds it produced and their average relative concentration.
Results
Our results indicate that chemical diversity of individual species has increased during Bursera's diversification as has been predicted by coevolutionary theory. We estimated changes in the number of individual secondary compounds (diversity) and kinds of secondary compounds (complexity). We used several approaches to determine whether Bursera's chemical diversity has increased over time. The first consisted of reconstructing the presence of each compound on every internal node in the phylogeny by using the chemical information that we obtained for every extant species (29). Then we counted the total number of compounds for each node, and, finally, for each branch of the phylogeny we compared descendant versus ancestral taxa for number of compounds, using a sign test (30). A significant excess of one sign would indicate a tendency for descendants to have a higher or lower number of compounds, the signature of an evolutionary time. The second approach to measure change in chemical diversity obviated the need for reconstruction of ancestral characters by taking advantage of the fairly large range in the time since divergence of extant species. We looked at the regression between time since divergence and the number of compounds produced by extant species and used maximum likelihood to compare a model of trait evolution that assumes a constant-variance random walk to the likelihood of the same model, altered to assess a directional trend in chemical diversity (31). Because the rate of evolution of the two subgenera has been different, and this affects the assumptions of the models, each subgenus was analyzed separately.
The sign test indicated that descendant taxa tended to have a higher number of chemical compounds than ancestral taxa (P < 0.0001). These results get more complex when the squirt response is accounted for, showing that trends in chemical diversity can be affected by whether plants also have mechanical defenses. The subgenus Bullockia only has one species with highly pressurized resins (and very simple chemistry) that are released as a squirt when leaves are damaged, whereas the subgenus Bursera includes 10 species with pressurized resins. For Bullockia the tendency to have more compounds in descendant nodes is much more consistent and significant (P < 0.00001) than it is for Bursera (P = 0.01).
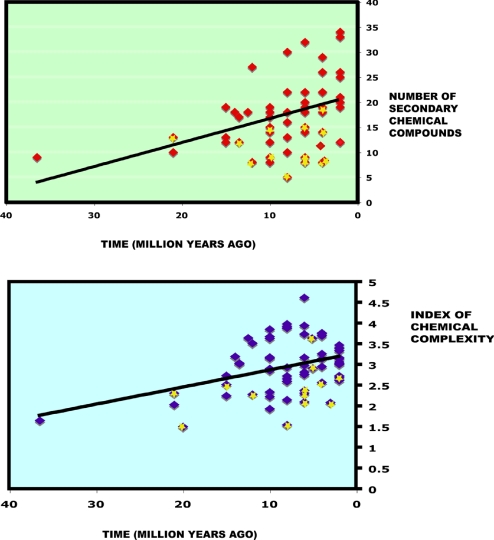
There was also an increase in chemical diversity of species over time when only the data for extant species were included (Fig. 2). However, as with our previous analysis, results differ for the two subgenera. For Bullockia the model that best fitted the data was the one that included a trend toward an increased chemical diversity and low phylogenetic conservatism [β(slope) = 1,805.17; λ = 0.0; P < 0.005); Table S1]. Two species in the subgenus have a strong opposing influence in this relationship. Bursera tecomaca, one of the early diverging species of the genus, has very low chemical diversity. If excluded from the analysis the difference in likelihoods between the random walk and the directional model ceases to be significant (P = 0.18). When Bursera infiernidialis, the only squirting Bursera in this section, is excluded the relationship increases in significance (P = 0.0028).
Fig. 2.
Diversity of secondary compounds (Upper) and complexity of chemical mixtures (Lower) of extant species of Bursera over time. Chemical diversity and complexity of individual species have increased during the genus' diversification as predicted by coevolutionary theory. The yellow stars indicate values of squirting Burseras. Significant P values for directional evolutionary trends are reported in the text.
In contrast, for the subgenus Bursera the likelihoods for the two alternative models, with and without the directional trend, were not significantly different (P < 0.46). However, when the squirting burseras were excluded from the analysis, the model with a directional trend became significant (P = 0.047; β = 8.79; λ = 0.11; Table S1).
As the maximum-likelihood models suggest a low phylogenetic signal in the data (λ = 0.00–0.10 for each section of the genus) we also performed a standard nonphylogenetic regression of number of compounds versus age of species divergence for the whole genus. This analysis showed again that more recently derived species had a higher chemical diversity [P = 0.002; R2 = 0.14; df (1,68)]. Without the squirting burseras the significance of the relationship was even higher [P < 0.0001; R2 = 0.32; df (1,55)]. For both analysis, with and without the squirting Bursera data, the relationship remained highly significant even if B. tecomaca was excluded from the dataset (P = 0.004 and < 0.0001, respectively).
Estimates of chemical complexity took into account both the kind of secondary compounds produced and their average relative concentration. Compounds were divided according to their biochemical pathway into four main categories: monoterpenes plus diterpenes, sesquiterpenes, aromatic compounds, and alkanes. First, a Shannon's diversity index was calculated for each species based only on whether they produce (score 1) or not (score 0) any of each of these four kinds of compounds. A second Shannon's diversity index was also calculated for each species based on the relative concentration of all individual compounds produced, independently of the biochemical pathway they belong to. Last, an index of chemical complexity for each species was obtained by adding the two Shannon's indexes (Tables S2–S4). To test for an increase in chemical complexity we regressed this complexity index against the time of divergence for each extant species and compared the likelihood of models with and without a directional trend as was done above to assess a directional trend in chemical diversity.
Results show that chemical complexity appears to have escalated as well (Fig. 2) and that the presence of mechanical defenses affects the evolutionary pattern. Log-likelihood comparisons for species of the subgenus Bullockia indicated that, as with chemical diversity, the best fit was achieved by a model that assumed increased chemical complexity over time and low phylogenetic constraint (β = 3,375.98; λ = 0.0; P = 0.008; Table S1). However, when B. tecomaca was excluded from the analysis the likelihood difference between the two models is nonsignificant, suggesting again that, at least for this analysis, a very early branching event might have contributed substantially to changes in Bullockia's chemical complexity. For the subgenus Bursera results also indicate increase in complexity (P = 0.046), except that when the squirting burseras are omitted from the analysis, the level of significance increases considerably (P = 0.002; β = 23.25; λ = 0.05).
Because likelihood models of evolution again suggested low phylogenetic signal for both subgenera, a standard, nonphylogenetical regression analysis was performed on complexity versus age of species divergence for both subgenera. The results again suggest that as species diversified over time their volatile chemistry became more complex [P = 0.001; R2 = 0.13; df (1,68)]. If the early diverging B. tecomaca is excluded in the analysis, the relationship remains significant [P = 0.01; R2 = 0.09; df (1,67)]. When the squirting burseras are removed the regression is still highly significant, but does not increase in significance [P = 0.004; R2 = 0.14; df (1,55)].
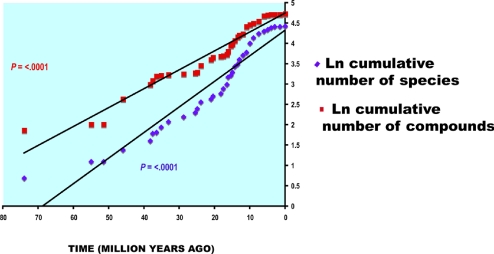
To examine whether the number of compounds in the genus as a whole has increased over time, as predicted by the arms-race hypothesis we used the data obtained from the phylogenetic reconstruction of compounds. For every node in the phylogeny we had information on which compounds were likely to have been new (not present before in the genus). Using these data we plotted the cumulative number of secondary compounds found in the genus as a whole. On the same graph we plotted the cumulative number of Bursera species over time, taken from ref. 22. To compare the rate of production of new secondary compounds with the rate of species diversification we fitted a linear model to each of the accumulation curves. The difference in rate of accumulation of compounds vs. taxa was also expressed by dividing at each time the total number of compounds by the total number of species.
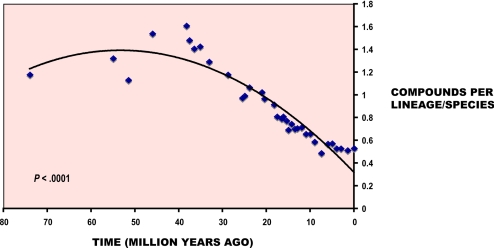
The results show a positive continuous trend in production of new secondary compounds through time, consistent with the idea that the arms race against herbivores has resulted in an incremental elaboration of new plant compounds (Fig. 3; P < 0.0001). However, the trend of accumulation of new compounds has proceeded at a lower pace than the accumulation of new species, as indicated by a lower slope for new compound production (m = 0.0464) when compared with the slope of new species accumulation (m = 0.0628). Interestingly, because the extensive diversification of the genus took place during the last 25 My, the contribution of new lineages/species to the production of new compounds has decreased to reach record low numbers in the last 5 My (Fig. 4; P < 0.0001). As a result, although many more Bursera species have arisen in the last 25 My and the number of compounds per taxon has increased, the new compounds are more and more likely to already be produced by another species in the genus.
Fig. 3.
Accumulation of Bursera lineages (blue diamonds) and compounds (red squares) through time.
Fig. 4.
The cumulative number of compounds divided by the cumulative number of species is plotted against time. In the last 30 My Bursera species have tended to contribute fewer new compounds.
Discussion
By finding a positive evolutionary trend in incremental secondary chemical diversity and complexity we are able to confirm predictions of classic coevolutionay theory. Previous studies have suggested that throughout evolution Blepharida beetles have attacked plants that share common chemical compounds (16) and that these herbivores may impose selective pressures for divergence of secondary compounds in their host plants (17). This study now shows that as new species emerge over time they tend to be armed not only with more compounds, but also with compounds that could potentially be more difficult for herbivores to adapt to because they belong to an increasing variety of chemical pathways and may require different detoxificative catalytic activities and enzymes (32).
Squirting Bursera species tend to have highly volatile simple mixtures composed mostly of one or two monoterpenes, whereas nonsquirting species have more complex mixtures. This relationship affects the macroevolutionary trends in chemistry because even very recently derived squirting species have low diversity and complexity of chemistry. Because the pressurized resins can be a formidable challenge for herbivores, the association of this trait with very simple chemistry has been interpreted as a relaxation of selection for the production of complex mixtures of compounds (33, 34). Accumulation of terpenoids, the most abundant compounds in Bursera, is more expensive per gram than accumulation of most other primary and secondary metabolites (35). Their high cost is caused by extensive chemical reduction mediated by ATP and NADPH and the expense of producing specific enzymes that catalyze the reactions along the biosynthetic pathway. Producing mixtures with more individual compounds often also requires production of more enzymes and cofactors. Thus, a squirting strategy may allow plants to reduce the high metabolic costs of terpenoid synthesis. Lower investment in chemical defenses when another defense mode is present has been observed in other systems (36, 37). However, the macroevolutionary interactions between different defense modes have not been documented before.
Interestingly, despite species becoming more chemically complex and diverse over time, production of new compounds in the genus as a whole has advanced at a relatively lower pace than speciation. One explanation for this result is that as the number of species and compounds has increased there is a higher opportunity for plants to be more chemically diverse and complex without including compounds new to the genus in their defensive arsenal. The majority of Bursera species are highly endemic, with ranges <50,000 Km2 that translates into high β diversity values among communities of these plants (and their associated herbivores). Thus, as previous research (17) has shown, interactions between these plants and insects occur only between subsets of species where a compound can be “new” even if it already occurs in a species in another community. Another way to get this result would be either because of incomplete taxon sampling in Bursera's phylogeny or nonexhaustive inclusion of extant species or extinction. If many speciation events were missed and these species had produced distinctive compounds results of reconstruction of ancestral chemical traits could be distorted (and so would conclusions of evolutionary models based on maximum likelihood) (24–26). However, this does not seem to be the case because the molecular phylogeny used includes the majority of extant species and extinction in the genus has been low.
By documenting historical trends of incremental secondary chemical diversity and complexity in the old Bursera–Blepharida interaction our study provides evidence consistent with an escalation in secondary chemistry although time. This study also demonstrates that, as predicted by the classical theory, as plant species diversify in time there is a net accumulation of new compounds. The weakening of these patterns for plants with alternative mechanical defense (pressurized resins) reinforces the interpretation that these patters are the result of interactions with herbivores.
Materials and Methods
Collection of Plant Tissues.
Samples of leaves of 70 Bursera species were collected from live plants in natural populations in Mexico and immediately extracted in dichloromethane. Leaves were collected from 3–5 individuals in each of 1–2 populations for species of restricted geographic distributions and up to five populations for species of more widespread distributions. For a few of the species, sampling consisted of only two individuals because the species are rare and difficult to find in a limited amount of time. Sampling was restricted to mature, full-grown individuals, concentrating on new, fully developed leaves during time of active growth of plants (June, July, and August).
Chemical Analysis.
All extracts were analyzed by gas chromatography and mass spectrometry performed on a Hewlett-Packard 5890 gas chromatograph linked to a Hewlett-Packard 5970B mass selective detector at 70 eV, m/z 40–600 full scan with a DB-5 column (J & W Scientific) 15 m long, 0.32 mm i.d., and 0.25-μm film. Helium was the carrier gas at a linear velocity of 20 cm/s. The splitless injector temperature was 200 °C, the flame ionization detector held at 240 °C, and the oven temperature was from 40 °C for 4 min then increasing 8 °C per min to 230 °C and held for 5 min. Detected volatile compounds in each species were identified by matching the obtained spectra with standard mass spectral libraries (U.S. National Bureau of Standards 75.K). The majority of the major compounds were also identified by comparing obtained spectra with spectra and retention times of authentic standards. More details of chemical methodology and results can be found in ref. 17.
Chemical Variation Within Species.
For most of the species, there was little variation among individuals in the same population and among populations, confirming previous results that showed high chemical cohesiveness within species (17). For this reason, for each species the relative abundance of every compound was averaged across individuals and populations.
Reconstruction of Ancestral Characters for the Sign Test and the Analyses of Cumulative Number of Compounds in the Genus.
The presence/absence of each chemical compound regardless of its chemical pathway was reconstructed in ancestral nodes of the phylogeny one at a time by using maximum parsimony (29). Any compounds that were present in a relative concentration of 0.03% or more were reconstructed. There are a few species for which there was no chemical information. In that node the information was coded as missing. When reconstruction gave ambiguous results, presence of a compound was recorded as 0.5 instead of 1. The presence or absence of each compound at each node was used to calculate chemical diversity at each node that was used for the sign test described below.
Calculation of a Complexity Index.
To construct an index of chemical complexity for each species compounds were divided into four main categories according to differences in biochemical pathways: monoterpenes plus diterpenes, sesquiterpenes, aromatic compounds, and alkanes. Monoterpenes and diterpenes were put together in one same category because these compounds are preferentially formed using precursors from the 2C-methyl-d-erythrito-4-phosphate pathway in plastids [sesquiterpenoids are preferentially formed using precursors from the mevalonic acid pathway in the cytosol (38)]. We also analyzed data separating monoterpenes from diterpenes and results (statistical significance) did not change. First, a Shannon's diversity index was calculated for each species based only on whether they produce (score 1) or not (score 0) any of each of these four kinds of compounds (Table S2). A second Shannon's diversity index was also calculated for each species based on the relative concentration of all individual compounds produced, independently of the biochemical pathway they belong to (Table S3). Last, an index of chemical complexity for each species was obtained by adding the two Shannon's indexes (Table S4).
Evolutionary Tendencies of Plant Chemical Diversity and Complexity.
Several different statistical analyses were used to determine whether Bursera's chemical diversity has increased over time.
Sign test.
We reconstructed the presence each compound on every internal node and counted the total number of compounds for each node. For each branch in the phylogeny we compared the descendant versus ancestral node for number of compounds, recording the sign of their difference (+, −, or 0) and subsequently conducted a one-tailed sign test (30) to test the hypothesis of a tendency to increase the number of compounds/taxon.
Maximum-likelihood tests for directional trends of chemical evolution.
The second statistical analysis used to test for macroevolutionary trends of chemical diversity or complexity involved the use of the computer program BayesTraits (31, 39). This program implements generalized least squares to examine random-walk versus directional-change models. One advantage of this approach is that it uses only extant species and thus does not rely on character reconstruction. The likelihood of a model of trait evolution that assumes a constant-variance random walk was compared with the likelihood of the same model, including an additional parameter to assess a directional trend. This parameter measures the regression of trait values across species against total path length from the root of the species to the tip and detects general trends toward a dominant direction of evolutionary change. Thus, a positive regression of number of compounds against total path length of extant species would indicate that recently derived species have more compounds whereas more ancestral species have fewer. We also tested for phylogenetic independence of the data by including the parameter λ into the models (31, 39). The program automatically excludes taxa with missing data.
To compare the goodness of fit of a model to the data with that of a simpler model that lacks one or more of the parameters we used the LR statistic defined as LR = −2loge [H0/H1], where H0 represents the simpler (null) model and H1 represents the (alternative) model containing the parameters representing the evolutionary processes. Because the simpler model is a special case of the more complicated one, the LR statistic is asymptotically distributed as a χ2 variable with degrees of freedom equal to the difference in the number of parameters between the two models. For each subgenus, a simpler model that lacked the directionality parameter and included the λ parameter was compared with one that included both parameters. Thus, the LR statistic used 1 df.
Standard, nonphylogenetic regression analyses.
Because log-likelihood models of evolution suggested low phylogenetic signal for both subgenera, standard, nonphylogenetical regression analyses were performed. One regression tested for a relationship between chemical diversity and age of species divergence and another regression tested for a relationship between chemical complexity and age of divergence.
Supplementary Material
Acknowledgments.
This work was supported by National Science Foundation CAREER Grant DEB-9815648 and a Young Investigator Award from the Beckman Foundation (to J.X.B.). D.L.V. was supported by National Science Foundation Grants DEB 0453781, DEB-0717466, DEB-0717380, and DEB-0817121.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.A.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904456106/DCSupplemental.
References
- 1.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 2.Vermeij GJ. The evolutionary interaction among species: Selection, escalation, and coevolution. Annu Rev Ecol Syst. 1994;25:219–236. [Google Scholar]
- 3.Berenbaum M. Coumarins and caterpillars: A case for coevolution. Evolution. 1983;37:163–179. doi: 10.1111/j.1558-5646.1983.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 4.Feeny P. The evolution of chemical ecology: Contributions from the study of herbivorous insects. In: Rosenthal GA, Berenbaum MR, editors. Hervivores: Their Interactions with Secondary Metabolites. Vol 11. San Diego: Academic; 1992. pp. 1–46. [Google Scholar]
- 5.Mitter C, Farrell B, Futuyma DJ. Phylogenetic studies of insect plant interactions: Insights into the genesis of diversity. Trends Ecol Evol. 1991;6:290–293. doi: 10.1016/0169-5347(91)90007-K. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JN. The Coevoutionary Process. Chicago: Univ Chicago Press; 1994. [Google Scholar]
- 7.Berenbaum M, Feeny P. Toxicity of angular furanocoumarins to swallowtail butterflies: Escalation in a coevolutionary arms race. Science. 1981;212:927–929. doi: 10.1126/science.212.4497.927. [DOI] [PubMed] [Google Scholar]
- 8.Farrell BD, Mitter C. The timing of insect/plant diversification: Might Tetraopes (Coleoptera:Cerambycidae) and Asclepias (Asclepiadaceae) have coevolved? Biol J Linn Soc. 1998;63:553–577. [Google Scholar]
- 9.Agrawal AA, Lajeunesse MJ, Fishbein M. Evolution of latex and its constituent defensive chemistry in milkweeds (Asclepias): A phylogenetic test of plant defense escalation. Entomol Exp Appl. 2008;128:126–138. [Google Scholar]
- 10.Agrawal AA, Salminen J, Fishbein M. Phylogenetic trends in phenolic metabolism of milkweeds (Asclepias): Evidence for escalation. Evolution. 2009;63:663–673. doi: 10.1111/j.1558-5646.2008.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Brielmann HLJ. Phytochemicals: The chemical components of plants. In: Kaufman PB, Cseke LJ, Warber S, Duke JA, Brielmann HL, editors. Natural Products of Plants. New York: CRC; 1999. pp. 1–36. [Google Scholar]
- 12.Hartmann T. Diversity and variability of plant secondary metabolism: A mechanistic view. Entomol Exp Appl. 1996;80:177–188. [Google Scholar]
- 13.Rzedowski J. Vegetación de México. México, D.F.: Limusa; 1978. [Google Scholar]
- 14.Becerra JX. Adaptations to ecological interactions. Tucson: University of Arizona; 1993. Dissertation. [Google Scholar]
- 15.Becerra JX, Venable DL. Rapid-terpene-bath and squirt-gun defense in Bursera schlechtendalii and the counterploy of chrysomelid beetles. Biotropica. 1990;22:320–323. [Google Scholar]
- 16.Becerra JX. Insects on plants: Macroevolutionary chemical trends in host use. Science. 1997;276:253–256. doi: 10.1126/science.276.5310.253. [DOI] [PubMed] [Google Scholar]
- 17.Becerra JX. The impact of plant–herbivore coevolution on plant community structure. Proc Natl Acad Sci USA. 2007;104:7483–7488. doi: 10.1073/pnas.0608253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans PH, Becerra JX. Nonterpenoids essential oils from Bursera chemapodicta. Flavor Fragrance J. 2006;21:616–618. [Google Scholar]
- 19.Becerra JX. Historical correlations of defenses and counterdefenses in an ancient plant–insect interaction. Am Zool. 1999;39:286. [Google Scholar]
- 20.Becerra JX. Molecular systematics of the New World Blepharida (Chrysomelidae:Alticinae) and relatives. Mol Phylogenet Evol. 2004;30:107–117. doi: 10.1016/s1055-7903(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 21.Becerra JX. Synchronous coadaptation in an ancient case of herbivory. Proc Natl Acad Sci USA. 2003;100:12804–12807. doi: 10.1073/pnas.2133013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becerra JX. Timing the origin and expansion of the Mexican tropical dry forest. Proc Natl Acad Sci USA. 2005;102:10919–10923. doi: 10.1073/pnas.0409127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becerra JX, Venable DL. Sources and sinks of diversification and conservation priorities for the Mexican tropical dry forest. PLoS ONE. 2008;3:e3436. doi: 10.1371/journal.pone.0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy CR. Reconstructing ancestral ecologies: Challenges and possible solutions. Diversity Distributions. 2005;12:7–19. [Google Scholar]
- 25.Salisbury BA, Kim J. Ancestral state estimation and taxon sampling. Syst Biol. 2001;50:557–564. [PubMed] [Google Scholar]
- 26.Zwickl DJ, Hillis DM. Increase taxon sampling greatly reduces phylogenetic error. Syst Biol. 2002;51:588–598. doi: 10.1080/10635150290102339. [DOI] [PubMed] [Google Scholar]
- 27.Becerra JX. Evolution of Mexican Bursera (Burseraceae) inferred from ITS, ETS, and 5S nuclear ribosomal DNA sequences. Mol Phylogenet Evol. 2003;26:300–309. doi: 10.1016/s1055-7903(02)00256-7. [DOI] [PubMed] [Google Scholar]
- 28.Becerra JX, Venable DL. Nuclear ribosomal, DNA phylogeny, and its implications for evolutionary trends in Mexican Bursera (Burseraceae) Am J Bot. 1999;86:1047–1057. [PubMed] [Google Scholar]
- 29.Maddison DR, Maddison WP. MacClade: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer; 2000. [DOI] [PubMed] [Google Scholar]
- 30.Sokal RR, Rohlf FL. Biometry. New York: Freeman; 1995. [Google Scholar]
- 31.Pagel MD. Inferring the historical patterns of biological evolution. Nature. 1999;402:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiontics. Annu Rev Entomol. 2006;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 33.Becerra JX. Squirt-gun defense in Bursera and the Chrysomelid counterploy. Ecology. 1994;75:1991–1996. [Google Scholar]
- 34.Becerra JX, Venable DL, Evans PH, Bowers WS. Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am Zool. 2001;41:865–876. [Google Scholar]
- 35.Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol. 1994;20:1281–1328. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- 36.Pisani JG, Distel RA. Inter- and intraspecific variations in production of spines in Prosopis caldenia and Prosopis flexuosa. J Chem Ecol. 1998;24:26–36. [Google Scholar]
- 37.Hanley ME, May OC. Relationships between mechanical and chemical attributes of congeneric seedlings: How important is seedling defense? Funct Ecol. 2002;16:216–222. [Google Scholar]
- 38.Bohlmann J, Keeling CI. Terpenoid biomaterials. Plant J. 2008;54:656–669. doi: 10.1111/j.1365-313X.2008.03449.x. [DOI] [PubMed] [Google Scholar]
- 39.Pagel MD. Inferring evolutionary processes from phylogenies. Zool Scripta. 1997;26:331–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.