Abstract
Stroke survivors often exhibit abnormal motoneuron excitability, manifested clinically as spasticity with exaggerated stretch reflexes in resting muscles. We examined whether this abnormal excitability is a result of increased activation of intrinsic voltage-dependent persistent inward currents (PICs) or whether it is a result of enhanced synaptic inputs to the motoneuron. This distinction was made by recording firing rate profiles of pairs of motor units during isometric contractions of elbow flexor muscles. To estimate PIC amplitude, the discharge of the lower-threshold (reporter) motor unit of the pair was used to estimate the synaptic input to the higher-threshold (test) motor unit. The estimated synaptic input required to recruit the test unit was compared with the synaptic input when the test unit was derecruited (ΔF) and this served as an estimate of the intrinsic (PIC) contribution to motoneuron firing. We found that PIC estimates were not larger in spastic-paretic motoneurons (ΔF = 4.0 ± 1.6 pps) compared with contralateral (4.6 ± 1.4 pps) and age-matched healthy control motoneurons (3.8 ± 1.7, all P > 0.1). Instead, following the voluntary contractions, the majority of lower-threshold motor units in spastic-paretic muscles (83%) exhibited spontaneous discharge, compared with 14% of contralateral and 0% of control motor units. Furthermore, there was strong co-modulation of simultaneously active units in spastic muscle. The presence of ongoing, correlated unit activity at “rest,” coupled with firing behavior at recruitment unique to lower-threshold motor units in spastic muscles, suggested that firing changes are likely a result of a low-level depolarizing synaptic drive to the resting motoneuron pool.
INTRODUCTION
Individuals who have sustained a stroke often exhibit spasticity and muscular weakness that limit daily function. Clinically, spasticity in stroke is characterized by increased stretch reflex responses that can be recorded with the muscles at rest (Lance 1980), suggesting enhanced excitability of the motoneuron pool (Chung et al. 2008). In addition, stroke survivors often exhibit an impaired ability to relax the muscle, especially following simple imposed movements such as extending the joint (Lewek et al. 2007). Their muscles often exhibit prolonged (spontaneous) firing of motor units following either voluntary or reflex muscle activation, suggesting that there is impaired control of motor unit firing at rest (Lewek et al. 2007; Lukacs 2005; Mottram et al. 2007).
One possible explanation for this abnormal motoneuron firing is that there may be a tonic increase in excitatory synaptic input to the motoneurons, rendering them more depolarized and thus resting closer to firing threshold. An increase in synaptic input, which may arise from either descending or segmental pathways (Burke and Ashby 1972; Burke et al. 1972; Hultborn 2003; Katz and Rymer 1989; Mazzaro et al. 2007), is consistent with the observed lower threshold for stretch reflexes (Chardon et al. 2008; Powers et al. 1988) that occur following a stroke.
Alternatively, it is also possible that the motoneurons themselves become intrinsically more excitable in stroke and this increase in excitability could appear as an enhancement of stretch and tonic vibration reflexes (TVRs). The mechanism of this increase could be via increasing persistent calcium currents, which are known to enhance long-lasting reflexes (Lee and Heckman 1996). The amplitude of these persistent inward currents (PICs) is also known to be proportional to the level of monoaminergic input from the brain stem (Heckman et al. 2005). To explain these changes, we suggest that following a stroke, inhibitory input from the corticobulbar pathways to the reticular formation may be interrupted, so that excitatory projections from the pontine reticular formation and brain stem to the spinal cord are disinhibited (Kline et al. 2007). This may enhance monoaminergic drive from the brain stem and thus PICs in stroke survivors. Thus it remains unclear whether changes in synaptic inputs or changes in intrinsic properties of the motoneurons contribute to enhanced reflex excitability after stroke. Our purpose in this study was to distinguish these two possibilities.
As mentioned earlier, there may be changes in the intrinsic excitability of motoneurons in stroke, including increases in the magnitude of the PICs in the motoneurons of these patients. The PIC is a depolarizing current, generated by voltage-sensitive Na+ and Ca2+ channels (termed Na and Ca PICs), that persists for many seconds after activation and thus promote self-sustained discharge of the motoneuron (Hounsgaard and Kiehn 1989; Lee and Heckman 1998a,b; Li and Bennett 2003; Schwindt and Crill 1982). Persistent inward currents—especially the Ca PIC—could be activated below action potential (AP) threshold and would thereby enhance motoneuron recruitment for a given input (Li et al. 2004) and, following this, promote self-sustained discharge of the motoneuron (Bennett et al. 1998a; Hounsgaard and Kiehn 1989; Lee and Heckman 1998a,b). In addition, during the initial activation of the PIC, the depolarizing effect of synaptic inputs is amplified to produce a steep rise in discharge rate of the motoneuron at recruitment, as shown by a high gain of the frequency–current (F–I) relation in this region (Bennett et al. 1998a; Lee et al. 2003). Thus PICs can exert a strong influence on motoneuron recruitment and excitability.
Accordingly, the first objective of our study was to examine the hypothesis that increased PICs contribute to the abnormal motoneuron excitability observed in spastic-paretic stroke survivors. To this end, we used an established technique (Bennett et al. 2001a,b) to estimate the amplitude of the PICs by examining the firing profiles of pairs of motor units in the spastic-paretic biceps brachii of stroke survivors during isometric voluntary ramp contractions of elbow flexor muscles and comparing this to the firing profiles in the subject's contralateral biceps and in biceps muscles of age-matched healthy control subjects. In the paired motor unit analysis technique, the firing frequency of a lower-threshold motor unit of the pair (reporter unit) is used to estimate the synaptic drive to the motoneuron pool, including the drive to a second, higher-threshold (test) motor unit of the pair. The degree to which a motoneuron PIC helps to sustain the discharge of the motor unit (in this case the test unit) is determined from the reduction in reporter unit firing at the derecruitment of the test unit compared with recruitment of the test unit (ΔF). This ΔF value corresponds to the reduction in synaptic drive needed to counteract the intrinsic PIC and thus is used as an indirect measure of this current (for assumptions used with this technique, see Gorassini et al. 2004; Powers et al. 2008).
The second objective was to examine the alternate hypothesis that low-level depolarizing synaptic drive to the resting motoneuron pool is enhanced in spastic-paretic muscle. Here, we used the finding of high levels of spontaneous motoneuron discharge in spastic-paretic muscle to explore the origins of this abnormal motoneuron excitability. In particular, we examined whether the firing rates of simultaneously active motor units were co-modulated, which would serve as evidence for the presence of a continued, common synaptic input (De Luca et al. 1982).
Finally, we also explored whether there were systematic differences in the recruitment behavior of lower-threshold (reporter) and higher-threshold (test) motor units in spastic muscles. Here, we found that the patterns of firing of lower-threshold units at recruitment in spastic-paretic muscles were systematically different from those of higher-threshold units. These differences support the possibility of a low-level depolarizing synaptic drive to the spastic-paretic motoneuron pool, although other explanations are certainly tenable. Some of these data were previously presented in abstract form (Mottram et al. 2006, 2007).
METHODS
Ten stroke survivors (62.3 ± 4 yr; range, 55–77 yr), with a unilateral brain lesion resulting in spastic hemiparesis of >6-mo duration, and 10 age- and sex-matched healthy subjects (61.9 ± 4 yr; range, 53–73 yr) participated in the study. Demographic and clinical measures for the stroke subjects are detailed in Table 1. Clinical assessments included spasticity measures at the elbow using the Modified Ashworth Scale (0–4) (Gregson et al. 2000) and magnitude of the biceps tendon jerk (0–4+) (Litvan et al. 1996).
Table 1.
Subject characteristics for the 10 stroke survivors who participated in the experiments
| Subject ID | Stroke Type | Sex | Age | Age of Sex-Matched Control Subject | Ashworth | Fugl-Meyer | Chedoke | Lesion Location | Manual Muscle Test Scores (0–5) | Involved Limb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ischemic | M | 68 | 71 | 2 | 27/66 | Stage 3 | Left cerebral cortex | 4 | R |
| 2 | Hemorrhagic | F | 57 | 56 | 1+ | 30/66 | Stage 3 | Encephalomalacia in the lateral aspect of the right basal ganglia | 4+ | L |
| 3 | Hemorrhagic | M | 66 | 60 | 3 | 21/66 | Stage 2 | Not available | 4 | L |
| 4 | Ischemic | M | 77 | 74 | 3 | 24/66 | Stage 3 | Left parietal lobe | 4 | R |
| 5 | Hemorrhagic | F | 58 | 60 | 2 | 7/66 | Stage 2 | Left middle cerebral artery aneurysm | 1 | R |
| 6 | Hemorrhagic | M | 57 | 58 | 3 | 28/66 | Stage 3 | Left middle cerebral artery | 3− | R |
| 7 | Ischemic | F | 54 | 53 | 1+ | 33/66 | Stage 3 | Left carotid artery | 4 | L |
| 8 | Ischemic | F | 66 | 72 | 3 | 28/66 | Stage 3 | Infarction of the left centrum semiovale | 4− | R |
| 9 | Hemorrhagic | F | 55 | 56 | 1+ | 13/66 | Stage 3 | Intracerebral hemorrhage in right parietal lobe | 2− | R |
| 10 | Hemorrhagic | F | 61 | 55 | 2 | 31/66 | Stage 3 | Right parietal lobe | 2 | L |
Upper arm impairment was assessed using the Fugl-Meyer test and the Chedoke–McMaster assessment. The lower boundary for spasticity was an Ashworth score of 1+ and a tendon jerk score of ≥3+. Subjects were excluded if they were unable to maintain the testing position, perform ramp isometric contractions with the elbow flexor muscles, or remain alert during testing. All subjects were withdrawn from antispasticity medications for ≥2 wk prior to testing. All procedures were performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at Northwestern University. Prior to participation in the study, all subjects gave written informed consent.
Experimental arrangement
Subjects were seated comfortably in a chair with their forearm, wrist, and fingers secured in a cast. The arm was abducted 30–40° from the sagittal plane and the elbow flexed to 90°. The casted forearm was fixed to a ring–mount interface attached to a six degrees-of-freedom load cell (FT-4227; ATI, Woodland Hills, CA). The load cell apparatus was connected to a plastic elbow rest mounted on a steel table. Each subject's shoulder and waist were secured tightly to the chair to minimize accessory trunk and shoulder movements.
Forces about the elbow joint were recorded on-line using a Power 1401 A/D converter and Spike2 software (version 5.12; Cambridge Electronics Design [CED], Cambridge, UK) and the elbow force (resultant of Fx and Fz) was displayed on a computer monitor.
Motor unit action potentials in the biceps brachii were recorded with Teflon-coated double-stranded wires (bifilar 50-μm diameter; California Fine Wire, Grover Beach, CA). The double-stranded wire was inserted into a 27-gauge hypodermic needle and the tip was bent back to form a barb. The recording ends of the electrode were cut, so the pair served as a differential recording electrode. The electrodes were connected to preamplifiers located at the muscle that connected to an eight-channel amplifier system (Delsys, Boston, MA). Single motor unit recordings were amplified (×1,000 to ×2,000), band-pass filtered (20–2,000 Hz), displayed on a computer monitor, and digitized for later analyses.
Surface electromyograms (EMGs) of the biceps brachii and triceps brachii were monitored simultaneously with the unitary recordings. Active surface EMG electrodes (Delsys) were placed on the biceps brachii short and long heads and the triceps brachii, in avoidance of the innervation zone to minimize signal cancellation (Merletti et al. 2001). All surface EMG signals were led to the same preamplifiers as the intramuscular EMG recordings (Delsys).
Experimental procedures
Each stroke survivor participated in one to two sessions for the contralateral limb and one to two sessions for the spastic-paretic limb, with testing sessions separated by >1 week. The order of testing was randomized. Control subjects participated in one to two sessions for the matched limb to ensure adequate trials for comparison to their spastic-paretic counterpart.
In all, 110, 118, and 108 pairs of motor units were collected during the voluntary ramp contractions for the spastic-paretic, contralateral, and control limb, respectively. We used published techniques to help us interpret changes in motor unit discharge rate as an index of intrinsic motoneuron excitability (Gorassini et al. 2002a; Kiehn and Eken 1997). During each session, subjects performed the following ordered tasks.
MVC force.
Subjects first performed three isometric maximum voluntary contraction (MVC) trials with both the elbow flexor and extensor muscles. Subjects were asked to gradually increase their voluntary effort to maximal levels, over 3 s, with forces held for 3 s. The greatest force achieved by the subject was defined as the MVC force.
Motor unit isolation.
To isolate two single motor units, subjects were verbally cued to slowly increase their force until two units were visualized in the intramuscular record. We refer to the lower-threshold motor unit as the “reporter unit” and the higher-threshold motor unit as the “test unit” (Gorassini et al. 2002a; Kiehn and Eken 1997; Powers et al. 2008). Single motor unit potentials were monitored on-line and on a digital oscilloscope during data collection. Up to three fine-wire electrodes were inserted in widely separated locations of the biceps brachii muscle to help the experimenter locate suitable pairs of motor units. Once two suitable single motor units were isolated, a target force was set just slightly above the higher-threshold (test) motor unit.
Isometric voluntary ramp contractions.
Next, the subject performed the triangular isometric voluntary ramp contractions by viewing his/her exerted resultant elbow flexion on the computer monitor and increasing the elbow flexion force at a predetermined rate to the target force slightly above the threshold force level of the higher-threshold motor unit. Subjects were instructed to make the rate of increase in force similar to the rate of relaxation. Given the variability in recruitment and derecruitment thresholds at higher rates, force rates that exceeded 3–5% MVC/s were discarded (Desmedt and Godaux 1977; Freund 1983).
The desired target force and desired rate of contraction were controlled by program software written in Matlab (The MathWorks, Natick, MA). All trials were separated by about 30 s to avoid frequency-dependent facilitation of the motor units or a reduction in the level of estimated synaptic input (as measured by the firing rate of the reporter motor unit) required to recruit a unit (Gorassini et al. 2002b). Sessions lasted 2–3 h depending on the ease with which the motor units were isolated.
Final MVC.
A post-task MVC was measured to verify that the results were not contaminated by fatigue.
Data analysis
Force, intramuscular, and surface EMGs were collected on-line and subsequently digitized (A/D converter, 16-bit resolution) and analyzed off-line using the Spike2 (version 5.12) data-analysis system (CED). The surface and intramuscular EMGs were digitized at 2,013 and 18,000 Hz, respectively. The elbow force signals (resultant of Fx and Fz) were digitized at 200 Hz.
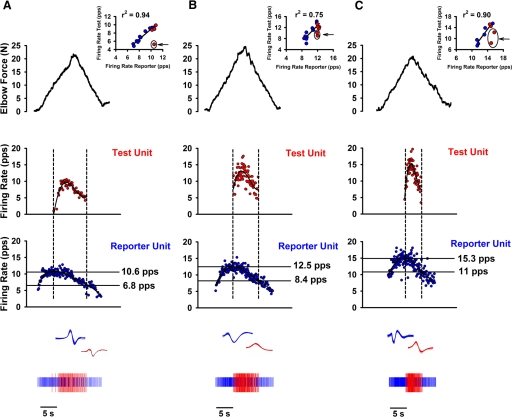
Action potentials discharged by single motor units in biceps brachii were discriminated using a computerized, spike-sorting algorithm (Spike2, version 5.12) (Fig. 1). To ensure discrimination accuracy, the interspike intervals (ISIs) and waveforms of identified motor units were visually examined for every trial.
Fig. 1.
Lowering of estimated synaptic drive at derecruitment compared with recruitment (delta firing rate; ΔF) during volitional contractions was similar in the spastic-paretic (A) and contralateral (B) limb of a stroke survivor and matched limb of a healthy control subject (C). A: 2 biceps brachii motor units recorded during a triangular isometric contraction with the elbow flexor muscles for the spastic-paretic limb of a stroke survivor. Vertical dashed lines indicate times of recruitment and derecruitment of the test unit; horizontal lines indicate the corresponding reporter unit firing rates at these times. Bottom panels denote representative trains of action potentials (APs) for the reporter and test units with corresponding overlayed waveforms; middle panels denote the instantaneous frequency of the reporter (bottom) and test (top) units; top panel denotes force of elbow flexor muscles during the voluntary ramp contractions. B and C: 2 biceps brachii motor units recorded from the contralateral limb of the stroke survivor (B) and from the limb of an age- and sex-matched healthy control subject (C) during the same protocol as that in A. Note the similar lowering of estimated synaptic drive at derecruitment compared with recruitment (ΔF) for the spastic-paretic (A; 3.8, pulses/s [pps]) and contralateral limb of the stroke survivor (B; 4.1 pps), and the limb of an age- and sex-matched control subject (C; 4.3 pps). Insets above figures: reporter and test unit firing profiles plotted against one another in 500-ms bins during the duration of the test unit firing for the ascending (red dots) and descending (blue dots) portion of the ramp contractions. The arrows and circles pointing to or surrounding the dots respectively indicate nonlinear startup frequencies that were not included in the calculation of the coefficient of determination (r2) from the linear regression fit through the rate–rate plots.
For the firing rate analyses, a pair of clearly distinguishable motor unit potentials was selected from each trial. The lower-threshold (reporter) unit fired during recruitment and derecruitment of the test unit (Fig. 1, second panel, test unit; third panel, reporter unit), with the firing rate of the reporter unit used as a monitor of synaptic input to the test motor unit (Gorassini et al. 1998, 2002a). Initial discharge of test and reporter units corresponded to the time of the first discharge in a train of spikes during the ascending ramp of the triangular contraction for which the ISIs were <500 ms (Fuglevand et al. 2006). The initial firing rates of the reporter and test motor units were determined from the average of three spikes (two ISIs). The force at recruitment of the reporter and test motor units corresponded to the resultant elbow flexor force at the time of the first discharge as determined earlier.
A fifth-order polynomial was fit to the reporter unit firing rate profile, with reporter unit firing rates at recruitment and derecruitment of the test unit determined from the intersection of the polynomial with the y-axis (Fig. 1, third panel). The difference in firing rate of the reporter unit when the higher-threshold test unit is recruited and derecruited is called the “delta firing rate” (ΔF) and it is an indirect estimate of the amplitude of the PIC during the voluntary ramp contractions.
The pattern of firing rate modulation between each reporter and test unit pair was also compared to determine whether both units were responding to common synaptic drive (De Luca and Erim 1994). To do this, the mean firing rates of the reporter and test units were calculated by binning the data every 500 ms (so there would be at least three to five frequency points in each bin) and averaging the frequency values in each bin. The mean firing rate of the reporter unit was then plotted against the mean firing rate of the test unit at the same time points (rate–rate plot; insets above Fig. 1, A, B, and C) and a linear regression was fit to the data. In some cases, the first one or two mean rate estimates of the test unit were excluded because the fast acceleration at the onset of recruitment likely represents firing when the PIC is first being recruited (secondary range firing; Bennett et al. 2001a,b; Gorassini et al. 2004; Kiehn and Eken 1997; Li et al. 2004). This was done to assess whether the test and reporter unit firing rates were modulated in a similar manner during the time that both units were firing linearly in response to synaptic input. Thus we wanted to compare the firing rate modulations when both the reporter and test units were firing in their tertiary ranges (after full PIC activation). For each rate–rate plot, the coefficient of determination (r2), slope, and P value of the linear regression were calculated with significance set at P < 0.05. The above-cited procedure was also conducted for dually spontaneously firing motor unit pairs that were observed following the voluntary ramp contractions.
Additional analyses focused on the increase in firing rate following recruitment and the incidence of spontaneous firing of motor units following cessation of the voluntary ramp contractions. The acceleration in firing rate during the first 500 ms following recruitment was analyzed separately for each unit as follows: in each instantaneous frequency plot for the reporter or test unit firing profile, the first through the fifth discharge rates were identified (this corresponded to approximately the first 500 ms following recruitment). The difference between the first discharge rate and the maximum discharge rate within these first five discharge rates was determined. The initial discharge rate, normalized to the subsequent four discharge rates (initial instantaneous frequency value subtracted from the averaged subsequent four instantaneous frequency values), was also determined. For the spontaneous motor unit firing analyses, any motor units that were still discharging 10 s after termination of the voluntary ramp contraction (Zijdewind and Thomas 2001) were considered to be firing spontaneously and were thus recorded.
All force measurements were presented relative to baseline force. Initial and final forces were calculated when the force left and returned to baseline after the rising and falling ramp contractions. The time to peak force and time to final force for each trial were determined for each subject to ensure similar times for the ascending and descending ramps within and across subjects. The rates of increase and decrease in force during the ramp contractions were also determined for each muscle type; trials in which the rate of contraction during the ascending portion of the contraction was not similar to the rate of relaxation during the descending portion of the triangular isometric contraction were removed from further analyses.
Ensuring similarities in task performance across muscle types
Three measures were taken to allow for comparison across muscle types. First, because it has been shown that the PIC can take upwards of 2 s to activate (Bennett et al. 2001a,b; Hounsgaard and Kiehn 1989; Li et al. 2004), trials in which the test unit did not fire for ≥2 s or the reporter unit fired for <1 s before the test unit was recruited were removed from further analyses (10 trials removed for spastic-paretic limbs, 17 trials removed for contralateral limbs, 11 trials removed for control limbs). This was to ensure that the reporter and test units' PICs were fully activated if present (Bennett et al. 2001a,b; Li et al. 2004). The rationale is as follows: it was recently shown that ΔF values may vary widely for reporter–test unit combinations with similar recruitment thresholds (i.e., spaced <0.6 s apart; Powers et al. 2008). Thus it is critical that the reporter and test units are recruited ≥1 s apart to allow for full activation of the PIC in the reporter unit (Bennett et al. 2001a,b; Li et al. 2004) and thus a more stable measurement of synaptic input at the time of test unit recruitment. Similarly, to allow for a stable ΔF measurement, it is critical that the firing of the test unit is stable and past its secondary range firing (i.e., the test unit has been firing for 2 s) to ensure the PIC has been fully activated before the test unit is derecruited (Li et al. 2004).
Next, trials in which the r2 value of the rate–rate plots (see earlier detailed description) was not ≥0.6 (usually due to pronounced secondary range firing in the test unit) were removed from further analyses (31 trials removed for spastic-paretic limbs, 30 trials for contralateral limbs, and 33 trials removed for control limbs). Last, individual trials in which the rates of increase and decrease in force were significantly different from the mean rates of force increase and decrease were also removed (10 trials were removed for spastic-paretic limbs, 2 trials for contralateral limbs, 5 trials for control limbs). This was to ensure similar rates of force increase and decrease across limb types.
Following removal of the above-described trials, there were 59 motor unit pairs for the spastic-paretic limbs, 69 motor unit pairs for the contralateral limbs, and 59 motor unit pairs for the control limbs. On occasion, there were instances in which we identified the same motor unit pair (i.e., the same reporter and test unit) in multiple trials of the voluntary ramp contractions. When this occurred, these duplicate motor unit pairs from the reported numbers were averaged, such that 2 ± 0.3 trials were averaged for each identical motor unit pair for the spastic-paretic limb, 3 ± 0.6 trials for the contralateral limb, and 2 ± 0.9 trials for the control limb. Thus with duplicate pairs averaged, we report on the firing properties of 51, 47, and 44 motor unit pairs for the spastic-paretic, contralateral, and control limb, respectively.
Statistical analysis
Because the subjects were age- and sex-matched, separate paired Student's t-tests (SPSS version 15.0) were used to compare the dependent variables across limb types (Hopkins and Glass 1996). Dependent variables included the difference in firing rate of the lower-threshold (reporter) motor unit at the recruitment and derecruitment of a higher-threshold (test) motor unit (ΔF), increment in firing rate from onset to maximal rate within the first 500 ms, the force at recruitment of the reporter and test units, the rate of increase and decrease in force, and the incidence of spontaneous firing of motor units following the voluntary ramp contractions. The Wilcoxon signed-rank test for zero median (Matlab Version 7.7) was conducted on the initial discharge rate normalized to the subsequent four discharge rates (initial instantaneous frequency value subtracted from the averaged subsequent four instantaneous frequency values) for the reporter and test units in the spastic-paretic muscle. Within-subject comparisons of the pre- and post-task MVCs were examined with separate paired t-tests for each muscle type. Linear regression analyses were used to determine the coefficient of determination (r2) from the linear regression fit through the rate–rate plots of the test and reporter unit firing profiles and of pairs of lower-threshold reporter motor units that discharged spontaneously in parallel. Following Bonferroni corrections for two-way comparisons (spastic-paretic vs. contralateral and spastic-paretic vs. control), the alpha level required for statistical significance across limb types was P ≤ 0.03 (with the exception of the P values from the linear regression fit through the rate–rate plots that were set at P < 0.05). Data are reported as means ± SD within the text and displayed as means ± SE in the figures.
RESULTS
To assess the potential contribution of PICs to the altered motoneuron discharge patterns observed in spastic-paretic stroke survivors, we calculated ΔF values from the discharge rates of pairs of motor units recorded in the spastic-paretic biceps brachii during voluntary ramp contractions (n = 51 pairs). These data were compared with ΔF values calculated in the contralateral muscle (n = 47 pairs) and in neurologically intact (control) subjects (n = 44 pairs).
Figure 1 illustrates how the ΔF values from paired unit recordings were derived. For example, in the spastic-paretic muscle of a stroke survivor (Fig. 1A), a higher-threshold unit (test unit; middle trace) was recruited during the rising phase of the contraction, when the firing rate of the lower-threshold (reporter) motor unit (bottom trace) was 10.6, pulses/s (pps), as marked by the first dashed vertical line. In contrast, when the test unit was derecruited during the relaxation phase of the contraction (at the second dashed vertical line), the firing rate of the reporter motor unit was only 6.8 pps. This difference in reporter unit firing rate at the onset and offset of test unit activation (ΔF = 3.8 pps) corresponds to the reduction in putative synaptic drive needed to counteract the added depolarization from the PIC and is thus an indirect measure of the amplitude of the PIC (Bennett et al. 2001b; Gorassini et al. 2002a; Powers et al. 2008).
Similar ΔF values were observed from the pair of motor units recorded in the contralateral muscle of this subject (Fig. 1B: 4.1 pps) and from motor units in the representative healthy age- and sex-matched control subject (Fig. 1C: 4.3 pps). As summarized in Table 2, ΔF values for the group were similar across muscle types (paired t-test, P ≥ 0.10), indicating that similar reductions in synaptic drive were needed to counteract potential PIC actions during the triangular isometric ramp contractions.
Table 2.
Characteristics from averaged values for each of the 10 age- and sex-matched subjects during the triangular ramp contractions
| Parameter | Spastic-Paretic | Contralateral | Control |
|---|---|---|---|
| Delta firing rate (ΔF), pps | 4.0 ± 1.6 | 4.6 ± 1.4 (0.10) | 3.8 ± 1.7 (0.38) |
| Rate of increase in force (N/s)§ | 2.1 ± 0.8 | 2.2 ± 1.4 (0.16) | 2.3 ± 1.2 (0.09) |
| (% MVC/s) | 3.1 ± 2.7 | 1.1 ± 0.42 (0.01) | 1.4 ± 0.72 (0.04) |
| Rate of decrease in force (N/s)§ | 1.7 ± 0.7 | 2.1 ± 1.4 (0.06) | 2.0 ± 1.3 (0.15) |
| (% MVC/s) | 3.1 ± 2.5 | 1.1 ± 0.5 (0.02) | 1.3 ± 0.7 (0.03) |
| Initial firing rate, pps | |||
| Reporter motor unit | 8.9 ± 3.2 | 8.5 ± 2.1 (0.34) | 7.8 ± 2.0 (0.18) |
| Test motor unit | 8.1 ± 2.0 | 8.0 ± 1.5 (0.45) | 7.9 ± 3.5 (0.43) |
| Recruitment threshold, N | |||
| Reporter motor unit | 2.3 ± 0.7 | 5.8 ± 3.0* | 11.3 ± 7.6* |
| Test motor unit | 9.2 ± 5.9 (0.0005)† | 11.1 ± 3.7 (0.0001)† | 17.5 ± 8.9 (0.0001)†‡ |
| Recruitment threshold, % MVC | |||
| Reporter motor unit | 2.9 ± 3.5 | 3.6 ± 2.4 (0.23) | 5.4 ± 3.3 (0.45) |
| Test motor unit | 8.7 ± 8.4† | 6.4 ± 2.5 (0.33)† | 8.5 ± 3.6 (0.09)† |
Values are means ± SD. P values compared with spastic-paretic muscle, are in parentheses.
P ≤ 0.001, compared with spastic-paretic muscle.
P ≤ 0.001, compared with reporter motor unit of respective muscle type.
P = 0.01, compared with spastic-paretic muscle.
For comparisons across muscle types, rates of increase and decrease in force were matched using absolute force levels. Values expressed as % MVC/s are also shown for comparison across muscle types.
Because the rate of muscle contraction and relaxation can influence the time of recruitment and derecruitment, respectively, of motor units (Freund 1983) and thus the measurement of ΔF, the speeds of isometric force increases and decreases were compared to ensure similar values across all three muscle types. The isometric force profiles across different muscle types of the representative trials in Fig. 1 were quite similar, as noted by the similar rates of increase (2.1, 2.1, 2.0 N/s) and decrease (−2.0, −2.2, and −1.7 N/s) in force for the spastic-paretic, contralateral, and control muscles, respectively. The rates of increase and decrease in force were also similar for the group data (Table 2), corresponding to 1–3% MVC/s.
Note also that the initial firing rates of the reporter units for the spastic-paretic, contralateral, and control muscle were also similar in Fig. 1 (7 to 9 Hz). This pattern also held true for the group data; the initial firing rate of the reporter (and test) motor units did not differ across muscle types (Table 2).
Furthermore, subjects did not report fatigue either during or following the voluntary ramp contractions, as shown by the similar or greater MVC values before and after data collection sessions for each group (spastic-paretic muscle MVC: pre 89 ± 38 N, post 95 ± 39 N; P = 0.02; contralateral muscle MVC: pre 180 ± 43 N, post 179 ± 47 N; P = 0.42; control muscle MVC: pre 200 ± 58 N, post 196 ± 72 N; P = 0.24).
Analysis of common synaptic drive to reporter and test units: rate–rate plots
One critical assumption of the paired motor unit method is that the synaptic drive to both units of a pair, termed common drive, is similar (De Luca and Erim 1994). Following this premise, the firing rate of the lower-threshold (reporter) motor unit provides a plausible estimate of the synaptic drive to the higher-threshold (test) motor unit before and after recruitment.
To determine whether the reporter and test units were modulated in a similar manner, the firing rates of the reporter and test units in Fig. 1 were plotted against one another (rate–rate plots; insets above Fig. 1, A, B, and C) in 500-ms bins during the ascending (red dots) and descending (blue dots) portions of the voluntary ramp contractions. Note that a greater portion of the rate–rate plot occurred during the descending phase of the voluntary ramp contraction. The coefficient of determination (r2) for the linear regression fit through the averaged rate–rate plots, after removing nonlinear startup frequencies in the test unit (see methods), was high for the spastic-paretic (r2 = 0.94), contralateral (r2 = 0.75), and control muscles (r2 = 0.90), indicating that a large percentage of the modulation in the test unit was accounted for by modulation in the reporter unit.
This high correlation also held true for the group data: the average coefficient of determination for the linear regression line fit through the rate–rate plots was consistently high for spastic-paretic (r2 = 0.84 ± 0.07), contralateral (r2 = 0.81 ± 0.07), and control (r2 = 0.78 ± 0.06) muscles, indicating that the reporter and test units were modulated in a similar manner during the duration of the test unit firing. The average coefficient of determination for the linear regression line fit through the rate–rate plots was also high when not removing the nonlinear startup frequencies for spastic-paretic (r2 = 0.78 ± 0.07), contralateral (r2 = 0.76 ± 0.03), and control (r2 = 0.76 ± 0.03) muscles.
Ensuring accuracy of ΔF measurements
In 9, 13, and 10 trials for the spastic-paretic, contralateral, and control muscle respectively, the firing rate of the reporter motor unit remained flat or decreased slightly at the time of test unit recruitment. In such trials, it is possible that the ΔF was underestimated because of possible firing rate saturation of the reporter units. However, after removing these trials, although some of the ΔF values increased slightly (spastic-paretic ΔF from 4.0 ± 1.6 to 4.1 ± 1.5 pps; contralateral ΔF from 4.6 ± 1.4 to 4.5 ± 1.6 pps; control muscle ΔF from 3.8 ± 1.7 to 4.0 ± 1.2 pps), they remained fairly close to their original values (Table 2) and, again, did not differ across muscle types (P > 0.05).
Other novel features of the firing rate profiles in spastic-paretic muscle
1) Spontaneous discharge of reporter motor units in spastic-paretic muscle.
An unexpected, yet important, finding was that spastic-paretic muscles showed a very high incidence of spontaneous unitary discharge following termination of the voluntary ramp contractions—i.e., continued discharge of motor units not produced by voluntary effort by the subject. This provided an opportunity to examine the patterns of discharge of these spontaneously firing units and the firing rate modulations between dually firing units, to determine whether the firing properties were determined by intrinsic properties of the motoneurons or by externally driven synaptic inputs to the motoneuron pool. To achieve this objective, we examined whether the firing rates of simultaneously active motor units that continue to discharge following a voluntary muscle contraction were co-modulated, which is evidence for the presence of a continued, common synaptic input (De Luca et al. 1982).
The average percentage of trials exhibiting spontaneous discharge of reporter motor units at the end of the voluntary ramp contraction was 21 ± 28% in spastic-paretic muscles, compared with 1 ± 3% of trials in contralateral muscles (P = 0.03) and 0% of trials in healthy control muscles (P = 0.03).
Likewise, the percentage of additional lower-threshold motor units recorded (i.e., units not used in the paired motor unit analysis technique) exhibiting spontaneous unit discharge was 83.2 ± 5.9% in spastic-paretic muscles, compared with only 14.1 ± 3.7% in contralateral muscles (P < 0.001) and 0% in healthy control muscles (P < 0.001). In contrast, spontaneous motor unit discharge of higher-threshold test motor units following the ramp contractions was not observed in any of the muscle types.
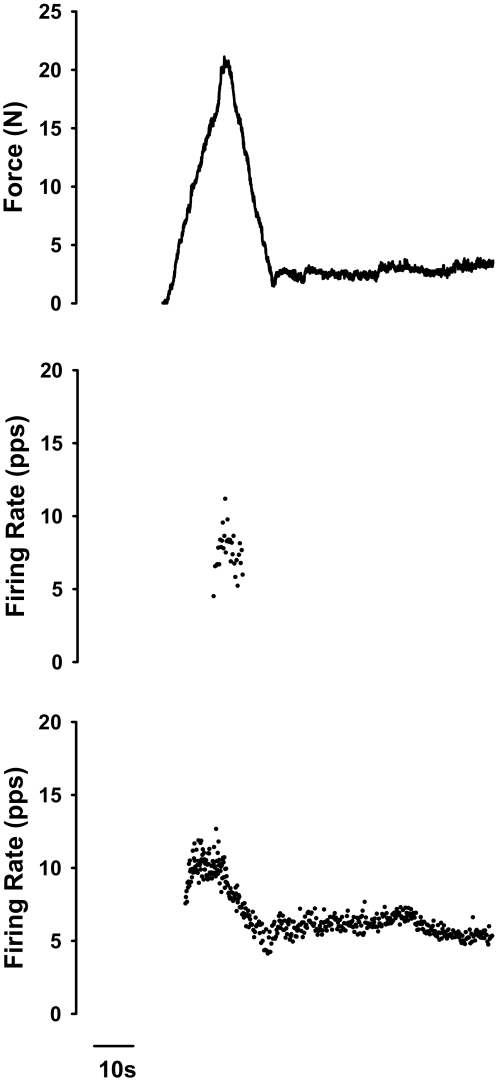
An example of this finding is portrayed in Fig. 2. Here, lower-threshold reporter motor unit discharge (bottom panel) continued for close to 60 s following the voluntary ramp contraction, whereas the higher-threshold test unit (middle panel) ceased firing before completion of the ramp contraction (Fig. 2).
Fig. 2.
The reporter motor unit in spastic-paretic muscle continued to fire following the voluntary ramp contraction, whereas the test unit did not. Top: force of the elbow flexor muscles from a spastic-paretic stroke survivor during and following the voluntary ramp contraction. Middle: instantaneous frequency of the test motor unit during the voluntary ramp contraction. Bottom: instantaneous frequency of the reporter motor unit during and following the voluntary ramp contraction. Note the continuation of force and reporter motor unit firing for about 60 s following the ramp contraction despite verbal cuing from the investigator to relax the muscle and the subject's report that he was relaxed. The higher-threshold test motor unit ceased firing before the end of the voluntary ramp contraction.
In 37 trials we observed spontaneous discharge of two lower-threshold motor units in parallel in spastic-paretic muscle, following the ramp contractions. One of these instances is portrayed in Fig. 3. Note the similar modulation of the two dually firing motor units as well as the high coefficient of determination (r2) from the linear regression fit through the rate–rate plots (inset of Fig. 3; P < 0.001, r2 = 0.64), suggesting common modulation of the two motor units. In 91% of instances of two dually firing motor units, the r2 from the linear regression fit through the rate–rate plots was significant (P ≤ 0.008), with visible co-modulation in the rate fluctuations across motor unit pairs.
Fig. 3.
Firing frequency profiles of 2 low-threshold motor units (below threshold of test unit) that continued to fire after a voluntary contraction in a spastic-paretic muscle. Overlayed waveforms denote the accuracy in discrimination of the motor units. Note that the firing rates of the 2 low-threshold units were modulated in a parallel manner. Inset shows the firing rate of the 2 motor units plotted against one another in 500-ms bins during the duration that they were firing in parallel. Note the high r2 value from the linear regression fit through the rate–rate plot, confirming that the 2 motor units were modulated in parallel (P < 0.001, r2 = 0.64).
In short, the presence of co-modulation in firing rates suggests that external synaptic input was driving the discharge of these motor units, rather than intrinsic properties, such as PICs, that would not be expected to display significant co-modulation of firing rates, although other explanations are tenable (see discussion).
2) Acceleration in motor unit firing following recruitment in spastic muscle.
In many recordings of the higher-threshold test motor unit from spastic-paretic muscle, we noted a sharp rise in firing rate during the first 500 ms after recruitment, followed by a more gradual increase as the discharge approached peak values. Similar findings have been reported in animal models (Heckman et al. 2005, 2008; Hornby et al. 2002) and in humans (Hornby et al. 2002; Kiehn and Eken 1997). In contrast, we often observed that the aforementioned sharp rise in firing rate of the higher-threshold (test) motor unit in spastic-paretic muscle was reduced for lower-threshold (reporter) motor units.
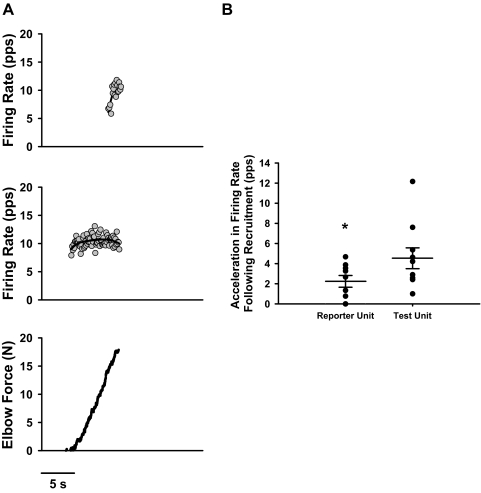
This finding is portrayed in Fig. 4A, which focuses on the rising phase of the contraction for the spastic-paretic muscle of one stroke survivor. The bottom panel portrays the force of the elbow flexor muscles during the rising phase of the contraction for the spastic-paretic stroke survivor, whereas the middle and top panels show the reporter and test unit firing profiles, respectively, that accompany the rising force profiles. Note that the sharp rise in lower-threshold reporter unit firing rate following recruitment was lacking in the spastic-paretic muscle. This is in contrast to the sharp rise in firing rate observed for the higher-threshold (test) unit in spastic-paretic muscle (Fig. 4A).
Fig. 4.
Acceleration in firing rate of the reporter motor unit following recruitment was less in the lower-threshold (reporter; middle panel) than that in the higher-threshold (test; top panel) unit in the spastic-paretic muscle of a stroke survivor (A). The acceleration in firing rate was quantified as the increment in firing rate from onset to maximal discharge within the first 5 discharges. The bottom panel portrays the force of the elbow flexor muscles during the rising phase of the contraction for one spastic-paretic stroke survivor, whereas the middle and top panels show the reporter and test unit firing profiles, respectively, that accompany the rising force profiles. Note the 5th-order polynomial fit through the firing rate data does not rise at contraction onset in the lower-threshold (reporter) motor unit in the spastic-paretic muscle, whereas it does in the higher-threshold (test) motor unit in the spastic-paretic, muscle (A). B: the average acceleration in firing rate following recruitment was less in the lower-threshold (reporter) than that in the higher-threshold (test) motor unit in spastic-paretic muscle. Each circle represents the average value from repeated trials of voluntary ramp contractions for each spastic-paretic muscle of the 10 subjects; wider horizontal bars denote the average value for the group data for the reporter and test units; smaller horizontal bars denote 1SE above and below mean values. Acceleration in the reporter unit firing rate (2.2 ± 1.8 pps) was less than the acceleration in the test unit firing rate (4.5 ± 3.3 pps) for the spastic-paretic muscle. *P = 0.02 compared with the test unit.
Although not observed in the lower-threshold reporter motor unit in Fig. 1, when all motor units were measured systematically, there was a marked difference in firing rate acceleration following recruitment between lower- and higher-threshold units in spastic-paretic muscles. To quantify the acceleration in firing rate following recruitment, we calculated the difference in reporter and test unit firing rates from onset to peak rate within the first five discharges. In the representative data in Fig. 4A, the difference in reporter unit firing rate within these first five discharges in the spastic-paretic muscle was only 2.0 pps for the reporter unit, whereas it was 3.9 pps for the higher-threshold test motor unit.
Indeed this trend for dissimilar rates of rise in firing for the reporter and test units in spastic-paretic muscle was maintained for the group data, as shown in Fig. 4B. This figure shows the average acceleration of firing rates in reporter and test units following recruitment in spastic-paretic muscle for each subject. The acceleration in firing rate of the reporter motor unit was substantially less in the spastic-paretic muscles (2.2 ± 1.8 pps) compared with that of the test unit for spastic-paretic muscles (4.5 ± 3.3 pps; P = 0.02).
Additional evidence for differences in the shape of the firing rate trajectory for the first five discharges of the reporter unit than that of the test unit in spastic-paretic muscle is manifested in the small increment in firing rate from the first instantaneous frequency value to the averaged subsequent four instantaneous frequency values for the lower- threshold reporter motor unit, yet not for the higher-threshold test motor unit firing profiles. Specifically, the reporter unit's initial instantaneous frequency value subtracted from the averaged subsequent four instantaneous frequency values was low in spastic-paretic muscle (0.01 ± 2.3 Hz) and did not differ from zero (P = 0.43), whereas the higher-threshold normalized test unit's instantaneous frequency value (1.9 ± 1.6 Hz) did differ from zero (P = 0.004).
DISCUSSION
The first major objective of this study was to examine the potential contribution of enhanced PICs to the abnormal motoneuron excitability observed in spastic-paretic biceps brachii muscles of stroke survivors. This aim was addressed by examining alterations in firing profiles of pairs of motor units in the biceps brachii during isometric voluntary ramp contractions. We hypothesized that PICs were enhanced in magnitude in spastic motoneurons and that this enhancement contributed to the abnormal motoneuron excitability (i.e., enhanced stretch reflex responses) observed in these patients. Contrary to our expectations, our findings were that the amplitude of the estimated PIC (ΔF) in spastic-paretic motoneurons was similar to that for motoneurons in contralateral nonspastic muscles, as well as for muscles of healthy controls.
A second objective of the study was to examine the hypothesis that a low-level depolarizing synaptic drive to the resting motoneuron pool is enhanced in spastic-paretic stroke survivors. This aim was addressed by examining whether the firing rates of simultaneously active motor units that continue to discharge following a voluntary muscle contraction were co-modulated, which is evidence for the presence of a continued, common synaptic input (De Luca et al. 1982), and by examining recruitment properties of lower-threshold units across the different muscle types.
We indeed observed several changes in motor unit firing behavior consistent with increased common low-level depolarizing synaptic inputs to the motoneuron pool. First, following a voluntary contraction when the subject was supposedly at rest, several motor units in the spastic-paretic muscle continued to discharge with firing rates that were modulated in a parallel manner. Second, the increase in initial firing rate of the lower-threshold reporter units following recruitment was reduced in spastic-paretic muscle.
As discussed in the following text, these novel findings together suggest that the lower-threshold for stretch reflexes observed in spastic-paretic stroke survivors, while at rest (Chardon et al. 2008; Powers et al. 1988), in addition to the enhanced tonic vibration reflex (TVR) (McPherson et al. 2008) may not be attributable to increases in motoneuron PIC amplitude, yet rather the result of a low-level depolarizing synaptic drive to the resting motoneuron pool.
Validity of ΔF measurements across muscle types
The similar ΔF values across muscle types suggested that there were similar PIC amplitudes in spastic, contralateral, and healthy control motoneurons and, by implication, that exaggerated PICs were not clearly responsible for the increased excitability in spinal motoneurons of spastic-paretic muscle.
As noted in the introduction, the estimate of PIC amplitudes in the test motor unit depends critically on whether the firing rate of the reporter unit is an accurate measure of synaptic drive to the motoneuron pool and, specifically, to the test motor unit under study This approach depends on the premise that the firing rate of a motoneuron is proportional to the underlying membrane potential, given the assumption that the frequency–current (F–I) relation for current reaching the soma is similar for injected current and for currents reaching the soma from synaptic inputs (effective somatic current) (Binder et al. 1996). If so, then the firing rate of a motor unit is potentially a fairly accurate measure of the drive to the motoneuron pool, including the test unit (assuming the two motor units are receiving common synaptic inputs; Bennett et al. 2001b).
The fact that during the duration of the test unit firing, the firing rate profile of the reporter unit closely matched that of the test unit in all muscle types, as indicated by the high coefficient of determination (r2) values for the linear regression fit through the averaged rate–rate plots, indicates that the test and reporter units likely received similar synaptic drive during the voluntary isometric contractions. This supports the claim that the firing rate profile of the reporter unit was a fairly accurate representation of synaptic drive to the test motor unit and that our relative estimates of PIC amplitude across muscle types were valid.
In contrast, the firing rate profiles often deviated from the force trace (see Fig. 2). Thus compensation from muscles in the same or other joints may have contributed to the force profile of the biceps brachii, especially in the spastic-paretic muscle, because activation of more proximal shoulder muscles is known to compensate for weak elbow activation after stroke (Roby-Brami et al. 2003). This observation underlines the importance of using the firing rate modulation of motor units from the muscle of interest, rather than force, to estimate the input to the motoneuron pool.
In certain key instances, the firing rate of a motoneuron may not accurately represent the synaptic drive to the cell. For example, the early, rapid acceleration in firing rate of a motoneuron at the onset of a voluntary contraction may be affected by the simultaneous activation of the PIC and the fast sodium channel at motoneuron recruitment (Hounsgaard and Kiehn 1989; Lee and Heckman 2000; Li et al. 2004). However, measurements of ΔF in the test motor unit of all muscle types were conducted well after this initial firing rate acceleration in the reporter motor unit and therefore could not complicate the ΔF measurements.
Incorrect estimates of the synaptic drive to the motoneurons could also arise because of possible saturation in the firing rates of the reporter unit—i.e., synaptic drive could increase, yet the firing rate of the motoneuron sometimes remains flat (Fuglevand et al. 2006). In some instances across all muscle types, the firing rate of the reporter unit flattened off as the firing rate of the test motor unit continued to increase. However, during these trials, the ΔF values were only slightly lower, suggesting that the synaptic input did not increase much above the flattened region of reporter unit firing.
In summary, the use of the firing rate profile of the reporter unit in calculating the ΔF of the test unit likely provided a relatively accurate estimation of PIC amplitude across muscle types. Accordingly, we cannot verify that the exaggerated and prolonged responses to muscle stretch or tendon taps observed in resting muscles of spastic-paretic stroke survivors are attributable to larger than normal PIC amplitudes in spinal motoneurons.
Alternative mechanisms for enhanced excitability: evidence for a low-level depolarizing synaptic drive to the resting spastic-paretic motoneuron pool
1) Greater incidence of co-modulated spontaneous unit firing in spastic-paretic muscle.
On termination of the voluntary ramp contractions in spastic-paretic muscle, we often noted continued firing of lower-threshold motor units not observed in contralateral or control muscle (Fig. 2). Importantly, almost all spontaneously firing motor units exhibited tight co-modulation of firing rates, as shown by significant correlations between their firing rates, with strong coefficient of determination values (Fig. 3). This suggests that there was substantial common synaptic drive to these simultaneously firing motor units. The co-modulated unit activity that occurred while the stroke survivors were at rest suggests that lower-threshold units of spastic-paretic muscle are under tonic depolarizing synaptic drive, potentially from descending or regional inputs. The suggestion of a tonically depolarized motoneuron pool in a “resting” spastic muscle is also supported by the enhanced levels of the Hoffman reflex (H-reflex) (Barzi and Zehr 2008; Crone et al. 2003), the reductions in the angular threshold of the stretch reflex (Powers et al. 1988), and the enhanced tonic vibration reflex (TVR) (McPherson et al. 2008) observed in the involved muscle of stroke survivors while at rest.
It is unlikely that the spontaneous unit activity at “rest” was due to an increase in PIC activation, as shown by the similar ΔF values across muscle types. Slow, regular spontaneous unit activity, as observed in the lower-threshold spastic-paretic motor units, has been suggested to be produced by the repetitive activation of a subthreshold Na PIC in patients with spinal cord injury (Gorassini et al. 2004) and in the chronic spinal rat (Li et al. 2004). For instance, following each AP, subthreshold activation of the Na PIC following the afterhyperpolarization depolarizes the membrane potential and triggers a fast sodium spike to produce steady, regenerative firing of the motoneuron. Importantly, this regenerative activation of the Na PIC cannot occur without some tonic, depolarizing bias current to the motoneuron (Li et al. 2004). It follows that the high numbers of lower-threshold, yet not higher-threshold, motor units exhibiting spontaneous activity whose firing rates were tightly co-modulated strongly suggest the presence of a common low-level depolarizing synaptic drive to the motoneuron pool to allow for the expression of Na PIC–triggered firing.
2) Lack of acceleration in firing following recruitment in lower-threshold spastic-paretic motor units: evidence for depolarizing drive.
The second novel feature observed in the firing rate profiles of spastic-paretic muscle suggesting a tonically depolarized motoneuron pool was the lack of acceleration in firing by the lower-threshold (reporter) motor units following recruitment (Fig. 4). Specifically, lower-threshold motor units from spastic-paretic muscle often displayed strikingly smaller rate increases over the first 500 ms following recruitment (Fig. 4), compared with those of higher-threshold test motor units in the spastic-paretic muscles.
If the lower-threshold motoneurons of spastic-paretic muscles are indeed depolarized from extrinsic synaptic sources, this might result in preactivation of the PIC because there is evidence that exogenous synaptic inputs assist in lowering PIC threshold when activated with subsequent current injection (Li et al. 2004). The sharp increase in the firing rate that occurs following recruitment is mediated by the coincident activation of the PIC and the fast sodium channel at the onset of cell firing. The PIC activation produces a rapid rate of change in the membrane potential and the subsequent steep region of firing that is observed (Hounsgaard and Kiehn 1989; Kuo et al. 2006; Li et al. 2004).
If a tonic depolarization activates the PIC before cell firing, the rate of change of membrane potential (a strong determinant of AP threshold; Kuo et al. 2006) is potentially reduced because the driving force between the sodium ion equilibrium potential and the prevailing membrane potential is also reduced (Li et al. 2004), thus preventing the sharp increase in firing rate that occurs when the PIC is being activated during motoneuron recruitment. Instead, it appears that a PIC is activated before recruitment, so motor units start to fire with a low F–I gain and lack of acceleration in firing following recruitment (Bennett et al. 1998a,b; Li and Bennett 2003; Li et al. 2004).
It is somewhat surprising to us that we did not observe reductions in the initial acceleration in firing rate in any of the higher-threshold units in spastic muscles. Presumably these features are manifested only in lower-threshold units, suggesting that a tonic synaptic drive did not bring these higher-threshold motor units sufficiently close to firing threshold. It is conceivable, however, that we may not have had a sufficiently large sample to observe these alterations in a range of units with widely disparate recruitment thresholds. Nonetheless, the loss of initial acceleration in firing rate might suggest that there are alterations in cellular mechanisms in lower-threshold spastic motoneurons.
Although tonic depolarizing drive to spastic motoneurons appears to us to be the most parsimonious explanation for our findings, our theories are based on indirect evidence and other explanations are certainly feasible. For example, it is likely that the descending pathways that mediate presynaptic inhibition of sensory feedback onto motoneurons (Seki et al. 2003) have been disrupted by stroke. Indeed there is evidence for reductions in presynaptic inhibition of flexor carpi radialis afferents in the affected side of stroke survivors (Aymard et al. 2000; Lamy et al. 2009); however, a correlation with spasticity was not found in these studies. Finally, reduced drive from the disrupted corticospinal tracts, increased postsynaptic inhibition from regional interneurons, or alterations in segmental afferent input might also contribute to the findings.
Role of monoamines in spasticity in stroke versus spinal cord injury
Based on the results from this study, there appear to be two different mechanisms producing spasticity in stroke and spinal cord injury (SCI). This postulate is not really surprising, given the different clinical presentations of spasticity in these two groups.
For example, in SCI spasticity is dominated clinically by the presence of prolonged, involuntary muscle spasms resulting (presumably) from the uncontrolled activation of Na and Ca PICs in the motoneuron (Li and Bennett 2003). Motoneuron PICs disappear swiftly after complete spinal cord transection, yet recover in the following months after a spinal cord injury despite the loss of serotonin (5-HT) and norepinephrine (NE), the two monoamines that are necessary for PIC activation (Bennett et al. 2001a,b). Despite this loss, 5-HT and NE receptors on the motoneuron remain active in chronic SCI (Harvey et al. 2006a). Furthermore, recent findings suggest that this preservation may be a result of the development of constitutive 5-HT receptor activity (i.e., receptors that become active without ligand binding; Murray et al. 2008) and from the activation of NE receptors by new peripheral sources of NE (Rank et al. 2008). Thus after SCI, the development of prolonged muscle spasms is apparently related to changes in how monoamine receptors are reactivated to facilitate PIC recovery.
In contrast, spasticity in stroke is characterized primarily by exaggerated stretch reflex responses, appearing as an enhanced resistance to externally imposed joint movement, and by an inability to relax the muscle after a contraction. Given that the ΔF values (our estimate of PIC amplitude) did not differ across the spastic and nonspastic muscles it is less likely that increased motoneuron PIC activation contributes to this increased motoneuron activity.
As stated in the introduction, we hypothesized that excessive PIC activation is possible after stroke, given the possible increase in monoaminergic drive to the spinal cord from brain stem disinhibition. It appears, however, that this is not the case. It remains possible that there are monoamine receptor adaptations to long-term changes in monoaminergic drive in chronic stroke. It does appear from this work that in stroke, both the enhanced reflex excitability at rest and the difficulty patients have in relaxing muscles may be due, in part, to the presence of a tonic, low-level ionotropic drive to the motoneuron pool that keeps a certain proportion of motoneurons close to firing threshold and thus more readily activated at rest.
In summary, estimated PIC amplitudes were similar across muscle types. In addition, spontaneously discharging lower-threshold motor units from spastic-paretic muscles exhibited co-modulated unit firing at rest. These findings, coupled with previous observations for enhanced stretch or H-reflexes at rest (Burne et al. 2005; Huang et al. 2006), yet not during a background contraction (Burne et al. 2005; Thompson et al. 2009), suggest that the enhanced reflex responses at rest in stroke survivors are explained by the presence of a low-level tonic depolarizing synaptic drive to the spastic-paretic motoneuron pool.
Clinical implications
Our current observations suggest that there are no demonstrable increases in PIC magnitude in spastic-paretic muscle and therefore this mechanism cannot readily explain the exaggerated motoneuron excitability that often occurs following a stroke. Instead, it is possible that depolarization of spastic-paretic motoneurons, attributable to low-level synaptic input from regional or supraspinal centers, might alter motoneuron threshold for afferent-mediated synaptic input. Therapeutic interventions to reduce tonic synaptic depolarizing drive to the resting motoneuron pool in spastic-paretic stroke survivors or to reduce resting membrane potential of motoneurons will allow the clinician to more effectively target spasticity when treating these patients.
GRANTS
This work was supported by the Brinson Foundation Post-Doctoral Fellowship awarded to C. J. Mottram and National Institute of Health and Human Development Grants 2T32 HD-007418-16 and 5R24 HD-050821-04 to W. Z. Rymer.
ACKNOWLEDGMENTS
We thank C. Wallace and C. Chikando for assistance with data collection and analysis; J. Moore and T. DeMott for assistance with data collection; J. Madoff for technical assistance and assistance with software development; Dr. Randy Powers and Dr. Dave Bennett for assistance with data interpretation; and Dr. George Hornby and Dr. Dave Bennett for reading a draft of the manuscript and providing insightful comments.
REFERENCES
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain 123: 1688–1702, 2000 [DOI] [PubMed] [Google Scholar]
- Barzi Y, Zehr EP. Rhythmic arm cycling suppresses hyperactive soleus H-reflex amplitude after stroke. Clin Neurophysiol 119: 1443–1452, 2008 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2038–2045, 1998b [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001b [DOI] [PubMed] [Google Scholar]
- Binder M, Heckman CJ, Powers RK. The physiological control of motor neuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, pt. 1, chapt. 1, p. 3–53 [Google Scholar]
- Burke D, Ashby P. Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? J Neurol Sci 15: 321–326, 1972 [DOI] [PubMed] [Google Scholar]
- Burke D, Knowles L, Andrews C, Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain 95: 31–48, 1972 [DOI] [PubMed] [Google Scholar]
- Burne JA, Carleton VL, O'Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry 76: 47–54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon MK, Suresh NL, Rymer WZ. Reflex threshold estimation in spastic muscles: a new method. Program No. 76.4/NN12. 2008 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Chung SG, van Rey E, Bai Z, Rymer WZ, Roth EJ, Zhang LQ. Separate quantification of reflex and nonreflex components of spastic hypertonia in chronic hemiparesis. Arch Phys Med Rehabil 89: 700–710, 2008 [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264: 673–693, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HJ. Motor unit and muscle activity in voluntary motor control. Physiol Rev 63: 387–436, 1983 [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA. Evaluation of plateau-potential-mediated “warm up” in human motor units. J Physiol 571: 683–693, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002a [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol 87: 1859–1866, 2002b [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 29: 223–228, 2000 [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins KD, Glass GV. Statistical Methods in Education and Psychology Needham Heights, MA: Pearson Allyn & Bacon, 1996, p. 296–299 [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve 25: 632–648, 2002 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Wang CH, Hwang IS. Characterization of the mechanical and neural components of spastic hypertonia with modified H reflex. J Electromyogr Kinesiol 16: 384–391, 2006 [DOI] [PubMed] [Google Scholar]
- Hultborn H. Changes in neuronal properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. J Rehabil Med Suppl 41: 46–55, 2003 [DOI] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989 [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997 [DOI] [PubMed] [Google Scholar]
- Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain 130: 159–169, 2007 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy JC, Wargon I, Mazevet D, Ghanim Z, Pradat-Diehl P, Katz R. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 132: 734–748, 2009 [DOI] [PubMed] [Google Scholar]
- Lance JW. The control of muscle tone, reflexes, and movement (Robert Wartenberg Lecture). Neurology 30: 1303–1313, 1980 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol 76: 2107–2110, 1996 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998a [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998b [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input–output processing in spinal motoneurons in vivo. J Neurophysiol 89: 27–39, 2003 [DOI] [PubMed] [Google Scholar]
- Lewek MD, Hornby TG, Dhaher YY, Schmit BD. Prolonged quadriceps activity following imposed hip extension: a neurophysiological mechanism for stiff-knee gait? J Neurophysiol 98: 3153–3162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Litvan I, Mangone CA, Werden W, Bueri JA, Estol CJ, Garcea DO, Rey RC, Sica RE, Hallett M, Bartko JJ. Reliability of the NINDS Myotatic Reflex Scale. Neurology 47: 969–972, 1996 [DOI] [PubMed] [Google Scholar]
- Lukacs M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin Neurophysiol 116: 1566–1570, 2005 [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 16: 135–144, 2007 [DOI] [PubMed] [Google Scholar]
- McPherson JG, Ellis MD, Heckman C, Dewald JP. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol 100: 3236–3243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merletti R, Rainoldi A, Farina D. Surface electromyography for noninvasive characterization of muscle. Exerc Sport Sci Rev 29: 20–25, 2001 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Chikando CN, Rymer WZ. The role of intrinsic motoneuron properties in stroke induced spasticity. Program No. 652.6. 2006 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2006. Online [Google Scholar]
- Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Mechanisms contributing to spontaneous firing of motor units in the spastic biceps brachii of stroke survivors. Program No. 726.10. 2007 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2007. Online [Google Scholar]
- Murray KC, Nakae A, Ballou T, Vavrek R, Fouad K, Rank M, Stephens M, Anelli R, Harvey PJ, Heckman C, Benentt DJ. Role of constitutively active 5-HT2C receptors following spinal cord injury in rats. Program No. 76.8/NN16. 2008 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrkiewicz M, Kudina L, Wang YL, Jakubiec M, Lin LN, Mierzejewska J, Chen J. Age-dependence of human motoneuron “fastness” in health and disease. In: Mechanisms of Plasticity and Disease in Motoneurons, June 26–29, 2008, Seattle, WA Seattle, WA: Univ. of Washington School of Medicine, 2008. Online [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank M, Vavrek R, Murray K, Sanelli L, Fouad K, Gorassini MA, Bennett DJ. Peripheral norepinephrine crosses the blood-brain barrier and contributes to spasms after spinal cord injury. Program No. 76.9/NN17. 2008 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Roby-Brami A, Feydy A, Combeaud M, Biryukova EV, Bussel B, Levin MF. Motor compensation and recovery for reaching in stroke patients. Acta Neurol Scand 107: 369–381, 2003 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982 [DOI] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003 [DOI] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23: 133–142, 2009 [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve 24: 952–962, 2001 [DOI] [PubMed] [Google Scholar]