Abstract
Ant-plant interactions represent a diversity of strategies, from exploitative to mutualistic, and how these strategies evolve is poorly understood. Here, we link physiological, ecological, and phylogenetic approaches to study the evolution and coexistence of strategies in the Acacia–Pseudomyrmex system. Host plant species represented 2 different strategies. High-reward hosts produced significantly more extrafloral nectar (EFN), food bodies, and nesting space than low-reward hosts, even when being inhabited by the same species of ant mutualist. High-reward hosts were more effectively defended against herbivores and exploited to a lower extent by nondefending ants than low-reward hosts. At the phenotypic level, secretion of EFN and ant activity were positively correlated and a mutualistic ant species induced nectar secretion, whereas a nondefending exploiter did not. All of these mechanisms contribute to the stable association of high-reward hosts with defending ant species. However, exploiter ants are less dependent on the host-derived rewards and can colonize considerable proportions of the low-reward hosts. Mapping these strategies onto phylogenetic trees demonstrated that the low-reward hosts represent the derived clade within a monophyletic group of obligate ant plants and that the observed exploiter ant species evolved their strategy without having a mutualistic ancestor. We conclude that both types of host strategies coexist because of variable net outcomes of different investment–payoff regimes and that the effects of exploiters on the outcome of mutualisms can, thus, increase the diversity within the taxa involved.
Keywords: ant plant, cheater, extrafloral nectar, indirect defense, parasite of mutualism
Mutualisms are interactions among different species that enhance the fitness of the involved partners and are often based on the exchange of rewards and services (1, 2). Other species can exploit such rewards without reciprocating, hence acting as “cheaters” or “parasites” of the mutualism (2–5). Such exploiters have been described, among others, for ant-plant mutualisms (6–9), cleaner fishes, lichens, pollination, mycorrhiza, and root–rhizobia symbiosis (see refs. 3, 4, and 10 for reviews). Exploiters do not invest in service provisioning and thus can be competitively superior to mutualists when receiving the same rewards (2, 3, 11–15). As a result, mutualists should tend to reduce service provisioning while optimizing the benefit obtained, leading to a strong “temptation to defect,” and exploitation has been predicted to be common in high-reward mutualisms (4). However, exploiters pose problems for the stability of mutualisms when competing with true mutualists for suitable hosts.
Several mechanisms can stabilize mutualisms against exploitation. For example, host sanctions act at the level of phenotypic variation, allowing the host to adjust reward provisioning to the current quality of the service received. Such mechanisms stabilize, for example, the mutualisms of host fishes with cleaner fishes (16) and plant roots with rhizobia (12, 17). Partner choice mechanisms, by contrast, depend on the recognition of species-specific cues during the phase of partner finding (18) and are known, among others, for ant-plant mutualisms (19, 20) and the early phases of signaling between plant roots and rhizobia (21, 22).
Exploiters evolving from former mutualists that reduce or cease reciprocation likely can retain the traits on which partner choice is based. Consequently, they are more efficiently controlled by host sanction mechanisms, which are based on the actual level of reciprocation. By contrast, exploiters with no history as a mutualist likely lack cues that specifically enable the onset of the interaction and can be excluded via partner choice (23). Because the phylogenetic history of an exploiter determines the mechanism that can stabilize a mutualism against its evolution and maintenance, we hereinafter use the term cheater explicitly for exploiters with mutualistic ancestors (24) and term those species parasites that invaded the system without having an evolutionary history as a mutualist (4, 23). Making this distinction it becomes apparent that few phylogenetic studies reported evidence for the existence of cheaters (3, 5), an obvious contradiction to theoretical expectations, which demonstrates the existence of mechanisms that can stabilize mutualisms.
We used a defensive ant-plant mutualism to investigate whether reward production by congeneric host plant species affects their level of exploitation. Such ant-plant mutualisms serve the “indirect defense” of plants against herbivores (25, 26) and allow studies of the ecology and evolution of mutualisms, because the partners involved can be experimentally separated (26). In detail we asked: (i) Do mutualists and exploiters compete for host plants? (ii) Do host species differ in their level of exploitation? (iii) Does reward production feed back to the level of exploitation and does the investment depend on the payoff received? (iv) Do the ants influence reward production? We used molecular phylogenies to understand the evolutionary history of the different strategies used by host plants and mutualistic and exploiting ants.
Results and Discussion
Competition for Host Plants.
Mesoamerican Acacia myrmecophytes (obligate ant plants) provide nesting space in hollow thorns and food rewards [extrafloral nectar (EFN) and food bodies (FBs)] (27–29) to specific, defending Pseudomyrmex ants (6). Several closely related species are involved in the mutualism on the side of both ants and plants. Nondefending ants exploit this system and workers of the nondefending ant species, Pseudomyrmex gracilis, prevent mutualist queens from colony founding (6), which makes P. gracilis an active competitor for host plants. To document the competition among colony-founding queens, we deprived branches of Acacia cornigera and Acacia hindsii plants of their resident ants and quantified foundresses in the newly developed thorns. Both plant species and ant species had a significant effect on the number of thorns occupied by queens [general linear model (GLM): plant species: F1,371 = 5.84, P = 0.016; ant species: F5,371 = 49.74, P < 0.001; interaction: F5,371 = 5.02, P < 0.001]. After 6 weeks, a total of 205 foundresses had arrived on A. cornigera and 448 on A. hindsii, occupying on average 82% (A. cornigera) and 121% (A. hindsii) of the available thorns (121% for A. hindsii because numerous thorns were occupied by 2 queens). Among all queens observed, 3% of those found on A. cornigera and 5% of those on A. hindsii belonged to P. gracilis, whereas the majority was queens of the mutualists, Pseudomyrmex ferrugineus, Pseudomyrmex mixtecus, and Pseudomyrmex peperi. Because Acacia plants usually are dominated by a single ant colony (27) these data indicate a massive competition for host plants.
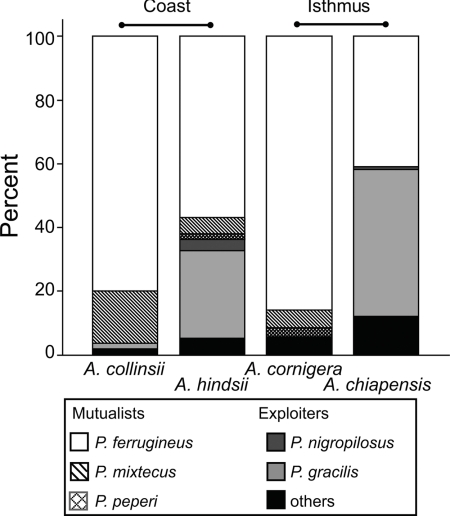
Next, we quantified the level of exploitation of 4 Acacia species: A. hindsii and Acacia collinsii were investigated at the Pacific coast, A. cornigera and Acacia chiapensis at a distance of ≈270 km in the Isthmus of Tehuantepec (both sites: state of Oaxaca, Mexico). In total, some 98% of the A. cornigera and A. collinsii plants were dominated by mutualistic Pseudomyrmex species. The most common ant was P. ferrugineus, which inhabited >80% of the plants of both species (Fig. 1). By contrast, P. ferrugineus inhabited only 55% of A. hindsii and 41% of A. chiapensis, whereas the parasite P. gracilis inhabited 26% and 44%, respectively, of plants of the latter 2 species (Fig. 1). Mutualists were significantly more frequent on A. cornigera and A. collinsii than on A. hindsii and A. chiapensis, both within each habitat and pooled over both habitats (likelihood ratio test, Gcoast = 14.1, df = 1, P < 0.0001; Gisthmus = 65.8, df = 1, P < 0.0001; Gpooled = 77.6, df = 1, P < 0.001, mutualists and parasites pooled within classes).
Fig. 1.
Occupancy of Acacia hosts by different ant species. The relative proportion of plants inhabited by the different ant species is given separately for the 4 species of Acacia myrmecophytes that were investigated at the 2 sites, site 1 (Coast) and site 2 (Isthmus). Sample sizes are 52 for A. collinsii, 61 for A. hindsii, 90 for A. cornigera, and 115 for A. chiapensis.
Plant-Derived Ant Rewards.
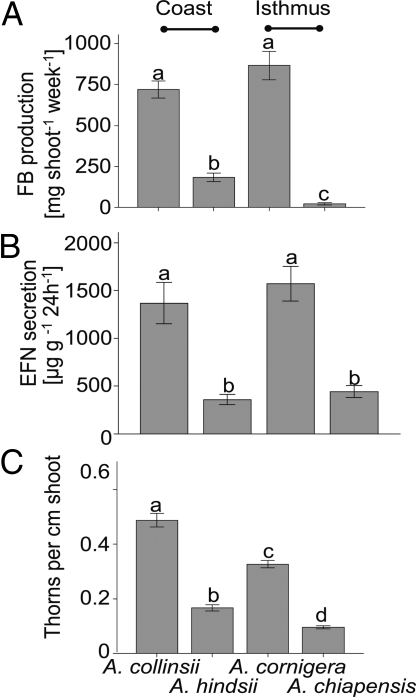
Reward production by the 4 myrmecophytes was quantified at the same 2 sites. To control for effects of the ant inhabitant, only plants inhabited by P. ferrugineus were used. Plant species was a significant source of variation in the FB production rates (GLM: F3, 21 = 101.57, P < 0.001). A. collinsii and A. cornigera produced on average 720 and 870 mg of FBs per shoot and week, whereas A. hindsii produced 180 mg of shoot−1·wk−1 and A. chiapensis produced only 25 mg of shoot−1·wk−1. The production rates of A. hindsii and A. chiapensis were significantly [P < 0.05, least significant difference (LSD) posthoc tests] lower than those of A. collinsii and A. cornigera (Fig. 2A). The same pattern was found for EFN secretion, because A. cornigera and A. collinsii secreted ≈3 times more EFN (1,400 and 1,600 μg of soluble solids g−1 leaf dry mass·24 h−1) than A. hindsii and A. chiapensis (<500 μg of g−1·24 h−1) (GLM: F3,92 = 19.176, P < 0.001; Fig. 2B). Similar to food provision, A. cornigera and A. collinsii had more thorns per cm shoot than A. hindsii and A. chiapensis (GLM: F3,120 = 142.05, P < 0.001; Fig. 2C).
Fig. 2.
Reward production by Acacia myrmecophytes. (A) FB production averaged for 8 plants per species is expressed in mg FB dry weight per shoot and week. (B) EFN secretion was averaged from 20–25 plants per species and is expressed in μg soluble solids per g leaf dry mass and day. (C) Nesting space was estimated for 30 plants per species as number of thorns present per cm shoot. Bars marked by different letters are significantly different (P < 0.05 according to LSD posthoc test after univariate ANOVA). Bars indicate means; error bars indicate standard errors.
In short, A. cornigera and A. collinsii can be characterized as high-reward hosts, whereas A. hindsii and A. chiapensis are low-reward hosts. All plants investigated here were inhabited by the same ant species, and 1 high-reward species and 1 low-reward species co-occurred in each of the sites. Neither site effects nor the ant inhabitant can therefore explain these differences, which obviously represent species-specific traits.
Reward Provisioning, Defense, and Exploitation.
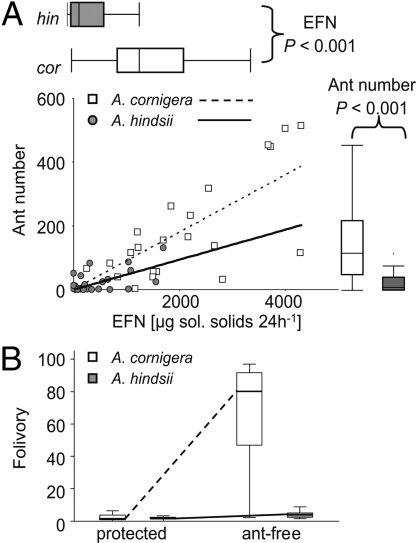
To investigate whether plants gain an enhanced payoff from higher investment rates we chose 23 A. cornigera and 24 A. hindsii plants, all being inhabited by P. ferrugineus. EFN secretion was quantified on the main shoot, and plant stems were then damaged to simulate attack. Numbers of ants recruiting to these lesion sites were quantified as a measure of colony aggressiveness during the next 2 h. Data on EFN secretion confirmed earlier results (Fig. 2A), because EFN secretion by A. cornigera was significantly higher than that by A. hindsii (Mann–Whitney U = 58.0, P < 0.001). For both species, numbers of ants recruited were positively correlated with EFN secretion (Fig. 3A; A. hindsii: Pearson's correlation coefficient = 0.574, n = 24, P = 0.003; A. cornigera: Pearson's correlation coefficient = 0.748, n = 23, P < 0.001), and significantly more ants were thus recruited on A. cornigera than on A. hindsii (Mann–Whitney U = 71.5, P < 0.001).
Fig. 3.
EFN secretion, ant activity, and defense. (A) Numbers of P. ferrugineus workers passing near fresh lesions are depicted as a function of EFN secretion of the respective plant separately for A. cornigera and A. hindsii. The distribution of data are indicated by box-whisker plots and differed significantly between the plant species (Mann–Whitney-U test: P < 0.001). (B) The higher activity of ants on the high-reward species A. cornigera translates to a higher defensive effect, because leaf damage (calculated as percentage of damaged leaflets) suffered by this species increased significantly more over 3 weeks of ant exclusion than for the low-reward species A. hindsii.
Different slopes of the regression lines describing the number of ants recruited as a function of EFN production (Fig. 3A) pointed to different defensive efficacies of EFN secretion for the 2 host species. This was tested by calculating the quotient of ant number per EFN secretion for each individual plant. These quotients for A. cornigera were significantly higher than for A. hindsii (Mann–Whitney U = 141.0, P = 0.018). Thus, a given increase in EFN provisioning increases ant recruitment to lesions more strongly on A. cornigera than on A. hindsii.
Resident ants being active on the plant surface should protect their host from competing ants (30) and A. cornigera was less often exploited than A. hindsii (Fig. 1), but does this ant activity translate to a stronger defense against herbivores? P. ferrugineus ants were excluded from 1 branch each of 10 A. hindsii and 10 A. cornigera plants. The resulting increase in folivory was indeed higher for A. cornigera than for A. hindsii (GLM on arcsin-transformed data: ant presence: F1,59 = 70.862, P < 0.001; plant species: F1,59 = 55.635, P < 0.001; interaction: F1,59 = 54.207, P < 0.001; see Fig. 3B), indicating that ants protect the high-reward host A. cornigera more strongly from folivory than A. hindsii.
Phylogenetic Relationships.
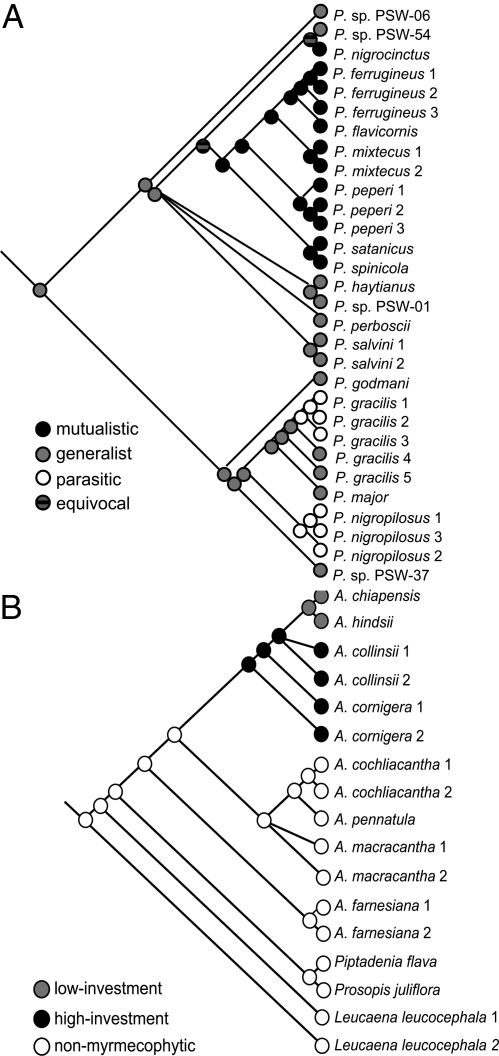
We used sequence data to reconstruct the relationships among mutualistic, exploiting, and generalist ant species and among low-reward and high-reward hosts. Two exploiting ant species, P. gracilis and Pseudomyrmex nigropilosus, belong to the same genus as the mutualists but formed a clade well separated from the mutualists (Fig. 4A). These species likely have no evolutionary history as a mutualist and represent parasites of the mutualism, which remains true also for a third exploiter of this system, Camponotus planatus (8). Thus, no evidence for a cheater exists so far, demonstrating the stability of the Mesoamerican Acacia–Pseudomyrmex mutualism against the evolution and persistence of exploiters that are derived from mutualists (but see refs. 31–33 for African ant acacias).
Fig. 4.
Phylogenetic histories. (A) Associations of ants with Acacia myrmecophytes (mutualistic, generalist, and parasitic) traced on a phylogeny of Pseudomyrmex ants, as inferred from a 5-gene fragments analysis (wg, abd-A, LW Rh, 28S, and mtCOI, totaling 3,224 bp). Redrawn from ref. 23. (B) The investment strategies of myrmecophytic acacias and their relation to nonmyrmecophytic species were traced on a phylogeny based on 2 gene fragments (trnK intron and trnL-trnF intrageneric spacer totaling 3,222 bp). Data were reevaluated from ref. 29. See Ref. 23 and Fig. S2 for branch support.
Character tracing for the hosts revealed that the low-reward species, A. hindsii and A. chiapensis, form a later diverging clade within the monophyletic group of obligate myrmecophytes (Fig. 4B) and led to the conclusion that the low-reward hosts exhibit the evolutionarily derived strategy. In fact, symbiotic mutualisms can show a tendency to relaxation or even be reversed toward a free-living lifestyle under certain external selection pressures (5).
The phylogeny of the ants used here is based on mitochondrial and nuclear genes and was consistent with morphological findings (23). By contrast, the current molecular phylogeny of Mesoamerican Acacia myrmecophytes is based on chloroplast genes only and thus represents exclusively the maternal line, not allowing the detection of hybridization processes. An alternative hypothesis would be that introgression of nonmyrmecophytic Acacia species into high-reward species gave rise to the species with intermediate levels of ant rewards, which we term low-reward hosts. Amplified fragment-length polymorphism (AFLP) data revealed high genetic similarity between the low-reward A. hindsii and the high-reward A. collinsii (29), and both species share elongate inflorescences as a typical morphological trait (27). Although sequence data are regarded more suitable to reconstruct phylogenetic trees than AFLP data, the current phylogenetic information does not allow the exclusion of alternative topologies to the one presented here (Fig. 4B). Most importantly in the context of the present study, high-reward and low-reward hosts formed part of the same major phylogenetic clade, and species using the different strategies were found to coexist at the same sites.
Stabilizing the Acacia–Pseudomyrmex Mutualism.
High-reward hosts were better protected from herbivory and exploitation than low-reward hosts and no cheaters could be detected. These observations empirically support that, for the evolution and stabilization of mutualisms, investment must depend on the payoff received (2) and that phenotypically plastic partners must exchange reciprocal responses (34).
Differences in the degree of reciprocal adaptations among the partners appear a key factor that tightens the association of high-reward hosts with defending ants. Mutualist Pseudomyrmex ants are physiologically adapted to live exclusively on host-derived food rewards (6, 20, 23) and were never found nesting outside an Acacia host (27, 35). By contrast, the common exploiter, P. gracilis, can nest independently of Acacia hosts (35) and consumes food that is captured off the host plant (6). We used Michaelis–Menten functions to describe how ant colony size increases as a function of reward level and the efficiency at which rewards are converted into workers. These functions demonstrate that the exploiter can reach competitive superiority when host-derived rewards limit colony growth, whereas the mutualist appears superior at higher reward levels (Fig. 5 and SI Text). Interestingly, models expressing the reproduction rate of a mutualist population in terms of costs and net effects as a function of its partner's population size revealed similar predictions when net effects to one partner were assumed to increase asymptotically and saturate with increasing abundance of the other partner (36).
Fig. 5.
Colony size as a function of plant reward level. Relations among reward level R, ant colony size S of species i, and the efficiency Ei at which rewards are converted into workers were inferred from models based on Michaelis–Menten functions (SI Text) with the intrinsic maximum colony size being Smaxi, and Rmini describing the minimum reward level at which a colony can exist. When both species compete for a high-reward host, the positive feedback of rewards to protecting behavior (Fig. S3) allows the mutualist (i = m) to displace the parasite (i = p), as indicated here by a term that exponentially increases with the mutualists' colony size, where a and C are constants. Black line indicates P. ferrugineus; dotted gray line indicates P. gracilis; gray line indicates P. gracilis in the presence of a competitively superior P. ferrugineus colony.
Net Effects of Exploitation and Species Coexistence.
If high-reward hosts are much more likely inhabited by defending mutualists, why do low-reward hosts exist at all, and does this facilitate the observed coexistence of both strategies? External selection pressures might force mutualisms to evolve toward independent lifestyles (5) and an expensive defense pays off best when there is a high herbivore pressure. The costs of ant rewards are illustrated by their rapid reduction in the absence of herbivores (33), their dependency on nutrient supply (37), and numerous mechanisms that regulate their production (38–42). EFN secretion was positively correlated with worker activity of the mutualist P. ferrugineus (Fig. S1A; A. hindsii: Pearson's correlation coefficient = 0.733, n = 41, P < 0.001; A. cornigera: Pearson's correlation coefficient = 0.879, n = 22, P < 0.001), whereas there was a negative, yet insignificant, relation with the presence of P. gracilis workers on A. hindsii (Fig. S1B; Pearson's correlation coefficient = −0.193, n = 41, P = 0.226). Both the correlation coefficient and the slope of the regression line were higher for A. cornigera than for A. hindsii (Fig. S1A), pointing to a stronger inducing effect of P. ferrugineus ants on EFN secretion by the first host species. An ant-exclusion experiment confirmed that Acacia myrmecophytes quickly reduce EFN secretion when mutualists are missing and this effect differs among plant species: P. ferrugineus workers were excluded from single branches of A. cornigera and A. hindsii and these branches secreted significantly less EFN than branches to which ants had access (Fig. S1C). Plant species and presence of ants had a significant effect on EFN secretion (GLM: plant species: F1,21 = 40.015, P < 0.001; ant presence: F1,21 = 24.884, P < 0.001; interaction: F1,21 = 7.072, P = 0.016) and the effect of mutualist ants on EFN secretion differed between the plant species (as indicated by the significant interaction), increasing EFN secretion more on A. cornigera than on A. hindsii.
In short, the presence of mutualists positively affects EFN secretion, although at different degrees among host species, whereas the exploiter lacks the reward-inducing features. Most likely, the plants somehow monitor the presence and/or defensive activity of the mutualist ants. In Mesoamerican Acacia myrmecophytes, leaf damage does not increase EFN secretion because resting levels of the wound hormone, jasmonic acid, are sufficient to elicit full secretion rates (29). Likewise, a regulation via EFN removal appears unlikely, because the parasite also feeds on EFN. The cue remains to be identified that is used by the mutualist ants to modulate EFN secretion or by the hosts to monitor the presence of reciprocating ants. However, it is clear that, as predicted before (34), phenotypic plasticity plays a key role in stabilizing the high-reward mutualisms in our study system. This interpretation is in line with a model showing that herbivore pressure poses selective pressure on the nesting space provided by myrmecophytic Tachigali species (43). In general, adaptive responses by mutualists to variation in partner quality can maintain diversity within mutualist guilds (31), and costs rather than benefits appear to be the central factor that shapes mutualisms (44). Spatial and temporal variation in herbivore pressure and the costs of defense apparently allow the coexistence of alternative strategies (31, 32).
Conclusions.
Exploiters might gain a competitive advantage from not reciprocating when reward provisioning by the host is not adjusted to the nature of the interacting partner. In the Acacia–Pseudomyrmex system, by contrast, rewards are preferentially provided to mutualist ants. Positive feedback mechanisms (Fig. S3) among phenotypically plastic and fitness-relevant traits of both partners facilitate associations of high-reward host plants with defending ant species and stabilize the Acacia–Pseudomyrmex interaction against the evolution of cheaters and the exploitation by parasites (Fig. 5).
Plants using the high-reward strategy appeared better protected against exploitation (see Fig. 1 and SI Text) and should be superior under conditions that make exploitation by nondefending ants particularly costly. By contrast, defense investment can become relaxed under conditions making defense function poorly (45). Because costs and benefits of defense depend on numerous and variable environmental factors such as nutrient availability and herbivore pressure, high-reward hosts and low-reward hosts can coexist at the same sites, at least in the current ecological situation (Figs. 1 and 2).
We found no hints that exploitation was more common in high-reward mutualisms and no phylogenetic evidence for the existence of cheaters. Thus, our study lends empirical support to the prediction that mutualisms are stabilized when increased investments in a partner increase reciprocation. Mutualisms can be stabilized by the reciprocal exchange of phenotypically plastic responses among partners.
Materials and Methods
Study System.
The study was conducted at 2 field sites in Oaxaca, Mexico. All plants were 1.0–2.0 m high. Site 1 (Pacific coast) was ≈15 km W of Puerto Escondido in the coastal area of the state (≈15°55 N and 097°09 W, elevation 15 m), and site 2 (Isthmus) was in the Isthmus of Tehuantepec ≈25 km N of Matias Romero (≈17°06 N and 094°55 W, elevation 150 m). A. collinsii Safford, A. hindsii Bentham, and A. cornigera (L.) Willdenow grow at site 1, and A. hindsii, A. cornigera and A. chiapensis Safford grow at site 2. The mutualist ant species P. ferrugineus F. Smith, P. mixtecus Ward, and P. peperi Forel cannot be found nesting apart from their host plants. Other ant species (at the study sites mainly P. gracilis Fabricius and P. nigropilosus Emery) inhabit the hollow thorns and consume FBs, EFN, and insect prey but show no defending behavior, hence acting as exploiters (6, 7).
Competition for Host Plants.
We investigated colonization by excluding ants from branches of 18 A. cornigera and 39 A. hindsii plants on site 1 in October 2007. Inhabited thorns were cut off and resident ants were excluded from the shoot tips by applying a ring of Tangletrap (Tanglefoot). After 6 weeks, newly produced thorns were counted and censused for founding queens.
Ant Occupancy.
A total of 316 plants at both sites were censused during April and May 2004 for ant inhabitants (n = 112 plants at site 1 and 204 plants at site 2). A colony was scored as inhabiting a plant only when thorns contained living brood and when no workers of other ant species were observed on the plant.
Reward Production.
Reward production was investigated at site 1 for A. collinsii and A. hindsii and at site 2 for A. cornigera and A. chiapensis. FB production was quantified for 8 individuals per species in September 2005. Ants were excluded from the main shoots, which were then placed into gauze bags. After 3 weeks, all newly produced leaves were collected and FBs were counted. A subsample of 100 FBs per plant was dried and weighed to determine average FB dry weight to calculate FB production in dry mass shoot−1·week−1. EFN secretion was quantified in September 2005 on 20–25 plants per species as described (29, 40). Thorn numbers were counted and overall shoot length was measured to the nearest 5 cm for 30 plants per species to calculate the number of thorns per cm shoot.
EFN Secretion and Ant Activity.
Ant activity in the undisturbed stage was quantified on 18 A. cornigera and 39 A. hindsii plants inhabited by P. ferrugineus at site 1 in September 2006 as follows. Six lines were drawn in the upper, central, and lower third of the main shoot of each plant and ants passing these lines were counted 3 times for 1 min each. Thereafter, EFN secretion was quantified for the same plants. To investigate the effect of mutualist ants on EFN secretion, P. ferrugineus ants were excluded from 1 branch of each of 5 A. cornigera and 6 A. hindsii plants (site 1, April 2008) and EFN secretion was quantified on the ant exclusion and the nearest untreated branch after 2 weeks.
The relation of EFN secretion with numbers of ants being recruited to lesions was studied at the same site in March 2007. EFN secretion of the main shoots of 24 A. hindsii and 23 A. cornigera plants was quantified and plants then were mechanically damaged (cutting a line ≈2 cm long and 3 mm deep) in the upper, central, and lower region of the main stem. To quantify the level of aggressiveness at which the plant is defended by its resident colony, workers passing lines ≈1 cm above and below each lesion site were censused 3 times for 1 min each with ≈1 h between censuses. Plant protection from herbivores was studied on site 1 in April 2008. Ants were excluded from 1 branch of each of 10 A. hindsii and 10 A. cornigera plants. Three leaves were collected from each of these and the neighboring ant-inhabited branches after 3 weeks, and the numbers of intact and damaged leaflets were counted to calculate folivory as percentage of damaged leaflets.
Reconstruction of Ancestral States.
We inferred the phylogeny of hosts by using a Bayesian approach and a maximum-likelihood analysis based on trnL-trnF intron and trnK intron sequence data (29). Nucleotide substitution models were selected by using Akaike Information Criterion (46). Branch support was assessed as Bayesian posterior probabilities and bootstrap support resulting from the maximum-likelihood analysis. Reconstruction of ancestral states (47) of the high-reward and the low-reward strategy was conducted as described (23). See SI Text for detailed information on phylogenetic methods. See ref. 23 and Table S1 for GenBank accession numbers, and see ref. 23 and Fig. S2 for branch support.
Supplementary Material
Acknowledgments.
We thank Judith Bronstein, Angela E. Douglas, Anurag A. Agrawal, and 3 anonymous referees for discussions and comments on earlier versions of this manuscript and Douglas W. Yu for invaluable statistical advice. This work was supported by Deutsche Forschungsgemeinschaft Grant He 3169/4-2, the Max-Planck-Gesellschaft, Consejo Nacional de Ciencia y Tecnología de México, and a PhD grant from the Deutscher Akademischer Austauschdienst (to M.G.-T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.A.A. is a guest editor invited by the Editorial Board.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession nos. AY574092–AY574121 and FJ436816–FJ436926).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904304106/DCSupplemental.
References
- 1.Bronstein JL. Our current understanding of mutualism. Q Rev Biol. 1994;69:31–51. [Google Scholar]
- 2.Doebeli M, Knowlton N. The evolution of interspecific mutualisms. Proc Natl Acad Sci USA. 1998;95:8676–8680. doi: 10.1073/pnas.95.15.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends Ecol Evol. 2006;21:585–592. doi: 10.1016/j.tree.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein JL. The exploitation of mutualisms. Ecol Lett. 2001;4:277–287. [Google Scholar]
- 5.Douglas AE. Conflict, cheats and the persistence of symbioses. New Phytol. 2008;177:849–858. doi: 10.1111/j.1469-8137.2007.02326.x. [DOI] [PubMed] [Google Scholar]
- 6.Clement LW, Köppen S, Brand WA, Heil M. Strategies of a parasite of the ant-Acacia mutualisms. Behav Ecol Sociobiol. 2008;26:953–962. doi: 10.1007/s00265-007-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janzen DH. Pseudomyrmex nigropilosa: A parasite of a mutualism. Science. 1975;188:936–937. doi: 10.1126/science.188.4191.936. [DOI] [PubMed] [Google Scholar]
- 8.Raine NE, Gammans N, Macfadyen IJ, Scrivner GK, Stone GN. Guards and thieves: Antagonistic interactions between two ant species coexisting on the same ant plant. Ecol Entomol. 2004;29:345–352. [Google Scholar]
- 9.Tillberg CV. Friend or foe? A behavioral and stable isotopic investigation of an ant-plant symbiosis. Oecologia. 2004;140:506–515. doi: 10.1007/s00442-004-1601-8. [DOI] [PubMed] [Google Scholar]
- 10.Yu DW. Parasites of mutualisms. Biol J Linn Soc. 2001;72:529–546. [Google Scholar]
- 11.Doebeli M, Hauert C, Killingback T. The evolutionary origin of cooperators and defectors. Science. 2004;306:859–862. doi: 10.1126/science.1101456. [DOI] [PubMed] [Google Scholar]
- 12.Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume–rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- 13.Hoeksema JD, Bruna EM. Pursuing the big questions about interspecific mutualism: A review of theoretical approaches. Oecologia. 2000;125:321–330. doi: 10.1007/s004420000496. [DOI] [PubMed] [Google Scholar]
- 14.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 15.Doebeli M, Hauert C. Models of cooperation based on the Prisoner's Dilemma and the Snowdrift game. Ecol Lett. 2005;8:748–766. [Google Scholar]
- 16.Bshary R, Grutter AS. Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol Lett. 2005;1:396–399. doi: 10.1098/rsbl.2005.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simms EL, et al. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc R Soc London Ser B. 2006;273:77–81. doi: 10.1098/rspb.2005.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster KR, Wenseleers T. A general model for the evolution of mutualisms. J Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 19.Brouat C, Garcia N, Andary C, McKey D. Plant lock and ant key: Pairwise coevolution of an exclusion filter in an ant-plant mutualism. Proc R Soc London Ser B. 2001;268:2131–2141. doi: 10.1098/rspb.2001.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heil M, Rattke J, Boland W. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science. 2005;308:560–563. doi: 10.1126/science.1107536. [DOI] [PubMed] [Google Scholar]
- 21.Mithöfer A. Suppression of plant defense in rhizobia–legume symbiosis. Trends Plants Sci. 2002;10:440–444. doi: 10.1016/s1360-1385(02)02336-1. [DOI] [PubMed] [Google Scholar]
- 22.Limpens E, Bisseling T. Signaling in symbiosis. Curr Opin Plant Biol. 2003;6:343–350. doi: 10.1016/s1369-5266(03)00068-2. [DOI] [PubMed] [Google Scholar]
- 23.Kautz S, Lumbsch HT, Ward PS, Heil M. How to prevent cheating: A digestive specialization ties mutualistic plant–ants to their ant-plant partners. Evolution. 2009;63:839–853. doi: 10.1111/j.1558-5646.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 24.Segraves K, Althoff D, Pellmyr O. Limiting cheaters in mutualism: Evidence from hybridization between mutualist and cheater yucca moths. Proc R Soc London Ser B. 2005;272:2195–2201. doi: 10.1098/rspb.2005.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain SA, Holland JN. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology. 2009;90:2384–2392. doi: 10.1890/08-1490.1. [DOI] [PubMed] [Google Scholar]
- 26.Heil M, McKey D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst. 2003;34:425–453. [Google Scholar]
- 27.Janzen DH. Swollen-Thorn Acacias of Central America. Washington, DC: Smithsonian Institution Press; 1974. [Google Scholar]
- 28.Heil M, Baumann B, Krüger R, Linsenmair KE. Main nutrient compounds in food bodies of Mexican Acacia ant plants. Chemoecology. 2004;14:45–52. [Google Scholar]
- 29.Heil M, et al. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature. 2004;430:205–208. doi: 10.1038/nature02703. [DOI] [PubMed] [Google Scholar]
- 30.Palmer TM. Wars of attrition: Colony size determines competitive outcomes in a guild of African acacia ants. Anim Behav. 2004;68:993–1004. [Google Scholar]
- 31.Palmer TM, Stanton ML, Young TP. Competition and coexistence: Exploring mechanisms that restrict and maintain diversity within mutualist guilds. Am Nat. 2003;162:S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- 32.Palmer TM. Spatial habitat heterogeneity influences competition and coexistence in an African acacia ant guild. Ecology. 2003;84:2843–2855. [Google Scholar]
- 33.Palmer TM, et al. Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African savanna. Science. 2008;319:192–195. doi: 10.1126/science.1151579. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal AA. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- 35.Ward PS. Systematic studies on Pseudomyrmex acacia ants (Hymenoptera: Formicidae: Pseudomyrmecinae) J Hym Res. 1993;2:117–168. [Google Scholar]
- 36.Holland JN, DeAngelis DL, Bronstein JL. Population dynamics and mutualism: Functional responses of benefits and costs. Am Nat. 2002;159:231–244. doi: 10.1086/338510. [DOI] [PubMed] [Google Scholar]
- 37.Heil M, et al. Nutrient allocation of Macaranga triloba ant plants to growth, photosynthesis, and indirect defense. Funct Ecol. 2002;16:475–483. [Google Scholar]
- 38.Heil M, et al. Food body production in Macaranga triloba (Euphorbiaceae): A plant investment in antiherbivore defense via mutualistic ant partners. J Ecol. 1997;85:847–861. [Google Scholar]
- 39.Risch SJ, Rickson F. Mutualism in which ants must be present before plants produce food bodies. Nature. 1981;291:149–150. [Google Scholar]
- 40.Heil M, Fiala B, Baumann B, Linsenmair KE. Temporal, spatial, and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Funct Ecol. 2000;14:749–757. [Google Scholar]
- 41.Heil M, et al. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mondor EB, Addicott JF. Conspicuous extra-floral nectaries are inducible in Vicia faba. Ecol Lett. 2003;6:495–497. [Google Scholar]
- 43.Fonseca CR. Nesting space limits colony size of the plant ant Pseudomyrmex concolor. Oikos. 1993;67:473–482. [Google Scholar]
- 44.Bronstein JL. The costs of mutualism. Am Zool. 2001;41:825–839. [Google Scholar]
- 45.Agrawal AA, Fishbein M. Phylogenetic escalation and decline of plant defense strategies. Proc Natl Acad Sci USA. 2008;105:10057–10060. doi: 10.1073/pnas.0802368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 47.Pagel M. The maximum-likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst Biol. 1999;48:612–622. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.