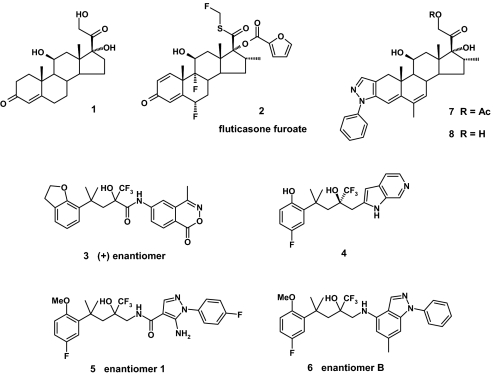
Abstract
Crystallography and computer modeling have been used to exploit a previously unexplored channel in the glucocorticoid receptor (GR). Highly potent, nonsteroidal indazole amides showing excellent complementarity to the channel were designed with the assistance of the computational technique AlleGrow. The accuracy of the design process was demonstrated through crystallographic structural determination of the GR ligand-binding domain–agonist complex of the D-prolinamide derivative 11. The utility of the channel was further exemplified through the design of a potent phenylindazole in which structural motifs, seen to interact with the traditional GR ligand pocket, were abandoned and replaced by interactions within the new channel. Occupation of the channel was confirmed with a second GR crystal structure of this truncated D-alaninamide derivative 13. Compound 11 displays properties compatible with development as an intranasal solution formulation, whereas oral bioavailability has been demonstrated with a related truncated exemplar 14. Data with the pyrrolidinone amide 12 demonstrate the potential for further elaboration within the “meta” channel to deliver compounds with selectivity for the desired transrepressive activity of glucocorticoids. The discovery of these interactions with this important receptor offers significant opportunities for the design of novel GR modulators.
Keywords: drug design, AlleGrow, glucocorticoid modulation, intranasal glucocorticoid
Since the discovery of the remarkable anti-inflammatory properties of the natural glucocorticoid cortisol 1 in the 1940s (1) this steroidal template has been the subject of extensive optimization to deliver compounds with enhanced potency, selectivity, and pharmacokinetic properties (Scheme 1) (2). The most recent derivative to emerge from this effort is fluticasone furoate 2, which as Veramyst/Avamys offers highly effective once-daily treatment for both nasal and ocular symptoms of seasonal and perennial allergic rhinitis (3, 4). The glucocorticoid receptor (GR) has proved to be very difficult to crystallize, but we have reported x-ray crystal structures of the glucocorticoid ligand-binding domain (LBD) containing the corticosteroid agonists dexamethasone, fluticasone propionate, and fluticasone furoate (5–7). These x-ray structures have provided a detailed picture of the interactions of steroidal ligands with this important nuclear receptor.
Scheme 1.
As with other steroid receptors there has also been increasing interest in recent years in the identification of novel nonsteroidal ligands for the GR (8–12). This effort has coincided with a more detailed understanding of the mechanism of glucocorticoids (13–15). These studies have raised the prospect of even safer glucocorticoids that target the transrepression (TR) activities associated with the desired anti-inflammatory activity over the transactivation (TA) activities more commonly associated with unwanted side effects of glucocorticoids. It is hoped that the structural diversity offered by nonsteroidal ligands may help deliver such “dissociated” glucocorticoids, and this has been borne out with the observation of improved side-effect profiles in preclinical models with nonsteroidal GR ligands, such as ZK 216348 3 (16) and BI-104 4 (17).
Recently we reported a series of nonsteroidal GR agonists, incorporating an aminopyrazole moiety (e.g., 5), displaying excellent selectivity for GR over other steroid hormone receptors (18, 19). Constraining the aminopyrazole amide afforded aminoindazole derivatives (e.g., 6) showing a further increase in GR agonist potency (20). Attempts to accommodate these pyrazole and indazole derivatives into the ligand-binding pocket as defined by the earlier steroidal GR crystal structures were unsuccessful. However, these derivatives share structural similarities to the steroidal glucocorticoid agonist cortivazol 7 and were found to be well accommodated in a GR binding-site model that had been developed for this extended corticosteroid (18) and that utilized side chain movements first postulated by Yoshikawa et al. (21).
This model required 2 residues (Arg-611 and Gln-570) to move substantially to accommodate the arylpyrazole moiety, and we noticed that this movement creates a new channel in the receptor extending from the meta- position of the phenyl ring. This expansion of the LBD and formation of the “meta” channel was supported by an in-house low-resolution GR crystal structure for a cortivazol analogue and has recently been confirmed by Suino-Powell et al. with a well-resolved crystal structure for deacetylcortivazol 8 (22). The predicted binding mode for the arylpyrazole ligands has recently been confirmed with a nonsteroidal GR crystal structure for compound 5 in which the same new channel is again observed (23). This channel presented an opportunity for the design of novel glucocorticoid ligands, and we can now report the discovery of highly potent nonsteroidal GR ligands on the indazole template occupying this previously unexplored region of the receptor. This design work was accomplished with the aid of the computational growth procedure, AlleGrow molecular modeling system (Boston De Novo).
Results
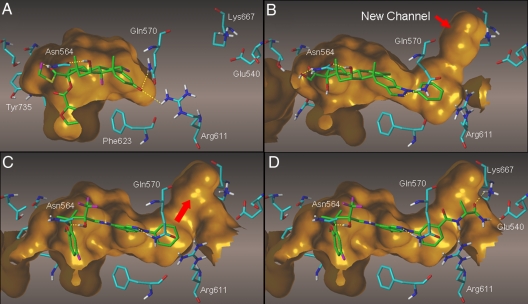
A comparison of the recent crystal structures (7, 22) of fluticasone furoate 2 and deacetylcortivazol 8 in the GR LBD (Fig. 1 A and B) illustrates how the binding pocket opens up to accommodate the extended cortivazol structure. Thus fluticasone furoate is seen to be completely enclosed by the receptor, with the partially hidden furoate ester neatly occupying the lipophilic 17α pocket on the left-hand side and the hydrogen bonds (Arg-611 and Gln-570) to the A-ring keto group defining the extremity of the binding pocket on the right-hand side (Fig. 1A). In contrast, the LBD is forced open in this direction by the phenyl pyrazole extension of the deacetylcortivazol structure, and a new channel is created adjacent to the N-phenyl group (Fig. 1B). We used the cortivazol model reported previously (18) to dock the arylindazole 6. Compound 6 shows good GR binding in vitro, displays potent and full GR agonist activity in in vitro functional assays of TR and TA, and shows good selectivity over the unwanted progesterone receptor (PR) agonist activity often seen with corticosteroids (Table 1). The cortivazol model shows the same channel observed in the deacetylcortivazol crystal structure (22), and it was found that the channel was still present after flexible docking of the arylindazole 6 using FLO+ computational methodology (Thistlesoft) (Fig. 1C). This methodology imposed hydrogen bonding restraints to guide the central alcohol toward Asn-564 and the pyrazole N-2 toward Gln-570 but allowed flexibility of all side chains lining the binding pocket, including side chains forming the channel itself. The new channel is seen to expand away from the meta position of the N-phenyl ring of 6 (Fig. 1C), and an exploration of this “meta” channel was undertaken in the search for additional receptor interactions that might deliver increased potency and/or selectivity. A meta-carboxylic acid synthon 9 was chosen to allow rapid exploration of these interactions through reactions with suitable amines to give a series of meta-amide derivatives (Scheme 2). The selection of amines was carried out using a 3-stage process involving AlleGrow computational growth of groups into the channel, followed by FLO+ scoring to eliminate those showing least complementarity to the site, and finally close visual examination of the virtual products within the channel to select those showing the most promising interactions. The meta-amide growth start point used for AlleGrow placed the amide manually in the 2 possible planar orientations relative to the phenyl ring. AlleGrow uses growth through incremental addition of atomic or small molecular fragments, with exploration of conformational space after each addition and finally evaluation of complementarity with the surrounding protein residues. For this work AlleGrow was run multiple times, setting at each occasion a different minimum number for the atom growth from 4 to 10 atoms and requesting 1,000 solutions for each run. In total AlleGrow proposed more than 7,000 compounds displaying a variety of interactions with the channel: hydrophilic, hydrophobic, and mixed. In particular, residues Lys-667, Glu-540, Arg-611, Tyr-663, and Gln-570 were used for hydrogen bonding interactions, whereas Leu-603 and Ala-573 were used for hydrophobic interactions.
Fig. 1.
(A) Fluticasone furoate–GR LBD crystal structure; (B) deacetylcortivazol–GR LBD crystal structure; (C) indazole 6–GR model showing access to “meta” channel; (D) AlleGrow alaninamide 10, showing growth into “meta” channel.
Table 1.
In vitro GR and PR activities for indazoles 6 and 10–12, compared with fluticasone furoate (2)
| Compound | GR binding pIC50* | GR NF-κB pIC50(% max)† | GR MMTV pEC50(% max)† | PR pEC50 |
|---|---|---|---|---|
| 2 | 8.2 ± 0.4 (n = 12) | 10.5 ± 0.3 (n = 57)‡ | 9.9 ± 0.3 (n = 11) | 9.1 ± 0.2 (n = 12)‡ |
| (108% ± 5%) | (128% ± 12%) | |||
| 6 | 7.7 ± 0.2 (n = 3) | 9.7 ± 0.3 (n = 7) | 9.1 ± 0.4 (n = 6) | 6.8 ± 0.8 (n = 11) |
| (104% ± 3%) | (97% ± 12%) | |||
| 10 | 8.2 ± 0.4 (n = 3) | 10.7 ± 0.2 (n = 11) | 9.9 ± 0.2 (n = 14) | 8.9 ± 0.3 (n = 4) |
| (106% ± 5%) | (130% ± 11%) | |||
| 11 | 8.2 ± 0.1 (n = 4) | 10.3 ± 0.4 (n = 18) | 9.6 ± 0.4 (n = 21) | 7.7 ± 0.4 (n = 10) |
| (107% ± 6%) | (132% ± 15%) | |||
| 12 | Not tested | 8.2 ± 0.1 (n = 5) | 7.7 ± 0.3 (n = 7) | <5 |
| (79% ± 5%) | (32% ± 6%) |
Scheme 2.
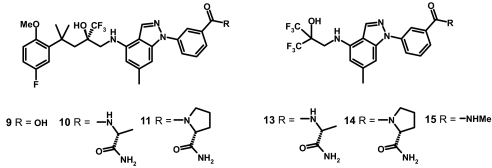
A small series of mainly polar amides designed using this process was prepared, with many showing potent GR agonist activity (24). One compound in particular, the D-alaninamide 10 was found to be 10-fold more potent than the unsubstituted parent 6 in the NF-κB TR assay. In common with the parent 6 and conventional corticosteroids, compound 10 displays full efficacy (≈106% response relative to the standard dexamethasone, 100%) in this TR assay and in a mouse mammary tumor virus long terminal repeat (MMTV) reporter assay of TA (≈130% response) (Table 1). This remarkable potency makes the nonsteroidal agonist 10 comparable to the most potent corticosteroids, such as fluticasone furoate 2. It was noted that AlleGrow predicted favorable interactions between the alaninamide moiety and 2 residues (Lys-667 and Glu-540) in the “meta” channel (Fig. 1D). Interestingly, introduction of the alaninamide substituent also enhanced PR agonist activity, with compound 10 showing inferior GR/PR selectivity to the unsubstituted parent 6 (Table 1). This enhancement of PR activity on introduction of the meta-amide substituent is a noteworthy observation and indicates that a similar “meta” channel must also open up in the PR.
It was discovered that PR agonist activity could be reduced by substitution of the secondary amide NH to give tertiary amide derivatives. Thus, constraining the D-alaninamide in a 5-membered ring gave the D-prolinamide 11, which showed only slightly lower GR potency than 10 but significantly reduced PR activity, resulting in GR/PR selectivity of >100-fold. In addition, compound 11 shows excellent selectivity over other steroid receptors, with no significant activity (pXC50 <5.6) seen in in vitro assays of androgen receptor (AR) and mineralocorticoid receptor (MR) agonism, MR antagonism, and AR and estrogen receptor (ERα and -β) binding. Compound 11 was also shown to display very low oral bioavailability (rat 1.5%, dog 5%), offering the ideal pharmacologic and pharmacokinetic profile for intranasal/inhaled development. Furthermore, whereas the highly insoluble steroidal glucocorticoids need to be delivered as intranasal suspensions, the more flexible nonsteroidal ligand 11, with its polar prolinamide functionality, was shown to possess sufficient aqueous solubility to allow formulation as an intranasal solution (24).
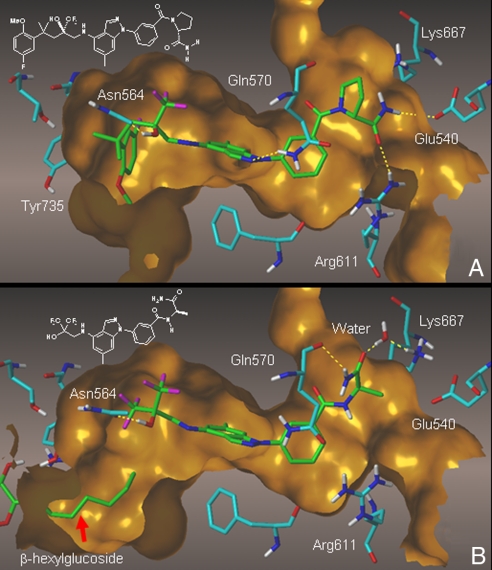
The design cycle was closed with the crystallographic determination of compound 11 in the GR LBD at a resolution of 3.0 Å. As before (7), crystallization was achieved in the presence of a 12-residue TIF-2 coactivator peptide. The x-ray structure (Fig. 2A) demonstrated clearly the expected interactions and the success of the design strategy. Thus, the structure confirmed the ability of the arylindazole to expand the GR binding pocket in the same way as cortivazol and showed good agreement with the binding mode predicted from the modeling studies. Thus, movement of the “charge clamp” residues Gln-570 and Arg-611 provides this increased volume and opens up access to the “meta” channel. Compound 11 is found to extend 7 to 8 Å into the “meta” channel, with Gln-570 forming a hydrogen bond to the indazole nitrogen (as predicted) and Arg-611 now interacting with the prolinamide group. The prolinamide group can also be seen to hydrogen bond to Glu-540, an interaction predicted by AlleGrow for the alaninamide (Fig. 1D). The remainder of the prolinamide group neatly occupies the “meta” channel. Within the classic binding pocket, the typical alcohol interaction with Asn-564 is present, and positioning of the 2-methoxy-5-fluoroaryl group in a favorable edge–face interaction with Tyr-735 is revealed, whereas the indazole 6-methyl group is accommodated in a small pocket at the center rear of this representation.
Fig. 2.
(A) D-prolinamide 11 GR LBD crystal structure; (B) truncated D-alaninamide 13 GR LBD crystal structure, showing at bottom left the tail of β-hexylglucoside pushing into unoccupied portions of the 17α pocket.
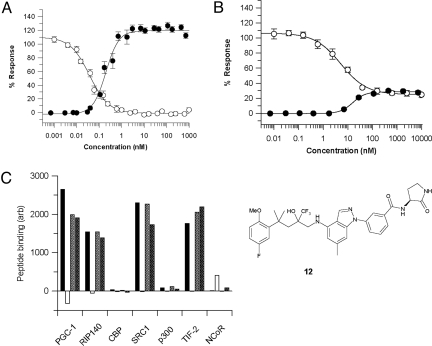
The high-potency meta-amides 10 and 11, like their unsubstituted parent 6, show full efficacy for both TR and TA (Table 1). However, further elaboration of the meta-amide substituent has provided evidence that the intrinsic pharmacology around this template can also be manipulated via the “meta” channel. Thus the (3S)-2-pyrrolidinone amide 12, although less potent than the D-prolinamide 11, retains much of the desired TR activity, inhibiting TNF-induced NF-κB activation by ≈79% (compared with dexamethasone). However, this compound shows a greatly reduced TA activity, inducing only a quarter of the MMTV activity achieved by compound 11 (Fig. 3 A and B and Table 1). This pharmacologic profile is very different from that of standard corticosteroids, and the downstream consequences of such pharmacology have yet to be fully explored.
Fig. 3.
(A) TR (NF-κB, open circles) and TA (MMTV, closed circles) dose responses for compound 11 and (B) for compound 12. (C) Peptide binding profiles for compounds 11 (hatched bars) and 12 (checked bars) compared with fluticasone propionate (black bars) and mifepristone (RU-486) (white bars).
A key component of the activation of the GR is the recruitment of coactivator proteins, such as PGC-1, SRC-1, and TIF-2, which mediate the positive and negative effects on transcription (25). This interaction is driven by the ligand-induced formation of a surface on the GR referred to as AF2 (activation function 2). This surface binds to a short helical LXXLL-containing sequence on the coactivators, which is known as the NR (nuclear receptor) box. These cofactor-derived peptides can be used as tools to probe the “shape” of the AF2 region and define the potential for the protein to interact with a given cofactor. In the presence of standard steroidal ligands, such as fluticasone propionate, the LBD of the GR binds to NR box peptides derived from known GR coactivator proteins PGC-1, RIP140, SRC-1, and TIF-2 but shows no interaction with NR box peptides derived from CBP or p300 and no interaction with a related sequence from the NR corepressor NCoR (Fig. 3C). In contrast, when mifepristone (RU-486) is bound to the GR LBD, no interaction with any of the coactivator peptides is detected, whereas some binding of the NCoR peptide is observed. Mifepristone contains an extension from the 11-position of the steroid core, which disrupts the formation of the AF2 surface, favoring interaction with peptides from corepressors rather than coactivators, and mifepristone generally acts as an antagonist. Interestingly, despite displaying very different pharmacologic profiles, both compound 11 and compound 12 induce an NR box peptide recruitment similar to that of the classic steroid fluticasone propionate (Fig. 3C). This suggests that cofactor interactions are not affected by the “meta” channel extensions and that a mechanism other than disruption of the AF2 surface must be driving the reduced efficacy observed with compound 12.
Having gained additional interactions with the receptor through this unique channel, we hypothesized that this might reduce the necessity for some of the interactions in the traditional binding pocket and provide simpler ligands more compatible with oral delivery. To test this hypothesis we explored a series of truncated compounds retaining the favored D-alaninamide substituent. One such compound, the bis-trifluoromethyl analogue 13, was indeed found to retain potent GR agonist activity (NF-κB pIC50 8.9) and good GR/PR selectivity (Table 2). Although very much less potent than the larger D-alaninamide 11, the truncated analogue 13 still displays in vitro potency superior to the gold standard oral corticosteroid prednisolone and retains full TR and TA activities (Table 2). This result demonstrates the potential of “meta” channel occupation to deliver novel GR agonists in which the traditional ligand pocket is now only partially occupied. Interestingly, the corresponding D-prolinamide analogue 14 retains GR agonist activity (NF-κB pIC50 7.4), but there is a bigger drop-off in potency than between 10 and 11. Encouragingly, this more Lipinski-compliant (26) truncated analogue 14 (Mr 517, cLogP 3.15) now shows measurable oral bioavailability of 10.5% in the rat, confirming the potential of this strategy to deliver novel oral nonsteroidal glucocorticoids. As in the full-length series, preliminary evidence for modulation of the pharmacologic profile by manipulation within the “meta” channel is also observed in the truncates. Thus compound 14 shows evidence for a partial agonist response, with reduced efficacy for both TR (≈89%) and TA (≈73%), whereas the simple meta-acetamide derivative 15 shows no agonist activity in either assay. Compound 15 clearly demonstrated the ability to displace labeled dexamethasone in an in vitro GR binding assay, and indeed was shown to antagonize the ability of dexamethasone to transactivate the MMTV reporter gene (pIC50 5.8 ± 0.1; maximum response 102% relative to RU-486).
Table 2.
In vitro GR and PR activities for truncated indazoles 13–15, compared with prednisolone
| Compound | GR binding pIC50* | GR NF-κB pIC50 (% max)† | GR MMTV pIC50 (% max)† | PR pEC50 |
|---|---|---|---|---|
| 13 | 7.9 ± 0.1 (n = 3) | 8.9 ± 0.2 (n = 8) | 7.3 ± 0.1 (n = 7) | 6.5 ± 0.2 (n = 6) |
| (101% ± 8%) | (99% ± 11%) | |||
| 14 | 7.4 ± 0.3 (n = 3) | 7.4 ± 0.2 (n = 6) | 6.3 ± 0.2 (n = 4) | <5 (n = 2) |
| (89% ± 8%) | (73% ± 6%) | |||
| 15 | 6.9 (n = 1) | <5.2 (n = 2) | <5.2 (n = 2) | <5 (n = 2) |
| Prednisolone | 7.7 ± 0.1 (n = 4) | 8.0 ± 0.1 (n = 5) | 7.3 ± 0.4 (n = 5) | <5 (n = 7) |
| (101% ± 6%) | (92% ± 10%) |
*Assay format B: pIC50 values ≈0.5 lower than for assay format A.
†Maximum response relative to dexamethasone (100%).
Crystals of the truncated alaninamide 13 GR LBD/TIF-2 complex were obtained that provided a 2.5 Å resolution crystal structure (Fig. 2B), which again confirmed the expected occupation of the “meta” channel. The alaninamide amide group makes alternative H-bonding interactions to those seen for the prolinamide 11. Thus, hydrogen bonds are now seen to be made to Lys-667 via a water bridge and also to the backbone carbonyl of Gln-570. The indazole nitrogen is once again seen to be within H-bonding distance to Gln-570, and the usual hydrogen bond from the alcohol to Asn-564 is again evident. As expected, the truncated structure does not reach the region of the receptor occupied by the D-ring and 17α ester of fluticasone furoate 2 and the 2-methoxy-5-fluorophenyl group of compound 11. Interestingly, a molecule of the crystallographic adduct β-hexylglucoside is incorporated in the crystal structure. This can be seen to sit with the carbohydrate portion on the outside of the receptor and the hexane chain pushing into the unoccupied 17α pocket (Fig. 2B).
Discussion
Computational modeling of the GR revealed the existence of a previously unexplored channel extending away from the typical steroidal A-ring region of the GR ligand-binding pocket, and this channel has been exploited for drug design on a nonsteroidal indazole template with the aid of an automated virtual design tool, AlleGrow. A series of amides were designed and prepared to rapidly explore the occupation of this channel, and the D-alaninamide 10 was found to show remarkable GR agonist potency comparable to that seen with the most potent corticosteroids. This result clearly confirms the potential of the “meta” channel to deliver enhanced GR potency. However, compound 10 also shows enhanced PR agonist activity, but this could be tempered, for example, by constraining the D-alaninamide to give the corresponding D-prolinamide 11. Compound 11 displays a very attractive potency, selectivity, and pharmacokinetic profile for topical delivery. Furthermore, unlike potent steroidal GR agonists, compound 11 displays sufficient aqueous solubility to allow delivery as an intranasal solution, which may offer enhanced efficacy in rhinitis at a reduced dose and more rapid onset of action. X-ray crystal structure determination of compound 11 in the GR LBD confirmed the existence and occupation of the “meta” channel and thereby validated the modeling and design methodology used.
The enhanced PR agonist activity seen with the D-alaninamide 10 compared with the unsubstituted parent 6 clearly suggests the presence of a similar channel in the PR. This is an important observation that could provide new opportunities in the design of modulators of the PR.
A major challenge of current nonsteroidal glucocorticoid research is the discovery of novel oral agents for the treatment of systemic inflammatory diseases, such as rheumatoid arthritis. Although the high molecular weight and high lipophilicity of compounds such as 11 (Mr 665, clogP 5.7) are acceptable for topical indications, these are clearly not suitable for oral delivery. However, occupation of the “meta” channel offers the prospect of simpler GR ligands by removing the reliance on interactions in the traditional steroid-binding pocket, as illustrated by the potent GR agonist activity seen with the truncated D-alaninamide derivative 13. This compound was designed to leave unoccupied a large proportion of the typical binding pocket in favor of “meta” channel occupation. Crystal structure determination of the receptor–ligand complex for compound 13 confirmed this binding mode and demonstrated clearly that full occupation of the typical steroidal binding pocket is not essential for potent agonist activity. This result offers the prospect of simplified GR ligands with physicochemical properties more compatible with oral administration, and an early indicator of this opportunity is provided by the modest oral bioavailability seen in the rat with the truncated D-prolinamide 14. Whereas the potent analogues 10, 11, and 13 show full TR and TA activities, data with the less-potent (3S)-2-pyrrolidinone amide 12 clearly demonstrate the potential for further elaboration of the meta-substituent to deliver novel glucocorticoid pharmacology, and this area is continuing to be investigated. Finally, the chance incorporation of β-hexylglucoside in the crystal structure of the compound 13 has revealed yet another channel away from the steroid ligand-binding pocket, this time via the 17α pocket, which could offer further possibilities for novel GR ligand design.
In summary, x-ray crystal structures and molecular modeling have been used to design highly potent nonsteroidal glucocorticoids. Occupation of the “meta” channel has been confirmed through crystallographic determination of GR LBD complexes with the amide derivatives 11 and 13. The highly potent and selective D-prolinamide analogue 11 displays properties compatible with development as an intranasal solution formulation, whereas the truncated D-alaninamide derivative 13 demonstrates activation of the receptor with only partial occupation of the steroid-binding pocket. Finally, data with the pyrrolidinone amide 12 show the potential to separate TR and TA activities via further exploration within the “meta” channel. The discovery of these additional interactions with the GR offers significant opportunities for the design of novel GR modulators, including orally bioavailable analogues.
Materials and Methods
Synthesis.
The meta-amide derivatives 6 and 10-12 were prepared from 2-{2-[5-fluoro-2-(methyloxy)phenyl]-2-methylpropyl}-2-(trifluoromethyl)oxirane, whereas the truncated analogues 13-15 were derived from 2,2-bis(trifluoromethyl)oxirane (Scheme S1). For details, see SI Materials and Methods.
Crystallographic Methods.
The GR LBD protein was the same F602Y and C638G construct used for fluticasone furoate and was purified and complexed with a 12-residue TIF-2 coactivator peptide using the published protocol (7). Compound 11 was crystallized in 28% PEG 5,000 monomethyl ether and 0.1 M Mes 6.5, and the hexagonal crystals (longest edge, 20 μm) formed over 1 month. The initial crystals of GR LBD complexed with compound 13 were obtained from 0.1 M Mes 6.5 and 1.6 M magnesium sulfate and were optimized after a second preparation of protein to 2.0 M magnesium sulfate with 0.1 M Mes 6.5 and the additive β-hexylglucoside, and the crystals (longest edge, 400 μm) formed overnight. For details, see SI Materials and Methods. The crystal structures have been deposited in the Research Collaboratory for Structural Bioinformatics (RCSB) database as 3K23 for the D-prolinamide 11 and 3K22 for the D-alaninamide 13.
GR Binding Assay.
The ability of compounds to bind to the GR was determined by assessing their ability to compete with either an Alexa 555 fluorescently labeled (assay format A) or a Cy3b fluorescently labeled (assay format B) dexamethasone. For details, see SI Materials and Methods.
GR-Mediated Transrepression of NF-κB Activity.
Human A549 lung epithelial cells were engineered to contain a secreted placental alkaline phosphatase gene under the control of the distal region of the NF-κB–dependent endothelial–leukocyte adhesion molecule (ELAM) promoter, as previously described by Ray et al. (27). Incubation with test compounds for 16 h was followed by measurement of alkaline phosphatase activity. Dose–response curves were constructed, from which pIC50 values were estimated and from which maximal responses are calculated relative to dexamethasone (100%). For details, see SI Materials and Methods.
Glucocorticoid-Mediated Gene Transactivation: MMTV Assay.
Human A549 lung epithelial cells were engineered to contain a Renilla luciferase reporter gene construct under the control of MMTV. Incubation with test compounds for 6 h was followed by measurement of luciferase activity. Dose–response curves were constructed, from which pEC50 values were estimated and from which maximal responses were calculated relative to dexamethasone (100%). For details, see SI Materials and Methods.
Assay for PR Agonist Activity.
A T225 flask of CV-1 cells at a density of 80% confluency was washed briefly with PBS, detached from the flask using 0.25% trypsin, and counted using a Sysmex KX-21N. Cells were diluted in DMEM containing 5% HyClone and 2 mM L-glutamate and transduced with 100 multiplicity of infection (MOI) of PRb-BacMam and 100 MOI of MMTV-BacMam. Cells were either plated in a flask for 24 h and then frozen or processed immediately. In either case, ≈6 × 103 cells were dispensed to each well of white Nunc 384-well plates, containing compounds at the required concentration. After 24 h, 10 μL of Steady-Glo was added to each well of the plates. Plates were incubated in the dark for 10 min before being read on a Viewlux reader. Dose–response curves were constructed, from which pEC50 values were estimated.
Details of glucocorticoid antagonism and androgen, mineralocorticoid, and estrogen assays, as well as the peptide binding assay, are provided in SI Materials and Methods.
Pharmacokinetic Studies.
Pharmacokinetic studies were conducted in GlaxoSmithKline laboratories in the United Kingdom under licence restrictions imposed by the United Kingdom Home Office. Rat studies were performed using adult male Sprague-Dawley rats, and studies in the dog used adult male beagles. For details, see SI Materials and Methods.
Computational Methods.
All molecular modeling images were prepared using SYBYL, version 7.3 (Tripos). Two computational methods, AlleGrow and FLO+, were used to assist the design process. For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank the following for their valuable contributions to this work: David Brown, Matilde Caivano, Margaret Clackers, Anette Miles-Williams, and Rosemary Sasse (screening and compound profiling); Bhavesh Patel, Heather Barnett, Karl Collins, and Natalie Wellaway (chemistry); James Gray, David Lugo, and Cesar Ramirez-Molina (drug metabolism); Eugene Stewart (computational chemistry); and Eric Hortense and Steve Jackson (analytical chemistry).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3K22 and 3K23).
This article contains supporting information online at www.pnas.org/cgi/content/full/0909125106/DCSupplemental.
References
- 1.Hench PS, Kendall EC, Slocomb CH, Polley HF. The effect of a hormone of the adrenal cortex, cortisone (17-hydroxy-11-dehydrocorticosterone: compound E), and of pituitary adrenocorticotropic hormone on rheumatoid arthritis and acute rheumatic fever. Trans Assoc Am Physicians. 1949;62:64–68. [Google Scholar]
- 2.Avery MA, Woolfrey JR. Anti-inflammatory steroids. In: Abraham DJ, editor. Burger's Medicinal Chemistry and Drug Discovery. Hoboken, NJ: John Wiley; 2003. pp. 747–853. [Google Scholar]
- 3.Salter M, et al. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L660–L667. doi: 10.1152/ajplung.00108.2007. [DOI] [PubMed] [Google Scholar]
- 4.Sorbera LA, Serradell N, Bolos J. Fluticasone furoate. Drugs Future. 2007;32:12–16. [Google Scholar]
- 5.Bledsoe RK, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110:93–105. doi: 10.1016/s0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 6.Apolito CJ, et al., inventors. WO 03/015692. Patent. 2003
- 7.Biggadike K, et al. X-ray crystal structure of the novel enhanced-affinity glucocorticoid agonist fluticasone furoate in the glucocorticoid receptor-ligand binding domain. J Med Chem. 2008;51:3349–3352. doi: 10.1021/jm800279t. [DOI] [PubMed] [Google Scholar]
- 8.Schäcke H, Berger M, Hansson TG, McKerrecher D, Rehwinkel H. Dissociated non-steroidal glucocorticoid receptor modulators: An update on new compounds. Expert Opin Ther Patents. 2008;18:339–352. [Google Scholar]
- 9.Hudson AR, Roach SL, Higuchi RI. Recent developments in the discovery of selective glucocorticoid receptor modulators (SGRMs) Curr Topics Med Chem. 2008;8:750–765. doi: 10.2174/156802608784535048. [DOI] [PubMed] [Google Scholar]
- 10.Mohler ML, He Y, Wu Z, Hong SS, Miller DD. Dissociated non-steroidal glucocorticoids: Tuning out untoward effects. Expert Opin Ther Patents. 2008;17:37–58. doi: 10.1517/13543776.17.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Miner JN, et al. Antiinflammatory glucocorticoid receptor ligand with reduced side effects exhibits an altered protein-protein interaction profile. Proc Natl Acad Sci USA. 2007;104:19244–19249. doi: 10.1073/pnas.0705517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bosscher K, et al. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA. 2005;102:15827–15832. doi: 10.1073/pnas.0505554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cato ACB, Schäcke H, Sterry W, Asadullah K. The glucocorticoid receptor as target for classic and novel anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy. 2004;3:347–353. doi: 10.2174/1568010042634479. [DOI] [PubMed] [Google Scholar]
- 14.Schäcke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 15.Newton R, Holden NS. Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 16.Schäcke H, et al. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA. 2004;101:227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Razavi H, Thomson D. Recent progress in the discovery of novel glucocorticoid receptor modulators. Curr Topics Med Chem. 2008;8:521–530. doi: 10.2174/156802608783955737. [DOI] [PubMed] [Google Scholar]
- 18.Clackers M, et al. Non-steroidal glucocorticoid agonists: The discovery of aryl pyrazoles as A-ring mimetics. Bioorg Med Chem Lett. 2007;17:4737–4745. doi: 10.1016/j.bmcl.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 19.Barnett HA, et al. Aryl aminopyrazole benzamides as oral non-steroidal selective glucocorticoid receptor agonists. Bioorg Med Chem Lett. 2008;19:158–162. doi: 10.1016/j.bmcl.2008.10.128. [DOI] [PubMed] [Google Scholar]
- 20.Eldred CD, House D, Inglis GGA, Macdonald SJF, Skone PA, inventors. WO 06/108699. Patent. 2006
- 21.Yoshikawa N, et al. The distinct agonistic properties of the phenylpyrazolosteroid cortivazol reveal interdomain communication within the glucocorticoid receptor. Mol Endocrinol. 2005;19:1110–1124. doi: 10.1210/me.2004-0264. [DOI] [PubMed] [Google Scholar]
- 22.Suino-Powell K, et al. Doubling the size of the glucocorticoid receptor ligand binding pocket by deacylcortivazol. Mol Cell Biol. 2008;28:1915–1923. doi: 10.1128/MCB.01541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madauss KP, et al. The first x-ray crystal structure of the glucocorticoid receptor bound to a non-steroidal agonist. Bioorg Med Chem Lett. 2008;18:6097–6099. doi: 10.1016/j.bmcl.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Biggadike K, Cooper AWJ, House D, McLay IM, Woolam GR, inventors. WO 07/122165. Patent. 2007
- 25.Lemon BD, Freedman LP. Nuclear receptor cofactors as chromatin remodelers. Current Opin Genet Dev. 1999;9:499–504. doi: 10.1016/s0959-437x(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 26.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 27.Ray KP, Farrow S, Daly M, Talabot F, Searle N. Induction of the E-selectin promoter by interleukin 1 and TNFα and inhibition by glucocorticoids. Biochem J. 1997;28:707–715. doi: 10.1042/bj3280707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.