Abstract
Alterations in neuronal Ca2+ homeostasis are important determinants of age-related cognitive impairment. We examined the Ca2+ influx, buffering, and electrophysiology of basal forebrain neurons in adult, middle-aged, and aged male F344 behaviorally assessed rats. Middle-aged and aged rats were characterized as cognitively impaired or unimpaired by water maze performance relative to young cohorts. Patch-clamp experiments were conducted on neurons acutely dissociated from medial septum/nucleus of the diagonal band with post hoc identification of phenotypic marker mRNA using single-cell RT-PCR. We measured whole cell calcium and barium currents and dissected these currents using pharmacological agents. We combined Ca2+ current recording with Ca2+-sensitive ratiometric microfluorimetry to measure Ca2+ buffering. Additionally, we sought changes in neuronal firing properties using current-clamp recording. There were no age- or cognition-related changes in the amplitudes or fractional compositions of the whole cell Ca2+ channel currents. However, Ca2+ buffering was significantly enhanced in cholinergic neurons from aged cognitively impaired rats. Moreover, increased Ca2+ buffering was present in middle-aged rats that were not cognitively impaired. Firing properties were largely unchanged with age or cognitive status, except for an increase in the slow afterhyperpolarization in aged cholinergic neurons, independent of cognitive status. Furthermore, acutely dissociated basal forebrain neurons in which choline acetyltransferase mRNA was detected had the electrophysiological profiles of identified cholinergic neurons. We conclude that enhanced Ca2+ buffering by cholinergic basal forebrain neurons may be important during aging.
INTRODUCTION
The basal forebrain (BF) contains a heterogeneous population of neurons, including cholinergic, GABAergic, and glutamatergic cell types. The BF integrates multimodal sensory information and interacts with cortical and subcortical sites to control cognitive processes such as attention, arousal, and some forms of memory (Sarter et al. 2003). Disruption of the BF cholinergic system during aging is thought to be a critical determinant of age-related cognitive impairment, dementia, and pathology (Sarter and Bruno 2004). In humans, BF cholinergic projection cells are lost during aging, senile dementia, and Alzheimer's disease (Bartus 2000). BF cholinergic neurons are lost in aged rats, as well (Fischer et al. 1992; Smith and Booze 1995). Properties specific to BF cholinergic neurons are thought to render them susceptible to age-related changes that influence cognitive function (McKinney 2005; Sarter and Bruno 2004; Wenk and Willard 1999). In our efforts to describe these changes, we have found that intracellular calcium (Ca2+) buffering values increase with age in rat BF neurons (Murchison and Griffith 1998) and that this buffering change can be reversed in cholinergic BF neurons by caloric restriction (Murchison and Griffith 2007). Alterations in Ca2+ buffering lead to altered Ca2+ signaling and from that to the initiation of a cascade of effects that influence neuronal function (Berridge et al. 2000; Petersen et al. 2005). Indeed, such a scenario has been considered as a primary mechanism of age-related neuronal dysfunction (reviewed in Kelly et al. 2006) in the past and evidence of age-related dysfunction of Ca2+ signaling continues to accumulate (Bezprozvanny and Mattson 2008). Currently, it is unknown how increased Ca2+ buffering in neurons of the aged rat BF alters function or how relevant this increase is to cognitive impairment during aging.
In the present study, we categorize aged rats as impaired or unimpaired, based on a learning index score in the water maze task (Bizon et al. 2009; Gallagher et al. 1993). The advantages of this testing paradigm are that a graded measure of the severity of age-related cognitive impairment can be determined for individual subjects and that the BF is involved in the learning of this task (Fischer et al. 1989, 1992). We show that elevated intracellular Ca2+ buffering values from BF cholinergic neurons of aged rats are associated with impaired cognition, suggesting that altered Ca2+ signaling with age in the BF cholinergic system is relevant to cognitive decline. Furthermore, we show that the change in buffering occurs in middle-aged rats prior to the onset of cognitive impairment. Additionally, we suggest that the mechanism by which altered buffering affects cognition may involve synaptic function rather than membrane firing properties.
METHODS
Animal Subjects
Male Fisher 344 rats were obtained from the National Institute on Aging colony (Harlan, Indianapolis, IN) and were the following average ages at the onset of behavioral testing: 6 mo (young, n = 35), 13 mo (middle-aged, n = 26), and 23 mo (aged, n = 46). Rats were housed in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, maintained at a consistent 25°C with a 12-h:12-h light/dark cycle and with unrestricted access to food and water. Most rats used for electrophysiological experiments were killed between 1 wk and 2 mo posttraining, but none later than 3 mo. Training and experiments were conducted on groups of rats of mixed ages. All animal procedures were conducted in accordance with Institutional Animal Care and Use Committee–approved institutional animal care practices and National Institutes of Health guidelines.
Behavioral Characterization
Rats were trained on a spatial-memory task in the Morris water maze. The water maze consisted of a large circular tank (diameter, 183 cm; wall height, 58 cm) painted white and filled with water (27°C), made opaque by the addition of nontoxic tempura paint. A retractable escape platform (diameter, 12 cm; HVS Image, Buckingham, UK) was submerged 2 cm below the surface in the southwest quadrant of the maze. Black curtains with large white geometric designs provided extramaze cues. Data were acquired via a video camera mounted above the maze that was connected to a DVD recorder and computer with a video tracking system (Water 2020, HVS Image). The white F344 rats had a black patch dyed onto their backs so that they could be resolved by the motion-capture software.
The spatial training protocol has been previously described in detail (Bizon et al. 2009; Gallagher et al. 1993; LaSarge et al. 2007). Rats received three training trials/day for 8 consecutive days using a 60-s intertrial interval. On each training trial, rats were placed in the water and allowed to swim until finding the platform or for 90 s, at which time they were placed on the platform by the experimenter. Rats remained on the platform for 30 s before removal from the maze and the start of the intertrial interval.
For spatial learning assessment, the location of the platform remained constant in one quadrant of the maze and the starting position for each trial was varied among four equally spaced positions around the perimeter of the maze. Every sixth trial was a probe trial, during which the platform was retracted to the bottom of the pool for the first 30 s of the 90-s trial. Training and probe trials assessed acquisition and search strategy, respectively (Bizon et al. 2009). Groups of five training trials and one probe trial over 2 days constituted one training block.
To assess sensorimotor skills and motivation independent of spatial learning ability, rats received one session of six trials of cue training after the last day of spatial training. Rats were trained to escape to a visible black platform located 2 cm above the water that varied in position from trial to trial. Rats were given 30 s to reach the platform and were allowed to remain there briefly before a 30-s intertrial interval.
Accuracy of performance was assessed using pathlength on training trials and a learning index (LI) score (computed from proximity to the platform) on probe trials. As detailed previously (Bizon et al. 2009), pathlengh is the distance from the start position to the platform represented in centimeters. On probe trials, the distance of the rat from the platform was sampled 10 times/s and averaged into 1-s bins that were summed and then divided by the duration of the probe trial (i.e., 30 s). The LI was calculated from the average proximity on the second, third, and fourth probe trials. Scores from these trials were weighted and summed to provide an overall measure of spatial learning ability. Lower scores on the LI indicate a more accurate search.
Age comparisons were made using a two-way ANOVA (age × training block) to compare pathlength and a one-way ANOVA to compared LI scores from probe trials and mean pathlengths from cue (visible) training trials. Fisher's least-significant difference test was used for post hoc analyses. In this study, performance of middle-aged and aged rats is referred to as “cognitively unimpaired” or “cognitively impaired.” “Cognitively impaired” rats possessed LIs outside the range of young rats (>280), whereas cognitively unimpaired middle-aged and aged rats had LI scores within the range of young rats (<280). Note that in preliminary experiments, a few animals (five young, four aged) were trained using a similar protocol, but LIs were not calculated. In this assessment, impairment in aged rats was defined as performance that fell 2SDs outside the mean of young performance on the final probe trial. All neurobiological data from these subjects were consistent and comparable to those obtained using the more refined LI score calculation to determine cognitive status. In all statistical comparisons, values of P < 0.05 were considered significant. Beginning 1 wk after cued (visible) platform training, electrophysiological experiments were conducted on acutely dissociated basal forebrain neurons to relate somatic calcium buffering and membrane properties to cognitive status. Recorded neurons were identified as cholinergic or noncholinergic using single-cell reverse transcription–polymerase chain reaction (scRT-PCR), as described in the following text.
Acutely Dissociated Neurons
Briefly, isoflurane (Anaquest, Liberty Corner, NJ) anesthetized rats were decapitated and coronal brain slices (440 μm) were microdissected to isolate the medial septum and nucleus of the diagonal band (MS/nDB) and enzymatically treated (trypsin 0.7–1.0 mg/ml; Sigma Type XI). Cells were dispersed and allowed to settle onto the glass floor of a recording chamber and then perfused at a rate of about 2 ml/min. Experiments were performed at room temperature (20–21°C) within 5 h of dispersal.
Electrophysiology Experiments
Standard whole cell and perforated patch-clamp techniques were used to measure high-voltage–activated (HVA) Ca2+ currents, cellular calcium buffering values, and membrane properties in acutely dissociated basal forebrain neurons, as detailed in Murchison and Griffith (1996, 1998). For Ca2+ current measurements, 2 mM Ba2+ was used as charge carrier and voltage clamp was obtained with the perforated-patch method. We used amphotericin B (Rae et al. 1991) or β-escin (Sarantopoulos et al. 2004) as the perforating agents. For Ca2+ buffering experiments, Ca2+ was used as the charge carrier and voltage-clamped neurons were depolarized to activate voltage-gated Ca2+ channels (VGCCs) and produce intracellular Ca2+ loads of different magnitudes. The fluorescent calcium indicator fura-2 was used to determine the spatially averaged intracellular calcium ion concentration ([Ca2+]i) in the soma. Calcium current and buffering experiments used an Axopatch 200A amplifier (Axon Instruments). Membrane properties were assessed by whole cell current-clamp recordings using a Multiclamp 700B amplifier (Axon Instruments). Neurons were then processed for scRT-PCR to identify them.
Solutions and Drugs
For recording buffering values, the extracellular solution contained (in mM): 132 NaCl, 2 CaCl2, 2 MgCl2, 33 d-glucose, 10 tetraethyl ammonium chloride (TEA), 0.00035 tetrodotoxin (TTX, Calbiochem, La Jolla, CA), and 10 HEPES (pH was adjusted to 7.4 with NaOH and osmolarity was about 315 mOsm). For recording HVA Ca2+ channel currents, the bath was identical, except BaCl2 was substituted for CaCl2. The conventional whole cell patch pipette solution contained (in mM): 124 Cs-acetate, 20 HEPES, 15 CsCl, 10 TEA, 4 ATP, 0.05 fura-2 Ks+ (Molecular Probes, Eugene, OR). The internal pipette solution for perforated-patch recordings contained (in mM): 120 CsAc, 15 CsCl, 20 HEPES, 0.4 EGTA, and amphotericin B (240 μg/ml) or β-escin (25 μM). Amphotericin B was initially dissolved in dimethyl sulfoxide (final concentration 0.4). For current-clamp experiments, the recording bath contained (in mM): NaCl 140, KCl 3, CaCl2 2, MgCl2 1.2, glucose 33, and HEPES 10 (pH = 7.4). The internal pipette solution contained (in mM): K-gluconate 136, NaCl 25, HEPES 10, MgCl2 2, ATP 4, and EGTA 0.05. All pipette solutions were adjusted to pH 7.2 with CsOH and to about 290 mOsm. The pipette solutions were prepared with RNAse-free water under sterile conditions and contained 50 μl/ml RNase inhibitor (10 U/μl; Invitrogen, Carlsbad, CA). Nifedipine (10 μM) was initially dissolved in ethanol/ethylene glycol at a final concentration of 0.2 and 0.1, respectively. ω-Conotoxin (CTX) MVIIC (500 nM, Alomone Labs) was dissolved in the bath solution. All chemicals were obtained from Sigma (St. Louis, MO) unless indicated.
Ca2+ Buffering Values
Patch-clamp techniques for recording VGCCs (Murchison and Griffith 1996) were combined with Ca-sensitive ratiometric microfluorimetry (Grynkiewicz et al. 1985; Murchison and Griffith 1998). Background and autofluorescence were subtracted at each wavelength (340 and 380 nm) prior to computation of the fluorescence ratio. In vitro and in vivo calibrations were used to estimate [Ca2+]i according to the standard equations. Values for buffering value (β) and maximum resistance (Rmax) were determined by in vitro measurement. Intracellular solutions identical to those used for recording were prepared with calculated concentrations of EGTA and Ca2+ to generate solutions with nominal [Ca2+] of 10 nM (minimum) and 36–50 μM (maximum). Fura-2 was added at a concentration (3 μM) that produced fluorescence in a 1-μl drop that approximated the fluorescent intensity of 50 μM fura in vivo. The value for minimum resistance (Rmin) was taken from in vivo recordings in which 10 mM EGTA was included in the normal intracellular solution. In vivo Kd for fura-2 was estimated at 250 nM (Murchison and Griffith 1998).
Buffering values of individual neurons were calculated as described previously (Murchison and Griffith 1998). Ca2+ influx was determined by integrating ICa to yield total charge movement, then dividing by 2 to obtain the ionic Ca2+ flux, dividing by Avogadro's number to find the molar flux and dividing by the estimated cell volume. Cell volume was estimated from the membrane capacitance, assuming the capacitance of biological membranes is 1 μF/cm2, and the cell was modeled as a hollow sphere. Ca2+ entry was plotted against the peak amplitude of the Δ[Ca2+]i measured with fura-2. The slope (determined by linear regression) of this graph reflected the ratio of free to bound (buffered) Ca2+ ions and the reciprocal of the slope (−1) was the rapid buffering value.
Single-Cell RT-PCR
Neurons were aspirated into the recording pipette and the contents were ejected into a sterile 0.5-ml siliconized tube (Ambion, Austin, TX) containing the initial RT solution (Griffith et al. 2006; Han et al. 2005). The tube was frozen in dry ice then stored overnight (−80°C). Standard RT was performed with SuperScript II reverse transcriptase (Invitrogen).
Real-time PCR was performed with an ABI Prism 7700 or an ABI 7500 Fast sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan (Applied Biosytems) detection probes (Han et al. 2005). Primer Express 1.5 (Applied Biosystems) was used to design forward and reverse primers and TaqMan probes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), choline acetyltransferase (ChAT), and glutamic acid decarboxylase 67 (GAD). Positive controls (whole brain cDNA) were run on each reaction plate and negative controls included PCR without cDNA template, aspirated bath solution, and cDNA template without RT. Data were expressed as an amplification plot and detection of a transcript was confirmed if the linear phase of the amplification plot (parallel to the positive marker GAPDH) crossed the threshold fluorescence intensity (detection threshold [CT], set above the fluorescent noise) before reaching the 36th amplification cycle. Nonparallel amplifications or plots crossing the CT after the 36th cycle were considered not detected. This limit was imposed because false-positive signals from GAPDH (in negative controls) were occasionally observed to cross the CT after the 36th cycle.
Electrophysiological Analyses
Data were compared using an independent two-tailed t-test, a Mann–Whitney test or a one-way ANOVA with Dunnett's test for multiple comparisons, or a Kruskal–Wallis rank test with a Dunn's method multiple comparison for nonparametrically distributed data, as appropriate with significance determined by P < 0.05 (SigmaStat, SPSS, Chicago, IL). Values are reported as means ± SE. Analyses of behavior, of electrophysiology, and of mRNA expression were conducted blind with respect to the results of the other analyses.
RESULTS
Behavioral Assessment
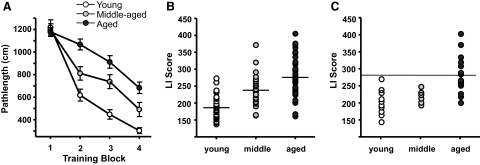
The results of spatial learning assessment in young, middle-aged, and aged cohorts were similar to those of previous studies using the same training paradigm (Bizon et al. 2009; LaSarge et al. 2007). Performance of young, middle-aged, and aged rats improved during the course of training [F(3,312) = 119.485, P < 0.001]. Although the pathlength to the platform did not differ on the first training block [F(2,106) = 0.15, ns], as shown in Fig. 1A, middle-aged and aged rats were subsequently impaired in the acquisition of this spatial task relative to young rats. This was evident in a two-way repeated-measures ANOVA that demonstrated a significant main effect of age during training [F(2,104) = 23.228, P < 0.001] and an interaction of age and pathlength [F(6,312) = 7.746, P < 0.001]. Post hoc analyses revealed that as a group, middle-aged rats were impaired relative to young cohorts and aged rats were impaired relative to both young and middle-aged rats (P < 0.01 in all cases).
Fig. 1.
Behavioral testing and assessment of cognitive status of individual F344 rats. Rats were trained in the hidden platform version of the Morris water maze and tested by probe trials as described in methods. A: learning curves of all rats. The graph shows mean cumulative pathlength during each of the successive training trials for young, middle-aged, and aged rats. B: scatterplot shows the Learning Index (LI) score distribution of individual subjects within the 3 age groups for all of the rats in the sample used for electrophysiology experiments. Horizontal lines indicate the mean for each age group. LI scores were calculated as described in methods. C: scatterplot of LIs of rats in the calcium buffering experiments. The horizontal line at the LI score of 280 indicates the cutoff between rats considered cognitively impaired and those considered unimpaired and represents a score that is outside the range of the young animals. Subjects earning an LI score >280 were considered cognitively impaired. The learning curves and mean LI scores of each age group were significantly different from one another, as described in the text.
Individual learning index (LI) scores of all animals used for electrophysiological experiments and of the subset of animals used in Ca2+ buffering studies are shown in Fig. 1,B and C, respectively. Lower LI scores indicate better performance. Analysis of spatial LIs revealed a main effect of age on LI [F(2,106) = 36.558, P < 0.001], such that middle-aged rats were impaired compared with young and aged rats compared with both middle-aged and young rats (P < 0.001 in all cases). Note however, that approximately half of the aged rats and most middle-aged rats had LI scores comparable with those of young rats (i.e., <280), whereas others fell outside this range. In contrast to the effects of age on spatial reference learning abilities, there was no impairment in the ability of aged rats to locate a visible escape platform during cue (visible platform) training. A one-way ANOVA revealed no significant difference between pathlength of young, middle-aged, or aged rats [F(2,89) = 1.72, ns].
High-Voltage–activated Ca2+ Currents in Behaviorally Characterized Rats
Age-related increases in Ca2+ currents, particularly the HVA L-type current (Campbell et al. 1996; Moyer Jr and Disterhoft 1994; Thibault and Landfield 1996), have been implicated in age-related cognitive impairment (Disterhoft et al. 1996; Tombaugh et al. 2005). Increased HVA Ca2+ channel function, in the form of reduced inactivation, has been described in BF neurons from aged rats (Murchison and Griffith 1996). However, the relationship between Ca2+ channel function in identified cholinergic BF neurons and cognitive status during aging has not been investigated. We used perforated patch voltage-clamp techniques coupled with scRT-PCR to measure the properties of HVA Ca2+ currents in cholinergic BF neurons acutely dissociated from behaviorally characterized rats in each of the three age groups. The perforated patch method, as opposed to the whole cell patch, prevents HVA current rundown (Gillis et al. 1991; Murchison and Griffith 1996). HVA current fractions arising from different channel types were assessed by pharmacological blockade with the N-type or P/Q-type channel blocker ω-conotoxin (CTX) MVIIC and the dihydropyridine L-type channel blocker nifedipine. Neurons were identified as cholinergic if ChAT sequence was detected by post hoc scRT-PCR (see following text for justification).
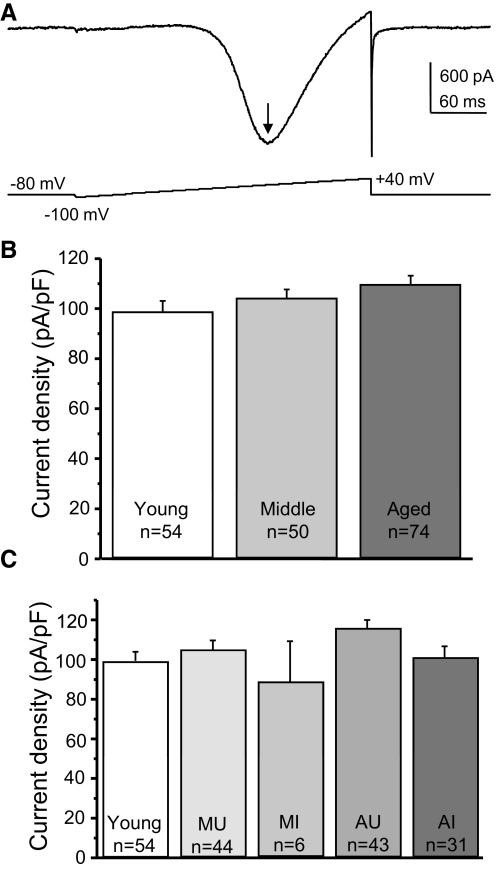
Peak current densities (pA/pF) with 2 mM Ba2+ as charge carrier were determined from voltage-ramp protocols, as shown in Fig. 2A. There was no difference in the peak Ca2+ current densities of young, middle-aged, or aged rat BF cholinergic neurons (Fig. 2B), nor was there any difference between cognitively impaired or unimpaired individuals (Fig. 2C). These data confirm previous observations that the function of BF neuron Ca2+ channels does not decline with age (Murchison and Griffith 1995, 1996) and show that the density of whole cell HVA current, per se, does not contribute to cognitive impairment in this model.
Fig. 2.
Perforated-patch recording of high-voltage–activated (HVA) Ba2+ current densities in cholinergic basal forebrain (BF) neurons from behaviorally characterized rats. Acutely dissociated voltage-clamped BF neurons were assessed for HVA voltage-gated Ca2+ channel (VGCC) function by measuring the peak current density (pA/pF) generated by a standard ramp voltage in perforated-patch configuration with 2 mM Ba2+ as charge carrier. Solutions for the isolation of HVA Ba2+ current are described in methods and neurons were determined to be cholinergic by detection of choline acetyltransferase (ChAT) sequence by post hoc single-cell reverse transcription/polymerase chain reaction (scRT-PCR). A: example HVA current in response to the standard voltage ramp from a holding potential of −80 mV. The arrow indicates the peak inward Ba2+ current. B: graphs of mean Ba2+ current densities by age show that there were no significant age-related differences. C: there were also no significant differences in the HVA current densities between BF cholinergic neurons of subjects with different cognitive status. “n” values refer to the number of cholinergic neurons in the sample. Error bars represent SE. In this and other figures: MU, middle-aged cognitively unimpaired; MI, middle-aged impaired; AU, aged unimpaired; AI, aged impaired.
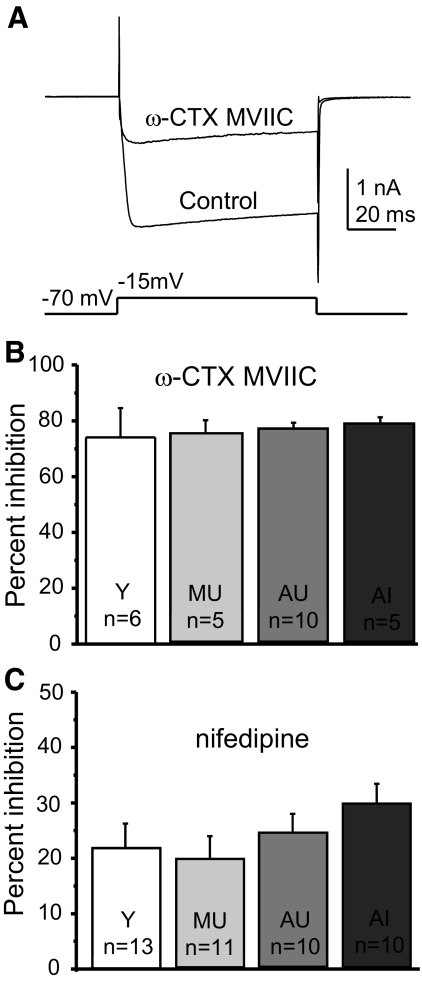
Although total HVA current density did not change with age or cognitive status, this did not eliminate the possibility that the subtypes of HVA currents could differentially contribute to the overall current density across aging and cognitive status. To test this directly, we used perforated-patch recording and pharmacological blockade of HVA channel subtypes. Subtypes of HVA currents were measured using a voltage-step protocol in which the neurons were briefly depolarized from −70 mV to the predetermined peak voltage (between −5 and −15 mV) at 20-s intervals during the bath application of one of the Ca2+ channel blockers. Peak blockade occurred when the current amplitude stabilized (ceased declining). We used 500 nM ω-CTX MVIIC (combined P/Q-type and N-type, or Cav2.1 and Cav2.2 antagonist) and 10 μM nifedipine (L-type or Cav1channel antagonist) to separate current subtypes. Figure 3A shows the control HVA current amplitude and the amplitude at the point of peak blockade by ω-CTX MVIIC in a typical BF cholinergic neuron. Summary data for all ω-CTX MVIIC experiments are shown in Fig. 3B. No change was observed in this Ca2+ channel fraction across age or cognitive status. Likewise, we tested the effects of nifedipine on the HVA current density. There were no age- or cognitive status–related differences in the amount of block by nifedipine (Fig. 3C). These results suggest that the proportions of current types mediating the HVA current in cholinergic BF neurons are not significantly altered with age or cognitive status.
Fig. 3.
Perforated-patch recording of components of the HVA current in cholinergic BF neurons from behaviorally characterized rats. Ba2+ currents were generated in acutely dissociated voltage-clamped neurons by a voltage step from −70 mV to the peak current voltage determined by voltage ramp (as in Fig. 2). Control currents were compared with those in the presence of the HVA antagonists 10 μM nifedipine (blocks L-type current) and 500 nM ω-conotoxin (CTX) MVIIC (blocks N- and P/Q-type currents). A: example of calcium current block by CTX in a BF cholinergic neuron with peak HVA current occurring at −15 mV. The control HVA current is superimposed over the current in ω-CTX MVIIC. B: summary data show that there were no age- or cognition-related differences in the amount of HVA current block by ω-CTX MVIIC. C: summary data for inhibition by nifedipine. There were no significant differences in the amount of block by nifedipine. “n” values refer to the number of cholinergic neurons in the sample. Error bars represent SE.
Calcium Buffering
Our lab has shown previously (Murchison and Griffith 1998) that aged male F344 rats have significantly increased rapid calcium buffering in the somatic compartment of basal forebrain neurons and that this increase can be prevented by caloric restriction (Murchison and Griffith 2007). Now, we sought to determine whether this increase in buffering is relevant to the cognitive status of rats assessed in a task in which the BF is known to be involved. Ca2+ buffering values were measured (as described in methods) in acutely dissociated BF neurons from rats that had been behaviorally characterized. Following buffering measurement, individual neurons were processed for scRT-PCR (see methods), to detect transcripts for ChAT, GAD, and GAPDH.
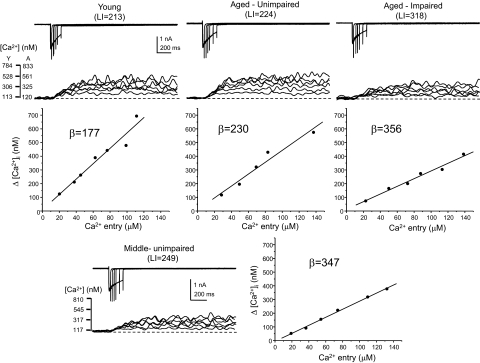
The Ca2+ buffering values represent the difference between the [Ca2+] expected in the somatic cytoplasm from a measured Ca2+ current through VGCCs and that which is observed with the ratiometric Ca-sensitive fluorescent probe fura-2. The larger the buffering value, the greater the buffering. Examples of Ca2+ currents, fura-2 fluorescence ratio records (background and autofluorescence subtracted), and buffering curves are shown in Fig. 4. For each cell, the data are composed of a set of superimposed Ca2+ current recordings (top) and the corresponding fura-2 fluorescence ratio records with calibrated [Ca2+] (middle), with the buffering curves shown at the bottom. In each example, the calculated buffering value (β) is given, as is the LI score for that individual subject. Buffering curves plot the [Ca2+] expected from the integrated Ca2+ current in the estimated cell volume (see methods) against the [Ca2+] measured in the cytoplasm with a calibrated fura-2 signal. The slope of the buffering curve is essentially the reciprocal of the buffering value β. ChAT sequence was detected in all neurons shown in Fig. 4. Note that the slopes of the buffering curves of the neurons from a middle-aged unimpaired and an aged impaired rat are flatter and thus the buffering values are higher than those of the young and aged unimpaired neurons.
Fig. 4.
Determination of neuronal calcium buffering values in individual cholinergic basal forebrain neurons from behaviorally characterized rats. Acutely dissociated BF neurons were whole cell voltage-clamped at −60 mV and depolarized to 0 mV for several different durations to provide several levels of Ca2+ influx via VGCCs using 2 mM Ca2+ as charge carrier. Simultaneously, the intracellular Ca2+ concentration ([Ca2+]i) in the soma was monitored with fura-2–based ratiometric microfluorimetry. Buffering curves were constructed to relate the Ca2+ influx to the calibrated fura signal as described in methods. Examples of data from single cholinergic neurons are shown for each of the age/cognitive status groups: young (top left), aged unimpaired (top middle), aged impaired (top right), and middle-aged unimpaired (bottom). The data for each cell are composed of a set of superimposed Ca2+ current recordings (top), the corresponding fura-2 fluorescence ratio records with calibrated [Ca2+] (middle), and the buffering curves constructed from the data (bottom). For each neuron, the calculated buffering value (β) is shown, as is the LI score in parentheses for that individual rat. All neurons included in the buffering analysis were found to express ChAT by post hoc scRT-PCR.
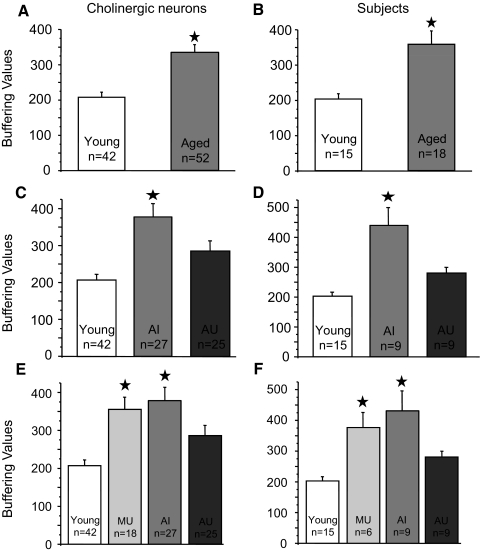
As justified in the following text (see Current-clamp experiments), neurons in which ChAT sequence was detected were considered to be identified as cholinergic. For all cholinergic neurons examined, the mean buffering value was significantly increased with age, whether comparing neurons (Fig. 5A) or when averaging neurons within each individual subject (Fig. 5B). Mean buffering values for young rat neurons were 207 ± 15 (n = 42) and for aged neurons were 334 ± 23 (n = 52, P < 0.05). When all the cholinergic cells were averaged within subjects, the β value in young was 203 ± 14 (n = 15 rats) and in aged it was 358 ± 78 (n = 18 rats, P < 0.05). When aged rats were divided according to cognitive status, it could be seen that the Ca2+ buffering values of cholinergic BF neurons were significantly elevated in aged cognitively impaired rats (Fig. 5, C and D). The β values for aged unimpaired neurons were 286 ± 56 (n = 25) and for aged impaired neurons they were 379 ± 72 (n = 27, P < 0.05). The values for aged unimpaired subjects were 281 ± 19 (n = 9 rats) and for aged impaired subjects 435 ± 63 (n = 9 rats, P < 0.05). The young β values were not significantly different from those of the aged unimpaired.
Fig. 5.
Ca2+ buffering values of cholinergic BF neurons in behaviorally characterized F344 rats during aging. Summary data showing the mean buffering values of all young and aged ChAT+ neurons (A) and the mean buffering values of ChAT+ neurons averaged for each young and aged subject (B). The mean buffering values of cholinergic neurons with the aged subjects divided into impaired and unimpaired cognitive status groups are shown in C, whereas the buffering values averaged within each subject are shown in D. Summary graphs incorporating the mean buffering values of ChAT+ neurons (E) and subjects (F) from middle-aged unimpaired rats are also shown. Note that β values for middle-aged unimpaired neurons and subjects are similar to those of the aged-impaired group. Stars indicate a significant difference (P < 0.05) from starless means. Error bars represent SE and statistical comparisons were by ANOVA for multiple comparisons.
Because neuronal biomarkers relevant to age-related cognitive decline are expected to appear prior to the onset of overt cognitive impairment, we examined middle-aged subjects that were not displaying definitive impairment. Interestingly, when the data from the cholinergic BF neurons of cognitively unimpaired middle-aged rats were compared, as in Fig. 5, E and F, the buffering values were significantly elevated relative to those of young (P < 0.05), but were not different from those of the aged impaired. The values for middle-aged unimpaired neurons were 356 ± 32 (n = 18) and for subjects 377 ± 49 (n = 6 rats). We interpret this to mean that altered Ca2+ homeostasis occurs in BF cholinergic neurons prior to the onset of distinct cognitive impairment.
Current-Clamp Experiments
Our results demonstrate that increased intracellular Ca2+ buffering in cholinergic basal forebrain (BF) neurons is associated with age-related cognitive impairment, but it is unclear whether this change alters the basic firing properties of BF neurons or whether changes in the firing properties could be responsible for the enhanced buffering. Therefore current-clamp experiments were performed on acutely dissociated BF neurons from young and aged rats that had been behaviorally characterized. Neurons were identified by scRT-PCR as described earlier. The goal of these experiments was to test two hypotheses: 1) that the age- and cognitive status–related changes in Ca2+ signaling have a significant impact on the firing properties of the neurons; and 2) that acutely dissociated BF neurons in which both ChAT and GAD sequence are detected (ChAT/GAD) are cholinergic and have firing properties characteristic of ChAT+ neurons rather than of GAD+ neurons. We measured membrane capacitance (Cm), spike threshold, action potential (AP) amplitude relative to spike threshold, AP duration at threshold, amplitude, and duration of the slow afterhyperpolarization (sAHP), spontaneous resting membrane potential (Em), maximum firing frequency during a 500-ms current step (Fmax), and input resistance (Rin). These data are summarized in Table 1. Patch-clamp recordings were conducted on 37 BF neurons from young rats (Rin = 640 ± 58 MΩ) and 36 BF neurons from aged rats (Rin = 620 ± 83 MΩ). Data were not retained unless the neuron exhibited a spontaneous Em that was more polarized than −50 mV. Whenever possible, measurements were made from spontaneously occurring spikes with resting Em near −60 mV. In other instances, small holding currents were applied to move the Em to −60 mV. In cases where the neuron would not display spontaneous spikes, minimal amplitude and duration current steps were applied to trigger spikes.
Table 1.
Membrane properties of acutely dissociated basal forebrain neurons
| n | Cap, pF | Spike |
AHP |
Em, mV | Thresh, mV | Freq, Hz | |||
|---|---|---|---|---|---|---|---|---|---|
| Amp, mV | Dur, ms | Amp, mV | Dur, ms | ||||||
| Young C/G | 26 | 18.4 ± 1.0 | 66.3 ± 1.6 | 5.1 ± 0.2 | 19.9 ± 1.4 | 101.0 ± 14.0 | −62.5 ± 1.8 | −34.0 ± 1.7 | 8.5 ± 1.3 |
| Young ChAT | 5 | 19.9 ± 1.9 | 70.0 ± 8.0 | 5.3 ± 0.3 | 24.2 ± 3.1 | 100.8 ± 45.1 | −68.4 ± 3.1 | −25.7 ± 3.7a | 12.8 ± 6.7 |
| Aged C/G | 22 | 15.2 ± 1.0 | 71.6 ± 1.4 | 4.7 ± 0.2 | 23.3 ± 1.6 | 160.3 ± 18.5 | −66.7 ± 2.3 | −34.2 ± 1.6 | 9.1 ± 1.4 |
| Aged ChAT | 7 | 16.4 ± 1.5 | 65.2 ± 2.7b | 4.9 ± 0.2 | 26.1 ± 2.5 | 143.1 ± 21.6 | −69.0 ± 4.5 | −34.3 ± 2.1 | 8.4 ± 1.9 |
| Young Cholinergic | 31 | 18.6 ± 0.9 | 66.9 ± 1.8 | 5.1 ± 0.2 | 20.6 ± 1.3 | 101.0 ± 13.5 | −63.5 ± 1.6 | −32.7 ± 1.6 | 9.2 ± 1.5 |
| Aged Cholinergic | 29 | 15.5 ± 0.8c | 70.0 ± 1.3 | 4.7 ± 0.2 | 24.0 ± 1.3d | 156.0 ± 14.8c | −67.3 ± 2.0 | −34.3 ± 1.3 | 9.0 ± 1.2 |
| Young Noncholinergic | 6 | 10.6 ± 0.8c | 79.0 ± 4.3c | 3.8 ± 0.4c | 25.7 ± 6.4 | 13.1 ± 1.5c | −61.7 ± 3.3 | −42.0 ± 1.5c | 56.3 ± 13.4c |
| Aged Noncholinergic | 7 | 11.8 ± 1.9e | 84.1 ± 6.3f | 4.7 ± 0.8 | 27.9 ± 2.4 | 18.6 ± 6.5f | −58.0 ± 1.4f | −32.4 ± 2.3g | 42.9 ± 4.6f |
| Unimpaired Cholinergic | 17 | 15.8 ± 1.2 | 71.4 ± 1.5 | 4.8 ± 0.3 | 23.4 ± 1.7 | 147.2 ± 21.2 | −68.5 ± 2.7 | −35.5 ± 1.6 | 10.2 ± 1.7 |
| Impaired Cholinergic | 12 | 14.9 ± 1.0 | 68.9 ± 2.4 | 4.8 ± 0.3 | 24.6 ± 2.1 | 160.9 ± 19.2 | −66.8 ± 3.1 | −33.0 ± 1.9 | 7.9 ± 1.3 |
Values are means ± SE; n, number of neurons. C/G = ChAT+/GAD+; ChAT = ChAT+; Cholinergic = ChAT and C/G combined. Student's t-test comparisons were made.
P = 0.06 with young C/G
P = 0.03 with aged C/G
P ≤ 0.01 with young cholinergic
P = 0.06 with young cholinergic
P = 0.06 with aged cholinergic
P ≤ 0.03 with aged cholinergic
P < 0.01 with young noncholinergic.
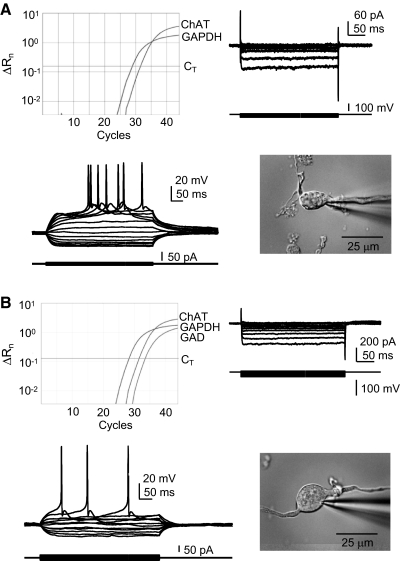
For both young and aged neurons, ChAT/GAD cells had properties that were not significantly different from those of ChAT cells, but that were significantly different from those of GAD cells. For example, in young BF neurons, the Cm of ChAT/GAD cells was 18.4 ± 1.0 pF, which was not different from the Cm of ChAT cells (19.9 ± 1.9 pF), but was significantly different (P < 0.001) from that of GAD cells (10.6 ± 0.8 pF). Likewise, the Fmax of ChAT/GAD and ChAT cells was not different (8.5 ± 1.3 and 12.8 ± 6.7 Hz, respectively), but was significantly different (P < 0.001) from the Fmax of GAD cells (56.3 ± 13.4 Hz). Therefore we are able to conclude unequivocally that acutely dissociated ChAT/GAD BF neurons have properties typical of BF cholinergic neurons. Figure 6 shows data from ChAT (Fig. 6A) and ChAT/GAD (Fig. 6B) neurons, each displaying properties typical of BF cholinergic neurons (Gorelova and Reiner 1996; Griffith 1988; Markram and Segal 1990), such as reluctance to spike, pronounced afterdepolarization (ADP), and sAHP and inward rectification. Note the fast inward rectification that can be seen under voltage clamp for both the ChAT+ (Fig. 6A) and ChAT/GAD neurons in Fig. 6B. This inward rectification is one of the most prominent features of BF cholinergic neurons (Griffith 1988).
Fig. 6.
Properties of ChAT+/GAD+ neurons resemble those of ChAT+ neurons rather than GAD+ neurons. BF neurons in which ChAT sequence is detected by RT-PCR resemble one another and stereotypical BF cholinergic neurons regardless of the detection of GAD sequence. Each example shows the real-time PCR amplification plot (top left), voltage-clamp records of the inward rectifier current in response to hyperpolarizing voltage steps from a holding potential of −60 mV (top right), current-clamp data of superimposed membrane voltage records during a series of small positive and negative current steps from −60 mV (bottom left), and a photo of the acutely dissociated neuron (bottom right). A: ChAT+ neuron. B: ChAT+/GAD+ neuron. Both examples are from aged rats. Current-clamp solutions (see methods) were used for this set of experiments. ChAT, choline acetyltransferase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GAD, glutamic acid decarboxylase; CT, detection threshold; ΔRn, change in reporter fluorescence.
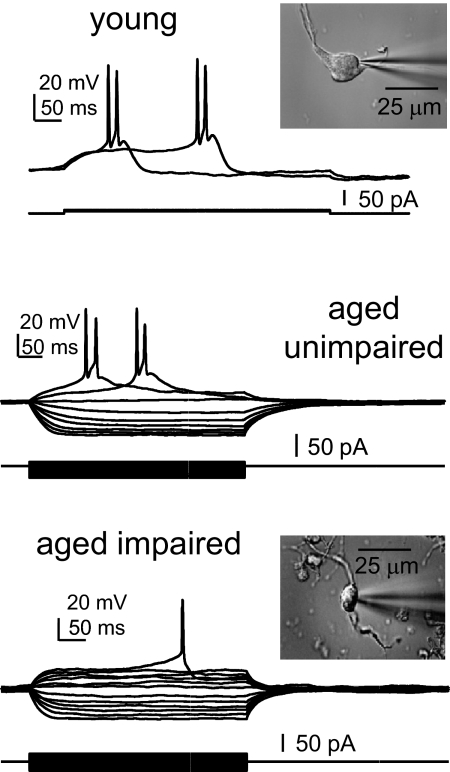
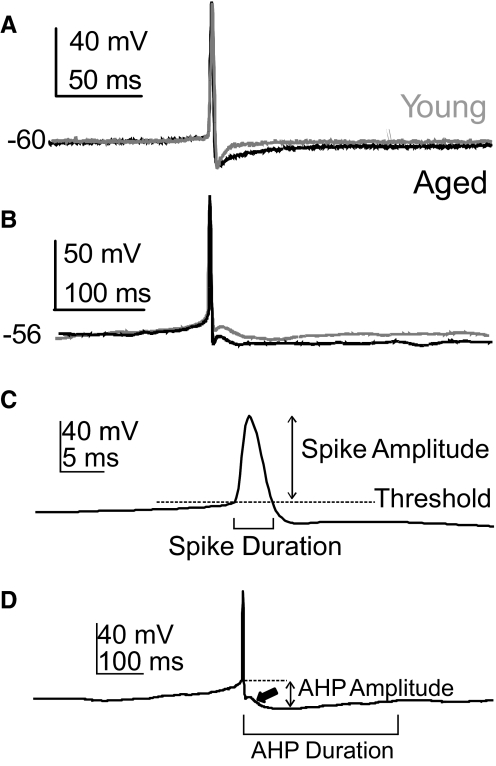
Figure 7 shows that the electrophysiology of cholinergic BF neurons from young, aged cognitively impaired, and aged unimpaired were similar and typical of previously described BF cholinergic neurons. Most of the properties of BF neurons were well preserved during aging, although some significant age-related differences were observed in both cholinergic and noncholinergic BF neurons (Table 1). Measurements of the electrophysiological properties are illustrated in Fig. 8, C and D. For cholinergic neurons, the Cm of young cells was significantly greater than that of aged cells, as shown previously (18.6 ± 0.9 vs. 15.5 ± 0.8 pF, P = 0.01). Moreover, the duration of the slow AHP was significantly longer in aged cholinergic neurons (156.0 ± 14.8 vs. 101.0 ± 13.5 ms, P = 0.008), similar to findings in aged hippocampal CA1 neurons. The amplitude of the sAHP was larger in aged cholinergic neurons, with a strong trend toward significance (P = 0.06). The sAHP durations are highlighted in Fig. 8; note that the sAHP is increased with age in cholinergic neurons lacking the ADP (Fig. 8A) and in cholinergic neurons displaying the ADP (Fig. 8B). There was no age-related difference in the prevalence of the ADP in cholinergic BF neurons; about 60 showed the ADP (arrow in Fig. 8D). These changes in the sAHP of aged BF neurons are not readily explained by the buffering results and will be addressed in the discussion. For GAD cells, the spike threshold was significantly increased with age, which could contribute to a reduced firing frequency in these cells. There were not enough GAD cells in the aged unimpaired sample to compare between cognitive states; however, when all aged cholinergic neurons were compared between cognitively impaired and unimpaired individuals, no significant differences were observed. We conclude from this evidence that the changes in Ca2+ signaling in aged cholinergic BF neurons do not mediate their effects on cognition by altering the intrinsic firing properties of these cells. Instead, it seems likely that the impact on cognition may be mediated at the synaptic level.
Fig. 7.
Cholinergic BF neurons display stereotypical properties that remain very similar during aging and are unaffected by cognitive status. Examples shown are from current-clamped ChAT+ BF neurons from a young, an aged cognitively unimpaired and an aged cognitively impaired rat. Each neuron's resting membrane potential (Em) was near −60 mV, and small depolarizing and hyperpolarizing current steps were applied to produce action potentials and inward rectification. Insets are photos of the acutely dissociated neurons.
Fig. 8.
The slow afterhyperpolarization (sAHP) duration is increased in aged cholinergic BF neurons. A: superimposed current-clamp records showing an action potential (AP) from a young and an aged cholinergic BF neuron without afterdepolarizations (ADPs: young:gray; aged:black). The spontaneous resting potential is given. B: superimposed current-clamp records as above, except from neurons displaying an ADP. In each case the sAHP of the aged neuron is of longer duration. APs occurred spontaneously without depolarizing current pulses and examples were selected for APs of nearly identical amplitude and duration. Quantitative data are presented in Table 1. C: measurement of the AP amplitude, duration, and threshold is illustrated. Amplitude was measured relative to threshold and duration was measured at threshold. D: an example of the measurement of the sAHP amplitude and duration is shown. Amplitude was measured relative to threshold and duration was measured from the time that the repolarizing phase of the AP crossed the threshold voltage to the time that the potential returned to the pre-AP level. The thick arrow indicates the ADP.
DISCUSSION
One of the most important tasks in research on brain aging is to identify physiological mechanisms relevant to age-related cognitive decline. These mechanisms can then be targeted for therapeutic interventions designed to enhance the quality of life in elderly humans by delaying, reducing, or preventing cognitive impairment. We used the Morris water-maze–trained rat model to investigate the relationships of several physiological properties of BF neurons to the cognitive status of individual young, middle-aged, and aged animal subjects. Cognitive status was assessed by the assignment of learning index (LI) scores (Bizon et al. 2009; Gallagher et al. 1993) to each subject based on performance in the water-maze learning task. Middle-aged and aged subjects were categorized as cognitively impaired if their LI scores were greater than any in the young cohort. We determined that increased cellular Ca2+ buffering in cholinergic BF neurons was clearly associated with cognitive decline in aged rats and that some other physiological properties, such as sAHP duration, were altered with age but were not correlated with cognitive status. We also found that this buffering increase is manifest in middle-aged rats not yet evincing profound cognitive dysfunction.
Behavioral Results
It is generally agreed that age-related cognitive impairment can be detected in a subset of the aged population, such that there is a significant deficit in the mean cognitive performance of the entire population. However, there is also a subset of the aged population whose cognitive performance is indistinguishable from that of the young population (Bizon et al. 2009; Gallagher et al. 2003; Rowe et al. 2007). These two subsets of the aged population are referred to as cognitively impaired and cognitively unimpaired. The physiological bases of these cognitive status subsets are the targets of our experiments. This is the first study to link a physiological property of individual cholinergic BF neurons to age-related cognitive impairment in the ability of rats to learn the Morris water-maze task. Previously in rat BF, cholinergic cell size and number, levels of nerve growth factor, and mRNA expression have been linked to impaired performance of this task (Fischer et al. 1991, 1992; Higgins et al. 1990; Sugaya et al. 1998).
Ca2+ Signaling and Aging
There is a growing consensus in the cognitive aging field that relatively subtle changes in Ca2+ signaling and synaptic function are responsible for the majority of age-related cognitive deficits (Foster 2007; Toescu and Verkhratsky 2007). Given the critical role of Ca2+ for both pre- and postsynaptic function, it is easy to imagine how increased neuronal buffering could influence synaptic plasticity and cognitive function (Cavazzini et al. 2005). In Alzheimer's disease, reduced cholinergic input from BF areas to cortical areas is thought to mediate much of the profound dementia associated with this disease (Bartus et al. 2000). It is quite possible that a less-severe disruption of cholinergic transmission could underlie the milder cognitive impairment associated with normal aging (Decker 1987; Sarter and Bruno 2004). Such a reduction in BF cholinergic input to hippocampal interneurons recently has been shown to reduce the plasticity of GABAergic synaptic inputs in aged rat CA1 neurons (Potier et al. 2006). Our results strongly suggest that the change in Ca2+ buffering does not influence firing properties; rather, Ca2+ buffering in aged BF neurons may influence cognition by altering synaptic function.
There is a clear rationale to suggest that changes in Ca2+ buffering may contribute to altering synaptic function. In the hippocampus, exogenous application of the Ca2+ buffer BAPTA-AM [bis(O-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid–acetoxymethyl ester] changed the amplitude of field potentials recorded in the CA1 region of both young and aged rats (Ouanounou et al. 1999). Likewise during aging, synaptic long-term potentiation could be regulated by BAPTA-AM (Tonkikh et al. 2006) or by regulating intracellular calcium release mechanisms (Kumar and Foster 2004). There is also considerable evidence that altered activity of L-type VGCCs underlies deficits in hippocampal synaptic plasticity with age (Norris et al. 1998; Thibault et al. 2001). There is a growing awareness that shifts in intracellular Ca2+ homeostasis regulate cell excitability and synaptic function during aging (for reviews see Burke and Barnes 2006; Foster 2007; Toescu and Verkhratsky 2007). The consequences of a change in Ca2+ buffering with age may depend on many factors such as cell type, intracellular release mechanics, type of synapse, and functional properties of the network.
The increase in BF cholinergic neuron Ca2+ buffering that is associated with cognitive deficits in aged rats was also observed in middle-aged rats that were not classified as cognitively impaired. These middle-aged rats, however, did perform significantly less well in the water-maze task, as a group, than did the young rats, but not so poorly as to reach criterion for cognitive impairment. This result suggests that increased Ca2+ buffering in rat cholinergic BF neurons results in a progressive dysfunction that gradually and increasingly impairs cognition during aging. Aged rats that avoid cognitive decline either never have increased buffering induced or possess some mechanism to rejuvenate buffering. Progressive age-related behavioral impairment in rats is known to begin in middle age (12–18 mo) with the frequency of impairment increasing with age (Fischer et al. 1992; Frick et al. 1995). Biomarkers associated with age-related cognitive impairment are known to appear during middle-age or earlier, prior to the onset of profound cognitive impairment (Baxter et al. 1999; Gant et al. 2006; Sarter and Bruno 2004). Thus prophylactic therapies introduced at middle-age should be able to prevent or delay cognitive deficits later in life. Such an approach has been applied successfully with nerve growth factor (NGF) in rat basal forebrain (Martinez-Serrano and Bjorklund 1998). We would predict that NGF properly administered in the BF of middle-aged rats could prevent or reverse the increased Ca2+ buffering in BF cholinergic neurons that is associated with cognitive decline in older animals.
Comparative Aging
There are several interesting differences in the age-related changes that have been described between hippocampal and BF neurons. For both neuronal types, there is evidence for an age-related increase in VGCC function, although the mechanisms appear to be different. In hippocampus, there is a well-documented increase in L-type currents with age (Campbell et al. 1996; Moyer Jr and Disterhoft 1994; Thibault and Landfield 1996) that can be related to cognitive impairment (Disterhoft et al. 1996; Tombaugh et al. 2005). In BF neurons, we have not observed a significant increase in L-type currents with age (Murchison and Griffith 1996; Fig. 3C), but we have found an age-related reduction in HVA current inactivation and an increase in low-voltage–activated (LVA; T-type) currents (Han et al. 2005; Murchison and Griffith 1995, 1996; Murchison et al. 2004). Furthermore, unlike cholinergic BF neurons, Ca2+ buffering does not seem to be increased and may even be reduced with age in hippocampal CA1 neurons, given that Ca2+ transients are increased in amplitude (Gant et al. 2006; Hemond and Jaffe 2005; Thibault et al. 2001) and addition of exogenous Ca2+ buffers reverses hippocampal dysfunction and cognitive deficits (Ouanounou et al. 1999; Tonkikh et al. 2006).
This reduced or unchanged Ca2+ buffering and increased L-type Ca2+ channel activity is believed to partially underlie an enhanced sAHP with age in CA1 neurons (Landfield and Pitler 1984; Power et al. 2002) that alters the firing properties of these cells and contributes to cognitive impairment (reviewed in Disterhoft and Oh 2007). The sAHP is sensitive to the intracellular [Ca2+] because it is mediated by a Ca2+-dependent K+ conductance (Sah 1996). Recent evidence in CA1 neurons has implicated increased Ca2+-induced Ca2+ release (CICR) from the ryanodine receptors of the endoplasmic reticulum with age as part of the mechanism for enhanced sAHP (Kumar and Foster 2004; Thibault et al. 2007).
The AHPs that we measured in this investigation occurred following single spontaneous APs. For cholinergic neurons, we have followed the convention of calling this a slow AHP (sAHP) in distinction to the fast AHP caused by the delayed inactivation of voltage-gated K+ channels (Gorelova and Reiner 1996; Hille 2001). Noncholinergic BF neurons do not display a sAHP and have only a fast AHP. We are aware that some investigators (e.g., Williams et al. 1997) define the sAHP as occurring after a depolarizing step and burst of APs and distinguish the AHP following a single AP as a medium AHP (mAHP) that can be identified as apamin sensitive. However, the functional distinction between mAHP and sAHP is not entirely clear (Disterhoft and Oh 2007) and the resolution of this issue was not within the scope of this investigation. In most cases, BF cholinergic neurons cannot be made to fire a “burst” of APs and the depolarizing step that is generally used to trigger a burst of APs is likely to activate VGCCs that may not be recruited during physiological burst generation. Nevertheless, the clear relationship between the amplitude/duration of the postburst sAHP, CA1 excitability, aging, and learning (Disterhoft and Oh 2007) establishes the physiological importance of the mechanisms mediating the postburst sAHP. BF cholinergic neurons can display both an apamin-sensitive postspike sAHP and an apamin-insensitive postburst sAHP (Gorelova and Reiner 1996), both of which are thought to be controlled by different Ca2+-activated K+ channels. It seems probable that under physiological conditions, both mechanisms contribute to the control of neuronal firing pattern.
Although the sAHP in CA1 neurons may differ somewhat from the sAHP in cholinergic BF neurons, we observed a similar increase in sAHP with age, but it was increased in all aged subjects independent of cognitive status. That this property did not influence cognition might be because the magnitude of the change was not sufficient to significantly affect the firing rate of the neurons (Table 1). The proposed mechanisms of sAHP increase in CA1 are unlikely to be at work in this case because increased buffering in cholinergic BF neurons, if it extends to near-membrane microdomains, would be expected to reduce the activation of Ca2+-dependent K+ conductance and thus reduce the resulting sAHP. However, there is some evidence that native Ca2+ binding proteins are ineffective buffers at near-membrane microdomains (Muller et al. 2005) and that increased exogenous Ca2+ buffering is able to prolong the duration of the sAHP (Velumian and Carlen 1999). Presumably, this is possible because an increase in a high-affinity Ca2+ buffer is expected to slow the clearance of Ca2+, thereby prolonging the Ca2+ signal available to activate the Ca2+-dependent K+ conductance thought to underlie the sAHP. On the other hand, an increase in Ca2+ buffering by the estrogen receptor in CA1 neurons of prion protein null mice appears to be responsible for a reduced sAHP (Powell et al. 2008). In addition, increased CICR is not observed in aged cholinergic BF neurons (Murchison and Griffith 1998, 1999) and so could not contribute to an enhanced sAHP. Alternatively, if our sAHP is similar to the mAHP described by Williams et al. (1997), increased LVA channel function with age (Murchison and Griffith 1995) could be an important mechanism for enhancing the AHP.
Recently, Luebke and Chang (2007) showed in layer 5 cortical pyramidal neurons of monkey that most membrane properties are unchanged with age, except for a prominent increase in sAHP that was not associated with declines in cognitive performance, paralleling our findings in this investigation. If increased sAHP with age is common to numerous cell types in the brain and has a negative impact on cognition in the hippocampus, it would appear to be an excellent target for a pharmacological agent designed to ameliorate age-related cognitive dysfunction (Disterhoft and Oh 2006).
Properties of Basal Forebrain Neurons
The electrophysiological properties reported here for acutely dissociated cholinergic BF neurons of adult and aged F344 rats are similar to results obtained on these same cells in BF slices from juvenile Sprague–Dawley rats (Garrido-Sanabria et al. 2007; Sotty et al. 2003). One notable difference appears to be the decreased excitability of the acutely dissociated neurons that displayed higher AP thresholds and lower maximum firing frequencies. An important point of concurrence in these studies is that there are different classes of BF neurons that present a spectrum of electrophysiological properties and firing patterns, some of which do not fit neatly into subjectively determined categories. In other words, there is considerable variation in the electrophysiological profiles even within a sample of clearly cholinergic BF neurons. Combined with the neurochemical complexity displayed by BF neurons, it is clear that to understand the full functional impact of this brain region, we will have to move beyond the concept of clearly demarcated cholinergic/GABAergic physiology.
Identification of Cholinergic Neurons
Basal forebrain neurons that appear to express both cholinergic and GABAergic markers have been reported in several studies (Brashear et al. 1986; Castaneda et al. 2005; Han et al. 2002, 2005; Kosaka et al. 1988; Puma et al. 2001; Tkatch et al. 1998; Williams et al. 2002), although it is thought that there are few or no BF neurons actually synthesizing both acetylcholine and γ-aminobutyric acid (Gritti et al. 1993). This contrasts with the situation for glutamatergic markers in BF cholinergic neurons, where coexpression with cholinergic markers appears to support actual release of acetylcholine and glutamate from the same neurons (Allen et al. 2006; Huh et al. 2008).
When RT-PCR is used to detect ChAT and GAD sequence, the rate of codetection in BF neurons can be considerable (Han et al. 2002; Sotty et al. 2003; Williams et al. 2002). We have previously argued that the detection of ChAT sequence, regardless of the detection of GAD sequence, is sufficient to identify a basal forebrain neuron as cholinergic. This conclusion was based partially on semiquantitative PCR results that showed much lower levels of GAD expression in neurons that also expressed ChAT, compared with neurons that expressed GAD only (Han et al. 2002). Additional support for this conclusion included the finding that ChAT+ basal forebrain neurons did not display a TTX-resistant Na+ current that was observed in ChAT− neurons (Han et al. 2005). The present study provides definitive evidence that this conclusion is justified because the membrane properties of the ChAT/GAD neurons resemble those of well-described basal forebrain cholinergic neurons, rather than the distinctly different properties of noncholinergic neurons (Gorelova and Reiner 1996; Griffith 1988; Markram and Segal 1990). This conclusion differs from that of Sotty et al. (2003) who suggested that BF neurons in which both ChAT and GAD were detected tended to resemble noncholinergic neurons. This discrepancy could arise from age, strain, and preparation differences because the Sotty et al. (2003) study was performed on BF slices from juvenile Sprague–Dawley rats.
Buffering and Cognitive Impairment
The findings presented here show that a robust increase in BF cholinergic neuron Ca2+ buffering is strongly associated with age-related cognitive impairment in male F344 rats. In contrast, the electrophysiological properties of BF neurons were little altered with age and were not clearly related to cognitive impairment. This suggests that the buffering enhancement in BF cholinergic neurons contributes to age-related cognitive decline by influencing Ca2+-dependent signaling involved in neuronal plasticity and synaptic function. It is now fairly clear that much age-related cognitive impairment is due to relatively subtle changes in physiology, rather than overt mechanisms, such as cell death (Burke and Barnes 2006; Foster 2007; Toescu and Verkhratsky 2007). A recent investigation by Roussel et al. (2006) showed that increased Ca2+ buffering could dramatically alter the firing pattern of cerebellar granule cells from a rapid tonic pattern to a bursting mode. This pattern was not observed in aged BF cholinergic neurons. However, it may be that altered buffering with age in noncholinergic BF neurons could alter their firing patterns in ways that could contribute to cognitive decline. In another recent study, the direction of synaptic plasticity in dentate gyrus granule neurons could be changed from long-term depression to long-term potentiation by simply reducing the Ca2+ buffering (Harney et al. 2006). In future experiments, we hope to show that the age-related increase in Ca2+ buffering in cholinergic BF neurons has both pre- and postsynaptic effects on synaptic transmission and plasticity.
GRANTS
This work was supported in part by National Institute on Aging Grant AG-007805.
ACKNOWLEDGMENTS
Present address of A. N. McDermott: P&G Beauty Care and Materials Science & Technology, Sharon Woods Innovation Center, 11520 Reed Hartman Highway, Cincinnati, OH 45241.
REFERENCES
- Allen TG, Abogadie FC, Brown DA. Simultaneous release of glutamate and acetylcholine from single magnocellular “cholinergic” basal forebrain neurons. J Neurosci 26: 1588–1595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 163: 495–529, 2000 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Frick KM, Price DL, Breckler SJ, Markowska AL, Gorman LK. Presynaptic markers of cholinergic function in the rat brain: relationship with age and cognitive status. Neuroscience 89: 771–780, 1999 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson M. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci 31: 454–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging 30: 646–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear HR, Zaborszky L, Heimer L. Distribution of GABAergic and cholinergic neurons in the rat diagonal band. Neuroscience 17: 439–451, 1986 [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the aging brain. Nat Rev Neurosci 7: 30–40, 2006 [DOI] [PubMed] [Google Scholar]
- Campbell LW, Hao S-Y, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J Neurosci 16: 6286–6295, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda MT, Sanabria ER, Hernandez S, Ayala A, Reyna TA, Wu JY, Colom LV. Glutamic acid decarboxylase isoforms are differentially distributed in the septal region of the rat. Neurosci Res 52: 107–119, 2005 [DOI] [PubMed] [Google Scholar]
- Cavazzini M, Bliss T, Emptage N. Ca2+ and synaptic plasticity. Cell Calcium 38: 355–367, 2005 [DOI] [PubMed] [Google Scholar]
- Decker MW. The effects of aging on hippocampal and cortical projections of the forebrain cholinergic system. Brain Res Rev 12: 423–438, 1987 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease. J Physiol (Paris) 99: 180–192, 2006 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6: 327–336, 2007 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci 59: 413–420, 1996 [DOI] [PubMed] [Google Scholar]
- Fischer W, Bjorklund A, Chen K, Gage FH. NGF improves spatial memory in aged rodents as a function of age. J Neurosci 11: 1889–1906, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging 13: 9–23, 1992 [DOI] [PubMed] [Google Scholar]
- Fischer W, Gage FH, Bjorklund A. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur J Neurosci 1: 34–45, 1989 [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6: 319–325, 2007 [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging 16: 149–160, 1995 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp Geront 38: 71–77, 2003 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107: 618–626, 1993 [DOI] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci 26: 3482–3490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Sanabria ER, Perez MG, Banuelos C, Reyna T, Hernandez S, Castaneda MT, Colom LV. Electrophysiological and morphological heterogeneity of slow firing neurons in medial septal/diagonal band complex as revealed by cluster analysis. Neuroscience 146: 931–945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis KD, Pun RY, Misler S. Long-term monitoring of depolarization-induced exocytosis from adrenal medullary chromaffin cells and pancreatic islet B cells using “perforated patch recording” Ann NY Acad Sci 635: 464–467, 1991 [DOI] [PubMed] [Google Scholar]
- Gorelova N, Reiner PB. Role of the afterhyperpolarization in control of discharge properties of septal cholinergic neurons in vitro. J Neurophysiol 75: 695–706, 1996 [DOI] [PubMed] [Google Scholar]
- Griffith WH. Membrane properties of cell types within guinea-pig basal forebrain nuclei in vitro. J Neurophysiol 59: 1590–1612, 1988 [DOI] [PubMed] [Google Scholar]
- Griffith WH, Han SH, McCool BA, Murchison D. Molecules and membrane activity: single-cell RT-PCR and patch-clamp recording from central neurons. Neuroanatomical Tract Tracing: Molecules-Neurons-Systems, edited by Zaborszky L, Wouterlood FG, Lanciego JL. New York: Springer, 2006, vol. 3142–174 [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-sensitizing neurons in the basal forebrain of the rat. J Comp Neurol 329: 438–457, 1993 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- Han S-H, McCool BA, Murchison D, Nahm S-S, Parrish AR, Griffith WH. Single-cell RT-PCR detects shifts in mRNA expression profiles of basal forebrain neurons during aging. Mol Brain Res 98: 67–80, 2002 [DOI] [PubMed] [Google Scholar]
- Han S-H, Murchison D, Griffith WH. Low voltage-activated calcium and fast tetrodotoxin-resistant sodium currents define subtypes of cholinergic and noncholinergic neurons in rat basal forebrain. Mol Brain Res 134: 226–238, 2005 [DOI] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci 26: 1128–1132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemond P, Jaffe DB. Caloric restriction prevents aging-associated changes in spike-mediated Ca2+ accumulation and the slow afterhyperpolarization in hippocampal CA1 pyramidal neurons. Neuroscience 135: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- Higgins GA, Oyler GA, Neve RL, Chen KS, Gage FH. Altered levels of amyloid protein precursor transcripts in the basal forebrain of behaviorally impaired aged rats. Proc Natl Acad Sci USA 87: 3032–3035, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes Sunderland, MA: Sinauer, 2001 [Google Scholar]
- Huh CY, Danik M, Manseau F, Trudeau LE, Williams S. Chronic exposure to nerve growth factor increases acetylcholine and glutamate release from cholinergic neurons of the rat medial septum and diagonal band of Broca via mechanisms mediated by p75NTR. J Neurosci 28: 1404–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res 68S: S5–S20, 2006 [DOI] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 70: 605–617, 1988 [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol 91: 2437–2444, 2004 [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent after-hyperpolarizations in hippocampal neurons of aged rats. Science 226: 1089–1092, 1984 [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging 28: 928–936, 2007 [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang Y-M. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience 150: 556–562, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the rat medial septum-diagonal band complex. Brain Res 513: 171–174, 1990 [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A, Bjorklund A. Ex vivo nerve growth factor gene transfer to the basal forebrain in presymptomatic middle-aged rats prevents the development of cholinergic neuron atrophy and cognitive impairment during aging. Proc Natl Acad Sci USA 95: 1858–1863, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol 70: 1115–1124, 2005 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Disterhoft JF. Nimodipine decreases calcium action potentials in rabbit hippocampal CA1 neurons in an age-dependent and concentration-dependent manner. Hippocampus 4: 11–18, 1994 [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Low-voltage-activated calcium currents increase in basal forebrain neurons from aged rats. J Neurophysiol 74: 876–887, 1995 [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. High-voltage-activated calcium currents in basal forebrain neurons during aging. J Neurophysiol 76: 158–174, 1996 [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Increased calcium buffering in basal forebrain neurons during aging. J Neurophysiol 80: 350–364, 1998 [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Age-related alterations in caffeine-sensitive calcium stores and mitochondrial buffering in rat basal forebrain. Cell Calcium 25: 439–452, 1999 [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell 6: 297–305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison D, Zawieja DC, Griffith WH. Reduced mitochondrial buffering of voltage-gated calcium influx in aged rat basal forebrain neurons. Cell Calcium 36: 61–75, 2004 [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci 18: 3171–3179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouanounou A, Zhang L, Charlton MP, Carlen PL. Differential modulation of synaptic transmission by calcium chelators in young and aged hippocampal CA1 neurons: evidence for altered calcium homeostasis in aging. J Neurosci 19: 906–915, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium 38: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- Potier B, Jouvenceau A, Epelbaum J, Dutar P. Age-related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience 142: 187–201, 2006 [DOI] [PubMed] [Google Scholar]
- Powell AD, Toescu EC, Collinge J, Jefferys JGR. Alterations in Ca2+-buffering in prion-null mice: association with reduced afterhyperpolarizations in CA1 hippocampal neurons. J Neurosci 28: 3877–3886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci 22: 7234–7243, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puma C, Danik M, Quirion R, Ramon F, Williams S. The chemokine interleukin-8 acutely reduces Ca(2+) currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J Neurochem 78: 960–971, 2001 [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 37: 15–26, 1991 [DOI] [PubMed] [Google Scholar]
- Roussel C, Erneux T, Schiffmann SN, Gall D. Modulation of neuronal excitability by intracellular calcium buffering: from spiking to bursting. Cell Calcium 39: 455–466, 2006 [DOI] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci 27: 3098–3110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- Sarantopoulos C, McCallum JB, Kwok W-M, Hogan Q. β-Escin diminishes voltage-gated calcium current rundown in perforated patch-clamp recordings from rat primary afferent neurons. J Neurosci Methods 139: 61–68, 2004 [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging 25: 1127–1139, 2004 [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem 80: 245–256, 2003 [DOI] [PubMed] [Google Scholar]
- Smith ML, Booze RM. Cholinergic and GABAergic neurons in the nucleus basalis region of young and aged rats. Neuroscience 67: 679–688, 1995 [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 551: 927–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Greene K, Personett D, Robbins D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging 19: 351–361, 1998 [DOI] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell 6: 307–317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci 21: 9744–9756, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272: 1017–1020, 1996 [DOI] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Basal forebrain neurons adjacent to the globus pallidus co-express GABAergic and cholinergic marker mRNAs. Neuroreport 9: 1935–1939, 1998 [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 6: 267–273, 2007 [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkikh A, Janus C, El Beheiry H, Pennefather PS, Samoilova M, McDonald P, Ouanounou A, Carlen PL. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol 197: 291–300, 2006 [DOI] [PubMed] [Google Scholar]
- Velumian AA, Carlen PL. Differential control of three after-hyperpolarizations in rat hippocampal neurones by intracellular calcium buffering. J Physiol 517: 201–216, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Willard LB. The neural mechanisms underlying cholinergic cell death within the basal forebrain. Int J Dev Neurosci 16: 729–735, 1998 [DOI] [PubMed] [Google Scholar]
- Williams S, Serafin M, Muhlethaler M, Bernheim L. Distinct contributions of high- and low-voltage-activated calcium currents to afterhyperpolarizations in cholinergic nucleus basalis neurons of the guinea pig. J Neurosci 17: 7307–7315, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Souchelnytskyi S, Danik M. TGFβ2 mediates rapid inhibition of calcium influx in identified cholinergic basal forebrain neurons. Biochem Biophys Res Commun 290: 1321–1327, 2002 [DOI] [PubMed] [Google Scholar]