Abstract
Tissue injury in early life can produce distinctive effects on pain processing, but little is known about the underlying neural mechanisms. Neonatal inflammation modulates excitatory synapses in spinal nociceptive circuits, but it is unclear whether this results directly from altered afferent input. Here we investigate excitatory and inhibitory synaptic transmission in the rat superficial dorsal horn following neonatal hindlimb surgical incision using in vitro patch-clamp recordings and test the effect of blocking peripheral nerve activity on the injury-evoked changes. Surgical incision through the skin and muscle of the hindlimb at postnatal day 3 (P3) or P10 selectively increased the frequency, but not amplitude, of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) recorded 2–3 days after injury, without altering miniature inhibitory postsynaptic current frequency or amplitude at this time point. Meanwhile, incision at P17 failed to affect excitatory or inhibitory synaptic function at 2–3 days postinjury. The elevated mEPSC frequency was accompanied by increased inward rectification of evoked α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)–mediated currents, but no change in AMPAR/N-methyl-d-aspartate receptor ratios, and was followed by a persistent reduction in mEPSC frequency by 9–10 days postinjury. Prolonged blockade of primary afferent input from the time of injury was achieved by administration of bupivacaine hydroxide or tetrodotoxin to the sciatic nerve at P3. The increase in mEPSC frequency evoked by P3 incision was prevented by blocking sciatic nerve activity. These results demonstrate that increased afferent input associated with peripheral tissue injury selectively modulates excitatory synaptic drive onto developing spinal sensory neurons and that the enhanced glutamatergic signaling in the dorsal horn following neonatal surgical incision is activity dependent.
INTRODUCTION
Human infants can experience considerable pain as a result of disease, surgery, or intensive care therapy. However, it is still unclear how tissue damage at various stages of postnatal development modulates synaptic function within emergent pain circuits in the CNS. Since the synaptic integration of nociceptive signals within the CNS begins in the superficial dorsal horn (SDH) of the spinal cord (Todd and Koerber 2006), a detailed characterization of how synaptic networks in the SDH are affected by tissue injury during the early postnatal period represents a logical first step toward addressing this issue.
Neonatal SDH neurons become hyperexcitable following early tissue damage (Ririe et al. 2008; Torsney and Fitzgerald 2002) and peripheral inflammation during the first, but not third, postnatal week transiently facilitates glutamatergic signaling in the immature rat SDH (Li and Baccei 2009). Although these data suggest that the balance of synaptic excitation and inhibition is altered by excess or aberrant input from nociceptive afferents, there is no direct evidence that these synaptic changes are activity dependent. The aim here was to test whether the facilitation of excitatory synaptic transmission caused by neonatal tissue injury is dependent on enhanced afferent input from the periphery.
Sensory experience is known to rapidly modulate developing synaptic networks in other regions of the immature CNS. For example, early visual experience results in significant modifications in the properties of synaptic circuits in the visual cortex, including a decrease in the proportion of N-methyl-d-aspartate receptors (NMDARs) expressing the NR2B subunit (Philpot et al. 2001) and an acceleration in the maturation of GABAergic synapses (Morales et al. 2002). However, specific mechanisms of experience-dependent synaptic plasticity vary between sensory systems and CNS regions; for instance, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)/NMDAR ratio is modulated by sensory input in the developing lateral olfactory tract (Franks and Isaacson 2005), but not the somatosensory barrel cortex (Mierau et al. 2004). This highlights the need to examine how sensory input influences synaptic function within central pain networks during early postnatal development.
Here we use in vitro electrophysiology to characterize the effects of surgical incision in early life on excitatory and inhibitory synaptic signaling in the immature SDH and test whether these effects are dependent on sensory afferent activity following tissue damage. The data suggest that glutamatergic synaptic drive in the developing dorsal horn is selectively modulated by tissue injury during the early postnatal period and is dependent on the level of sensory input under pathophysiological conditions.
METHODS
All experiments adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee and the United Kingdom Animal (Scientific Procedures) Act 1986.
Surgical incision
Sprague–Dawley rat pups (postnatal day 3 [P3] to P17) were anesthetized with isoflurane (2–3%); an incision was made in the lateral midthigh region and the underlying muscle was separated via blunt dissection. The wound was immediately closed with a series of muscle (6-0 Vicryl; Ethicon, Cornelia, GA) and skin (5-0 Ethilon; Ethicon) sutures. This model of tissue damage provides access to the sciatic nerve to manipulate the level of sensory input to the developing spinal cord in vivo.
In vivo block of sciatic nerve with bupivacaine hydroxide
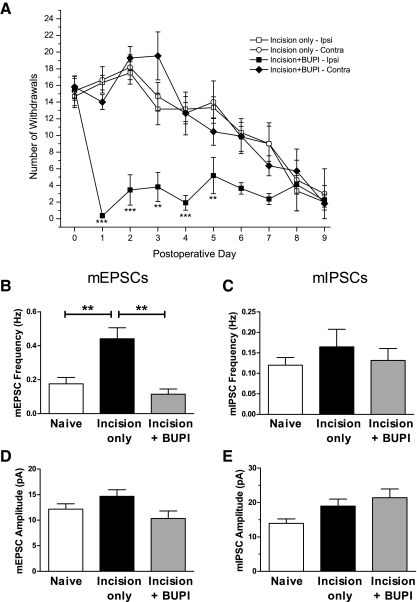
The local anesthetic bupivacaine hydrochloride (Sigma, St. Louis, MO) was converted to bupivacaine hydroxide (BUPI) to obtain an insoluble depot form as previously described (Xie et al. 2005). Following the exposure of the sciatic nerve at P3, BUPI powder (3–4 mg) was loosely packed along the nerve and the wound closed with muscle and skin sutures. To quantify the degree of sciatic nerve block achieved by the implantation of BUPI, a series of calibrated von Frey hair filaments (vFh numbers 6–12) were applied (five times each) to the ipsilateral and contralateral hindpaws and the total number of hindpaw withdrawals was plotted as a function of postoperative time (see Fig. 3A).
Fig. 3.
Reducing sensory input to the developing SDH in vivo with bupivacaine hydroxide (BUPI) prevents the increase in excitatory synaptic signaling following incision. A: the efficacy of the sciatic nerve block was tested using mechanical stimulation of the hindpaw with von Frey hairs (vFh 6–12; each applied 5 times). Plot shows the total number of hindpaw withdrawals evoked by mechanical stimulation following midthigh surgical incision at P3 (postoperative day 0) with or without subsequent implantation of BUPI. BUPI application to the sciatic nerve (n = 11) led to a significant decrease in mechanical sensitivity on the ipsilateral paw (**P < 0.01; ***P < 0.001; 2-way ANOVA), which lasted for about 5 days. Mechanical sensitivity on the paw contralateral to the BUPI was similar to that seen following incision alone (n = 6), suggesting that the reduction in reflex sensitivity by BUPI does not result from the systemic uptake of the drug. B: surgical incision at P3 failed to significantly change mEPSC frequency at P5–P6 when accompanied by implantation of BUPI to the sciatic nerve at the time of injury (**P < 0.01; Kruskal–Wallis test). C–E: there were no significant differences in mean mEPSC amplitude (D), mIPSC frequency (C), or mIPSC amplitude (E) between the 3 groups at this time point (P > 0.05; Kruskal–Wallis test).
Immunohistochemistry
To determine whether prolonged blockade with BUPI produced neuronal injury, dorsal root ganglia (DRG) were evaluated for immunoreactivity to activating transcription factor-3 (ATF3). In P3 pups, the sciatic nerve was exposed and either BUPI block was performed or the sciatic nerve was sectioned, given that axotomy was previously shown at this age to provide a positive control for ATF3 activation (Walker et al. 2003). Three days later, animals were terminally anesthetized with intraperitoneal (ip) administration of pentobarbitone and perfused with 4% paraformaldehyde; the L4 and L5 DRGs were removed, postfixed, and then stored at 4°C in 30% sucrose overnight. Frozen sections (10 μm) were mounted on slides and 0.1 M PBS washes were performed between incubation at 4°C overnight with 1:250 rabbit anti-ATF3 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then 1:500 AlexaFluor goat anti-rabbit IgG (Molecular Probes, Eugene, OR) for 2 h at room temperature. Slides were coverslipped with GelMount (Sigma) and examined using the appropriate wavelength filter with a Nikon E800 microscope.
To evaluate sensory input to the spinal cord during BUPI block, the degree of c-Fos activation in the dorsal horn following hindpaw injection of capsaicin was evaluated. Under halothane (2–4%) in oxygen anesthesia, 1 μl of 0.1% capsaicin (Sigma) in 10% Tween, 10% ethyl alcohol, and 80% saline was injected in the midplantar surface of the hindpaw. This dose was sufficient to produce a behavioral response with flicking and licking of the paw following recovery from anesthesia. In naïve animals, capsaicin was injected into the left hindpaw. In separate animals, capsaicin was injected into both hindpaws at 2, 4, or 6 days after the onset of BUPI block. Two hours after injection, rats were terminally anesthetized with ip pentobarbitone and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The lumbar spinal cord was removed, a marker pin was placed through the dorsal segment of the right (contralateral) side, and cords were postfixed and then stored at 4°C in 30% sucrose. Spinal cord sections (40 μm) were cut on a freezing microtome and reactions were performed at room temperature on floating sections agitated on a shaker. Sections were washed in 0.1 M PB between the following steps: 1) 3% hydrogen peroxide to quench endogenous peroxidase activity; 2) 3% normal goat serum and 0.3% Triton X-100 in 0.1 M PB for 1 h; 3) 1:60,000 rabbit polyclonal antibody anti-c-Fos (Calbiochem, La Jolla, CA) overnight at room temperature; 4) 1:500 goat anti-rabbit biotinylated antibody (Vector Laboratories, Burlingame, CA) for 2 h; 5) avidin–biotin–peroxidase complex (ABC Elite; Vectastain, Vector Laboratories) for 2 h; 6) 2–4 min in a solution containing 0.05% of 3,3′-diaminobenzidine and 0.2% ammonium nickel sulfate in 0.15 M Tris buffer and, once visible, the reaction was stopped by washes in Tris buffer. The sections were mounted on gelatin-coated slides and coverslipped. Under bright-field microscopy, c-Fos-IR neurons in the superficial dorsal horn were counted.
In vivo block of sciatic nerve with tetrodotoxin microcapillaries
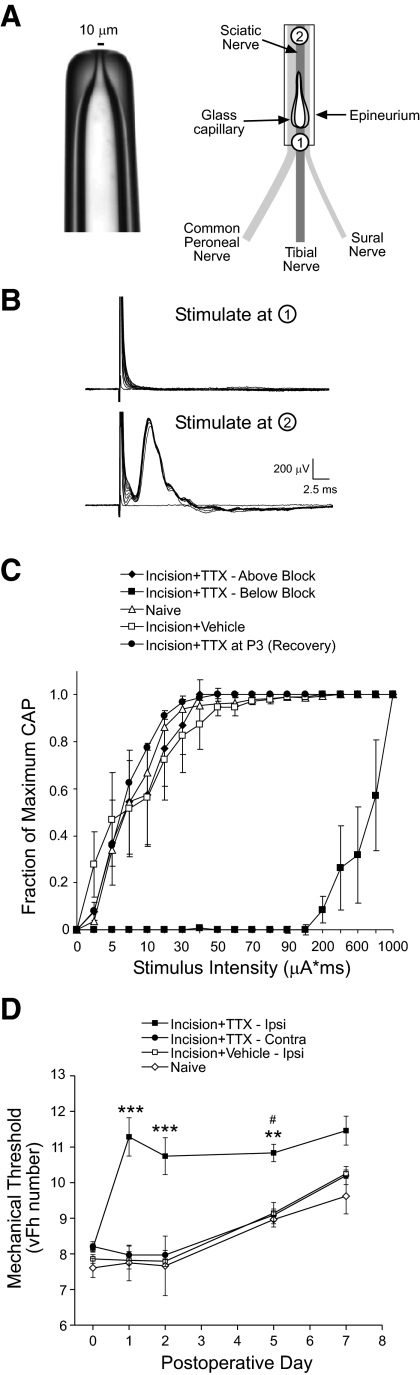
A protocol previously used to block the adult sciatic nerve in vivo (Bray et al. 1979; Martinov and Nja 2005; Wall et al. 1982) was modified for younger animals. Briefly, borosilicate glass capillaries (0.75 mm OD/0.40 mm ID; A-M Systems, Carlsborg, WA) were pulled on a microelectrode puller (P-97; Sutter Instruments, Novato, CA). The tip was scored with a diamond pencil and broken to obtain an external tip diameter of about 100 μm, then fire-polished with a microforge (MF-900, Narishige, Tokyo) to obtain a final pore size of 10–15 μm (Fig. 4A). The back end of the capillary was scored and broken to obtain a microcapillary with a length of 4–5 mm. The back end of the microcapillary was then fire-polished to produce a rounded end with a slight opening to allow back-filling of the capillary with either tetrodotoxin (TTX, 7.5–30 mM; Tocris, Ellisville, MO) or equivalent citrate buffer (vehicle), after which the back end was sealed with bone wax (Ethicon) and silicone sealant (3140 RTV, Dow Corning, Midland, MI).
Fig. 4.
Localized block of neonatal sciatic nerve activity in vivo with tetrodotoxin (TTX) microcapillaries. A, left: picture of microcapillary with pore diameter of about 10 μm. Right: diagram showing insertion of microcapillary under epineurium of sciatic nerve near point of trifurcation. B: 3 days after TTX microcapillaries were implanted at P17, stimulation of the sciatic nerve (0–1 mA at 100 μs) below the site of TTX delivery (point #1 in A) reveals no compound action potentials (CAPs) in the L4/L5 dorsal roots in vitro. However, stimulation at a more proximal location (∼5 mm above the capillary tip; point #2 in A) restores conduction in the nerve, suggesting that TTX evokes a highly localized block of sciatic nerve fibers in vivo. C: summary plot of normalized CAP amplitude vs. stimulus strength at P20, showing that the thresholds of TTX-treated nerve fibers are similar to vehicle and naïve controls if stimulation occurs proximal to the TTX delivery site. Nerves that had been treated with TTX from P3 exhibited a similar stimulus–response relationship as vehicle and naïve controls by P20, arguing against any permanent alterations in the excitability of sciatic nerve fibers by the TTX application. D: plot of mechanical withdrawal thresholds (vFh number evoking flexion withdrawal in 50% of trials) vs. postoperative day after insertion of microcapillaries containing TTX or vehicle into the sciatic nerve at P3. TTX (n = 8) significantly elevated mechanical thresholds on the ipsilateral paw compared with the contralateral side, vehicle (n = 7) or naïve (n = 2) controls (**P < 0.01, ***P < 0.001; 2-way ANOVA; #P < 0.05 compared with naïve at this particular time point).
Pups (P3 or P17) were anesthetized with isoflurane and the sciatic nerve was exposed at the midthigh level. Microcapillaries containing TTX (7.5 mM at P3; 30 mM at P17) or equivalent citrate buffer (vehicle) were inserted under the epineurium into the space between the diverging sural, tibial, and common peroneal nerves and the wound was closed as described earlier. The efficacy of the TTX block at different postoperative times was assayed by measuring the mechanical withdrawal thresholds (vFh which evokes a flexion withdrawal reflex in 50% of trials) or recording in vitro compound action potentials (CAPs) from the dorsal root. All patch-clamp recordings and subsequent analysis of synaptic function in the SDH (see following text) were performed by an experimenter who was blinded to the contents of the microcapillary.
In vitro recording of dorsal root CAPs
Three days after the implantation of microcapillaries containing either TTX or vehicle solution, pups were deeply anesthetized with isoflurane (5%) and the ipsilateral sciatic nerve, L4–L5 DRGs, and L4–L5 dorsal roots were harvested intact; placed in a submersion-type recording chamber (RC-22; Warner Instruments, Hamden, CT); and mounted on the stage of an upright microscope (BX51WI, Olympus, Center Valley, PA). The chamber was filled with oxygenated artificial CSF (aCSF) solution containing the following (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose. The L4 or L5 dorsal root was inserted into a glass suction electrode and extracellular CAPs were recorded using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) in response to stimulation of the sciatic nerve at various points along its length with a second suction electrode coupled to a Master-8 constant-current stimulator (AMPI, Jerusalem, Israel).
Preparation of spinal cord slices
Pups (P5–P20) were deeply anesthetized with sodium pentobarbital (30 mg/kg) and then perfused transcardially with ice-cold dissection solution consisting of (in mM): 250 sucrose, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, and 25 glucose continuously bubbled with 95% O2-5% CO2. The lumbar spinal cord was isolated and immersed in low-melting-point agarose (3% in the above-cited solution; Invitrogen, Carlsbad, CA) and parasagittal slices (350–400 μm) were cut from the ipsilateral side using a Vibroslice tissue slicer (HA-752; Campden Instruments, Lafayette, IN). The slices were placed in a chamber filled with oxygenated dissection solution for 30 min, then allowed to recover in an oxygenated aCSF solution for ≥1.5 h at room temperature.
Patch-clamp recordings
After recovery, slices were transferred to a submersion-type recording chamber, mounted on the stage of an upright microscope, and perfused at room temperature with oxygenated aCSF at a rate of 1.5–3 ml/min.
Patch electrodes were constructed from thin-walled single-filamented borosilicate glass (OD, 1.5 mm; World Precision Instruments, Sarasota, FL) using a microelectrode puller. Pipette resistances ranged from 5 to 7 MΩ and seal resistances were >1 GΩ. Patch electrodes were filled with a solution containing the following (in mM): 130 Cs-gluconate, 10 CsCl, 10 HEPES, 11 EGTA, 1.0 CaCl2, and 2.0 MgATP (pH 7.2; 305 mOsm).
Dorsal horn neurons were visualized with infrared–differential interference contrast and patch-clamp recordings were obtained as previously described (Baccei and Fitzgerald 2004) using a Multiclamp 700B amplifier. Excitatory postsynaptic currents (EPSCs) were isolated at a holding potential (Vh) of −70 mV, whereas inhibitory postsynaptic currents (IPSCs) were recorded at a Vh of 0 mV, thus minimizing the contribution of NMDAR and AMPA/kainate receptor–mediated events (Yoshimura and Nishi 1993). Miniature postsynaptic currents (mPSCs) were isolated via the bath application of 500 nM TTX and recorded for a period of 3 min at each Vh. In some experiments, monosynaptic EPSCs were evoked via focal electrical stimulation (0–100 μA, 100-μs duration) delivered via a second patch electrode placed near the cell of interest that was connected to a constant-current stimulator. In all experiments, the threshold to evoke an EPSC was defined as the current intensity (at a duration of 100 μs), which evoked a measurable EPSC in 50% of the trials. EPSCs were classified as monosynaptic based on their ability to follow repetitive stimulation (five stimuli at twofold threshold delivered at 10 Hz) with a constant latency and absence of failures. To examine the current–voltage relationship of evoked AMPAR-mediated currents, spermine (Fisher Scientific, Florence, KY) was added to the intracellular solution at 100 μM and EPSCs were evoked (at twofold threshold) from a variety of holding potentials (−70 to +40 mV) in the presence of 50 μM d-2-amino-5-phosphonopentanoic acid (AP5, Tocris), 10 μM gabazine (Tocris), and 0.5 μM strychnine (Sigma) to block NMDARs, γ-aminobutyric acid type A receptors (GABAARs), and glycine receptors, respectively. To quantify the degree of rectification, a rectification index (RI) was calculated as RI = I at Vh+40/I at Vh−40 as described previously (Clem and Barth 2006). To investigate whether surgical incision altered the probability of glutamate release in the dorsal horn, pairs of identical stimuli (at twofold threshold at a frequency of 0.10 Hz) were delivered at various interstimulus intervals (ISIs, 50–250 ms; 10 trials each) and the paired-pulse ratio (PPR) was calculated as: PPR = mean EPSC2/mean EPSC1. To calculate the ratio of AMPAR/NMDAR currents, EPSCs were evoked from a Vh of +50 mV at a frequency of 0.10 Hz in the presence of 10 μM gabazine and 0.5 μM strychnine. On verification of a stable baseline current amplitude, AP5 was bath applied at 50 μM to block the NMDAR component of the composite current and the NMDAR-mediated response was subsequently obtained via electronic subtraction.
Membrane voltages were adjusted for liquid junction potentials (approximately −14 mV) calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices). Currents were filtered at 4–6 kHz through a −3-dB, four-pole low-pass Bessel filter, digitally sampled at 20 kHz, and stored on a personal computer (ICT, Cincinnati, OH) using a commercially available data-acquisition system (Digidata 1440A with pClamp 10.0 software; Molecular Devices).
Data analysis and statistics
mPSCs were analyzed via visual inspection using Mini Analysis (version 6.0.3; Synaptosoft, Decatur, GA), whereas evoked EPSCs were analyzed using Clampfit software (Molecular Devices). The threshold for mPSC detection was set at twice the mean amplitude of the background noise. The positive skew in the distribution of mPSC frequencies in newborn dorsal horn neurons makes the use of the Kolmogorov–Smirnov test (to compare the cumulative interevent intervals or amplitudes between groups) problematic because the events measured in a small number of cells would disproportionately dominate the analysis. As a result, we measured a mean mPSC frequency in each neuron of a given experimental group, as widely done in studies of dorsal horn synaptic physiology following tissue or nerve injury (Kohno et al. 2003; Moore et al. 2002; Muller et al. 2003), and used nonparametric statistical tests to determine whether significant differences in mPSC frequency existed between various groups (Mann–Whitney test for two groups; Kruskal–Wallis test for more than two groups; Prism 5.0 software, GraphPad Software, La Jolla, CA). Nonparametric tests were also used in cases in which the number of observations was insufficient (n < 24) to definitively conclude that the data were distributed in a Gaussian manner. t-Tests were used for two-group comparisons in which parametric tests were appropriate. Data are expressed as means ± SE; n refers to the number of neurons sampled in a given group.
RESULTS
Surgical incision during the early postnatal period potentiates glutamatergic signaling in the immature spinal dorsal horn at 2–3 days postinjury
A midthigh surgical incision was performed at a variety of postnatal ages (P3, P10, and P17) and spinal cord slices were prepared from the ipsilateral side 2–3 days later to characterize synaptic function in the SDH using patch-clamp techniques. Patch-clamp recordings were obtained from neurons located in the superficial dorsal horn (laminae I–II), as judged by visual inspection. Because sampled neurons were located about 50–150 μm from the edge of the dorsal white matter, we concluded that the majority resided in lamina II (Lorenzo et al. 2008).
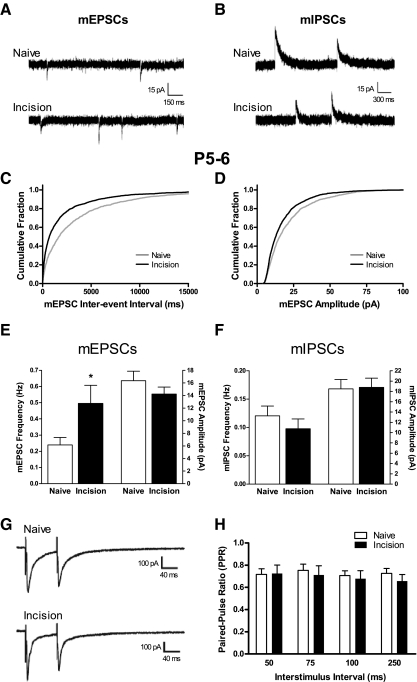
Miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs, respectively) were recorded in the same cells (Fig. 1, A and B), to examine the functional balance between synaptic excitation and inhibition in individual dorsal horn neurons after tissue injury. Figure 1 shows that an incision at P3 led to a significant increase in the frequency of glutamatergic mEPSCs compared with naïve littermate controls 2–3 days later at P5–P6 (Naïve: 0.24 ± 0.05 Hz, n = 30; Incision: 0.50 ± 0.11 Hz, n = 27; P = 0.030; Mann–Whitney test; Fig. 1E), whereas mEPSC amplitude was not significantly affected (Naïve: 16.36 ± 1.51 pA; Incision: 14.24 ± 1.10 pA; P = 0.334). This increase in mEPSC frequency was not accompanied by significant alterations in the PPR of evoked EPSCs across a range of ISIs (P > 0.05, two-way ANOVA; Fig. 1, G and H). Meanwhile, there was no effect of tissue damage on either the mIPSC frequency (Naïve: 0.12 ± 0.02 Hz; Incision: 0.10 ± 0.02 Hz; P = 0.185) or mIPSC amplitude (Naïve: 18.51 ± 1.81 pA; Incision: 18.78 ± 1.78 pA; P = 0.940; Fig. 1F).
Fig. 1.
Surgical incision during early life potentiates excitatory synaptic transmission in the developing rat superficial dorsal horn (SDH) at 2–3 days postinjury. A: examples of miniature excitatory postsynaptic currents (mEPSCs) recorded at a holding potential (Vh) of −70 mV in postnatal day 5 (P5) to P6 SDH neurons from naïve pups or littermates undergoing surgical incision at P3 (Incision). B: representative traces of miniature inhibitory postsynaptic currents (mIPSCs) isolated at a Vh of 0 mV in the Naïve and Incision groups at P5–P6. C and D: cumulative probability plots showing the distribution of mEPSC interevent intervals (C) and amplitudes (D) in the Naïve (gray) and Incision (black) groups at P5–P6. E: plot of mean frequency (left) and amplitude (right) of mEPSCs recorded in P5–P6 SDH neurons following surgical incision at P3, illustrating a selective increase in mEPSC frequency after tissue injury (*P = 0.030; Mann–Whitney test). F: incision at P3 failed to alter the mean frequency (left) or amplitude (right) of mIPSCs in the same population of P5–P6 SDH cells. G: examples of EPSCs evoked by paired-pulse stimulation at an interstimulus interval (ISI) of 75 ms in P5–P6 SDH neurons from the Naïve or Incision groups. Each sweep represents the average of 10 evoked EPSCs. H: the average paired-pulse ratio (PPR, defined as mean EPSC2/mean EPSC1) was similar in the Naïve (n = 18) and Incision (n = 19) groups across a range of ISIs at P5–P6 (P > 0.05, 2-way ANOVA).
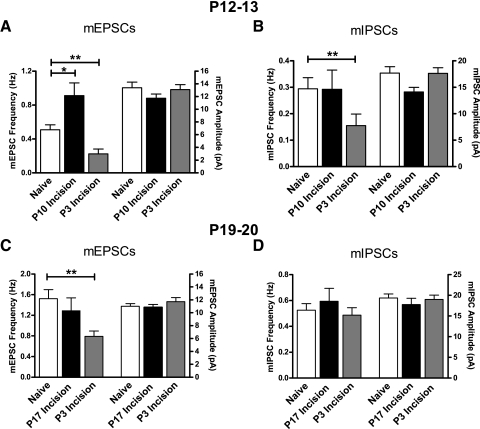
Surgical incision at P10 evoked similar changes in SDH synaptic function as seen at P3, with a selective increase of mEPSC frequency 2–3 days after injury (Naïve: 0.51 ± 0.06 Hz, n = 47; P10 Incision: 0.91 ± 0.15 Hz, n = 24; P < 0.05; Kruskal–Wallis test; Fig. 2 A), but this effect was not seen when the incision was performed at P17 (Fig. 2C). mEPSC amplitude (Fig. 2, A and C) and mIPSC frequency and amplitude (Fig. 2, B and D) were not affected by incision at the 2- to 3-day time point at any postnatal age (i.e., P3, P10, or P17). The observed developmental increase in mEPSC frequency, but not mEPSC amplitude (Figs. 1 and 2), is consistent with our previous findings in the immature SDH (Baccei et al. 2003). Collectively, these results indicate that tissue damage during a restricted period of early postnatal development facilitates excitatory but not inhibitory synaptic transmission in the immature SDH 2–3 days after injury.
Fig. 2.
Excitatory synaptic function in the P19–P20 SDH is unchanged by tissue damage during the 3rd postnatal week, but decreased by injury during the neonatal period. A: incision at P10 (black bars) increased the frequency (*P < 0.05; Kruskal–Wallis test; left), but not amplitude (right), of mEPSCs recorded at P12–P13 compared with naïve controls (white). Meanwhile, mEPSC frequency in P12–P13 SDH neurons was significantly reduced by P3 incision (gray) compared with the naïve group (**P < 0.01). B: whereas P10 incision failed to affect mIPSC frequency at P12–P13 compared with naïve littermates, incision at P3 significantly decreased mIPSC frequency at this age (**P < 0.01; Kruskal–Wallis test). C: plot of mean frequency (left) and amplitude (right) of mEPSCs recorded in SDH neurons at P19–P20 following surgical incision at P3 (gray bars) or P17 (black) compared with naïve littermates (white). P3 surgical incision selectively decreased mEPSC frequency at P19–P20 compared with naïve littermates (**P < 0.01; Kruskal–Wallis test), whereas incision at P17 had no significant effect at the same time point. D: mIPSCs in the same population of P19–P20 SDH cells were not significantly affected by incision at P3 or P17.
Neonatal tissue injury causes a delayed reduction in glutamatergic synaptic drive in the developing SDH
To investigate the effects of early tissue damage on synaptic transmission within the SDH at later stages of postnatal development, we performed midthigh incision at P3 (as described earlier) and subsequently characterized synaptic function in the SDH at P12–P13 and P19–P20, by which times the incision had visibly healed. Interestingly, the P3 incision evoked a significant and persistent decrease in mEPSC frequency compared with naïve littermates when measured at P12–P13 (Naïve: 0.51 ± 0.06 Hz, n = 47; P3 Incision: 0.22 ± 0.06 Hz, n = 24; P < 0.01; Kruskal–Wallis test; see Fig. 2A) and P19–P20 (Naïve: 1.53 ± 0.17 Hz, n = 83; P3 Incision: 0.79 ± 0.10 Hz, n = 55; P < 0.01; Fig. 2C) without accompanying changes in mEPSC amplitude (Fig. 2, A and C). In addition, P3 incision caused a transient reduction in mIPSC frequency, which was apparent by P12–P13 (Naïve: 0.29 ± 0.04 Hz, n = 50; P3 Incision: 0.16 ± 0.04 Hz, n = 24; P < 0.01; Fig. 2B), but had resolved by P19–P20 (Fig. 2D). This suggests that early tissue injury modulates glutamatergic synapses in a biphasic manner within the developing dorsal horn.
Role of primary afferent activity in the regulation of injury-evoked changes in synaptic transmission within the developing SDH
To determine whether the potentiation of glutamatergic signaling seen 2–3 days after injury requires ongoing peripheral input, we used two different approaches to reduce primary afferent drive to the spinal cord in vivo during the postinjury period and subsequently characterized synaptic function in the SDH as described earlier.
Immediately after the surgical incision at P3, bupivacaine hydroxide (BUPI) was implanted into the wound site around the sciatic nerve (Xie et al. 2005). BUPI effectively blocked sciatic nerve conduction because a profound motor and sensory block was apparent 30 min following the procedure. Figure 3 A shows a continued and pronounced reduction in withdrawal reflexes in response to mechanical stimulation of the ipsilateral paw compared with the contralateral paw or littermates undergoing midthigh incision without sciatic block. A prolonged unilateral sensory block was also confirmed by a reduction in capsaicin-evoked c-Fos activation in the dorsal horn (Supplemental Fig. S1, C and D).1 Importantly, the mechanical responsiveness of the ipsilateral paw recovered to match that of the contralateral (unoperated) side within 7–10 days. In addition, neonatal DRG neurons failed to express ATF3, a marker of neuronal stress (Hai et al. 1999), which is significantly up-regulated in these cells after damage to their axons (Supplemental Fig. S1B), following BUPI treatment (Supplemental Fig. S1A). Collectively, these results strongly suggest that BUPI administration reduces primary afferent input to the developing dorsal horn without damaging sensory fibers in the sciatic nerve.
To test whether the short-term increase in mEPSC frequency following the surgical incision required ongoing primary afferent input to the SDH, the properties of synaptic transmission onto SDH cells were compared between the Naïve, Incision only, and Incision + BUPI littermates at P5–P6 (2–3 days after surgery at P3). As before, the incision led to a significant increase in the mEPSC frequency compared with that of naïve controls (Naïve: 0.18 ± 0.04 Hz, n = 18; Incision only: 0.44 ± 0.07 Hz, n = 9; P < 0.01, Kruskal–Wallis test; see Fig. 3B). However, in the presence of sciatic nerve blockade with BUPI, this increase in mEPSC frequency failed to occur (Incision + BUPI: 0.11 ± 0.03 Hz, n = 8; P < 0.01 compared with the Incision only group; Fig. 3B). No significant differences in mEPSC amplitude (Fig. 3D) or mIPSC frequency or amplitude (Fig. 3, C and E) were observed between the three groups, suggesting a selective effect of nerve block on the frequency of excitatory synaptic currents in the developing SDH.
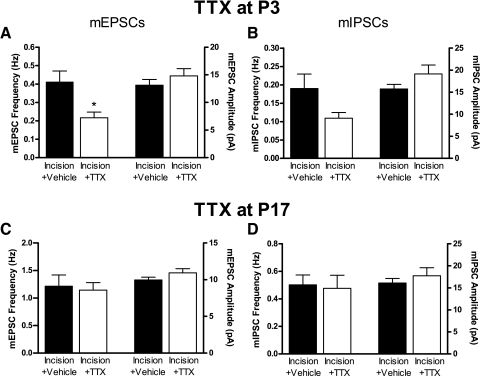
To confirm the role of afferent activity, we developed an additional method for prolonged sensory blockade in vivo. TTX was slowly released from microcapillaries inserted under the epineurium of the sciatic nerve (see methods), and as earlier, the efficacy of sciatic nerve conduction block was confirmed with behavioral testing. TTX application at P3 produced a significant increase in the mechanical withdrawal threshold on the ipsilateral paw compared with that of naïve and vehicle-treated littermate controls, which lasted for roughly 5 days (Fig. 4 D). The mechanical sensitivity on the contralateral paw was not significantly affected by the TTX microcapillary, suggesting that the decrease in mechanical responsiveness on the ipsilateral side reflects a spatially restricted block of sciatic nerve conduction by TTX (Fig. 4D).
To confirm that the TTX microcapillaries evoke a highly localized conduction block in the sciatic nerve, in vitro compound action potentials (CAPs) were recorded from sensory fibers in the L4/L5 dorsal roots 3 days after the capillaries were inserted (see methods). In the majority of TTX-treated pups examined, electrical stimulation of the sciatic nerve below (i.e., more distal to) the site of the microcapillary failed to evoke CAPs in the dorsal roots. However, in the same nerves, dorsal root CAPs could be readily evoked by identical stimulation at a location above (i.e., more proximal to) the site of the microcapillary (Fig. 4, A and B), suggesting that TTX delivery to the immature sciatic nerve in vivo produces a highly localized conduction block. The average stimulus–response curves for dorsal root CAPs from the naïve, vehicle, and TTX-treated groups at P20 (following microcapillary implantation at P17) are illustrated in Fig. 4C. These population data also demonstrate that TTX application causes a significant decrease in the excitability of sciatic nerve fibers (as evidenced by the rightward shift in the stimulus–response curve) when stimulation occurs below the location of the TTX microcapillary (n = 5); however, when the electrical stimulation is delivered above the site of the microcapillary the stimulus–response relationship for the TTX-treated nerves is similar to that of naïve (n = 3) and vehicle-treated (n = 4) controls (Fig. 4C). In addition, P20 nerves that had been treated with TTX at P3 (n = 4) also exhibited excitability similar to that of naïve and vehicle-treated controls, suggesting that conduction in the developing sciatic nerve was not permanently altered by the TTX application (Fig. 4C).
Surgical incision at P3 was combined with the insertion of microcapillaries containing either TTX (7.5 mM) or vehicle into the sciatic nerve and synaptic function in the SDH was examined 2–3 days later at P5–P6. First, we observed that the properties of spontaneous synaptic signaling in the vehicle-treated group (Incision + Vehicle; see Fig. 5, A and B) were similar to those previously documented for pups receiving incision alone (see Figs. 1 and 3), suggesting that neither the presence of the microcapillary itself nor perfusion with the vehicle solution caused significant modifications at immature SDH synapses. Importantly, as might be predicted from the BUPI experiments, block of sciatic nerve conduction with TTX caused a significant decrease in mEPSC frequency (Incision + Vehicle: 0.41 ± 0.06 Hz, n = 34; Incision + TTX: 0.22 ± 0.03 Hz, n = 23; P = 0.046; Mann–Whitney test) without an accompanying change in mEPSC amplitude (Incision + Vehicle: 13.12 ± 1.03 pA; Incision + TTX: 14.81 ± 1.31 pA; P = 0.202; Fig. 5A) or statistically significant alterations in mIPSC frequency (P = 0.124) or mIPSC amplitude (P = 0.142; Fig. 5B). These results suggest that excitatory synaptic function in the neonatal SDH depends on the level of primary afferent input under pathological conditions.
Fig. 5.
TTX delivery to the sciatic nerve in vivo during the 1st postnatal week decreases glutamatergic synaptic function in the SDH. A: reduction in primary afferent drive to the developing SDH from P3 decreased mEPSC frequency at P5–P6 compared with vehicle controls (*P = 0.046; Mann–Whitney test) without altering mEPSC amplitude. B: mIPSC frequency and amplitude were not significantly affected by TTX block of sciatic nerve activity from P3. C: in contrast, TTX application at P17 failed to alter mEPSC frequency (P = 0.527; Mann–Whitney test) or amplitude (P = 0.153; t-test) at P19–P20 compared with vehicle controls. D: TTX treatment during the 3rd postnatal week similarly had no effect on mIPSC frequency (P = 0.333; Mann–Whitney test) or amplitude (P = 0.590).
As demonstrated earlier (Fig. 2), in contrast to the effects of tissue injury during the first 10 postnatal days, surgical incision during the third postnatal week failed to evoke changes in spontaneous excitatory or inhibitory synaptic signaling in the SDH at the 2- to 3-day time point. If this reflects fundamental differences in the way older SDH neurons respond to alterations in sensory input, then one might predict that blocking the sciatic nerve in vivo during the third postnatal week would also fail to modulate synaptic transmission in the SDH. To address this issue, microcapillaries containing either TTX (30 mM) or vehicle solution were implanted at P17 and patch-clamp recordings were obtained from SDH neurons at P19–P20 under blinded conditions. No significant differences were observed in either excitatory or inhibitory synaptic function between the vehicle (n = 38) and TTX-treated (n = 36) groups at this age (Fig. 5, C and D).
Collectively, the results suggest that excitatory, but not inhibitory, synaptic drive onto SDH neurons strongly depends on the level of primary afferent input to the spinal cord during a limited period of early postnatal development.
Surgical incision alters the rectification of AMPAR-mediated currents, but not the AMPAR/NMDAR ratio, in neonatal SDH neurons
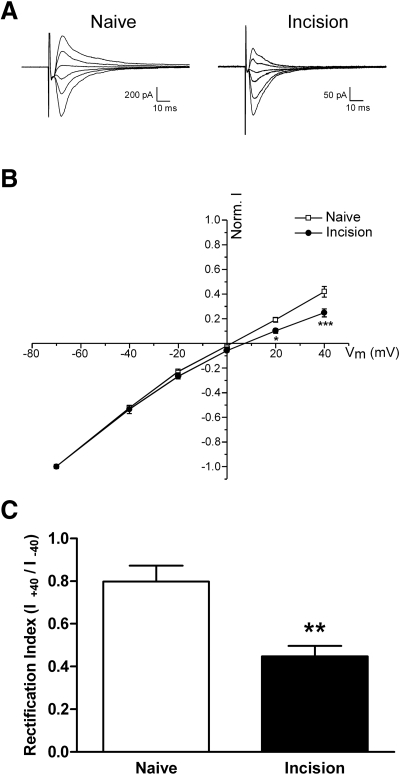
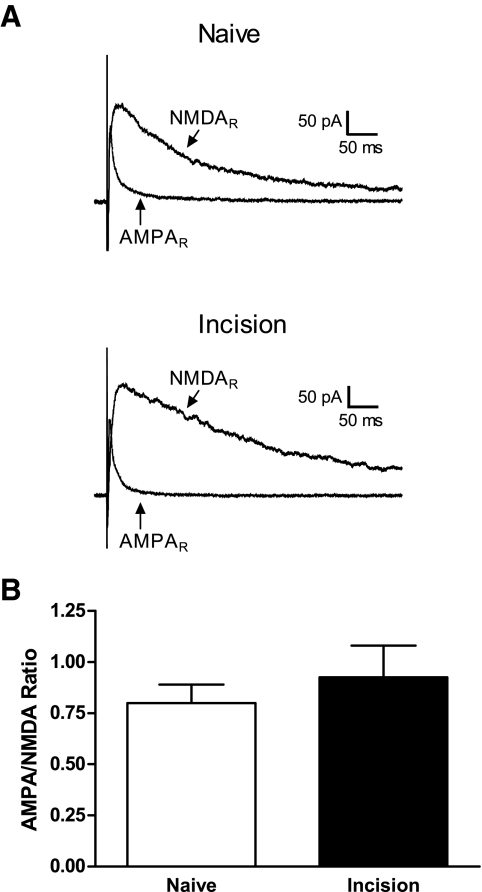
To determine whether tissue damage during the early postnatal period evokes changes in AMPAR function in neonatal SDH neurons, we characterized the current–voltage relationship of AMPAR-mediated EPSCs in P5–P6 SDH neurons following surgical incision at P3. Figure 6 A illustrates representative EPSCs evoked by focal stimulation at different holding potentials (from −70 to +40 mV) in the absence or presence of tissue injury. Incision at P3 increased the inward rectification of AMPAR currents in neonatal SDH neurons, as evidenced by the significantly lower current amplitudes seen at holding potentials of +20 mV (n = 15, *P < 0.05; two-way ANOVA) and +40 mV (***P < 0.001) compared with those of naïve littermate controls (n = 15; Fig. 6B). The degree of rectification of AMPAR responses can also be quantified by calculating a rectification index (RI), defined as RI = I at Vh+40/I at Vh−40 (Clem and Barth 2006). Surgical incision at P3 led to a significant decrease in this RI at P5–P6 (Naïve: 0.80 ± 0.07, n = 15; Incision: 0.45 ± 0.05, n = 15; P = 0.0011; Mann–Whitney test; Fig. 6C), suggesting the possibility that tissue damage may modulate the composition of postsynaptic AMPARs in neonatal SDH neurons. We also examined the effect of tissue injury at P3 on the AMPAR/NMDAR ratio at P5–P6 (see methods). As demonstrated in Fig. 7, we observed no significant difference in this ratio between the naïve and incision groups at the time point examined (Naïve: 0.80 ± 0.09, n = 20; Incision: 0.93 ± 0.15, n = 16; P = 0.738; Mann–Whitney test).
Fig. 6.
Surgical incision increases the inward rectification of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)–mediated currents in neonatal SDH neurons. A: representative traces corresponding to pharmacologically isolated AMPAR-mediated currents evoked by focal stimulation in lamina II from different holding potentials (−70 to +40 mV) in P5–P6 SDH neurons in the absence (left) or presence (right) of surgical incision at P3. Each sweep represents the average of 10 evoked EPSCs. B: current–voltage plot of the normalized amplitude of AMPAR-mediated currents (normalized to the mean amplitude of current evoked from a holding potential of −70 mV) as a function of holding potential (Vh). Tissue damage at P3 (n = 15) significantly increased the inward rectification of AMPAR responses compared with naïve (n = 15) controls at P5–P6 (*P < 0.05, ***P < 0.001; 2-way ANOVA). C: the mean rectification index (defined as I at Vh+40/I at Vh−40) of AMPAR currents at P5–P6 was significantly decreased by incision at P3 (**P = 0.0011; Mann–Whitney test).
Fig. 7.
Tissue damage fails to modulate AMPAR/N-methyl-d-aspartate receptor (NMDAR) ratios in immature SDH neurons. A: representative traces illustrating AMPAR-mediated and NMDAR-mediated currents evoked from a holding potential of +50 mV in SDH neurons from the Naïve (top) or Incision (bottom) groups at P5–P6 following injury at P3. Each sweep represents the average of 10 evoked EPSCs. B: there was no significant difference in the average AMPAR/NMDAR ratio between the Naïve (n = 20) and Incision (n = 16) groups at P5–P6 (P = 0.738; Mann–Whitney test).
DISCUSSION
Improving the clinical treatment of pain in infants and children requires knowledge of how tissue injury during the early postnatal period modulates immature pain circuitry at the cellular and molecular levels. Using an incision through the skin and muscle of the midthigh as a model of surgical injury (Flatters 2008), the present study illustrates that synapses within the spinal superficial dorsal horn (SDH) respond in an age-dependent manner: surgical injury during early life first potentiates, but later depresses, glutamatergic signaling in the SDH. Meanwhile, the same injury during the third postnatal week failed to regulate synaptic function in the SDH at the same 2- to 3-day time point. These results demonstrate a developmental shift in the way SDH synapses respond to tissue damage and thus are in agreement with our previous findings following neonatal inflammation (Li and Baccei 2009). In addition, we have now confirmed that the observed facilitation in glutamatergic transmission is activity dependent because blocking primary afferent activity after early tissue injury prevented the effects on excitatory synaptic drive within the neonatal dorsal horn.
Previous studies have revealed developmental differences in the response of the dorsal horn network to tissue injury. For example, hindpaw incision acutely increases both spontaneous and evoked firing, as well as receptive field size, in dorsal horn neurons at P7 but not at P28 (Ririe et al. 2008). The age-dependent, selective facilitation of excitatory SDH synapses in the short-term period following neonatal surgical injury (Fig. 1) or inflammation (Li and Baccei 2009) could contribute to these alterations in spinal nociceptive processing during early life. Thus although tissue damage clearly evokes behavioral hyperalgesia throughout postnatal development (Marsh et al. 1999; Ren et al. 2004; Ririe et al. 2003), it may enhance the excitability of the immature versus mature dorsal horn network via distinct underlying synaptic mechanisms. Although spontaneous excitatory neurotransmission in the mature SDH is unaffected by peripheral inflammation (Baba et al. 1999), the efficacy of glycinergic synaptic inhibition is reduced (Harvey et al. 2004; Muller et al. 2003). By contrast, we failed to observe changes in mIPSCs 2–3 days following neonatal surgical incision, although we cannot exclude the possibility that early tissue injury does modulate inhibitory synaptic efficacy in the immature SDH via changes in chloride homeostasis, as reported in the adult after peripheral inflammation or nerve injury (Coull et al. 2003; Zhang et al. 2008).
Mounting evidence suggests that tissue damage during a critical period of early postnatal development results in a delayed decrease in mechanical and thermal sensitivity in both rats (Chu et al. 2007; Lidow et al. 2001; Ren et al. 2004) and children (Hermann et al. 2006; Schmelzle-Lubiecki et al. 2007; Walker et al. 2009). These prolonged behavioral effects may partly reflect alterations in spinal cord function, given that distinct changes in gene expression are known to occur within the adult dorsal horn following neonatal tissue damage (Ren et al. 2005). Since the maturation of the dorsal horn network is highly activity dependent (Beggs et al. 2002; Granmo et al. 2008; Waldenstrom et al. 2003), even transient changes in glutamatergic transmission following neonatal tissue injury could evoke persistent effects on nociceptive processing by triggering intracellular signaling cascades that subsequently modulate the organization of emergent pain circuits within the SDH. It should be noted that the range of postnatal ages at which we observe these synaptic changes correlates well with the critical period as defined at the behavioral level in the rat (LaPrairie and Murphy 2007; Ren et al. 2004). It remains to be seen whether the delayed reduction in excitatory synaptic drive within the developing SDH following early surgical injury (Fig. 2, A and C) is also activity dependent and contributes to the above-cited reduction in pain sensitivity. Interestingly, a similar delayed decrease in mEPSC frequency was not observed following subcutaneous inflammation with carrageenan (Li and Baccei 2009), suggesting the possibility that the slower alterations in glutamatergic function are dependent on the severity or location (i.e., superficial vs. deep) of the tissue damage.
The SDH is comprised of several morphological cell types (Grudt and Perl 2002; Lu and Perl 2003, 2005) and the functional implications of the synaptic modifications reported here will depend on whether excitatory and/or inhibitory interneurons are affected by the tissue damage (Graham et al. 2007). Cell-type–specific changes in synaptic transmission, based on firing patterns, have been reported in the adult dorsal horn under pathological conditions (Balasubramanyan et al. 2006), although such classification is not possible using our cesium-based intracellular solution. In any case, the rapid postnatal dendritic growth (Bicknell Jr and Beal 1984) and the predominance of tonic action potential discharge in young rat SDH neurons (Baccei and Fitzgerald 2005; Li et al. 2009) suggest that adult cell classification systems may be inappropriate. This issue could be addressed using selective green fluorescent protein–labeled transgenic mice (Daniele and MacDermott 2009; Huang et al. 2006).
The activity dependence of the enhanced glutamatergic function within the SDH seen 2–3 days following neonatal incision was confirmed in two models of prolonged primary afferent blockade. Although the absence of ATF3 expression in the L4/L5 DRGs argues against a toxic effect of bupivacaine (BUPI) in our experiments (Supplemental Fig. S1, A and B), previous studies have suggested that BUPI can cause neurotoxicity (Myers et al. 1986; Radwan et al. 2002) and block fast axonal transport (Lavoie 1983). To confirm that the effect of BUPI on excitatory SDH synapses resulted from blocking electrical activity in the sciatic nerve, we used a second method of reducing sensory input to the developing spinal cord using TTX microcapillaries (Bray et al. 1979; Martinov and Nja 2005; Wall et al. 1982), which provides clear advantages over the implantation of local anesthetics. First, TTX application to the rat sciatic nerve does not interrupt axonal transport or cause axonal degeneration, even at concentrations that completely block action potential conduction (Bray et al. 1979; Martinov and Nja 2005; Pasino et al. 1996). Also, the insertion of identical microcapillaries containing pH-matched vehicle solution provides for a more stringent control group than is currently available with the BUPI approach, due to the lack of an inert form of bupivacaine hydroxide.
The selective increase in mEPSC frequency observed 2–3 days after surgical incision predicts changes in presynaptic function within the SDH. Since the majority of mEPSCs recorded in lamina II neurons likely reflect glutamate release from local excitatory interneurons (Baccei et al. 2003), we examined whether tissue damage increases the probability of glutamate release (Pr) at these synapses using paired-pulse analysis of EPSCs evoked by focal stimulation within the SDH (Li and Baccei 2009). Incision at P3 failed to alter the PPR of the evoked EPSCs (Fig. 1, G and H), which agrees with our previous findings that hindpaw inflammation with carrageenan potentiates mEPSC frequency without altering the PPR of EPSCs evoked via either focal or dorsal root stimulation (Li and Baccei 2009). However, the carrageenan study did not distinguish between different classes of primary afferents, which is challenging in the neonate since the relative lack of myelination (Friede and Samorajski 1968) and the ongoing growth in axonal diameter (Sima 1974) prevent the clear separation of sensory inputs that is observed in the adult (Baccei et al. 2003). As a result, we cannot exclude the possibility that subtype-selective changes in the PPR of primary afferent-evoked EPSCs occur after injury. Nonetheless, the available results collectively suggest that an increased Pr is unlikely to explain the enhanced mEPSC frequency following early tissue injury. Whereas the PPR analysis depends only on Pr, measurements of mEPSC frequency are dependent on both Pr and the number of glutamate release sites (n). Thus the simplest explanation for an increased mEPSC frequency without changes in the PPR is that early tissue damage elevates n in the SDH. Interestingly, extensive sprouting of nociceptive primary afferent fibers selectively occurs within the neonatal dorsal horn following peripheral inflammation during the early postnatal period (Ruda et al. 2000; Walker et al. 2003). As a result, we hypothesize that tissue damage during the first, but not third, postnatal week causes the expansion of C-fiber synaptic inputs within the SDH, resulting in an elevated mEPSC frequency.
Nonetheless, we cannot exclude other possible explanations for the increased mEPSC frequency in the absence of changes in the evoked PPR. For example, the enhanced mEPSC rate could reflect the conversion of “silent” (“NMDAR only”) synapses, which are present in the SDH during the first two postnatal weeks (Baba et al. 2000; Bardoni et al. 1998; Li and Zhuo 1998), to functional connections via the insertion of AMPARs into the postsynaptic membrane (Isaac et al. 1997). However, the evidence suggests this is unlikely to explain the elevated mEPSC frequency after neonatal incision. First, a significant increase in the number of synapses expressing AMPARs following tissue damage would be expected to produce a concomitant change in the AMPAR/NMDAR ratio, which was not observed (Fig. 7). Second, previous studies of immature rat SDH neurons have estimated that pure NMDAR-only synapses account for about 20% of the total number of glutamatergic synapses (Bardoni et al. 1998). It is difficult to envision how recruitment of AMPARs to these synapses can explain the 100% increase in mEPSC frequency evoked by incision at P3. Alternatively, early tissue injury could selectively modulate spontaneous excitatory neurotransmission in the developing SDH. Different subtypes of voltage-gated Ca2+ channels are known to regulate miniature versus evoked EPSCs in the adult dorsal horn (Bao et al. 1998). Recent work has also demonstrated that spontaneous and evoked glutamate release originate from separate pools of synaptic vesicles in hippocampal neurons (Fredj and Burrone 2009; Sara et al. 2005).
Although the data point to a restricted developmental window during which tissue injury can transiently strengthen excitatory transmission in the SDH, the mechanisms regulating the timing of this window are unclear. Significant alterations in the properties of GABAergic and glycinergic synapses occur within the rat SDH during the early postnatal period (Baccei and Fitzgerald 2004; Cordero-Erausquin et al. 2005). Thus the maturation of GABAergic (or glycinergic) synapses within the SDH and the subsequent increase in inhibitory tone could prevent any potentiation of excitatory synaptic function after tissue damage during the third postnatal week. A second possibility is that the closure of the developmental window is initiated by the maturation of descending inhibitory pathways from supraspinal sites (Fitzgerald and Koltzenburg 1986; Hathway et al. 2009). These two possibilities are not mutually exclusive because a proportion of the descending inhibitory inputs to the adult dorsal horn are GABAergic (Kato et al. 2006).
Neonatal tissue damage also causes alterations in SDH synaptic function which may be conserved throughout postnatal development. For example, the increased inward rectification of AMPAR-mediated currents following P3 incision (Fig. 6) also occurs in young adult lamina I neurons after hindpaw inflammation with complete Freund's adjuvant (Vikman et al. 2008). The inward rectification of AMPAR currents is caused by a voltage-dependent block of Ca2+-permeable, glutamate receptor 2–lacking receptors by intracellular polyamines (Bowie and Mayer 1995; Donevan and Rogawski 1995; Hollmann et al. 1991; Washburn et al. 1997). As a result, increased inward rectification is normally associated with an enhanced contribution of Ca2+-permeable AMPARs to the overall synaptic response (Clem and Barth 2006; Vikman et al. 2008), which may facilitate activity-dependent synaptic strengthening (Gu et al. 1996) and spinal nociceptive plasticity (Hartmann et al. 2004). However, a shift in the proportion of Ca2+-permeable AMPARs may have additional functional significance in the neonatal dorsal horn, given the well-documented role of intracellular calcium in neuronal development.
It is now widely accepted that the neonatal spinal cord is capable of significant activity-dependent plasticity that can lead to central sensitization and subsequent hypersensitivity to pain, given that hyperalgesia and allodynia have been extensively documented in both human infants and rat pups after tissue injury (Fitzgerald et al. 1989; Guy and Abbott 1992; Jiang and Gebhart 1998). The present results suggest that the mechanisms underlying central sensitization may be developmentally regulated because the effects of tissue damage on synaptic function in the superficial dorsal horn are highly dependent on postnatal age. This study emphasizes the importance of considering the pathophysiology of pain from a developmental perspective.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke Grant NS-060858, University of Cincinnati Millennium Fund, and the Medical Research Council, Great Britain.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Baba H, Doubell TP, Moore KA, Woolf CJ. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. J Neurophysiol 83: 955–962, 2000 [DOI] [PubMed] [Google Scholar]
- Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci 19: 859–867, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol 549: 231–242, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci 24: 4749–4757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol 96: 579–590, 2006 [DOI] [PubMed] [Google Scholar]
- Bao J, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci 18: 8740–8750, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. J Neurosci 18: 6558–6567, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci 16: 1249–1258, 2002 [DOI] [PubMed] [Google Scholar]
- Bicknell HR, Jr, Beal JA. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol 226: 508–522, 1984 [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15: 453–462, 1995 [DOI] [PubMed] [Google Scholar]
- Bray JJ, Hubbard JI, Mills RG. The trophic influence of tetrodotoxin-inactive nerves on normal and reinnervated rat skeletal muscles. J Physiol 297: 479–491, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YC, Chan KH, Tsou MY, Lin SM, Hsieh YC, Tao YX. Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation: effects of N-methyl-d-aspartate receptor antagonists. Anesthesiology 106: 1204–1212, 2007 [DOI] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49: 663–670, 2006 [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. J Neurosci 25: 9613–9623, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938–942, 2003 [DOI] [PubMed] [Google Scholar]
- Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci 29: 686–695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res 389: 261–270, 1986 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 39: 31–36, 1989 [DOI] [PubMed] [Google Scholar]
- Flatters SJ. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain 135: 119–130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron 47: 101–114, 2005 [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci 12: 751–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol 27: 546–570, 1968 [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. Moving from an averaged to specific view of spinal cord pain processing circuits. J Neurophysiol 98: 1057–1063, 2007 [DOI] [PubMed] [Google Scholar]
- Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci 28: 5494–5503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol 540: 189–207, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature 381: 793–796, 1996 [DOI] [PubMed] [Google Scholar]
- Guy ER, Abbott FV. The behavioral response to formalin in preweanling rats. Pain 51: 81–90, 1992 [DOI] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr 7: 321–335, 1999 [PMC free article] [PubMed] [Google Scholar]
- Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron 44: 637–650, 2004 [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, bo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Muller U. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304: 884–887, 2004 [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem–spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol 587: 2711–2712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain 125: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science 252: 851–853, 1991 [DOI] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 95: 1656–1668, 2006 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron 18: 269–280, 1997 [DOI] [PubMed] [Google Scholar]
- Jiang MC, Gebhart GF. Development of mustard oil-induced hyperalgesia in rats. Pain 77: 305–313, 1998 [DOI] [PubMed] [Google Scholar]
- Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. J Neurosci 26: 1787–1794, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol 548: 131–138, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132, Suppl. 1: S124–S133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie PA. Inhibition of fast axonal transport in vitro by the local anesthetics prilocaine, mepivacaine, and bupivacaine. Can J Physiol Pharmacol 61: 1478–1482, 1983 [DOI] [PubMed] [Google Scholar]
- Li J, Baccei ML. Excitatory synapses in the rat superficial dorsal horn are strengthened following peripheral inflammation during early postnatal development. Pain 143: 56–64, 2009 [DOI] [PubMed] [Google Scholar]
- Li J, Xie W, Zhang JM, Baccei ML. Peripheral nerve injury sensitizes neonatal dorsal horn neurons to tumor necrosis factor-alpha. Mol Pain 5: 10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 393: 695–698, 1998 [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport 12: 399–403, 2001 [DOI] [PubMed] [Google Scholar]
- Lorenzo L-E, Ramien M, St Louis M, De Koninck Y, Ribeiro-da-Silva A. Postnatal changes in the Rexed lamination and markers of nociceptive afferents in the superficial dorsal horn of the rat. J Comp Neurol 508: 592–604, 2008 [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci 23: 8752–8758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci 25: 3900–3907, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D, Dickenson A, Hatch D, Fitzgerald M. Epidural opioid analgesia in infant rats. II. Responses to carrageenan and capsaicin. Pain 82: 33–38, 1999 [DOI] [PubMed] [Google Scholar]
- Martinov VN, Nja A. A microcapsule technique for long-term conduction block of the sciatic nerve by tetrodotoxin. J Neurosci Methods 141: 199–205, 2005 [DOI] [PubMed] [Google Scholar]
- Mierau SB, Meredith RM, Upton AL, Paulsen O. Dissociation of experience-dependent and -independent changes in excitatory synaptic transmission during development of barrel cortex. Proc Natl Acad Sci USA 101: 15518–15523, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 22: 6724–6731, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084–8090, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Heinke B, Sandkuhler J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience 122: 799–805, 2003 [DOI] [PubMed] [Google Scholar]
- Myers RR, Kalichman MW, Reisner LS, Powell HC. Neurotoxicity of local anesthetics: altered perineurial permeability, edema, and nerve fiber injury. Anesthesiology 64: 29–35, 1986 [PubMed] [Google Scholar]
- Pasino E, Buffelli M, Arancio O, Busetto G, Salviati A, Cangiano A. Effects of long-term conduction block on membrane properties of reinnervated and normally innervated rat skeletal muscle. J Physiol 497: 457–472, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29: 157–169, 2001 [DOI] [PubMed] [Google Scholar]
- Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg 94: 319–324, 2002 [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain 110: 588–596, 2004 [DOI] [PubMed] [Google Scholar]
- Ren K, Novikova SI, He F, Dubner R, Lidow MS. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats (Abstract). Mol Pain 1: 27, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG, Bremner LR, Fitzgerald M. Comparison of the immediate effects of surgical incision on dorsal horn neuronal receptive field size and responses during postnatal development. Anesthesiology 109: 698–706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG, Vernon TL, Tobin JR, Eisenach JC. Age-dependent responses to thermal hyperalgesia and mechanical allodynia in a rat model of acute postoperative pain. Anesthesiology 99: 443–448, 2003 [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 289: 628–631, 2000 [DOI] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45: 563–573, 2005 [DOI] [PubMed] [Google Scholar]
- Schmelzle-Lubiecki BM, Campbell KA, Howard RH, Franck L, Fitzgerald M. Long-term consequences of early infant injury and trauma upon somatosensory processing. Eur J Pain 11: 799–809, 2007 [DOI] [PubMed] [Google Scholar]
- Sima A. Studies on fibre size in developing sciatic nerve and spinal roots in normal, undernourished, and rehabilitated rats. Acta Physiol Scand Suppl 406: 1–55, 1974 [PubMed] [Google Scholar]
- Todd AJ, Koerber HR. Neuroanatomical substrates of spinal nociception. In: Wall and Melzack's Textbook of Pain ( 5th ed.), edited by McMahon SB, Koltzenburg M. Philadelphia, PA: Elsevier Churchill Livingstone, 2006, p. 73–90 [Google Scholar]
- Torsney C, Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. J Neurophysiol 87: 1311–1317, 2002 [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol 586: 515–527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci 23: 7719–7725, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 141: 79–87, 2009 [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain 105: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- Wall PD, Mills R, Fitzgerald M, Gibson SJ. Chronic blockade of sciatic nerve transmission by tetrodotoxin does not produce central changes in the dorsal horn of the spinal cord of the rat. Neurosci Lett 30: 315–320, 1982 [DOI] [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci 17: 9393–9406, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain 116: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience 53: 519–526, 1993 [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu LY, Xu TL. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience 152: 502–510, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.