Abstract
Hypoxia-induced short-term potentiation (STP) of respiratory motor output is manifested by a progressive increase in activity after the acute hypoxic response and a gradual decrease in activity on termination of hypoxia. We hypothesized that STP would be differentially expressed between physiologically defined phrenic motoneurons (PhrMNs). Phrenic nerve “single fiber” recordings were used to characterize PhrMN discharge in anesthetized, vagotomized and ventilated rats. PhrMNs were classified as early (Early-I) or late inspiratory (Late-I) according to burst onset relative to the contralateral phrenic neurogram during normocapnic baseline conditions. During hypoxia (FIO2 = 0.12–0.14, 3 min), both Early-I and Late-I PhrMNs abruptly increased discharge frequency. Both cell types also showed a progressive increase in frequency over the remainder of hypoxia. However, Early-I PhrMNs showed reduced overall discharge duration and total spikes/breath during hypoxia, whereas Late-I PhrMNs maintained constant discharge duration and therefore increased the number of spikes/breath. A population of previously inactive (i.e., silent) PhrMNs was recruited 48 ± 8 s after hypoxia onset. These PhrMNs had a Late-I onset, and the majority (8/9) ceased bursting promptly on termination of hypoxia. In contrast, both Early-I and Late-I PhrMNs showed post-hypoxia STP as reflected by greater discharge frequencies and spikes/breath during the post-hypoxic period (P < 0.01 vs. baseline). We conclude that the expression of phrenic STP during hypoxia reflects increased activity in previously active Early-I and Late-I PhrMNs and recruitment of silent PhrMNs. post-hypoxia STP primarily reflects persistent increases in the discharge of PhrMNs, which were active before hypoxia.
INTRODUCTION
The hypoxic ventilatory response is a complex, time-dependent process reflecting both acute and delayed onset mechanisms (Powell et al. 1998). The acute response includes a rapid increase in respiratory motor output (e.g., increased amplitude of respiratory muscle EMG bursts or tidal volume) coupled with an increase in breath frequency. The acute response is followed by short-term potentiation (STP) of respiratory output (Fuller et al. 2005; Powell et al. 1998), which is manifest by a progressive increase in respiratory activity during hypoxia (i.e., the “onset” of STP) followed by a gradual return toward pre-hypoxia baseline activity after termination of hypoxia (i.e., the “offset” of STP). Respiratory-related STP has been described using phrenic neurograms (e.g., compound, extracellular action potentials; Hayashi et al. 1993; Wagner and Eldridge 1991), diaphragm EMG (Mateika and Fregosi 1997) and ventilation measurements (Fregosi 1991; Georgopoulus et al. 1992; Kline et al. 2002). However, the in vivo behavior of respiratory motoneurons, including phrenic motoneurons (PhrMNs), has not been evaluated in the context of hypoxia-induced STP or other forms of respiratory neuroplasticity.
PhrMNs are classified based on their bursting patterns as early inspiratory (Early-I; neurons that begin bursting at the onset of inspiration) or late inspiratory (Late-I; cells initiating bursting activity later in the inspiratory effort) (Kong and Berger 1986; St John and Bartlett 1979). In addition, several studies have described “silent” PhrMNs that are inactive during quiet breathing but can be recruited during chemoreceptor stimulation and/or cough (Milano et al. 1992; St John and Bartlett 1979). However, it is unknown whether the expression of respiratory motor plasticity associated with hypoxia [e.g., STP and/or long-term facilation (LTF); Baker-Herman et al. 2004] is accompanied by persistently enhanced bursting of previously active Early- and Late-I PhrMNs, or alternatively, recruitment of previously silent PhrMNs.
The primary purpose of this study was to investigate PhrMN behavior, including discharge frequency, onset, duration, and total spikes per breath during hypoxia-induced STP of PhrMN output. The segregation of PhrMN responses after electrical activation of descending inputs in the cervical lateral funiculus (Hayashi et al. 2003; McCrimmon et al. 1997) led us to hypothesize that STP would be differentially expressed across physiologically defined groups of PhrMNs.
METHODS
Animals
Male Sprague-Dawley rats (n = 17; 397 ± 6 g) purchased from Harlan (Indianapolis, IN) were used in this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
General Animal Preparation
These procedures were similar to those described in our previous reports (Doperalski and Fuller 2006; Fuller et al. 2009). Animals were placed in a closed chamber and anesthetized by 3–4% isoflurane in oxygen. Stable anesthesia (2–3%) was maintained via a nose cone. A short length of PE-240 tubing was inserted into the trachea below the larynx, and rats were mechanically ventilated for the remainder of the experiment (model 683, Harvard Apparatus, South Natick, MA). A PE-50 catheter was inserted into the femoral artery for blood pressure measurement (Statham P-10EZ pressure transducer, CP122 AC/DC strain gauge amplifier, Grass Instruments, West Warwick, RI) and withdrawal of blood samples. Another catheter was placed into the femoral vein to enable conversion from the volatile anesthesia (isoflurane) to urethane (1.6 g/kg, iv, Sigma, St. Louis, MO) and injection of a paralytic drug (pancuronium bromide, 2.5 mg/kg, iv, Hospira, Lake Forest, IL). The vagus nerves were sectioned bilaterally in the cervical region to prevent entrainment of respiratory output with the ventilator. End-tidal CO2 partial pressure (PETCO2) was analyzed with a Capnogard neonatal CO2 monitor placed on the expired line of the ventilator circuit (Novametrix Medical Systems, Wallingford, CT). Arterial blood gases and pH were measured in 10/17 rats from 0.2-ml arterial blood samples (i-Stat, Heska, Fort Collins, CO) obtained at baseline and during the final minute of hypoxia. Rectal temperature was monitored by an electrical thermometer and maintained at 37.5 ± 1°C using a servo-controlled heating pad (model TC-1000, CWE, Ardmore, PA).
Phrenic Nerve and Motoneuron Recording
Both phrenic nerves were exposed in the neck region with a ventral surgical approach and sectioned peripherally. The methods for recording phrenic fibers were similar to our previous reports (Lee et al. 2007a,b, 2008). Briefly, the left phrenic nerve was stripped of connective tissue, desheathed, and separated into small filaments. The isolated filaments were placed across a monopolar silver electrode; the contralateral phrenic nerve was placed on a bipolar silver electrode. Neural signals were amplified (1,000×) and band-pass filtered (0.3–10 KHz) using a differential A/C amplifier (Model 1700, A-M Systems, Carlsborg, WA). Raw signals from the whole phrenic nerve were full-wave rectified and integrated (time constant, 100 ms; model MA-1000; CWE). Waveforms recorded from the phrenic fibers were confirmed to represent action potentials from a single PhrMN by tracking the waveform amplitude and shape using Spike 2 software (Fig. 1 B). We recorded a total of 32 PhrMN spikes from 17 rats. All neural signals were digitized using a CED Power 1401 data acquisition interface and record on a PC using Spike2 software (Cambridge Electronic Design, Cambridge, UK).
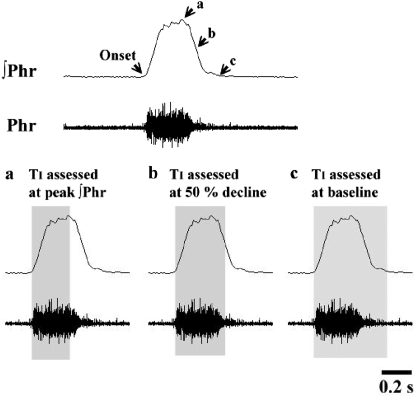
Fig. 1.
Analyses of inspiratory duration (TI) using the ∫Phr waveform. Top: an example of the raw (Phr) and integrated phrenic burst (∫Phr). Bottom: the impact of measuring TI at either the peak ∫Phr (A), at the point were ∫Phr declines by 50% of the peak (B), or at the return of ∫Phr to baseline (C). Assessing TI at peak ∫Phr clearly underestimates TI, whereas measuring TI at the return of ∫Phr to baseline results in the inclusion of postinspiratory activity. Accordingly, we assessed TI as the interval between phrenic burst onset and the point where the ∫Phr signal declined by 50% of the peak value (B).
Experimental Protocol
After adequate anesthesia and stable phrenic recordings were established, the PETCO2 apneic and recruitment threshold for inspiratory bursting in the whole phrenic nerve were determined. Ventilation was gradually increased until phrenic bursting ceased for 2 min. The recruitment threshold was defined by the reappearance of rhythmic phrenic activity when ventilation was gradually reduced. PETCO2 was maintained at 2–3 mmHg above the recruitment threshold during the experimental procedure. After individual PhrMNs were identified in the phrenic fiber recordings, STP was induced by exposing the rat to a 3-min bout of hypoxia (FIO2 = 0.12–0.14).
Data Analyses
Phrenic nerve signals including whole nerve and phrenic fiber recordings were analyzed using Spike 2 software. The integrated phrenic neurogram (∫Phr) was used to calculate respiratory frequency (bursts/min), inspiratory and expiratory phrenic burst duration (TI and TE), and peak amplitude. The TI was defined as the period between inspiratory phrenic onset and the time point when ∫Phr amplitude declined by 50% of the peak value (Fig. 1). The respiratory frequency was calculated as 60/(TI + TE). The waveforms recorded from phrenic fibers were analyzed for discharge duration (i.e., time from initial to final spike within each neural breath), total number of spikes per respiratory cycle, and frequency (total spike number divided by discharge duration). The discharge frequency of PhrMNs in the representative figures (e.g., Figs. 2, 4, and 6) were calculated in 100-ms bins. The onset time of PhrMN bursting was expressed as a percentage of TI as calculated from the contralateral ∫Phr signal. Phrenic nerve and PhrMN data were averaged over 30-s periods immediately before hypoxia (baseline), at both the onset and end of hypoxia, and 3 min after hypoxia. A one-way repeated-measures ANOVA and the Student-Newman-Keuls post hoc test (Sigma Stat 2.03, Jandel Scientific, St. Louis, MO) was used to analyze changes in the respiratory cycle, ∫Phr signal, blood pressure, and heart rate. Firing behaviors of Early-I and Late-I PhrMNs were compared using a two-way repeated-measures ANOVA and the Student-Newman-Keuls post hoc test. Blood gas data were compared between baseline and hypoxia using a paired t-test. Linear regression analyses were used to examine the relationship between discharge onset time (% TI) and PhrMN behavior (e.g., discharge duration, spike numbers, and discharge frequency) during baseline and hypoxia. P < 0.05 was considered statistically significant for all analyses. All data are presented as the mean ± SE.
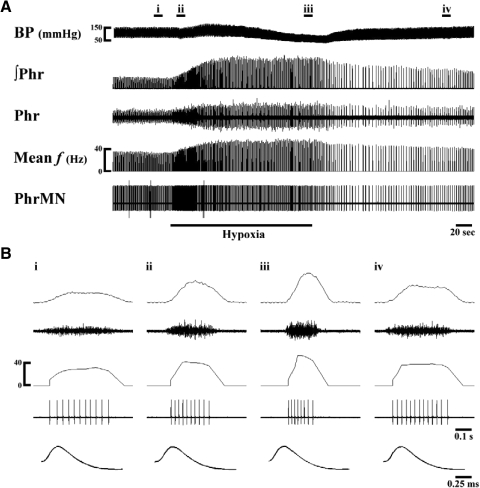
Fig. 2.
Representative phrenic neurogram and Early-I PhrMN bursting during hypoxia. A: electrical activity recorded from the whole phrenic nerve and a single fiber before, during, and after hypoxia. Whole phrenic activity is presented as both the unprocessed or “raw” signal (Phr) and the moving averaged or “integrated” signal (∫Phr). Phrenic motoneuron (PhrMN) discharge is shown as the raw signal (PhrMN) and the mean burst frequency (mean f) as calculated in 100-ms bins. B: expanded time scale traces showing a single neural breath from the areas marked i–iv in A. Bi: the single fiber recording reflects the bursting of an Early-I PhrMN (i.e., discharge onset occurring early during inspiration). The discharge frequency of this neuron was enhanced during both the onset (Bii) and end (Biii) of hypoxia. Discharge frequency remained above baseline at 3 min after hypoxia (Biv). The bottom traces of B show superimposition of the individual spikes from Bi to Biv. BP, blood pressure.
RESULTS
Arterial Blood Gases and Cardiovascular Responses
The arterial partial pressure of O2 (PaO2) was reduced during hypoxia as expected (Table 1). Hypoxia was accompanied by hypocapnia as the arterial partial pressure of CO2 (PaCO2) dropped by 5.5 ± 1.9 mmHg (Table 1). Thus we were concerned that the change in PaCO2 could influence phrenic STP. However, prior work indicated that moderate CO2 reductions during hypoxia do not prevent respiratory STP (Song and Poon 2009), and indeed, a robust STP of phrenic activity was observed during and after hypoxia. In addition, the magnitude of the acute hypoxic phrenic response reported here is both quantitatively and qualitatively similar to prior reports in which strict isocapnia was maintained (Fuller 2005; Golder et al. 2005). Finally, we confirmed in additional experiments that the drop in PaCO2 during hypoxia did not significantly alter the expression of phrenic STP.
Table 1.
Arterial blood gases and pH during baseline and hypoxia
Values are means ± SE.
P < 0.01.
P < 0.05 compared with values at baseline. PaCO2, arterial PCO2; PaO2, arterial PO2.
Arterial pH dropped by 0.03 ± 0.01 units during hypoxia despite the reduction in PaCO2. Decreases in arterial pH during hypoxia have been reported previously (Golder and Martinez 2008; Iizuka and Fregosi 2007), possibly reflecting an increase in plasma lactate concentration (Romeh and Tannen 1986). Mean arterial blood pressure (MAP) was reduced during hypoxia (P < 0.01; Table 2) as previously reported (Bavis and Mitchell 2003; Fuller 2005; Wilkerson et al. 2008). However, MAP returned to baseline values by 3 min after hypoxia (Table 2). Heart rate (HR) increased during hypoxia (P < 0.05) but also returned to baseline by 3 min after hypoxia (Table 2).
Table 2.
MAP and HR
| Baseline | Onset | End | 3 min | |
|---|---|---|---|---|
| MAP, mmHg | 124 ± 6 | 119 ± 5 | 94 ± 8* | 123 ± 5 |
| HR, beats/min−1 | 440 ± 7 | 451 ± 8† | 458 ± 10* | 445 ± 7 |
Values are means ± SE. Onset and end represent the initial and final 30 s of the hypoxic episode; 3 min represents 3 min after cessation of hypoxia.
P < 0.01 and
P < 0.05 compared with value during baseline.
MAP, mean arterial blood pressure; HR, heart rate.
Whole Phrenic Nerve
An example of phrenic motor output as assessed with whole nerve recordings is shown in Fig. 2. Baseline phrenic TI and TE were 0.41 ± 0.02 and 1.34 ± 0.14 s, respectively, and TI was progressively reduced over the course of hypoxia (P < 0.01; Fig. 3 A). In contrast, TE was shortened at hypoxia onset but returned toward baseline values by the end of the hypoxic exposure (Fig. 3B). At 3 min after hypoxia, TI was comparable to baseline (P > 0.05; Fig. 3A), but TE was significantly prolonged (P < 0.01; Fig. 3B). The increased TE was associated with a significant reduction in overall phrenic burst frequency after hypoxia as previously described [i.e., post-hypoxia frequency decline (PHFD); Bach et al. 1999; Coles and Dick 1996]. Inspiratory phrenic burst frequency was 39 ± 3 breaths/min at baseline, and this increased to a peak value of 74 ± 2 breaths/min (P < 0.01; Fig. 3C) at the onset of hypoxia. As hypoxia progressed, phrenic burst frequency gradually declined to 56 ± 2 breaths/min (Fig. 3C) and further declined to 28 ± 2 breaths/min at 3 min after hypoxia (Fig. 3C).
Fig. 3.
Effects of hypoxia on TI (A), TE (B), phrenic burst frequency (C), and ∫Phr burst amplitude (D). Both TI and TE were reduced, and burst frequency was enhanced during hypoxia. ∫Phr amplitude showed a progressive increase as hypoxia progressed. After hypoxia, TE was elongated, resulting in a decrease of phrenic burst frequency. ∫Phr amplitude was maintained above baseline at 3 min after hypoxia. *P < 0.05 and **P < 0.01 vs. baseline.
Hypoxia caused an initial rapid increase in ∫Phr burst amplitude (i.e., the acute response) followed by a progressive increase with a slower time course (i.e., STP onset; Poon et al. 1999) (Fig. 3D). After hypoxia was terminated, ∫Phr amplitude gradually declined but remained above baseline after 3 min (i.e., STP offset, Poon et al. 1999) (Fig. 3D). Because of concerns that reductions in PaCO2 during hypoxia could have influenced the magnitude of STP, additional experiments were performed (n = 10) in which strict isocapnia was maintained during hypoxia (PaCO2: baseline = 32 ± 1 mmHg, hypoxia = 32 ± 1 mmHg; P = 0.68) while phrenic neurograms (whole nerve) were recorded. Both the magnitude of the acute hypoxic phrenic response and phrenic STP were virtually identical to the earlier results [2-way repeated-measures ANOVA; factor 1: time (baseline, hypoxia onset, etc.), factor 2: treatment (isocapnic hypoxia or hypocapnic hypoxia); P = 0.72; data not shown). Accordingly, in this preparation, hypocapnia in the range of 5.5 ± 1.9 mmHg PaCO2 below baseline (32 ± 2 mmHg) does not impact phrenic STP. However, in an additional experiment (n = 1), we confirmed the earlier finding of Eldridge (1980) that more substantial reductions in PETCO2 (e.g., >10 mmHg from baseline) substantially reduces the acute phrenic response to hypoxia and virtually eliminates phrenic STP (data not shown).
Phrenic Motoneuron Discharge during Baseline
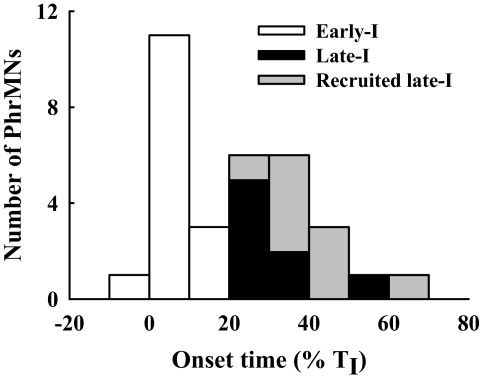
Twenty-three active PhrMNs were recorded during the baseline condition (FIO2 = 0.58 ± 0.01; see Figs. 2 and 4). The distribution of PhrMN discharge onset relative to inspiratory bursting recorded in the contralateral phrenic nerve is depicted in Fig. 5. Those PhrMNs that initiated bursting during the initial 20% of TI were classified as “Early-I PhrMNs” (Fig. 2; St John and Bartlett 1979). Of the 15 Early-I PhrMNs recorded, 11 ceased firing at the mid- to end-stage of TI; the other 4 neurons exhibited a few spikes during TE (1.0 ± 0.5 spikes/respiratory cycle). The remaining PhrMNs active at baseline (i.e., bursting initiated at >20% TI, n = 8) were classified as “Late-I PhrMNs” (Fig. 4). Baseline discharge frequency was similar between Early-I and Late-I PhrMNs (P > 0.05; Fig. 7 B); however, overall discharge duration and total spike number/breath were significantly less in Late-I PhrMNs (P < 0.01; Fig. 7, C and D). An additional nine PhrMNs were inactive during baseline conditions but were recruited during hypoxia.
Fig. 4.
Representative phrenic neurogram and Late-I PhrMN bursting during hypoxia. Both ∫Phr amplitude and Late-I PhrMN discharge frequency were progressively enhanced during hypoxia and remained above baseline levels at 3 min after hypoxia (A). B: expanded time scale traces showing a single neural breath from the areas indicated by i–iv in A. Late-I PhrMN onset time became earlier during hypoxia (e.g., Bi vs. Biii). The bottom traces of B show superimposed individual spikes from Bi to Biv. Labels are the same as in Fig. 2.
Fig. 5.
Distribution of PhrMN discharge onset. PhrMN discharge onset time was expressed as percentage of TI as measured from the contralateral phrenic neurogram. Early-I (white) and Late-I PhrMNs (black) were active during baseline conditions. The recruited late-I PhrMNs (gray) began bursting during hypoxia.
Fig. 7.
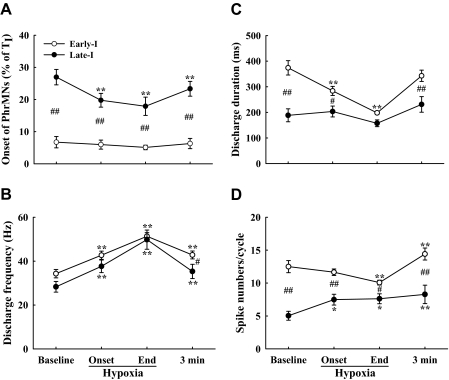
Mean discharge properties of Early-I and Late-I PhrMNs. The discharge onset time (%TI; A), frequency (Hz; B), overall duration (ms; C), and total number of spikes per neural breath (D) were quantified before, during, and after hypoxia. *P < 0.05 and **P < 0.01 vs. baseline. #P < 0.05 and ##P < 0.01, significant difference between Early-I and Late-I PhrMNs.
The relationship between onset of PhrMN bursting (expressed as % TI) and baseline burst parameters including spike numbers, overall discharge duration, and discharge frequency was examined using regression analysis. Both the total number of PhrMN spikes per respiratory cycle (r2 = 0.71) and the PhrMN discharge duration (r2 = 0.86) correlated significantly with discharge onset time (P < 0.01). In other words, PhrMNs recruited earlier in the breath had longer discharge durations and greater spike numbers compared with cells with delayed activation. However, the discharge frequency of PhrMNs was unrelated to the discharge onset time (P > 0.05). Thus baseline discharge frequency was similar between Early- and Late-I PhrMNs.
Phrenic Motoneuron Behavior during and after Hypoxia
The discharge frequency of Early-I PhrMNs was progressively enhanced over the duration of hypoxia (P < 0.01; Fig. 7B). In contrast, the overall discharge duration was gradually reduced during hypoxia (P < 0.01; Fig. 7C). As a result of the shorter burst duration, the total number of Early-I spikes per breath was reduced from 12.5 ± 0.9 (baseline) to 10.1 ± 0.4 at the end of hypoxia (P < 0.01; Fig. 7D). At 3 min after hypoxia, the overall discharge duration of Early-I PhrMNs had returned to baseline values (P > 0.05; Fig. 7C). In contrast, both the discharge frequency and total spike number/breath remained significantly above baseline values (P < 0.01; Fig. 7, B and D). Thus Early-I PhrMNs showed STP of burst frequency after hypoxia.
During hypoxia, Late-I PhrMNs began bursting earlier in the breath (i.e., reduced onset time, P < 0.05; Fig. 7A). However, the overall Late-I discharge duration was not altered during hypoxia (P > 0.05; Fig. 7C). Similar to Early-I PhrMNs, Late-I cells showed a gradual increase in burst frequency as hypoxia progressed (P < 0.01; Fig. 7B). However, in contrast to the Early-I PhrMN response, the total number of Late-I spikes/breath was increased during hypoxia (P < 0.05; Fig. 7D). After hypoxia, Late-I PhrMNs also showed evidence of STP. Specifically, Late-I cells showed a significantly higher discharge frequency and total spike number/breath at 3 min after hypoxia compared with baseline values (P < 0.01; Fig. 7, B and D).
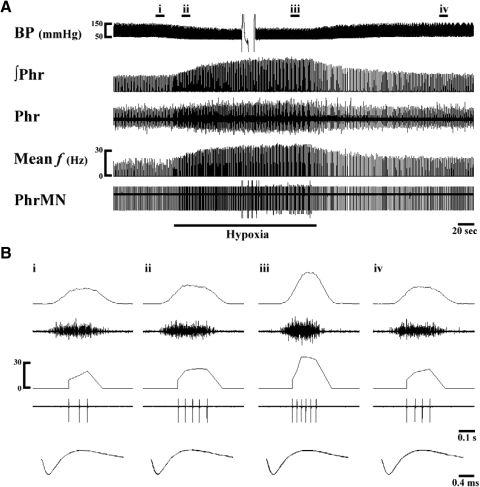
An additional nine PhrMNs were inactive during baseline conditions but were recruited during hypoxia. These initially silent PhrMNs all exhibited a late onset time (40 ± 4% TI) and were designated as “recruited late-I PhrMNs” (Fig. 6; Table 3). These recruited Late-I PhrMNs exhibited discharge frequency at 37.9 ± 2.8 Hz at the end stage of hypoxia, and all but one of these PhrMNs ceased firing on termination of hypoxia. The remaining “outlier” PhrMN continued to burst after hypoxia (frequency = 32 Hz at 3 min). Thus recruited PhrMNs do not seem to contribute substantially to post-hypoxia STP.
Fig. 6.
Representative example of hypoxia-induced recruitment of a silent Late-I PhrMN. A previously silent PhrMN was recruited after ∼45 s of hypoxia exposure (A). As shown in B, this recruited PhrMN burst with a Late-I pattern during but not after hypoxia. Labels are the same as in Figs. 2 and 4.
Table 3.
Firing behaviors of recruited Late-I PhrMNs during the onset and end of the hypoxic challenge
| Onset | End | |
|---|---|---|
| Discharge frequency, Hz | 5.4 ± 4.7 | 37.9 ± 2.8 |
| Discharge duration, ms | 16.5 ± 15.1 | 80.2 ± 13.8 |
| Spike numbers | 0.7 ± 0.6 | 3.4 ± 0.5 |
Values are means ± SE. These PhrMNs were silent during baseline recordings. PhrMN, phrenic motoneuron.
Regression analyses of the relationship between the onset of PhrMN bursting (i.e., % TI) and changes in burst behavior (i.e., spike numbers, discharge duration, and discharge frequency) during and after hypoxia is shown in Fig. 8. The increase in number of spikes/breath during hypoxia onset showed a robust correlation with onset time (r2 = 0.75; Fig. 8A, left). In other words, PhrMNs recruited earlier in the breath tended to have little change or even a decline in overall spike number during hypoxia. Conversely, cells recruited later in the breath showed an increase in spike numbers during hypoxia. This relationship was maintained at the end of hypoxic exposure (Fig. 8A, middle; r2 = 0.79) but was less robust (although still significant) during the post-hypoxia period (Fig. 8A, right; r2 = 0.45). PhrMN onset time and overall discharge duration showed a similar linear relationship during hypoxia onset, end, and the post-hypoxic period (Fig. 8B). Therefore the probability of having a greater number spikes during STP is greater for PhrMNs recruited later (Late-I) versus earlier in the breath (Early-I). In contrast to spike number and duration (Fig. 8, A and B), changes in PhrMN discharge frequency showed no correlation with onset time (P > 0.05; Fig. 8C). Thus changes in discharge frequency during and after hypoxia were similar between Early-I and Late-I PhrMNs.
Fig. 8.
The relationship between discharge onset time and PhrMN burst parameters during and after hypoxia. Onset time was expressed as %TI (abscissa), and PhrMN bursting was expressed relative to baseline values (ordinate). The results of linear regression analyses are presented for spike number (A), spike discharge duration (B), and spike discharge frequency (C).
DISCUSSION
The results of this study first show that STP can be evoked in most, if not all, PhrMNs that are active before hypoxia. Specifically, both Early-I and Late-I PhrMNs showed STP of discharge frequency during and after hypoxic challenge. Second, previously silent PhrMNs (i.e., recruited cells not active before hypoxia) are active during the onset of phrenic STP but do not seem to make a significant contribution to the post-hypoxia phase of phrenic STP. Accordingly, the onset and offset phases of respiratory STP are associated with distinct PhrMN recruitment strategies.
Critique of Methodology
We used a traditional “fiber-picking” approach (Kong and Berger 1986; Lee et al. 2007a,b, 2008; St John and Bartlett 1979) to record action potentials from PhrMN axons. Although the overall sample size of 32 PhrMNs is relatively small, we were able to record enough cells to statistically differentiate differences in firing behavior between Early-I, Late-I, and silent PhrMNs. In addition, the overall distribution of PhrMNs reported here is quite similar to what has been observed in previous reports (Kong and Berger 1986; St John and Bartlett 1979). It is nevertheless possible that our description of PhrMNs, and in particular silent PhrMNs, is not representative of the entire population of these cells. For example, Sieck and Fournier (1989) proposed that 10–15% of diaphragm motor units are active during eupneic breathing, and this number rises to only ∼30% during chemical challenge (e.g., hypoxia, hypercapnia). Accordingly, a considerable number of silent PhrMNs may become active only during expulsive behaviors such as emesis (Sieck and Fournier 1989). The population of silent PhrMNs described in this study is presumably representative of only those cells that can be recruited by hypoxia.
Other potential concerns include the integrity of the vagus nerves and the use of anesthesia (Hwang et al. 1983). Respiratory STP has been observed in both vagal intact (Xi et al. 1993) and vagotomized conditions (Golder et al. 2005; Hayashi et al. 1993; Wagner and Eldridge 1991). However, vagal afferent feedback probably attenuates the expression of phrenic STP. For example, blockade of vagal inputs slows the decay of ventilation after hypoxia in conscious dogs (Xi et al. 1993). Golder and Martinez (2008) reported that the overall increase in phrenic burst amplitude during hypoxia is more robust in vagal intact versus vagotomized rats. However, to our knowledge, the impact of vagotomy on STP has not been evaluated in the rat. Our data showed a robust phrenic STP in vagotomized rats (e.g., Fig. 2), and based on the data of Xi et al. (1993), we predict that STP would be attenuated in vagal intact rats. The potential impact of urethane anesthesia on STP is not certain. However, respiratory STP occurs in both anesthetized animals (e.g., current data; Golder et al. 2005; Hayashi et al. 1993) and conscious humans (Fregosi 1991) and animals (Kline et al. 2002; McGuire 2008). Urethane was selected as the anesthetic in this study because robust hypoxic phrenic responses occur in urethane anesthetized rats (Bach and Mitchell 1996; Baker-Herman et al. 2004). In summary, despite some caveats associated with the urethane anesthetized and vagotomized rat, this preparation provides an opportunity to examine PhrMN behavior during hypoxia-induced STP under controlled experimental conditions.
Phrmn Discharge during Baseline
Two populations of PhrMNs (i.e., Early-I and Late-I) were active during normocapnia as previously described (Kong and Berger 1986; Nail et al. 1972; St John and Bartlett 1979). The difference in burst onset between Early- and Late-I PhrMNs probably reflects both intrinsic motoneuron properties and differential regulation via presynaptic inputs (Hilaire et al. 1983; St John and Bartlett 1981). With regard to the former possibility, intracellular recordings in rats indicate that earlier onset PhrMNs have both larger membrane resistance and smaller rheobase current relative to Late-I cells (Hayashi and Fukuda 1995). Thus in accordance with Henneman's size principle (Henneman et al. 1955), Early-I PhrMNs may be more likely to depolarize for a given synaptic input. Nevertheless, any differences in intrinsic cellular properties did not seem to impact STP, because both Early-I and Late-I PhrMNs had similar changes in burst frequency during and after hypoxia. Several studies have provided evidence that differential presynaptic inputs contribute to PhrMN recruitment order (Hilaire et al. 1983; Saboisky et al. 2007; St John and Bartlett 1981). For example, Hilaire et al. (1983) used cross-correlation to show that common synaptic inputs to Early-I and Late-I PhrMNs seem to be relatively rare. Accordingly, the pattern of activation of these cell groups may reflect distinct innervation of Early-I and Late-I neurons versus exclusively intrinsic neuronal properties. Similarly, Saboisky et al. (2007) suggested that respiratory motoneuron bursting in humans is determined primarily by descending inputs (vs. intrinsic motoneuron properties). However, the neuroanatomical substrate underlying any potential differences in presynaptic input to PhrMNs is not defined. In this regard, we recently reported that some cervical spinal interneurons are interposed between the rostral ventral respiratory group (rVRG) and PhrMNs in the rat (Lane et al. 2008) as has been reported in other species (Bellingham 1999; Lois et al. 2008; Palisses et al. 1989; Yates et al. 1999). Given that at least some of these prephrenic interneurons show respiratory modulation (Hayashi et al. 2003; Lane et al. 2009), it is tempting to speculate that these cells may be involved in shaping the overall pattern of Early-I and Late-I PhrMN recruitment during breathing.
Phrmn Discharge during and after Hypoxia
Both Early-I and Late-I PhrMNs responded acutely to hypoxia with a brisk increase in discharge frequency (St John and Bartlett 1979). Moreover, both PhrMN types showed a progressive increase in discharge frequency as hypoxia progressed. We suggest that this gradual increase in discharge frequency represents activation of phrenic STP mechanisms. However, contrary to our initial hypothesis, the relative increases in discharge frequency were similar between PhrMN subtypes. Accordingly, “frequency STP” was similar between PhrMNs regardless of when the cells were recruited during the inspiratory cycle. Thus intrinsic differences in excitability between these PhrMN populations (Hayashi and Fukuda 1995) may not be a key factor in determining STP of phrenic motor output.
Similar to prior reports (St John and Bartlett 1979), hypoxia influenced the onset of bursting in Late-I but not Early-I PhrMNs. More specifically, PhrMNs normally activated later in the inspiratory cycle (e.g., Late-I) began to burst earlier during the hypoxic challenge (St John and Barlett 1979). In contrast, Early-I PhrMNs had a similar onset time during both baseline and hypoxic challenge. Early-I PhrMNs also had a reduction in overall discharge duration during hypoxia. The net result was that, despite the increased discharge frequency, the total number of action potentials (spikes) per breath was actually reduced for Early-I PhrMNs during STP onset. On the other hand, Late-I PhrMNs maintained their discharge duration during hypoxia, and thus the total spikes/breath was increased during the onset of STP. It is thus conceivable that Late-I cells make a relatively greater functional contribution to STP of diaphragm activity and ventilation during hypoxia.
In addition to previously active Early- and Late-I PhrMNs, a population of silent PhrMNs was recruited during hypoxia. Similar quiescent PhrMNs have been observed in neonatal and adult rats as well as adult cats (Hayashi and Fukuda 1995; Milano et al. 1992; Nail et al. 1972; St John and Bartlett 1979; Su et al. 1997). In our study, the recruited PhrMNs began bursting after the initial hypoxic response and thus were not observed until 48 ± 8 s after the initiation of hypoxia. This delayed onset suggests that recruitment of these neurons represents an important component of the onset of STP but not the initial acute hypoxic response. We suggest that silent PhrMNs represent a pool of cells that can be recruited during respiratory plasticity including STP, LTF, and perhaps the crossed phrenic phenomenon after high cervical spinal cord hemisection injury (El-Bohy and Goshgarian 1999). However, to our knowledge, there have been no previous formal studies of the role of silent PhrMNs in the expression of respiratory-related neuroplasticity. Because only a relatively small portion of the total PhrMN pool is active during “eupneic” breathing (Sieck and Fournier 1989), recruitment of previously inactive PhrMNs may represent a common mechanism associated with the expression of a variety of forms of respiratory neuroplasticity.
Stp Mechanism(S)
It is generally accepted that STP reflects a central neural mechanism because it can be evoked by electrical stimulation of the carotid sinus nerve (Wagner and Eldridge 1991). Thus, although the acute hypoxic response is triggered primarily by peripheral chemoreceptors (Bavis and Mitchell 2003), their continued activation is not necessary for STP expression. Within the CNS, there is evidence that both brain stem (Mifflin 1997; Young et al. 2003) and spinal cord (Hayashi et al. 2003; McCrimmon et al. 1997) mechanisms contribute to STP. For example, Mifflin (1997) showed that high-frequency stimulation of the carotid sinus nerve potentiated subsequent evoked responses in neurons within the nucleus of the solitary tract (NTS). Thus “integration” of peripheral inputs within the NTS may be enhanced during STP. On the other hand, spinal cord stimulation can evoke STP-like changes in respiratory motor output in spinalized rats (Hayashi et al. 2003; McCrimmon et al. 1997) and turtles (Johnson and Mitchell 2002). Accordingly, the mechanisms underlying phrenic STP may also include changes in PhrMNs and/or spinal circuits (Fuller et al. 2005), including respiratory interneurons (Lane et al. 2008). In other words, STP could reflect a (relatively) constant descending respiratory drive but a more responsive motor pool.
It is unknown if similar mechanisms contribute to the onset (i.e., during hypoxia) versus offset (i.e., after hypoxia) phases of respiratory STP. One hypothesis is that the gradual decrease of respiratory output after hypoxia represents a time-dependent “decay” of the STP mechanism (Powell et al. 1998). However, because the onset and decline of STP generally follow a different time course (Wagner and Eldridge 1991), it may be that distinct mechanisms contribute to each phase (Fuller et al. 2005). We suggest that our PhrMN discharge data are consistent with this latter suggestion. For example, the expression of STP during hypoxia occurred in parallel with increased discharge frequency of both Early-I and Late-I PhrMNs, as well as recruitment of previously silent late-I PhrMNs. Thus the increased bursting in each of these cell populations contributed to the observed STP of phrenic motor activity. After hypoxia, however, STP was associated with increased bursting of those Early-I and Late-I PhrMNs that were active before hypoxia, but the recruited, silent PhrMN population did not make a substantial contribution. The abrupt cessation of silent PhrMN bursting after hypoxia is consistent with a removal of descending drive to these cells. Taken together, the observed PhrMN behavior indicates that the decay of STP does not represent a nonspecific or “general” potentiation of all PhrMNs activated during hypoxia. Rather, the STP mechanism is specific between PhrMN types and is differentially regulated during onset and offset.
Other differences in PhrMN behavior were noted during the onset versus offset of STP. For instance, the enhanced discharge frequency of Early-I PhrMNs during the onset of STP was accompanied by a reduction in both overall discharge duration and total spike numbers. In contrast, during the recovery phase of STP, the number of Early-I PhrMN spikes per breath was increased without alteration in overall discharge duration. This is consistent with earlier observations that the time course of STP differs between the onset and offset phases (Fuller et al. 2005; Wagner and Eldridge 1991).
Conclusion
The purpose of hypoxia-induced respiratory STP is not precisely known. However, STP may serve as a mechanism to reduce variability in respiratory output and thereby enhance the stability of breathing during and after hypoxia (Fuller et al. 2005). In this regard, it is interesting that STP may be impaired in patients with congestive heart failure and obstructive sleep apnea (Ahmed et al. 1994; Georgopoulus et al. 1992).
Here we report that the initiation of STP involves three types of PhrMNs (i.e., Early-I, Late-I, and silent recruited Late-I). However, post-hypoxia STP primarily reflects discharge of Early-I and Late-I PhrMNs. Thus the induction of plasticity associated with persistent recruitment of silent PhrMNs may require more robust and/or repeated activation such as would occur with intermittent bouts of hypoxia.
GRANTS
This work was supported by National Institutes of Health Grants 1R01 HD-052682-01A1 to D. D. Fuller and 1R01 NS-054025 to P. J. Reier. Support was also provided by the Oscar and Anne Lackner Chair in Medicine to P. J. Reier. K.-Z. Lee was supported by a grant from the University of Florida.
REFERENCES
- Ahmed M, Serrette C, Kryger MH, Anthonisen NR. Ventilatory instability in patients with congestive heart failure and nocturnal Cheyne-Stokes breathing. Sleep 17: 527–534, 1994 [DOI] [PubMed] [Google Scholar]
- Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res 817: 25–33, 1999 [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol 94: 399–409, 2003 [DOI] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostals nerve stimulation. J Neurophysiol 82: 1224–1232, 1999 [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90, 1979 [DOI] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J Physiol 497: 79–94, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol 200: 74–81, 2006 [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Goshgarian HG. The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol 156: 172–179, 1999 [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Subthreshold central neural respiratory activity and afterdischarge. Respir Physiol 39: 327–343, 1980 [DOI] [PubMed] [Google Scholar]
- Fregosi RF. Short-term potentiation of breathing in humans. J Appl Physiol 71: 892–899, 1991 [DOI] [PubMed] [Google Scholar]
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol 98: 1761–1767, 2005 [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Mitchell GS. Respiratory plasticity: respiratory gases, development, and spinal injury. In: Pharmacology and Pathophysiology of the Control of Breathing, Ward DS, Dahan A, Teppema L. Boca Raton, FL: Taylor and Francis, 2005, 155–223 [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratoru motor recovery. Respir Physiol Neurobiol 165: 245–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulus D, Giannouli E, Tsara V, Arqiropoulou P, Patakas D, Anthonisen NR. Respiratory short-term poststimulus potentiation (after-discharge) in patients with obstructive sleep apnea. Am Rev Respir Dis 146: 1250–1255, 1992 [DOI] [PubMed] [Google Scholar]
- Golder FJ, Martinez SD. Bilateral vagotomy differentially alters the magnitude of hypoglossal and phrenic long-term facilitation in anesthetized mechanically ventilated rats. Neurosci Lett 442: 213–218, 2008 [DOI] [PubMed] [Google Scholar]
- Golder FJ, Zabka AG, Bavis RW, Baker-Herman T, Fuller DD, Mitchell GS. Differences in time-dependent hypoxic phrenic responses among inbred rat strains. J Appl Physiol 98: 838–844, 2005 [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs decerebellate rats. Am J Physiol 265: R811–R819, 1993 [DOI] [PubMed] [Google Scholar]
- Hayashi F, Fukuda Y. Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol 45: 69–83, 1995 [DOI] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CFL, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol 94: 1421–1430, 2003 [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitory of motoneurons of different sizes. J Neurophysiol 28: 599–620, 1956 [DOI] [PubMed] [Google Scholar]
- Hilaire G, Gauthier P, Monteau R. Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respir Physiol 51: 341–359, 1983 [DOI] [PubMed] [Google Scholar]
- Hwang JC, St John WM, Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol 55: 785–792, 1983 [DOI] [PubMed] [Google Scholar]
- Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol 157: 196–205, 2007 [DOI] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity in descending synaptic inputs to respiratory spinal motoneurons. Respir Physiol Neurobiol 131: 79–90, 2002 [DOI] [PubMed] [Google Scholar]
- Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol 539: 309–315, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol 61: 1999–2004, 1986 [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD, Lu IJ, Ku LC, Hwang JC. Pulmonary C-fiber receptor activation abolishes uncoupled facial nerve activity from phrenic bursting during positive end-expired pressure in the rat. J Appl Physiol 104: 19–129, 2008 [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD, Lu IJ, Lin JT, Hwang JC. Neural drive to tongue protrudor and retractor muscles following pulmonary C-fiber activation. J Appl Physiol 102: 434–444, 2007a [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD, Tung LC, Lu IJ, Ku LC, Hwang JC. Uncoupling of upper airway motor activity from phrenic bursting by positive end-expired pressure in the rat. J Appl Physiol 102: 878–889, 2007b [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106: 138–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol 82: 419–425, 1997 [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol 110: 161–176, 1997 [DOI] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Physiol 105: 942–950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Short-term potentiation of carotid sinus nerve inputs to neurons in the nucleus of the solitary tract. Respir Physiol 110: 229–236, 1997 [DOI] [PubMed] [Google Scholar]
- Milano S, Grélot L, Bianchi AL, Iscoe S. Discharge patterns of phrenic motoneurons during fictive coughing and vomiting in decerebrate cats. J Appl Physiol 73: 1626–1636, 1992 [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- Nail BS, Sterling GM, Widdicombe JG. Patterns of spontaneous and reflexly-induced activity in phrenic and intercostals motoneurons. Exp Brain Res 15: 318–332, 1972 [DOI] [PubMed] [Google Scholar]
- Palisses R, Perséqol L, Viala D. Evidence for respiratory interneurones in the C3-C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res 78: 624–632, 1989 [DOI] [PubMed] [Google Scholar]
- Pickering M, Jones JF. Comparison of the motor discharge to the crural and costal diaphragm in the rat. Respir Physiol Neurobiol 159: 21–27, 2007 [DOI] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS, Young DL, Eldridge FL. Short-term potentiation of carotid chemoreflex: an NMDAR-dependent neural integrator. Neuroreport 10: 2261–2265, 1999 [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- Romeh SA, Tannen RL. Amelioration of hypoxia-induced lactic acidosis by superimposed hypercapnea or hydrochloric acid infusion. Am J Physiol 250: F702–F709, 1986 [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Bulter JE. Differential activation among five human inspiratory motoneuron pool during tidal breathing. J Appl Physiol 102: 772–780, 2007 [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol 66: 2539–2545, 1989 [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol 65: 1–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM, Bartlett D., Jr Comparison of phrenic motoneuron responses to hypercapnia and isocapnic hypoxia. J Appl Physiol 46: 1096–1102, 1979 [DOI] [PubMed] [Google Scholar]
- St John WM, Bartlett D., Jr Comparison of phrenic motoneuron during eupnea and gasping. J Appl Physiol 50: 994–998, 1981 [DOI] [PubMed] [Google Scholar]
- Stuth EA, Dogas Z, Krolo M, Kampine JP, Hopp FA, Zuperku EJ. Dose-dependet effects of halothane on the phrenic nerve responses to acute hypoxia in vagotomized dogs. Anesthesiology 87: 1428–1439, 1997 [DOI] [PubMed] [Google Scholar]
- Su CK, Mellen NM, Feldman JL. Intrinsic and extrinsic factors affecting phrenic motoneuronal excitability in neonatal rats. Brain Res 774: 62–68, 1997 [DOI] [PubMed] [Google Scholar]
- Wagner PG, Eldridge FL. Development of short-term potentiation of respiration. Respir Physiol 83: 129–139, 1991 [DOI] [PubMed] [Google Scholar]
- Wilkerson JER, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28: 2949–2958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Smith CA, Saupe KW, Dempsey JA. Effects of memory from vagal feedback on short-term potentiation of ventilation in conscious dogs. J Physiol 462: 547–561, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience 90: 1501–1513, 1999 [DOI] [PubMed] [Google Scholar]
- Young DL, Eldridge FL, Poon CS. Integration-differentiation and gating of carotid afferent traffic that shapes the respiratory pattern. J Appl Physiol 94: 1213–1229, 2003 [DOI] [PubMed] [Google Scholar]