Abstract
Off-line learning is facilitated when motor skills are acquired under a random practice schedule and retention suffers when a similar set of motor skills are practiced under a blocked schedule. The current study identified the neural correlates of a random training schedule while participants learned a set of four-element finger sequences using their nondominant hand during functional magnetic resonance imaging. A go/no go task was used to separately probe brain areas supporting sequence preparation and production. By the end of training, the random practice schedule, relative to the block schedule, recruited a broad premotor–parietal network as well as sensorimotor and subcortical regions during both preparation and production trials, despite equivalent motor performance. Longitudinal analysis demonstrated that preparation-related activity under a random schedule remained stable or increased over time. The blocked schedule showed the opposite pattern. Across individual subjects, successful skill retention was correlated with greater activity at the end of training in the ipsilateral left motor cortex, for both preparation and production. This is consistent with recent evidence that attributes off-line learning to training-related processing within primary motor cortex. These results reflect the importance of an overlooked aspect of motor skill learning. Specifically, how trials are organized during training—with a random schedule—provides an effective basis for the formation of enduring motor memories, through enhanced engagement of core regions involved in the active preparation and implementation of motor programs.
INTRODUCTION
Contemporary research in the acquisition of motor skills demonstrates that performance improvements unfold over at least two separable timescales: a fast component that occurs within a training session and a delayed latent component that is present after a training session. This latent component, referred to as off-line learning, is often equated with memory consolidation, which is explained as a process of transforming memories into a stable and endurable form (McGaugh 2000). Considerable research has demonstrated both time (Robertson et al. 2005) and sleep (Korman et al. 2007; Walker et al. 2003) to be critical to off-line learning. In line with these behavioral features, experimental evidence from transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) have shown motor regions—with strong emphasis on the primary motor cortex and its subcortical targets—to be involved in off-line learning (Doyon and Benali 2005; Krakauer and Shadmehr 2006; Robertson and Cohen 2006). Although it is understood that off-line learning is a product of successful training and the activation of a cortical–subcortical motor network, it remains relatively unclear how the structure of training itself shapes activity within motor regions that support enduring motor memories. To approach this question, we manipulated the effectiveness of training through practice structure to identify motor areas that support off-line learning in humans.
For motor skills in which more than one specific action is being acquired, the structure of practice trials plays a critical role in the enhancement of off-line learning after practice. A robust behavioral effect, known as contextual interference (CI), represents a fundamental example of how practice can be manipulated to enhance newly formed memories. CI experiments typically present the same set of tasks to two groups of participants. The only difference between the two groups is how the specific actions are ordered during training. One group is given the actions in a blocked order so that all the trials for one particular action are repeated before the next is presented and the other group practices the different actions in a random order. Performance during training typically favors the blocked group. Critically, performance on a retention task following a substantial delay demonstrates greater off-line learning for the skills practiced with a random schedule. First described by Battig (1972) and Shea and Morgan (1979), the CI effect has been described in numerous motor and cognitive tasks (for reviews. see Brady 2004; Lee and Simon 2004; Magill and Hall 1990).
The CI effect shows that a random training schedule can benefit motor skill retention. Unlike the beneficial effects that time and sleep have on learning after training ends, CI is also dependent on processes occurring at the time of training. One dominant theoretical account suggests that more effortful processing must occur during random training because the motor program information related to the current trial is forgotten by intervening information that emerges after the previous trial (Lee and Magill 1983, 1985). The theory contends that increased preresponse processing linking the relevant stimulus information with the required motor output is what drives improved retention performance. Recent evidence from continuous sequence learning experiments appears to support this “active preparation” theory, demonstrating that random training leads to extensive processing during the preresponse interval prior to sequence execution (Immink and Wright 1998, 2001). Immink and Wright found that random training demands more time during acquisition relative to block training and that this additional processing time leads to improved performance retention, as established by greater accuracy and precision following a substantial delay. Behavioral evidence in support of an active planning model suggests that random training should lead to greater recruitment of neural systems supporting preparation and execution. Regions showing increased activation by the end of training might also support subsequent molecular changes that lead to performance improvements after training has ended.
Evidence that there is increased neural processing during a random training schedule was first provided by an fMRI study of contextual interference of sequence learning (Cross et al. 2007). One group of subjects practiced three keyboard sequences with a block training schedule and a second group practiced the same sequences with a random training schedule. A go/no-go paradigm was used to distinguish preparation from execution. By the end of training the random group demonstrated stronger blood-oxygen-level–dependent (BOLD) activation in sensorimotor and premotor regions during movement preparation when contrasted with the block group. Interestingly, random practice failed to produce any stronger activation during movement execution at the end of practice. A retention test, performed outside the scanning environment, showed greater off-line learning benefits for the random group. This suggests that the increased movement preparation activation by the end of practice led to improved performance retention.
The scope of this prior result is limited because subjects were given an unlimited amount of time to prepare a response, with random trials taking significantly longer to plan than block trials. Because the amount of time spent preparing sequences differed between groups, it is difficult to say whether the activation results were related to differences between the training schedules or were related to a more general effect of sequence preparation. Second, the study used a between-population design. Although this experimental design was consistent with approaches used in behavior studies, the between-groups design undermines statistical power in brain imaging.
To measure brain activity linked with subsequent off-line learning and also to address the shortcomings of the previous fMRI study, we used a within-subject design and measured brain activity during sequence learning under blocked and random training schedules. To achieve this goal we first completed a behavioral study demonstrating for the first time that the CI effect holds true when using a within-subject design (unpublished data, but replicated in the current fMRI study). A within-subject design affords the direct comparison of brain activation images from the same participant under each training schedule. We adopted a hypothesis that the random training schedule leads to increased processing, particularly during motor planning. As such, random training trials, relative to block training trials, should demonstrate greater activity in motor preparation and execution areas, particularly by the end of training. An additional aim was to characterize how the activation patterns for both training schedules evolve over the entire training period. This is an important consideration because it tells not only which regions are greater for random training at the end of practice, but also identifies which training effects are specific to random training compared with blocked training.
Candidate regions that might be more active during training under a random schedule and thought to support subsequent sequence retention were hypothesized to be those areas previously shown to demonstrate experience-dependent changes when learning arbitrary or sequential visuomotor associations during the fast component of motor skill learning (for reviews, see Ashe et al. 2006; Sanes and Donoghue 2000; Tanji 2001). A significant body of evidence from both humans and nonhuman primates suggests that a network involving the dorsal premotor cortex (PMd) and the posterior parietal cortex (PPC) is involved in the planning and selection of learned visuomotor associations (Bischoff-Grethe et al. 2004; Cavina-Pratesi et al. 2006; Diedrichsen et al. 2006; Grafton et al. 1998; 2002; Grol et al. 2006; Thoenissen et al. 2002). Recent evidence suggests that the primary motor cortex (M1) is also essential for the storage of newly learned motor skills (Ashe et al. 2006; Lu and Ashe 2005; Matsuzaka et al. 2007), a role also assumed by the basal ganglia (Jueptner et al.1997; Lehéricy et al. 2005; Toni et al. 2001). Additional evidence using TMS has implicated the primary motor cortex (M1) in the functional involvement of latent learning of several different motor skills (Muellbacher et al. 2002; Richardson et al. 2006; Robertson et al. 2005). Furthermore, the temporal representation of learned associations appears to be facilitated by the supplementary and presupplementary motor areas (SMA and pre-SMA), with the former having an emphasis on the fluid execution of movement and the latter having an emphasis on the grouping and organization of motor information (Isoda and Tanji 2004; Shima and Tanji 2000). Together, these areas form the core network of motor preparation and execution in which we would predict random training might lead to a greater recruitment of activity.
Training with a random schedule typically leads to performance enhancement on the following day. We also know that motor cortex plays a central role in skill consolidation. In a recent TMS study, we found that single-pulse TMS applied to left motor cortex during sequence preparation eliminated the beneficial effect of a random practice structure on subsequent consolidation of sequences learned by the left hand (Cohen et al. 2009). In the current study we sought to merge these observations by testing whether individual subject differences of motor cortex activity at the end of training could be correlated with skill consolidation.
METHODS
Participants
Twenty right-handed participants (11 female, mean age = 20.9 yr) volunteered with informed consent in accordance with the Committee for the Protection of Human Subjects, Dartmouth College, Hanover, New Hampshire. All participants had <4 yr of piano training or experience. Four participants were excluded from further analysis because behavioral response data exceeded a predetermined exclusion threshold (explained in the following text). All participants had normal vision and no history of neurological disease or psychiatric disorders. Participants were either paid or given coursework credit.
Each participated in two scanning sessions: a training session and a follow-up test session acquired 1 day after the training session. Two participants completed this test session 4 days after training, one because of technical difficulties and the other because of drowsiness during the initial follow-up session. The test session was performed within the identical scanner environment to maintain consistency with the initial training session.
Experiment setup and procedure
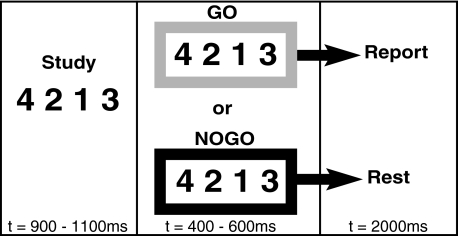
Participants lay supine in the MRI scanner. Stimuli were back-projected onto an adjustable mirror mounted inside of the headcoil. On both days, participants performed a visually cued sequence response task using their nondominant left hand on a custom-made fiber-optic response box placed on their lap. To make the response surface uniform and the button box stable during scanning, a board was placed between the participant's lap and the button box. Responses were made with all four fingers, excluding the thumb. Each visual cue, as illustrated in Fig. 1, displayed a string of four digits containing the numbers 1–4, randomly ordered, and without repetition. The digit strings excluded combinations that occur sequentially (e.g., “1234” and “4321”) or as runs (e.g., “4123”). The keys were matched to the numbers from left to right so that a “1” was reported with the smallest finger and a “4” was reported with the index finger. There are a total of 24 nonrepeating combinations of the numbers 1–4. Combinations that contained strings of three or four sequential digits (i.e., “1234” or “4123”) were excluded, leaving a total 18 sequences to choose from. The following sequences were hand-selected: “1423,” “2413,” “3142,” “3241,” “4213,” and “4231.” The same sequences were learned by all participants and were counterbalanced across training schedules.
Fig. 1.
Trial structure. Participants first prepared the number sequence in the study segment. Either a green (lighter shade) or a red (darker shade) box then appeared with the sequence still present on the screen. Trials with the green box (go) signaled the subject to execute the sequence. The red box (no go) cued subjects to disengage and wait for the next trial.
A trial began with the presentation of the sequence (900 to 1,100 ms) at the center point of the video screen (Fig. 1, left). Participants were instructed to prepare but not execute a response. Next, the sequence reappeared (400 to 600 ms) surrounded by either a green (go) or a red (no go) border cuing participants to execute or refrain from responding (Fig. 1, center). On the disappearance of the go cue, participants had 2,000 ms to generate their response; otherwise, it was counted as incorrect.
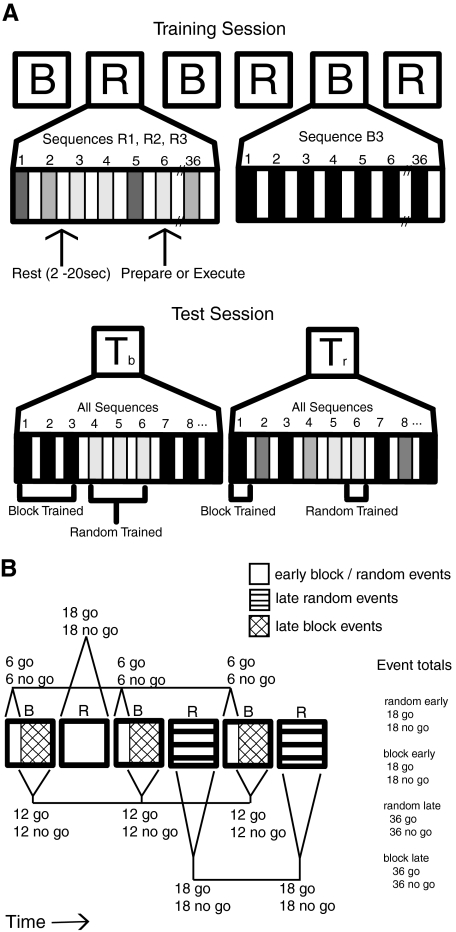
Figure 2A depicts the experiment timeline. Subjects trained on six sequences over the course of six scan epochs. The sequences were divided between two training schedules, with three of the sequences presented under a block training schedule and the other three sequences presented under a random training schedule. There were three block training scan epochs interleaved with three random training scan epochs as shown. Half the subjects started with a block epoch and the other half started with a random epoch, each alternating until all six epochs were completed. Sequences were unique to type of epoch, so that a sequence trained under a block schedule would not be trained under the random schedule and vice versa. The block schedule featured one sequence for an entire scan epoch. Each block epoch presented the same sequence over 36 trials, with half being preparation (i.e., no go trials) and the other half being production (i.e., go trials). The random schedule presented all three random sequences over the course of each scan epoch. Random epochs also contained 36 trials, with half being no go and the other half being go trials. The order of the three sequences was randomized within each epoch and each of the three sequences had the same amount of no go and go trials within the epoch. The same three random sequences were presented in each of the three random epochs. Event totals for the experiment are tabulated on the right in Fig. 2B. By the end of training, all sequences (three block, three random) were presented the same number of times. There are two critical points in this design. First, all sequences received the same amount of training, with the only difference being how the training was structured. Second, the training was performed with a within-groups design, so all participants trained on all sequences and under both practice schedules.
Fig. 2.
Experimental design and structure of event-related functional magnetic resonance imaging (fMRI) analysis. A: the training session presented participants with alternating trial epochs organized in both block (B) and random schedule (R) form. Each schedule epoch contained 18 sequence preparation (no go) and 18 sequence production (go) trials. Participants received 6 schedule epochs: 3 block schedules and 3 random schedules each lasting 139 scans. Presentation of block and random epochs were interleaved, such that following the completion of all trials contained in a block schedule, the next epoch of trials would be presented using a random schedule. For the test session participants received 2 epochs of trials comprised of all the practiced sequences, independent of initial practice schedule. One epoch contained trials organized into miniblocks of 3 trials of the same sequence (Tb) and the other epoch presented trials in a random order (Tr). For each epoch, trials could be either preparation or production events. Epochs continued until participants produced 18 correct sequences. B: to account for behavioral performance differences between schedules within the fMRI analysis, the training trials were grouped into thirds. The first third of trials, shown in solid white, are known as “early training events,” whereas the final two thirds of trials, shown with line patterns, are known as “late training events.” Early and late training events were defined by the amount of training time for each sequence. Such that, the first third of trials (6 trials) for a particular sequence were considered early events, and the final two thirds of trials (12 trials) for the same sequence were considered late events. This was done separately for both preparation (no go) and production events (go). Because only one sequence was presented in each block schedule, early events were considered to be the first third of each block epoch and late events were from the final two thirds of each block epoch. On the other hand, all 3 random schedule sequences were presented in each random schedule epoch, so that the early events were considered to be the first random epoch, whereas the final 2 random epochs were classified as late events. A total of 18 early events and 36 late events were designated for both block and random schedules.
Trials were spaced with an intertrial interval (ITI) lasting between 2 and 20 s. This variable ITI was used to improve fMRI model estimation of task relative to baseline. Following every six sequence execution trials, participants received feedback that displayed the average time to complete a sequence and the number of correct sequences. Each training epoch lasted a total of 139 scan repetition times (TRs, 348 s) and each training session a total of 834 scan TRs (∼35 min).
The test session presented two epochs, each containing all six sequences used in the training sessions. To compensate for possible differences in the order of sequence presentation during the training epochs and the subsequent test schedules, one test epoch presented all sequences in short blocks of trials and the other test epoch presented all sequences in a random order, as shown in the bottom of Fig. 2A. The order of blocked or random test epochs was randomized across subjects. Both test epochs contained preparation and execution trials and each continued until the subject made 18 correct responses. No performance feedback was given during the test session.
Prior to the start of the training session, participants were introduced to the task inside the scanner room. After being positioned in the scanner and while initial localizer and anatomical images were collected, participants practiced the sequences “4321” and “1234.” Feedback followed each practice trial. During the test session, participants were reminded of the task but received no additional practice.
Behavioral apparatus
Stimulus presentation was controlled with a PC running Matlab version 7.1 (The MathWorks, Natick, MA) in conjunction with Cogent 2000 (FIL 2000). Key-press responses and response times were collected using a fiber-optic custom button box transducer connected to a digital response card (DAQCard-6024e; National Instruments, Austin, TX).
Imaging procedures
The experiment was performed in a 3.0-T Philips Intera using an eight-channel SENSE head coil (Philips Medical Systems, Best, the Netherlands). BOLD imaging used single shot echo planar imaging. The scanning parameters were: 30 slices per repetition time (TR, 4.5-mm thickness, 0.5-mm gap, interleaving, axial scan plane), with a TR of 2,500 ms, echo time (TE) of 35 ms, a field of view (FOV) of 24 cm, and a 64 × 64 matrix. We acquired 834 functional volumes from each subject during training and a variable amount during follow-up, depending on how long a subject needed to make 18 correct responses. Full-brain T1-weighted images were acquired using a spoiled gradient recalled three-dimensional sequence (TR = 9.9 ms; TE = 4.6 ms; flip angle = 8°; FOV = 240 mm; slice thickness = 1 mm; matrix = 256 × 256).
Data analysis: behavior
Movement time (MT) and response time (RT) data were collected for all production trials. RT is the time elapsed from the disappearance of the response cue to the initial key-down press. MT is the time elapsed from the initial to the final key-down press. Training session trials with errors or other contaminated trials (due to malfunction of the response box) were first removed and then accounted for using an expectation maximization algorithm (Schneider 2001). The algorithm sampled the mean, SE, and covariance of the existing data points to generate replacement values. Following this step, MT values that fell outside a 2SD range were replaced with either the upper or lower bound of this range. Training data were sorted by schedule type and then segmented into groups of six temporally contiguous trials. The block training groups were then reordered to reflect sequence experience in terms of trials rather than time, a perspective adopted from the initial CI literature (Shea and Morgan 1979). Because three block sequences were presented during training, trials were reordered so that the initial 6 trials of the first block sequence were followed by the initial 6 trials of the second block sequence and, again, by the initial 6 trials of the third block sequence. This pattern was repeated for the trial group that contained production trials 7–12 and then, finally, by the group containing trials 13–18. The random trial groups maintained their original order in time. Subjects were excluded if behavior from two or more scanning epochs contained trial group values outside the ±2SD range from the population across each MT group. MTs for each subject, segment, and schedule were entered into a 2 × 9 repeated-measures ANOVA, with subject being entered as a random factor (SPSS, Chicago, IL). A probability of P < 0.05 was set as the threshold for rejection of the null for the training session.
To determine the effect of training schedule on off-line learning, MT data were selected from the initial half of trials from the first test session epoch. The rationale for selecting data only from the beginning of the test session was to isolate memory performance from the relearning of the sequences. Because test session trials presented all six sequences from the previous day's training session, test session trials were grouped according to what training schedule they were practiced under on the previous day. In other words, three of the sequences were classified as “block trained” and the other three were classified as “random trained.” The effect of schedule on off-line learning was determined by comparing test trials with the last four training trials from the same training schedule. As such, test session sequences originally learned under a block training schedule were compared with the final four block training trials. Four training trials from each schedule type allowed for an ideal match between the amount of data points needed to perform a paired-sample t-test comparison with the test session trials. Furthermore, results from the comparison would not be attributed to unequal variance between conditions because SEs for training (block = 22.4; random = 22.8) and test session trials (block = 27.4; random = 24.7) were equivalent for the group data. Finally, difference scores of latent learning were calculated for each participant and for each schedule by subtracting the mean of the early test trials from the mean of the final training trials. Positive difference scores reflect off-line learning. The random schedule difference scores were used as a covariate of interest in aim 4 of the fMRI analysis.
The analysis of test session was limited to the behavioral data. fMRI data were collected at the same time to closely replicate the conditions subjects experienced during training. However, because only a limited number of trials were collected per condition to minimize additional learning during the test session, analysis of test session fMRI data was not well suited for statistical modeling. Imaging results for the test session are not reported, to avoid the risk of making a type I error from the low statistical power of fMRI data in this session.
fMRI data analysis
The fMRI analyses addressed four aims. Aim 1 was to identify task-specific neural regions that supported the preparation and the production of visually cued finger sequences with the nondominant left hand. Aim 2 was to identify those regions supporting the preparation and execution of sequences late in training for the random schedule relative to the block schedule. Aim 3 was to identify differences in longitudinal changes of brain activation over the entire course of training as a function of the two training schedules. To avoid confounds of differing kinematics, this question was limited to preparation, no go trials only. Aim 4 was to test whether activity in motor cortex measured at the end of training and for just the random training schedule, correlated with individual differences in subsequent off-line learning.
Preprocessing and statistical analysis of the data were performed using FSL (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain [FMRIB], Oxford University, Oxford, UK) (Smith et al. 2004). Motion correction was performed by MCFLIRT (Jenkinson et al. 2002). Images were temporally high-pass filtered with a 50-s cutoff period. Spatial smoothing was applied with a Gaussian kernel of 8 mm (full width at half-maximum) and signal intensities were globally normalized to account for transient fluctuations in signal intensity.
Statistical analyses were performed at the single-subject level by using the general linear model (GLM) as implemented in FSL (FMRI Expert Analysis Tool). Training regressors were defined by schedule (block, random) and split into two sets based on exposure time (early training, late training).
Figure 2B shows how trials were defined for the formation of regressors used in the general linear model (GLM) to estimate BOLD effects. “Early” training events were defined as the first third of go and no go trials from each practice schedule. Similar to the analysis of the behavioral data, the first 6 trials from each block epoch were designated as early block trials. Early random trials also came from the first third of the random sequences. Because the first random epoch contained 6 go and 6 no go trials from each of the three random sequences trained on, all early random trials could be taken from this initial epoch. Both block and random schedules contained 18 go and 18 no go early events. “Late” training events were parcellated in a similar fashion. The final two thirds of each block epoch were defined as late block events, whereas the final two random epochs were used to define late random events. This resulted in 12 go and 12 no go trials for each of the sequences and thus a total of 36 go and 36 no go trials per practice schedule. By organizing the GLM regressors in this manner, the fMRI design is consistent with how the behavioral data were binned and thus is also consistent with the CI literature. Further, as discussed in greater detail in the following text, this organization allowed for the direct comparison of block and random events because MT was equivalent between schedules during late training.
Additional nuisance variables of noninterest were added as model regressors. These consisted of session means, error trials, and MT, which was used as a covariate by weighting each production trial by a value that was the percentage difference from the mean MT generated from all training trials. MT was included as a covariate of noninterest to ensure that brain activation differences at the end of training were not related to performance differences during sequence production.
This design matrix was convolved with the default gamma-shaped canonical hemodynamic response function (HRF) and temporal derivative in FSL. Each subject-specific design matrix was estimated using a fixed-effects approach, generating parameter estimates (PEs) for each regressor as well as contrasts of parameter estimates (COPEs) using the PEs convolved with the HRF, excluding the temporal derivative. The COPEs were then carried up to the second-level group analysis.
Individual brains were normalized to the Montreal Neurological Institute (MNI)–152 template with a three-stage, 12 degrees of freedom affine registration using FLIRT. A nonlinear registration was then carried out using FNIRT, with a warp resolution of 10 mm. Spatially normalized individual subject contrasts (COPEs) were submitted to a mixed-effects group analysis. Higher-level analysis was carried out using FLAME “1 + 2,” FMRIB's Local Analysis of Mixed Effects in FSL, with automatic outlier deweighting and with a whole-brain search volume (Beckmann et al. 2003; Woolrich et al. 2004). Type II error was minimized by using the FDR algorithm for multiple comparison correction with q = 0.05 (Genovese et al. 2002). One exception to this was the contrasts performed for aim 4, which was corrected using the Bonferroni method, so that voxels above the threshold P < 0.0125 were significant. For visualization purposes, the z-images were mapped to the partially inflated cortical surface of the Population Average Landmark and Surface-based (PALS-B12) atlas using the Caret software application (Van Essen 2005). The PALS-B12 atlas represents the surface registration of 12 normal adult high-resolution scans, which can be used as an unbiased template for displaying images from group fMRI analyses.
To address our first aim, we created separate contrasts for the main effects of sequence preparation and sequence execution, both independent of time and practice schedule, relative to baseline.
To address aim 2, that is to identify regions that were differentially activated during late training, the linear contrasts “block > random late” and “random > block late” were performed separately for both sequence preparation and sequence execution trials. The objective was to determine which brain regions showed greater activity for each practice schedule once all the sequences had already been practiced. Because there was a significant behavioral effect of practice schedule on the MTs, a contrast that included all of the execution trials would have introduced a potential confounding performance factor despite including MT as a nuisance variable in the design matrix. Instead, the contrast was restricted to only the final two thirds of the training session (block late, random late) when MTs were equivalent. To maintain consistency with the sequence execution trials and also to adhere to our hypothesis that imaging at the end of training would capture differential effects of the practice schedules, sequence preparation contrasts were also limited to the final two thirds of trials.
Results were then quantified by calculating the mean z-statistic, number of voxels, and the MNI space coordinates of the peak voxel within a series of MRI atlas anatomic templates. These templates are not the same as regions of interest. Instead, the regions in the current analysis were used to present an accurate anatomical description and labeling of the data. This approach was also carried out in aims 3 and 4 and a description of the template boundaries is described in the following section.
In the third aim, we addressed the effects of the block and random schedules on sequence-preparation–related brain activation over the course of training by testing for an interaction between type of training schedule (block vs. random) and time (early vs. late). We were particularly interested in the pattern of interaction in which (random late > block late) > (random early > block early). Results were first organized into the templates subsequently described and local peaks were then identified. Activity from the local peak and adjacent 26 voxels (a cube with a width of 3 voxels) were then used to calculate percentage signal change relative to baseline. This allowed us to plot task means and characterize the specific pattern of interaction for each region.
The fourth aim was to test whether activity in motor cortex at the end of random training, for both preparation and production events, correlated with successful off-line learning as determined by individual subject differences. Would greater activity in motor cortex as a function of practice structure lead to better consolidation? To test this, the difference scores calculated for each participant were applied to the linear contrast, “random late > baseline” as an additional covariate of interest in the group analysis. The difference score represented how efficiently each participant consolidated motor sequence information learned during random training. Subjects with above-average difference scores reflect a high amount of savings, whereas those with below-average difference scores reflect poor retention. Anatomical templates of right and left sensorimotor cortex were used to constrain the results. Statistical corrections for multiple comparisons were based on the size of this anatomic volume.
Standard-space anatomical templates
Anatomical templates were defined from either the Jülich histological atlas or the Harvard–Oxford cortical and subcortical structural atlas, both supplied with FSL (Eickhoff et al. 2005). The most inclusive probabilistic map pertaining to BA6 was first selected from the Jülich atlas. This region was then subdivided into more detailed premotor regions using gyral and sulcul landmarks taken from the group-averaged anatomical image. Medial premotor regions were drawn with respect to the intersection of the bicommissural line (anterior commissure–posterior commissure [AC–PC]), with the presupplementary motor area (pre-SMA) rostral and the supplementary motor area (SMA) caudal to the AC. The cingulate motor area (CMA) was drawn inferior to the SMA, extending from the superior bank of the cingulate sulcus to the superior cingulate gyrus (Picard and Strick 1996, 2001). Lateral premotor regions included the dorsal and ventral premotor cortex, their boundary defined by a gyral branch landmark on the precentral gyrus at the level z = 48, based on results from a recent diffusion-weighted imaging tractography-based parcellation of the precentral gyrus (Tomassini et al. 2007). In addition, the primary motor cortex (M1) was drawn by hand, from the anterior bank of the central sulcus to the postcentral gyrus. All other regions were determined using the Harvard–Oxford cortical and subcortical atlas. Included are those regions that demarcate the predicted sequence preparation network. These are: supramarginal gyrus (SMG), angular gyrus (ANG), superior parietal lobule (SPL), putamen, caudate, globus pallidus, and the lateral cerebellum. Further, cerebellar lobes were labeled following the demarcations provided by a recently developed three-dimensional MRI atlas, such that numerals I–X follow the cerebellar lobules superior–inferior, respectively, and Crus 1 and 2 refer to the lateral hemispheres (Schmahmann et al. 1999).
RESULTS
Training performance
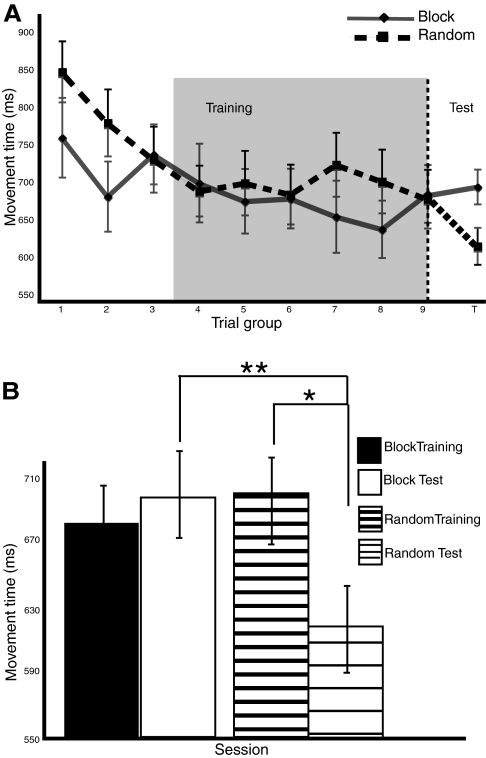
Performance was characterized by the amount of time needed to type out each sequence trial, referred to as movement time (MT). A group (block/random training schedule) × time (bins of six trials over the course of training) repeated-measures ANOVA revealed two main effects and a modest interaction. Consistent with the CI effect, performance was faster for sequences performed with a block schedule [main effect of group: F(1,15) = 5.81, P < 0.03] and, overall, both groups learned to perform the sequences at a faster rate over time [main effect of time: F(8,8) = 4.75, P < 0.02] (Fig. 3A). There was also a greater performance gain over the course of training for the random schedule [group × time interaction: F(8,8) = 3.42, P < 0.05].
Fig. 3.
Performance results. A: movement times demonstrate slower performance for random schedule trials during training. This cost diminishes over time, as indicated by a small interaction between practice schedule and time at the end of training. The shaded region reflects the final two thirds of production trials during which movement time between schedules was equivalent. These trials were used to compare brain activity between practice schedules during sequence production. The abscissa label, “Trial group” represents bins of 6 consecutive trials. The dashed vertical line following trial group 9 represents the transition from the end of training to the initiation of the test session. B: movement times show an effect of memory consolidation (**) for random practiced sequences. The standard contextual interference (CI) effect was also found, with faster retest performance for sequences practiced with a random schedule than a blocked schedule (*).
Performance was further characterized by the time needed to initiate a response, measured from the removal of the response cue (go/no go) to the first downstroke recorded from the button box. This response time (RT) was used to test for differences of sequence initiation for the different groups of sequences. RT performance was faster for the block schedule than for the random schedule [main effect of group: F(1,15) = 6.58, P < 0.02]. This suggests that when sequences are practiced in a random fashion, more time is needed to initiate the first movement of the sequence. Further, this suggests that if random MTs are slower than block MTs, this MT difference might be driven by continued preparation following the initial keypress. To test whether this was occurring, both dependent variables (RT, MT) were reanalyzed for group differences using only the final two thirds of trials, when MTs were equivalent. Again, RTs were faster for the block schedule [main effect of group, last two thirds trials: F(1,15) = 5.76, P < 0.03], but both groups had equivalent MT performance [main effect of group, last two thirds trials: F(1,15) = 2.05, P < 0.18]. This shows that although the random schedule led to slower response initiation throughout training, by the final two thirds of trials there were no differences in MT between groups. Thus it is unlikely that continued sequence preparation was occurring in the random group once the go imperative was presented. To avoid potential confounds of persistent sequence planning after the go imperative and to avoid an MT rate confound, only the last two thirds of go trials were used to compare brain activity as a function of type of training.
Test performance
Off-line learning was measured by comparing MT performance at the end of the training session with the start of the test session. This method has an advantage for characterizing off-line learning because it compares peak training performance with initial test performance prior to any additional practice. MTs from the last four training trials of each schedule were compared with the MTs during the first half of the first test session epoch. The test session trials were balanced between sequences learned with a block training schedule (n = 60) and those learned with a random training schedule (n = 61).
Paired-samples t-tests performed between the end of training and the start of the test session revealed an effect of off-line learning for the random trained sequences but not the block trained sequences. Random sequences were executed faster at the beginning of the test session than at the end of training, M (mean) = 89.24, t = 2.67, P < 0.01, whereas the block trained sequences were not: M = −0.87, t = −0.02, P = 0.98 (Fig. 3B). Furthermore, a direct comparison of MT performance during the initial test session revealed that random trained sequences are faster than block trained sequences: M = 77.7, t = 1.98, P = 0.05. By using a within-groups design, these results confirm that off-line learning is sequence specific, with performance enhancement for sequences practiced with a random schedule.
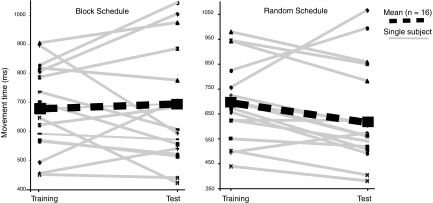
Individual differences in training and test session MT performance were also explored. The single-subject training and test session means that were used in the previous analysis are shown in Fig. 4. The group means for each training schedule, shown with thick dashed lines, are overlaid on the individuals and highlight off-line learning for the random trained sequences but not for the block trained sequences. Variability among block schedule subjects was high, with some subjects showing marked performance improvement, but others showing poor retention. On the other hand, variability across the same group of subjects was much lower for the random schedule. These trends suggest that random training does a better job at facilitating off-line learning and that block training does not in itself preclude off-line learning, but is less robust across subjects. The amount of off-line learning for each subject was expressed as a single numerical value, referred to as the performance delta (PD). The PD is taken by subtracting the mean MT from the start of the test session with the mean MT from the end of training (training mean − test mean). PD values provide a sensitive method for evaluating individual differences of performance retention. These values were implemented in a parametric analysis of the imaging data, allowing for the identification of those brain regions activated by random training that are critical for off-line learning.
Fig. 4.
Effects of schedule on off-line learning. Individual participant movement time means at the end of the training session and at the start of the test session. The random schedule shows an overall effect of off-line learning, whereas the block schedule does not. Both practice schedules induced considerable performance variability among individuals. However, participant variability was considerably higher for block schedule compared with the random schedule. Performance deltas (PDs) for each participant were calculated by subtracting the training session mean from the test session mean.
Comparisons were also performed on RT data from the same trials at the end of training and initial testing. Participants responded more quickly for both block (M = 25.87, t = 2.07, P < 0.05) and random (M = 49, t = 2.65, P = 0.01) sequences during the test session compared with training. However, the test session RTs for block and random were not different (M = −11, t = −0.71, P < 0.5). These results suggest there was a general effect of off-line learning on the RT, independent of the practice schedule used.
Imaging results
MAIN EFFECTS OF SEQUENCE PREPARATION AND EXECUTION.
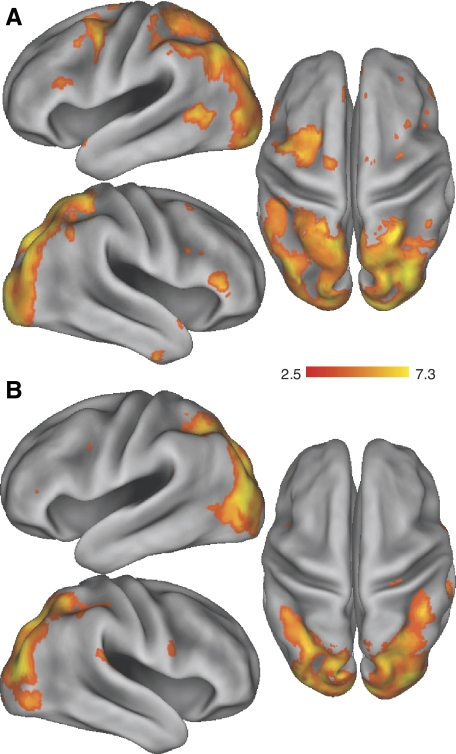
Brain areas associated with the preparation and execution of sequences with the left hand are illustrated in Fig. 5. Contrasts revealed activation in a broad network of classically defined motor sequence planning and production regions, including lateral and medial premotor regions, dorsal prefrontal regions, and the parietal cortex. In addition, there was extensive activation in primary and secondary occipital cortex, as well as inferior temporal cortex.
Fig. 5.
Main effects of task. Regions of greater blood oxygenation level–dependent (BOLD) activity for the main effects of sequence preparation (A) and sequence production (B) during training, generated using the contrast “task > baseline.” Results are displayed at the corrected threshold of P < 0.05.
DIFFERENCES BETWEEN BLOCK AND RANDOM SEQUENCE BOLD ACTIVITY DURING LATE TRAINING.
Training with a random practice schedule strengthens off-line learning. We hypothesized that the random schedule enhances the activity in a neural network used for both preparing and producing motor sequences and that these benefits would be manifest, relative to the block schedule by the end of training. To measure what effect practice schedule has on brain activation once the sequences were well practiced, data from the final two thirds of the training session were compared. The initial third of training session data was excluded from the analysis because there was a significant performance difference between practice schedules. Contrasts were made between these final two thirds of trials performed under random or block training schedule, separately for preparation and production events. The resulting contrasts random > block and block > random were performed on a whole-brain volume and corrected for multiple comparisons using FDR, at the q = 0.05 level.
Sequence preparation at the end of training.
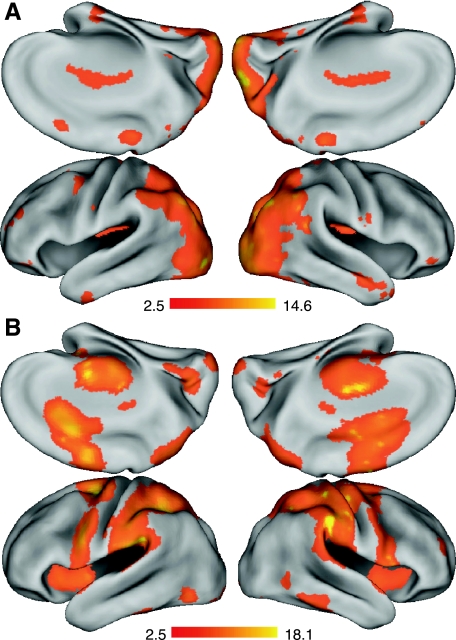
Relative increases in brain activation during sequence preparation after training with the block schedule compared with the random schedule were localized to subcortical structures as well as activation of the medial frontal and inferolimbic cortices (Table 1). Of particular note was the extensive activation of the retrosplenial anterior cingulate cortex (rsACC), which extended bilaterally and into the adjacent orbital frontal gyri. These areas are part of a network that is often more active at baseline relative to a broad range of stimulus dependent tasks and it is commonly referred to as the default network. Because these areas are more active for the block practice structure than the random practice structure, we speculate that during block training, these areas are responding more similarly to a baseline resting state, rather than a state of task engagement. Additional clusters of activation were found in the external globus pallidus, right putamen, left dentate cerebellar nucleus, right posterior superior temporal gyrus (STG), and the bilateral fusiform gyrus.
Table 1.
Brain regions differentially activated as a function of training structure during sequence planning
| Anatomical Region | Side | MNI Coordinates |
Functional Name | Voxels | Z-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||||||
| A. Random > Block | ||||||||||||||
| Cerebellum | R | 14 | −84 | −36 | Crus2 | 1,289 | 4.04 | |||||||
| Precuneus | R | 4 | −58 | 62 | 220 | 3.96 | ||||||||
| Occipital pole | R | 18 | −96 | 12 | 1,690 | 3.78 | ||||||||
| Middle occipital gyrus | R | 40 | −82 | 28 | sLO | 3,038 | 3.67 | |||||||
| Precentral gyrus | R | 50 | 16 | 34 | PMd | 133 | 3.64 | |||||||
| Angular gyrus | L | −60 | −50 | 14 | ANG | 651 | 3.61 | |||||||
| Precentral gyrus | L | −44 | 4 | 48 | PMd | 809 | 3.55 | |||||||
| Middle occipital gyrus | L | −38 | −72 | 42 | sLO | 2,780 | 3.53 | |||||||
| Inferior frontal gyrus | R | 52 | 32 | 2 | IFG | 54 | 3.50 | |||||||
| Occipital pole | L | −32 | −92 | −2 | 2,840 | 3.46 | ||||||||
| Superior parietal lobule | L | −14 | −56 | 64 | SPL | 1,657 | 3.44 | |||||||
| Cerebellum | R | 44 | −68 | −32 | Crus1 | 53 | 3.42 | |||||||
| Cerebellum | L | −18 | −84 | −42 | Crus2 | 235 | 3.37 | |||||||
| Inferior temporal gyrus | R | 46 | −4 | −42 | aITG | 39 | 3.36 | |||||||
| Superior parietal lobule | R | 28 | −56 | 62 | SPL | 999 | 3.35 | |||||||
| Middle occipital gyrus | R | 42 | −84 | 12 | iLO | 547 | 3.35 | |||||||
| Postcentral gyrus | L | −42 | −34 | 60 | 678 | 3.34 | ||||||||
| Percentral gyrus | L | −54 | 16 | 26 | PMv | 119 | 3.33 | |||||||
| Superior frontal gyrus | L | −4 | 38 | 52 | pre-SMA | 56 | 3.27 | |||||||
| Middle frontal gyrus | R | 14 | −4 | 68 | PMd | 11 | 3.26 | |||||||
| Supramarginal gyrus | L | −54 | −46 | 46 | SMG | 598 | 3.26 | |||||||
| Precentral gyrus | L | −2 | −30 | 82 | SMA | 42 | 3.22 | |||||||
| Cerebellum | L | −16 | −86 | −26 | Crus1 | 276 | 3.14 | |||||||
| Superior frontal gyrus | R | 0 | 56 | 32 | pre-SMA | 70 | 3.09 | |||||||
| Middle frontal gyrus | R | 24 | 4 | 62 | PMdr | 10 | 2.93 | |||||||
| Supramarginal gyrus | R | 46 | −50 | 52 | SMG | 138 | 2.93 | |||||||
| B. Block > Random | ||||||||||||||
| Putamen/insula | R | 26 | −8 | 20 | 331 | 3.80 | ||||||||
| Globus pallidus, external | R | 16 | −4 | −4 | GP | 200 | 3.70 | |||||||
| Superior temporal gyrus | R | 48 | −20 | −4 | pSTG | 456 | 3.74 | |||||||
| Fusiform gyrus | R | 36 | −32 | −30 | 663 | 3.65 | ||||||||
| Dentate nucleus | R | 16 | −42 | −36 | CBdn | 64 | 3.69 | |||||||
| Cerebellum | R | 14 | −50 | −10 | Lobule V | 151 | 3.71 | |||||||
| Red nucleus | R | −2 | −24 | −16 | 24 | 3.72 | ||||||||
| Transverse temporal gyrus | L | −50 | −14 | −2 | 180 | 3.91 | ||||||||
| Globus pallidus, external | L | −18 | −6 | −6 | GP | 168 | 3.92 | |||||||
| Fusiform gyrus | L | −42 | −26 | −20 | 269 | 3.95 | ||||||||
| Dentate nucleus | L | −28 | −54 | −44 | CBdn | 84 | 3.94 | |||||||
| Brain stem | L | −4 | −40 | −58 | 42 | 3.92 | ||||||||
| Middle frontal gyrus | R | 42 | 54 | 22 | VLPFC | 28 | 3.76 | |||||||
| Medial frontal gyrus | R | 10 | 66 | 4 | MeFG | 91 | 3.09 | |||||||
| Anterior cingulate/Orbital | R | 0 | 10 | −26 | rsACC/OFG | 2,529 | 3.62 | |||||||
| Anterior cingulate/Orbital | L | 44 | 69 | 23 | rsACC/OFG | 2,407 | 3.42 | |||||||
| Cingulate gyrus | L | −16 | −10 | 34 | pCing | 70 | 3.48 | |||||||
Significance for all voxels was tested with a group mixed-effects analysis—false discovery rate—corrected P < 0.05. M1, primary motor cortex; PMd(r), dorsal premotor cortex (rostral); PMv, ventral premotor cortex; (pre)SMA, (pre-)supplementary motor area; (r)CMA, (rostral) cingulated motor area; SMG, supramarginal gyrus; SPL, superior parietal lobule; sLO, superior lateral occipital cortex; iLO, inferior lateral occipital cortex; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex.
On the other hand, preparing a sequence that had been practiced under a random training schedule was associated with relatively greater activity throughout a broad network of cortical structures known to be involved in sequence preparation, as well as extensive activation of the cerebellar hemispheres (Fig. 6A). Predominant activation was found in bilateral premotor cortices, with a predicted leftward bias. In particular, there was substantial activation of the left dorsal premotor cortex (PMd) and ventral premotor cortex (PMv), as well as the left SMA. The right hemisphere also showed differential premotor activity when planning during the random schedule, with smaller premotor clusters localized to the PMd, rostral PMd (PMdr), and the pre-SMA. A similar left hemisphere bias was reflected in the parietal lobe, with extensive acivation in the superior parietal lobule (SPL), angular gyrus (ANG), and supramarginal gyrus (SMG). In addition, the random schedule led to greater activation of the lateral occipital cortex and the occipital pole (Table 1). These results show that, by the end of training, the random training schedule leads to extensive recruitment of regions associated with the preparation of learned motor skills compared with a blocked training schedule.
Fig. 6.
Effects of random schedule training. Greater BOLD activity for late (final two thirds) trials practiced under a random schedule compared with a blocked schedule for (A) sequence preparation and (B) sequence production. All cortical surface data have the same color scale and orientation as those in Fig. 3. Results are shown at the corrected threshold of P < 0.05.
Sequence production at the end of training.
Brain activity during sequence execution was significantly greater for trials practiced under a random training schedule in a largely posterior cortical and cerebellar network (Fig. 6B). This also included right hemisphere activation of the primary motor cortex, left inferior frontal gyrus (IFG) and PMv, and extensive activation of the superior and lateral parietal cortex (supramarginal gyrus, superior parietal lobule), the occipital pole, and the lateral occipital cortex (Table 2). Additional activation was found in the lateral (Crus 2) and the middle (VIIIA/VIIB) cerebellum. There were no regions above the corrected threshold that showed greater activation for the block training schedule compared with the random schedule by the end of training. These results demonstrate that training under a random schedule, relative to a block schedule, recruits a cortical–cerebellar network known to be involved in the mapping and production of skilled motor sequences. The differences in motor production areas occurring with random training compared with block training are likely to be independent of movement rate because measured MTs were equivalent at the time of fMRI measurements, and any residual MT effects for individual trials were added as a separate covariate of noninterest to the design matrix.
Table 2.
Brain regions differentially activated during sequence execution as a function of training structure
| Anatomical Region | Side | MNI Coordinates |
Functional Name | Voxels | Z-Value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle occipital gyrus | L | −44 | −76 | 14 | iLO/sLO | 2,409 | 4.29 |
| Middle occipital gyrus | R | 32 | −84 | 20 | iLO/sLO | 2,082 | 4.26 |
| Occipital pole | L | −10 | −94 | 8 | 752 | 4.11 | |
| Occipital pole | R | 4 | −96 | 12 | 863 | 4.04 | |
| Superior parietal lobule | R | 28 | −56 | 50 | SPL | 313 | 3.94 |
| Superior parietal lobule | L | −34 | −56 | 52 | SPL | 437 | 3.94 |
| Supramarginal gyrus | R | 44 | −42 | 46 | SMG | 172 | 3.88 |
| Cerebellum | R | 6 | −76 | −42 | Lobule VIIB | 659 | 3.82 |
| Cerebellum | L | −16 | −74 | −48 | Crus2 | 314 | 3.61 |
| Planum temporale | R | 48 | −30 | 10 | 224 | 3.57 | |
| Cerebellum | R | 10 | −68 | −14 | Lobule VIIIA | 219 | 3.53 |
| Inferior frontal gyrus | L | −50 | 40 | 12 | IFG | 34 | 3.46 |
| Precentral gyrus | R | 26 | −24 | 50 | M1 | 16 | 3.46 |
| Precentral gyrus | L | −34 | 4 | 30 | PMv | 64 | 3.43 |
See Table 1 for explanatory details.
INTERACTION OF PRACTICE SCHEDULE AND TIME ON SEQUENCE PREPARATION BOLD ACTIVITY.
The next analysis focused on how BOLD activity measured during sequence preparation changes with practice and whether this is different as a function of practice schedule. This takes into consideration how brain regions, critical to sequence preparation, change over the course of the entire training session. Because sequence-preparation events were short and held constant between subjects, the amount of time spent preparing block and random sequences was equivalent. Note that an analogous test could not be made for sequence production trials because of significant MT performance differences between the two groups during early training.
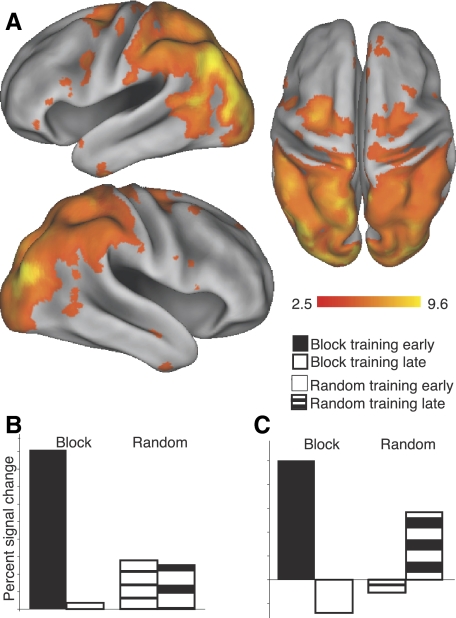
The interaction (random late > block late) > (random early > block early) was tested using all sequence preparation trials and is shown in Fig. 7. These results reveal an expansive cortical and subcortical network that is modulated by both practice schedule and training time (Table 3). To further characterize the interaction effect between the conditions, percentage signal change relative to baseline (e.g., block early > baseline) was extracted from those regions showing a significant interaction effect. For each region, mean percentage signal change was calculated by averaging the 26 voxels adjacent to and including the local maxima. Thus the interaction effect at each site could be characterized by a mean percentage signal value for each of the four conditions. Two primary observations can be made. First, there was a dramatic reduction in sequence-preparation–related activity over time for the block schedule trials. All of the regions listed in Table 3 show this pattern, indicating that little preparation activity occurs later in block training. Second, activation for the random trials demonstrated two distinct temporal patterns over training. Some regions maintained a pattern of constant activation over training, as exemplified in Fig. 7B and referred to as Type 1 in Table 3. Reflecting this stable pattern includes the occipital lobe (left lateral occipital, right fusiform, bilateral occipital pole), the cerebellar hemispheres (Crus 2), and the left caudate tail. In contrast, other regions demonstrated an increase in activation over time for randomly scheduled trials (Fig. 7C) and referred to as Type 2 in Table 3. Regions with this activation pattern include left M1, lateral (PMd, PMv) and medial premotor regions (pre-SMA, SMA), expansive activation in the posterior superior parietal lobe (SPL, ANG) as well as lateral occipital cortex, and posterior inferior temporal gyrus. Areas that were classified as Type 2, or increasing, were to have signal change values that were ≥50% greater during late training compared with early training. Otherwise, they were considered Type 1, or having stable activation across early and late training. These results indicate that, with random training, regions that are active during preparation trials remain engaged or actually increase in activity over training. This is substantially different from the block schedule regions, which show over time a dramatic reduction in recruitment.
Fig. 7.
Differences of schedule-related activity over the entire course of training. Regions demonstrating a significant interaction between time (early vs. late training) and practice schedule type (random or blocked). Data are for sequence preparation trials only. Extracted percentage signal change data from anatomical templates reflected a strong reduction in activation for the block schedule over time, and either stable (B) or increasing (C) activation for the random schedule over time. Bar plots reflect examples, and not actual data, of the trends shown among the regions evaluated. See Table 3 for areas that capture these trends over the course of training. Results are shown at the corrected threshold of P < 0.05.
Table 3.
Brain regions that reflect active preparation during random training
| Anatomical Region | Side | MNI Coordinates |
Functional Name | Voxels | Z-Value | Interaction Type | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Precuneus | R | 6 | −56 | 62 | 294 | 4.94 | 2 | |
| Cerebellum | R | 28 | −78 | −40 | Crus2 | 736 | 4.71 | 1 |
| Fusiform gyrus | R | 12 | −90 | −8 | 229 | 4.52 | 1 | |
| Tail of caudate | L | −20 | −36 | 14 | 138 | 4.47 | 1 | |
| Cerebellum | L | −16 | −84 | −30 | Crus2 | 893 | 4.29 | 1 |
| Occipital pole | R | 6 | −88 | 30 | 1,724 | 4.15 | 1 | |
| Precuneus | L | −10 | −66 | 58 | 701 | 4.15 | 2 | |
| Middle occipital gyrus | R | 36 | −84 | 20 | sLO | 3,947 | 4.13 | 2 |
| Occipital pole | L | −18 | −92 | 22 | 1,450 | 4.09 | 1 | |
| Angular gyrus | L | −58 | −52 | 14 | ANG | 1,264 | 4.07 | 2 |
| Middle occipital gyrus | L | −48 | −66 | 40 | sLO | 3,587 | 4.00 | 1 |
| Inferior occipital gyrus | L | −48 | −72 | 14 | iLO | 1,001 | 3.99 | 2 |
| Middle occipital gyrus | R | 44 | −78 | 14 | iLO | 929 | 3.83 | 2 |
| Superior parietal lobule | R | 46 | −42 | 58 | SPL | 4,305 | 3.78 | 2 |
| Superior parietal lobule | L | −44 | −38 | 56 | SPL | 4,701 | 3.78 | 2 |
| Precentral gyrus | L | 30 | −12 | 54 | PMd | 408 | 3.75 | 2 |
| Precentral gyrus | L | −10 | −30 | 72 | SMA | 165 | 3.74 | 2 |
| Inferior frontal gyrus | L | −56 | 18 | 22 | PMv | 221 | 3.32 | 2 |
| Angular gyrus | R | 65 | 37 | 60 | ANG | 628 | 3.32 | 2 |
| Inferior temporal gyrus | L | −48 | 0 | −42 | pITG | 85 | 3.29 | 2 |
| Inferior frontal gyrus | R | 52 | 12 | 30 | PMv | 98 | 3.27 | 2 |
| Precentral gyrus | R | 14 | −4 | 62 | PMd | 447 | 3.23 | 2 |
| Inferior temporal gyrus | R | 46 | 0 | −42 | pITG | 44 | 3.08 | 2 |
| Superior temporal gyrus | R | 58 | −6 | −18 | STG | 41 | 2.96 | 2 |
| Superior frontal gyrus | R | 2 | 34 | 50 | pre-SMA | 169 | 2.92 | 2 |
CORRELATES OF SUCCESSFUL CONSOLIDATION FROM RANDOM SEQUENCE PREPARATION AND PRODUCTION BOLD ACTIVITY IN M1.
The random schedule promoted off-line learning, yet there was considerable variability between participants (Fig. 4). This suggests that participants with exceptional off-line gains might recruit more activity in motor areas during random training. To investigate whether motor cortex activity is related to performance gains during off-line learning, BOLD estimates of brain activity obtained during late random sequence preparation and also production were correlated with individual participant PD scores. The PD score reflects the amount of performance gain (or loss) between the end of training and the start of the test session on the following day. Off-line learning was correlated with activation in left M1 for both random preparation and execution by the end of training under the random schedule (Table 4). This finding extends our recent TMS study, which showed stimulation to left (ipsilateral) M1 during preparation disrupts random schedule off-line learning (Cohen et al. 2009).
Table 4.
M1 activity correlated with individual differences of consolidation
| Condition | Side | MNI Coordinates |
Voxels | Z-Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Preparation | L | −38 | −30 | 60 | 151 | 2.76 |
| Production | L | −34 | −16 | 52 | 10 | 2.85 |
Shown are results that reflect correlations of off-line leaning in the sensorimotor cortex. Corrected, P < 0.05. See Table 1 for additional details.
DISCUSSION
Capitalizing on a beneficial effect of practice structure known as contextual interference (CI), we were able to modulate training-related brain activity and alter subsequent off-line learning. Our results clearly demonstrate the feasibility of a within-subject design to test for the relative benefits of a random practice schedule compared with a blocked practice schedule. This establishes the CI effect to be trial type specific. Furthermore, these results establish the efficacy of using a single-group approach and manipulation of training structure to manipulate off-line learning. Thus training structure itself is an important means of altering skill consolidation that can be applied to a broad range of learning problems. Three key observations can be made regarding the neural substrates of learning motor sequences under a random schedule. First, the random schedule, relative to the block schedule recruited greater neural activity in regions at the end of training that are known to be involved in the preparation and production of learned motor skills. Second, brain areas associated with sequence preparation either maintained a constant state of activity or even increased activity over the course of training under a random schedule. In contrast, the same regions all showed a dramatic longitudinal decrease in activation for the trials learned under a block schedule. This provides empirical support for the behavioral theory, suggesting that random training benefits off-line learning due to persistent active preparation. Third, we were able to show, based on individual differences of random sequence performance, that activity in the left ipsilateral M1 correlates with off-line learning, during both preparation and execution. This finding in ipsilateral M1 expands a large body of experimental evidence that has previously linked changes of contralateral M1 with off-line learning (Muellbacher et al. 2002; Richardson et al. 2006; Robertson et al. 2005).
Off-line learning and motor cortex
Our finding that activity in contralateral M1 was greater at the end of random schedule training while subjects generated a sequence is consistent with converging evidence indicating that M1 is critical for the storage and retrieval of motor sequence knowledge. This includes studies involving nonhuman primates (Ben-Shaul et al. 2004; Carpenter et al.1999; Lu and Ashe 2005; Matsuzaka et al. 2007) as well as human imaging experiments (Kansaku et al. 2005; Zang et al. 2003). Furthermore, numerous experiments using TMS have shown M1 to have a temporally specific role in performance retention (Muellbacher et al. 2002; Cohen et al. 2009; Richardson et al. 2006; Robertson et al. 2005). Disruption immediately following acquisition resulted in reduced performance during the day of training, but not following a night of sleep (Muellbacher et al. 2002; Robertson et al. 2005), and disruption prior to the start of acquisition resulted in diminished skill retention even after a night of sleep (Richardson et al. 2006).
We also found that activity in ipsilateral M1 for sequences learned under a random practice schedule correlated with subsequent off-line learning across subjects. This complements a recent TMS study using a CI task similar to our own, which also highlighted the importance of ipsilateral left M1 in off-line learning (Rice et al. 2009). Off-line learning was particularly vulnerable when left M1 was stimulated during sequence preparation prior to movement onset for sequences learned under a random practice structure. These two studies implicate ipsilateral (left) M1 in having a critical sequence planning role, independent of effector, that is not generally recognized in the skill learning literature. These results suggest that on-line recruitment of left M1 during training is necessary for effective off-line learning. Taken from this perspective, the disruption of left M1 by TMS likely interferes with the initial storage of motor sequences, which is probably lateralized to the left hemisphere that, in turn, interferes with latent learning during an extended retention interval.
Off-line learning and changes outside of motor cortex
The effects of a random practice schedule were observed beyond M1, both during sequence preparation and execution. During late training, executing random sequences led to greater activation in the posterior parietal cortex (PPC) extending along much of the IPS, as well as the cerebellum lobules. These regions have been described previously in an extensive literature of motor learning in which longitudinal changes during task execution are identified (Bischoff-Grethe et al. 2004; Doyon et al. 2002; Shadmehr and Holcomb 1997).
Preparing sequence movements late in random training led to greater recruitment of all of the classic premotor regions (PMd, PMv, pre-SMA, SMA), as well as a large area extending from PPC through the lateral occipital cortex. Additionally, there were large foci of activation within the posterolateral cerebellum (Crus 2). These differences were apparent even though task performance between block and random schedules was equated. The recruitment of a predominantly left premotor–parietal circuit is consistent with previous studies that suggest these regions are critical for the integration of spatial goals with effectors in visuomotor tasks (Cavina-Pratesi et al. 2006; Thoenissen et al. 2002; Toni et al. 1999, 2001). We suggest the increased recruitment of this premotor–parietal circuitry is a critical factor contributing to the superior savings for those sequences practiced with the random schedule.
Interestingly, block preparation trials, relative to random trials, showed greater activity in both the medial prefrontal cortex (retrosplinal ACC, medial prefrontal gyrus, orbital frontal gyrus) as well as the left posterior cingulate gyrus during late training. These areas constitute part of a default network of regions, including the medial prefrontal cortex and posterior cingulate, that have been reliably shown to be anticorrelated with task-related activity (Fox and Raichle 2007; Mason et al. 2007). Although this finding was not a particular focus a priori, it nevertheless presents an intriguing insight as to why block schedule off-line learning is so poor, by suggesting that block training leads to less active preparation and increased stimulus independent processing. This pattern is opposite of the active preparation fostered through the random training schedule.
The neural circuitry preferentially recruited during sequence planning under the random practice schedule is quite complementary with regions commonly reported in motor imagery experiments. Motor imagery is the ability to simulate movement without overt execution and is thought to underlie movement preparation (Jeannerod 1994). The classic finding in motor imagery experiments is the degree of overlap of brain activity between the conscious reenactment of a movement and the actual production of the same movement. Similarly, preparing to generate sequences with the random training schedule requires the active reconstruction of motor programs. This is supported by our results that show the random schedule engages sequence preparation regions throughout training, whereas the block schedule does not. Because both motor preparation and motor imagery are thought to retrieve, construct, and encode motor information they are often regarded as sharing similar processing elements. This was illustrated in a recent experiment that found activation for an imagery condition to overlap considerably with preparation activity, including activation of the lateral and medial premotor (PMv, PMd, pre-SMA, SMA), parietal (SMG, SPL) and the posterolateral cerebellum. Interestingly, these regions are similar to the preparation network sustained by the random schedule. We suggest this overlap indicates that both random sequence preparation and imagery share similar neural circuitry used to retrieve and reconstruct motor plans rather than conscious appraisal of the task.
Practice structure and the pharmacology of consolidation
By relating the current study to recent human learning experiments that characterize the role of sleep, several important links can be defined that lead to plausible mechanisms for future investigation. Our task is very similar to a motor learning paradigm that is known to result in overnight changes in behavior and increased corticospinal excitability for participants that learned simple motor sequences (Nishida and Walker 2007; Walker et al. 2003). These studies show that off-line motor learning is influenced by sleep and, in particular, the amount of stage 2 nonrapid eye movement (non-REM) sleep. This has been extended to studies not only of daytime napping but also of nocturnal sleep (Nishida and Walker 2007; Walker et al. 2003). Moreover, these increases have been localized using EEG to motor regions that are active during task performance (Morin et al. 2008; Nishida and Walker 2007). A key finding is the presence of increased sleep spindle activity in frontal (F3/F4), motor (C3/C4), and parietal (P3/P4) cortices (Morin et al. 2008; Nishida and Walker 2007). Sleep spindles are generated by reticular thalamic neurons, which lead to the propagation of synchronous 12- to 16-Hz neural activity of the thalamocortical loop. Further, spindle activity is linked to long-term potentiation (Rosanova and Ulrich 2005), a cellular mechanism known to be involved in learning. Importantly, the benefits of sleep spindle activity increase as a function of task complexity (Morin et al. 2008). The random schedule offered a more complex training regimen relative to the block schedule, suggesting a parallel mechanism between the tasks. Interestingly, regions that were predictive of successful off-line learning, including M1, are similar to those regions that have higher sleep spindle densities when recorded during consolidation. This indicates that the random schedule might be beneficial not simply because it offers a more complex version of the task, but because it maximally recruits those neural systems that are used to perform and store specific motor memories, which are further enhanced during sleep.
Recent evidence also shows that rapid eye movement (REM) sleep and related neurochemical processes are important for successful off-line learning and it appears that the different stages of sleep cooperate for successful consolidation to occur (Marshall and Born 2006). One of the neurochemical signatures of REM is the presence of high, wake-like levels of the neurotransmitter acetylcholine (ACh). High levels of ACh activity are integral for the encoding of memories in a wakeful state (Bartus et al. 1982; Rasch et al. 2006) and for long-term potentiation (Rasmusson 2000). Extensive animal research has shown that REM sleep is enhanced following procedural skill learning (for a review see Peigneux et al. 2001). A recent study has shown that blockade of muscarinic and nicotinic receptors significantly impaired off-line motor sequence consolidation but not the memory for word pairs also studied prior to sleep (Rasch et al. 2009). This suggests that, unlike declarative memory, memory for motor skills depends on the action of ACh during REM sleep. Further, this finding suggests that impaired REM sleep might block any gains from stage 2 sleep. Because ACh receptors are distributed throughout cortical and subcortical regions, the location of their effect is unknown. We speculate that the extensive differences in premotor, motor, and parietal cortices as a function of task scheduling could be mediated in large part via cholinergic projections during wakefulness, or possibly during posttraining REM sleep.
Neurophysiology of contextual interference
Our training results extend the findings of Cross et al. (2007), the first neuroimaging study examining the CI effect. They compared brain activity for the random schedule relative to the block schedule at the end of training to identify preferential recruitment of areas that might support performance retention. Their key neural differences were related to sequence preparation but not execution. They found greater activation in motor preparation areas for the random training schedule. Our results share a similar trend, but identify a much larger cortical network at a higher level of statistical certainty. The current results identified more training-schedule–related differences during sequence execution than were observed by Cross and colleagues (2007). One explanation is that they used a between-groups design, whereas the current design used a within-groups approach. This alone will significantly enhance statistical sensitivity for detecting a difference. Another key difference between the two neuroimaging CI studies was the amount of time given for subjects to prepare their response. Cross and colleagues (2007) allowed both groups an unlimited amount of time to prepare their responses. Although this is a useful precaution for a demanding task, the sequences were simple four-element versions performed with the dominant hand. Our task used short-timed durations for all preparation trials, allowing for a more constrained estimate of preparation-related brain activity. In terms of analysis, this was advantageous because we were able to compare the brain activation related to preparation between schedules because the latencies for each were held constant. The extensive behavioral literature of the CI effect suggests that block and random practice schedules differ in how they interact with the processes that determine how motor memories are consolidated. One dominant account for the CI effect suggests that random schedules promote deeper motor memory processing because, for each trial, a new motor program must be retrieved, interfering with the motor memory for the previous trial. Recent studies using the CI effect support this theory of active preparation (Immink and Wright 1998, 2001). They found the CI effect to be modulated by the amount of time subjects were given prior to generating sequences during training. When given enough time to process the previous trial outcome and retrieve the motor plan for the upcoming sequence, the performance for random and blocked sequences was similar by the end of training and random training was the most beneficial for performance retention. Interestingly, our study replicated their retention effect but did so with a fixed study interval for both blocked and random schedules. One potential explanation for this finding is that the amount of study time was sufficient for the random group. This is supported by the response time (RT) data measured over the duration of the training session. Because there was no change in RT with continued training, it is reasonable to suggest that subjects were given enough time to prepare upcoming movements. Further, our behavioral results are consistent with off-line learning research showing that behavioral performance can be modulated with a particular posttraining retention interval and sleep, (Korman et al. 2007; Walker et al. 2003) as well as the awareness of sequential material (Robertson et al. 2004). Critically, time and sleep were not factors of interest and were held constant in the current experiment. Instead, our off-line learning results were modulated by training schedule alone, which is consistent with other recent experimental evidence showing that the presentation of intermittent practice trials supports motor memory formation (Overduin et al. 2006).
Intermittent practice may also be beneficial because it reduces the amount of interference of previously learned contextual cues (Cothros et al. 2006; Krakauer and Shadmehr 2006; Krakauer et al. 2007). Cothros and colleagues (2006) tested whether prospective interference of a previously learned skill could be blocked by rTMS to contralateral M1 immediately following training. Indeed, following rTMS, performance was impaired on the skill learned prior to stimulation, but not the memory for a second force field learned after the rTMS session. This supports the role of M1 in retention and also that M1 is susceptible to interference if skills are learned in a block structure. This leads to the intriguing interpretation that the block schedule is inherently flawed because learning of the initial sequence interferes with the retention of the additional sequences. To date there is no imaging-based correlate of this interference effect including the current data. On the other hand, the random schedule circumvents this potential pitfall because to-be-learned sequences are presented in an unpredictable fashion. Because no one sequence is learned quicker than the others active preparation must be used. The current imaging results are consistent with and highly supportive of this view.
GRANTS
This research was supported by a National Science Foundation grant and National Institute of Neurological Disorders and Stroke/Public Health Service Grant NS-44393 to S. T. Grafton.
ACKNOWLEDGMENTS
We thank members of the Action Lab for helpful discussion and two anonymous reviewers for thoughtful comments on an earlier draft of this manuscript.
REFERENCES
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol 16: 213–221, 2006 [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414, 1982 [DOI] [PubMed] [Google Scholar]
- Battig WF. Intratask interference as a source of facilitation in transfer and retention. In: Topics in Learning and Performance, edited by Thompson RF, Voss JF. New York: Academic Press, 1972, p. 131–159 [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063, 2003 [DOI] [PubMed] [Google Scholar]
- Ben-Shaul Y, Drori R, Asher I, Stark E, Nadasdy Z, Abeles M. Neuronal activity in motor cortical areas reflects the sequential context of movement. J Neurophysiol 91: 1748–1762, 2004 [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci 16: 127–138, 2004 [DOI] [PubMed] [Google Scholar]
- Brady F. Contextual interference: a meta-analytic study. Percept Mot Skills 99: 116–126, 2004 [DOI] [PubMed] [Google Scholar]
- Carpenter AF, Georgopoulos AP, Pellizzer G. Motor cortical encoding of serial order in a context-recall task. Science 283: 1752–1757, 1999 [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Köhler S, Obhi SS, Marzi CA, Goodale MA. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci 26: 2704–2713, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Cross ES, Wymbs NF, Grafton ST. Transient disruption of M1 during response planning impairs subsequent offline consolidation. Exp Brain Res 196: 303–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Köhler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci 18: 2167–2176, 2006 [DOI] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST. Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci 19: 1854–1871, 2007 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Grafton S, Albert N, Hazeltine E, Ivry RB. Goal-selection and movement-related conflict during bimanual reaching movements. Cereb Cortex 16: 1729–1738, 2006 [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15: 161–167, 2005 [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335, 2005 [DOI] [PubMed] [Google Scholar]
- Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007 [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878, 2002 [DOI] [PubMed] [Google Scholar]
- Grafton S, Hazeltine E, Ivry R. Motor sequence learning with the nondominant left hand. Exp Brain Res 146: 369–378, 2002 [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci 18: 9420–9428, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol MJ, de Lange FP, Verstraten FA, Passingham RE, Toni I. Cerebral changes during performance of overlearned arbitrary visuomotor associations. J Neurosci 26: 117–125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink MA, Wright DL. Contextual interference: a response planning account. Q J Exp Psychol 51A: 735–754, 1998 [Google Scholar]
- Immink MA, Wright DL. Motor programming during practice conditions high and low in contextual interference. J Exp Psychol Hum Percept Perform 27: 423–437, 2001 [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Participation of the primate presupplementary motor area in sequencing multiple saccades. J Neurophysiol 92: 653–659, 2004 [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The representing brain. Neural correlates of motor intention and imagery. Behav Brain Sci 17: 187–245, 1994 [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002 [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337, 1997 [DOI] [PubMed] [Google Scholar]
- Kansaku K, Muraki S, Umeyama S, Nishimori Y, Kochiyama T, Yamane S, Kitazawa S. Cortical activity in multiple motor areas during sequential finger movements: an application of independent component analysis. Neuroimage 28: 669–681, 2005 [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 10: 1206–1213, 2007 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: e316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci 29: 58–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD, Magill RA. The locus of contextual interference in motor skill acquisition. J Exp Psychol Learn Mem Cogn 9: 730–746, 1983 [Google Scholar]
- Lee TD, Magill RA. Can forgetting facilitate skill acquisition? In: Differing Perspectives in Motor Learning, Memory, and Control, edited by Goodman D, Wilburg RB, Franks IM. Amsterdam: North-Holland, 1985, p. 3–22 [Google Scholar]
- Lee TD, Simon DA. Contextual interference. In: Skill Acquisition in Sport: Research, Theory and Practice, edited by Williams AM, Hodges NJ. London: Routledge, 2004, p. 29–44 [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102: 12566–12571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron 45: 967–973, 2005 [DOI] [PubMed] [Google Scholar]
- Magill RA, Hall KG. A review of the contextual interference effect in motor skill acquisiton. Hum Mov Sci 9: 241–289, 1990 [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci 11: 442–450, 2007 [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science 315: 393–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol 97: 1819–1832, 2007 [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory: a century of consolidation. Science 287: 248–251, 2000 [DOI] [PubMed] [Google Scholar]
- Morin A, Doyon J, Dostie V, Barakat M, Hadj Tahar A, Korman M, Benali H, Karni A, Ungerleider LG, Carrier J. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep 31: 1149–1156, 2008 [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002 [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2: e341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, Richardson AG, Lane CE, Bizzi E, Press DZ. Intermittent practice facilitates stable motor memories. J Neurosci 26: 11888–11892, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport 12: A111–A124, 2001 [DOI] [PubMed] [Google Scholar]
- Penhune VB, Doyon J. Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci 22: 1397–1406, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353, 1996 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672, 2001 [DOI] [PubMed] [Google Scholar]
- Rasch B, Gais S, Born J. Impaired off-line consolidation of motor memories after combined blockade of cholinergic receptors during REM sleep-rich sleep. Neuropsychopharmacology 34: 1843–1853, 2009 [DOI] [PubMed] [Google Scholar]
- Rasch BH, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci 18: 793–802, 2006 [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res 115: 205–218, 2000 [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci 26: 12466–12470, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Cohen D. Understanding consolidation through the architecture of memories. Neuroscientist 12: 261–271, 2006 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol 14: 208–212, 2004 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci 25: 6372–6378, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 25: 9398–9405, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10: 233–260, 1999 [DOI] [PubMed] [Google Scholar]
- Schneider T. Analysis of inomplete climate data: estimation of mean values and covariance matricies and imputation of missing values. J Climate 14: 853–871, 2001 [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997 [DOI] [PubMed] [Google Scholar]
- Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol Hum Learn Mem 5: 179–187, 1979 [Google Scholar]
- Shima K, Tanji J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol 84: 2148–2160, 2000 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl. 1: S208–S219, 2004 [DOI] [PubMed] [Google Scholar]
- Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci 24: 631–651, 2001 [DOI] [PubMed] [Google Scholar]
- Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci 22: 9024–9034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein J, Behrens T, Pozzilli C, Matthews P, Rushworth M, Johansen-Berg H. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci 27: 10259–10269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Ramnani N, Josephs O, Ashburner J, Passingham RE. Learning arbitrary visuomotor associations: temporal dynamic of brain activity. Neuroimage 14: 1048–1057, 2001 [DOI] [PubMed] [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE. Signal-, set- and movement-related activity in the human brain: an event-related fMRI study. Cereb Cortex 9: 35–49, 1999 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86, 2007 [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425: 616–620, 2003 [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem 10: 275–284, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21: 1732–1747, 2004 [DOI] [PubMed] [Google Scholar]
- Zang Y, Jia F, Weng X, Li E, Cui S, Wang Y, Hazeltine E, Ivry R. Functional organization of the primary motor cortex characterized by event-related fMRI during movement preparation and execution. Neurosci Lett 337: 69–72, 2003 [DOI] [PubMed] [Google Scholar]