Abstract
Cortical dysplasia (CD) is a common cause of intractable epilepsy in children and adults. We have studied rats irradiated in utero as a model of CD to better understand mechanisms that underlie dysplasia-associated epilepsy. Prior studies have shown a reduction in the number of cortical interneurons and in the frequency of inhibitory postsynaptic currents (IPSCs) in pyramidal cells in this model. They have also shown a reduced frequency of spontaneous and miniature excitatory postsynaptic currents (EPSCs) in the surviving cortical interneurons. However, the inhibitory synaptic contacts were not examined in that study. The current experiments were performed to assess inhibitory synaptic activity in fast-spiking (FS) interneurons in irradiated rats and controls and the balance of excitatory and inhibitory synaptic activity in these cells. Whole cell recordings were obtained from layer IV FS cells in controls and comparable FS cells in irradiated rats. The frequency of spontaneous and miniature IPSCs was reduced in dysplastic cortex, but the amplitude of these currents was unchanged. Stimulus-evoked IPSCs showed short-term depression in control and short-term facilitation in dysplastic cortex. Simultaneous recording of spontaneous EPSCs and IPSCs showed a shift in the ratio of excitation-to-inhibition in favor of inhibition in FS cells from dysplastic cortex. The same shift toward inhibition was seen when miniature EPSCs and IPSCs were examined. These results show that FS cells in dysplastic cortex have a relative lack of excitatory drive. This may result in an important class of inhibitory cells that are less able to perform their normal function especially in periods of increased excitatory activity.
INTRODUCTION
Epilepsy is a relatively common and often debilitating neurological condition that affects an estimated 2.5 million people in the United States (Begley et al. 2000). Although some types of epilepsy are well controlled with medications, ∼30% of cases are intractable (Kwan and Brodie 2000). Some people with intractable epilepsy may require more aggressive therapies, such as brain surgery, whereas others must suffer the effects of a lifetime of uncontrolled seizures. Epilepsy in children can be especially damaging due to the impact of seizures beginning earlier in a person's life and subsequent interference with normal patterns of learning and brain development. Cortical dysplasia (CD) is a common finding in children with intractable epilepsy (Farrell et al. 1992; Porter et al. 2003). CD is a developmental brain anomaly that is characterized by abnormalities of cortical cytoarchitecture and neuronal positioning and morphology (Palmini et al. 2004). Despite convincing clinical evidence for CD as a cause of epilepsy (Palmini et al. 1991, 1995), the specific mechanisms whereby a region of dysplastic cortex produces recurrent seizures are still poorly understood. Impaired inhibition has been demonstrated in some types of human CD (Calcagnotto et al. 2005).
In utero irradiation of rats is an animal model that mimics some aspects of human CD (Roper 1998). Animals exposed to external radiation as fetuses grow up and demonstrate microcephaly, diffuse areas of CD, areas of heterotopic gray matter, heterotopic neurons in the hippocampus, and hypoplasia/agenesis of the corpus callosum (Cowen and Geller 1960; McGrath et al. 1956; Roper et al. 1995). Irradiated rats can show spontaneous seizures in vivo (Kellinghaus et al. 2004; Kondo et al. 2001) and hyperexcitability in vitro (Roper et al. 1997). Dysplastic cortex in this model shows a reduced density of inhibitory interneurons (Roper et al. 1999) and reduced frequency of inhibitory synaptic currents when recording from pyramidal cells (Zhu and Roper 2000). More recently, the surviving interneurons were found to have a reduced excitatory drive (Xiang et al. 2006), suggesting that they might be less active than normal during periods of increased excitatory activity; but that paper did not examine inhibitory currents in these cells.
The current study was undertaken to examine synaptic connectivity in a specific subset of cortical interneurons (fast-spiking cells) in more detail. Specifically we aimed to study inhibitory synaptic transmission and quantify the relative balance of excitation versus inhibition that these neurons receive in an experimental setting where both excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) could be recorded from the same cell. We found that the frequency of IPSCs was reduced and short-term plasticity of IPSCs was altered in fast-spiking (FS) interneurons in dysplastic cortex. However, the reduction in excitatory activity was of a greater magnitude such that the ratio of EPSCs to IPSCs was significantly reduced in FS cells from irradiated rats compared with controls. This further supports the concept that FS cells in CD have an impaired synaptic drive, and this may result in an inhibitory neuron with a reduced capacity to fire when needed.
METHODS
Animals and irradiation
Timed-pregnant Sprague Dawley rats were purchased from Harlan Sprague Dawley (Indianapolis, IN). The day of insemination was designated embryonic day 0 (E0). Pregnant rats on E17 were placed in a well-ventilated Plexiglas box and were exposed to external radiation, 225 cGy, from a linear accelerator source. Control litters were obtained and housed in an identical fashion without irradiation. Offspring were weaned on postnatal day 21 (P21). Male offspring from P28 to P32 were used for experiments. All rats were maintained on 12-h light/dark cycles and were provided food and water ad libitum. All procedures used in this study followed guidelines approved by the Institutional Animal Care and Use Committee at the University of Florida.
Brain slice preparation
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with ice-cold cutting solution under 70 cm H2O pressure at a flow rate of 15 ml/min. The cutting solution contained (in mM) 220 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 10 d-glucose and was oxygenated with 95% O2-5% CO2 (pH 7.4 was adjusted with KOH, and osmolarity was maintained at 350–360 mOsm). Rats were decapitated, and their brains were quickly removed. Coronal brain slices (300 μm thickness) were prepared in an ice-cold cutting solution using a Vibratome (Leica VT1000 s, Leica Microsystems, Wetzlar, Germany). Sections were taken from 8.7 to 4.8 mm anterior to the interaural line (Paxinos and Watson 1986), which included somatosensory cortex (Lehohla et al. 2001). Slices were incubated in extracellular solution for ≥1 h in a storage chamber at room temperature (∼23°C) and were then transferred to a submerged chamber for recording. The extracellular solution contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 2 MgCl2, and 10 d-glucose and was constantly oxygenated (pH 7.4 was adjusted with KOH, and osmolarity was maintained at 300–310 mOsm). All electrophysiological recordings were carried out at 30°C under visual guidance using an inverted microscope (Nikon Eclipse E600FN) equipped with infrared DIC optics and an × 40 water-immersion lens.
Conventional whole cell patch-clamp techniques were performed. Patch pipettes were pulled from Wiretrol II capillary glass (Drummond Scientific, Broomall, PA) in a horizontal pipette puller (Model P-87 Flaming/Brown Micropipette puller, Sutter Instrument). Patch pipettes had resistances of 4–5 MΩ in the bath when filled with an internal solution containing (in mM) 135 K-gluconate, 0.1 CaCl2, 2 MgCl2, 10 HEPES, 2 MgATP, 0.3 Na3GTP, 0.2 EGTA, 4 Na2-phosphocreatine, and 0.1% biocytin (pH 7.25 was adjusted with KOH, and osmolarity was maintained at 280–290 mOsm). With this internal solution, IPSCs were recorded as outward currents, whereas EPSCs were recorded as inward currents at a holding potential of – 40 mV.
Recordings were made in FS interneurons in control (layer IV) and dysplastic somatosensory cortex (middle region of the dyslaminated cortex). FS interneurons were identified, initially, by their morphology and, second, by firing patterns with injected currents. After tissue fixation the recorded neurons were analyzed for the presence of parvalbumin (PV), a calcium-binding protein that is commonly found in FS cells (Cauli et al. 1997; Gibson et al. 1999; Kawaguchi and Kubota 1998). Miniature IPSCs and EPSCs (mIPSCs and mEPSCs) were recorded by adding 1 μM TTX to the bath solution. Input resistance of cells was monitored by frequently applying a 100-ms hyperpolarizing voltage step of 10 mV from a holding potential of – 40 mV. Monosynaptic IPSCs were evoked by extracellular electrical stimuli delivered through a glass electrode (3–5 MΩ when filled with extracellular solution) placed ∼100 μm away from the recorded cell bodies. Five-pulse trains at 10 Hz were delivered to elicit IPSCs every 10 s. Thirty trains were given to each cell, and the responses were averaged to measure the amplitude of evoked IPSCs. The stimulation strength was adjusted to twofold of threshold intensity (100–150 μA). Monosynaptic IPSCs were recorded in the presence of N-methyl-d-aspartate (NMDA) and AMPA/kainate receptor antagonists, d-2-amino-5-phosphonopentanoic acid (d-AP5) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo quinoxaline-2,3-dione (NBQX), respectively. After electrophysiological recording, slices with biocytin-filled neurons were fixed in paraformaldehyde (4% in 0.1 M phosphate buffer, PB) and kept at 4°C overnight. Without resectioning, slices were further processed for double labeling for PV and biocytin (described in detail in the following text).
The recordings were performed using a multiClamp 700B amplifier (Axon Instruments). Recording started 5–10 min after the whole cell patch was formed. Data acquisition and analysis were performed using pClamp 10.1 software with Digidata 1320A interface (Molecular Devices, Union City, CA). Signals were digitized at 5–20 kHz and analyzed off-line. Recordings were discarded if access resistance changed >10% during the experiment. Analysis of IPSCs and EPSCs was based on 5 min of continuous recording from each cell to obtain averaged data. Negative-going inward EPSCs and/or positive-going outward IPSCs were separately detected at the same baseline using the Mini Analysis Program (Synaptosoft, Leonia, NJ), and the instantaneous amplitude and frequency were acquired to obtain mean value and cumulative probability. The threshold for IPSC and EPSC detection was 6 pA, and the automatic detection was verified post hoc by visual inspection. For calculation of EPSC-to-IPSC ratios, averaged values of each measure for each cell were calculated and expressed as a ratio (e.g., frequency sEPSC/sIPSC). The ratios were then pooled within groups and compared between groups.
Immunohistochemistry
All fixed slices with biocytin-filled neurons were incubated in 30% sucrose/0.1 M PB/0.1% sodium azide solution at 4°C overnight for cryoprotection. Sections were then processed for immunofluorescence to detect PV and biocytin. Slices were quenched in 10% methanol and 3% H2O2 in 0.02 M phosphate buffered saline (PBS) for 5 min to remove endogenous peroxidase activity and incubated with 2% normal goat serum (NGS) and 1% bovine serum albumin (BSA) and 0.5% Triton-100 in 0.02 M PBS for 1 h to block nonspecific binding and render cell membranes more permeable. Slices were incubated with primary antibody, a mouse monoclonal anti-PA antibody (Sigma, diluted at 1:1,500), in 2% NGS and 1% BSA and 0.5% Triton-100 in 0.02 M PBS for 48 h at 4°C. After thorough rinsing, slices were incubated with secondary antibodies Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, diluted at 1:250 and used for labeling PV) and Alexa-fluor 594-conjugated streptavidin (Invitrogen, diluted at 1:250 and used for labeling biocytin) at room temperature (∼23°C) for 2.5 h. After staining, slices were mounted on glass slides, coverslipped with fluoromount aqueous mounting medium (Sigma), and the edges were sealed with nail polish. Sections were examined with an Olympus IX81-DSU Spinning Disk Confocal Microscope (Olympus America, Melville, NY). Fifteen series images from each section were acquired with a z step of 0.3 μm and an image size of 672 × 512 pixel. z-axis image stacks were prepared from the series images. We performed all image processing using ImageJ software version 1.37V (Wyne Rasband, National Institutes of Health).
Chemicals
d-AP5, NBQX, and TTX were purchased from Sigma. Suppliers of primary and secondary antibodies were described in the preceding text.
Statistical analysis
All values are expressed as means ± SE. One-way ANOVA was used to compare group data from different groups, and the Kolmogorov-Smirnov test was used to compare data for the distributions of synaptic events with the significance level set at P < 0.05.
RESULTS
Identification of PV-immunoreactive FS interneurons
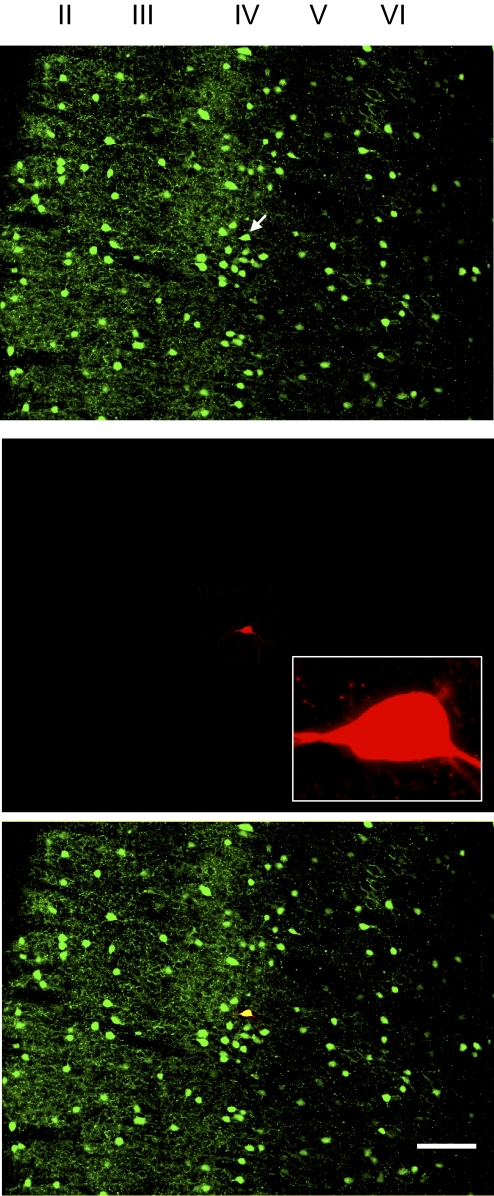
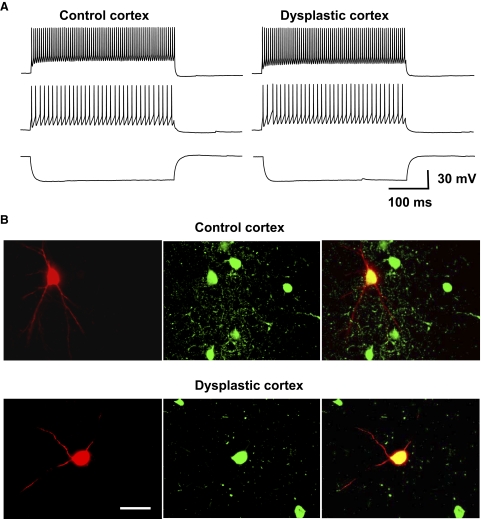
Using infrared videomicroscopy, layer IV of somatosensory cortex was detectable at low magnification (× 4) under bright-field illumination in live cortical slices. Recordings were performed in layer IV in controls and in the middle region in dysplastic cortex, which had no clearly defined lamina. As shown in Fig. 1, the recorded control FS interneuron was confirmed to reside in layer IV by double labeling with biocytin and PV. Putative interneurons under high magnification (×40) were initially selected for recording based on their relatively small size and the lack of a long, thick apical dendrite. Although neurons with round or ovoid soma are more likely interneurons, we found that some neurons with other morphologies (but still lacking a long, thick apical dendrite) proved to be interneurons. Therefore in this study, we did not classify interneurons by soma shape. Pyramidal cells were easily distinguished from interneurons based on morphology with a long, thick apical dendrite, a slower firing rate, and smaller afterhyperpolarizations (AHPs). FS interneurons were separated from other types of interneurons based on their electrophysiological properties. As shown in Fig. 2 A, FS interneurons fired continuously without frequency adaptation in response to depolarizing currents. Their firing rates increased sharply in response to increasing the injection current and, at higher levels of injected current, rates of >120 Hz could be seen. FS cells did not display rebound spikes after hyperpolarizing current step injections. They had an average input resistance of 181.4 ± 11.2 MΩ (n = 47) in control cortex and 191.6 ± 16.2 MΩ (n = 25) in dysplastic cortex. FS interneurons were further characterized by double staining of biocytin and PV. We electrophysiologically characterized 47 FS interneurons in controls and 25 from irradiated rats. We attempted visualization in 19 of 47 biocytin-filled neurons in control and 14 of 25 in dysplastic cortex. Of these, 16 of 19 biocytin-filled neurons in control and 10 of 14 in dysplastic cortex were successfully visualized. The successfully visualized biocytin-filled neurons were then examined for PV labeling. Twelve of 16 biocytin-filled neurons in control and 7 of 10 in dysplastic cortex were PV-positive (Fig. 2B). FS interneurons demonstrated a variety of soma shapes (round, ovoid, stellate or fusiform; Fig. 1). Twelve cells (4 putative pyramidal cells and 8 putative interneurons) without electrophysiological and morphological properties of FS interneurons were selected at random to perform double labeling of biocytin and PV. None of these biocytin-labeled cells were PV-immunoreactive.
Fig. 1.
Representative photomicrographs showing that a parvalbumin (PV)-immunopositive, fast-spiking (FS) interneuron in layer IV of control somatosensory cortex was recorded. The FS interneuron resided in layer IV (arrow) and double-labeled for biocytin (red) and PV (green). Scale bar: 100 μm.
Fig. 2.
Electrophysiological and immunocytological properties of FS interneurons. A: representative traces illustrating the firing pattern of FS interneurons from control and dysplastic cortex. Depolarizing current injection (600 ms) evoked high-frequency spike firing (54 and 125 spike/s in control and 54 and 123 spike/s in dysplastic cortex) without adaptation using currents of 100 and 300 pA, respectively. Hyperpolarizing current injection (600 ms, –300 pA did not elicit rebound spikes. The membrane potential was – 63.7 mV in the control FS interneuron and – 63.2 mV in the FS cell from dysplastic cortex. B: representative double-labeling photomicrographs of FS interneurons with biocytin (red) and PV (green) in control and dysplastic cortex, showing that electrophysiologically characterized and biocytin-filled FS interneurons were immunoreactive for PV. Scale bar: 25 μm.
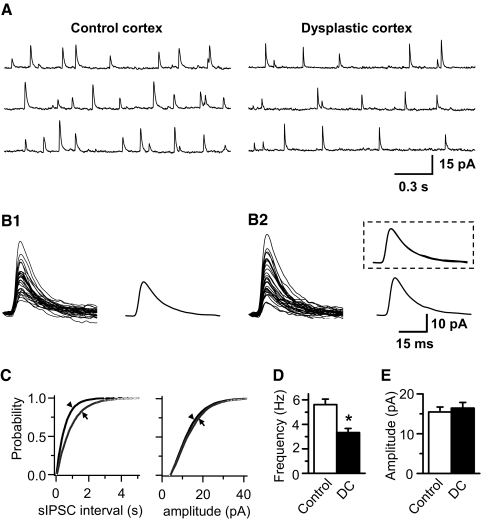
Frequency of both sIPSCs and mIPSCs in FS interneurons is decreased in dysplastic cortex
Under baseline recording conditions, without blocking AMPA and NMDA receptors, IPSCs were outward currents, whereas EPSCs were inward currents at a holding potential of – 40 mV. To isolate IPSCs and better analyze IPSC kinetic properties, NBQX (10 μM) and d-AP5 (50 μM) were added to block AMPA and NMDA receptors. Under these conditions, as shown in Fig. 3 A, sIPSCs were outward currents and all sEPSCs were blocked. Quantitative analysis showed that the cumulative probability of interevent interval (the inverse of frequency) of sIPSCs in dysplastic cortex was significantly different from controls (Fig. 3C). The mean frequency of sIPSCs was significantly lower in dysplastic cortex (3.33 ± 0.34 Hz, n = 11) compared with control cortex (5.62 ± 0.04 Hz, n = 21, P < 0.01; Fig. 3, A and D). In contrast, the cumulative probability of sIPSC amplitude in dysplastic cortex was not different from controls, and mean amplitude of sIPSCs also showed no difference between control and dysplastic cortex (17.6 ± 1.35 pA in control; 18.4 ± 1.43 pA in dysplastic cortex; Fig. 3E). The kinetic properties of sIPSCs were also compared and showed no differences (Fig. 3B). The mean 10–90% rise time and decay time constant of sIPSCs were 2.11 ± 0.14 and 16.52 ± 1.38 ms in controls, respectively, and 2.06 ± 0.19 and 17.14 ± 1.55 ms in dysplastic cortex, respectively.
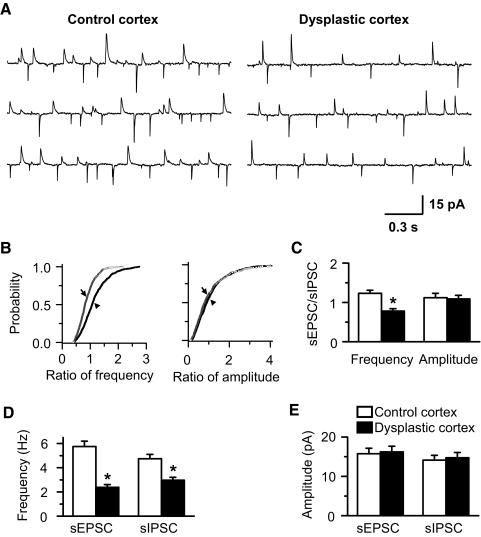
Fig. 3.
Frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) is reduced in FS interneurons in dysplastic cortex. A: representative sIPSCs recorded from FS interneurons in control and dysplastic cortex. B: 30 consecutive individual sIPSCs, selected at random, from control (B1) and dysplastic cortex (B2) were superimposed and averaged, and 2 averaged traces were scaled to the same amplitude and superimposed (B2, inset), showing there was no difference of sIPSC kinetics in FS interneurons from control and dysplastic cortex. C: cumulative probability curve showing an increased interevent interval (inverse of frequency, left; Kolmogorov-Smirnov test, P < 0.01) and no difference in amplitude of sIPSCs (right; Kolmogorov-Smirnov test, P > 0.05) from the representative FS interneurons in dysplastic cortex (→) compared with control cortex (▴). D and E: pooled data showing a decrease of sIPSC frequency (D, P < 0.01) and no difference in sIPSC amplitude (E) of FS interneurons from dysplastic cortex (n = 11) compared with control cortex (n = 21). Spontaneous IPSCs were recorded in the presence of 10 μM NBQX and 50 μM d-AP5.
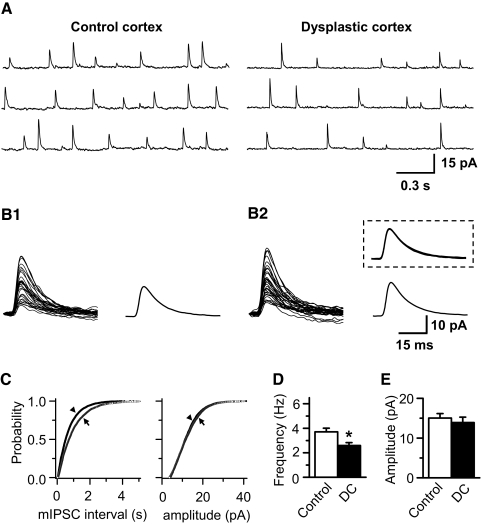
We added 1 μM TTX in bath solution and mIPSCs were recorded. As illustrated in Fig. 4, the cumulative probability of interevent interval for mIPSCs in dysplastic cortex was clearly different from controls (C), and the mean frequency of mIPSCs was found to be significantly reduced in dysplastic cortex (2.59 ± 0.24 Hz, n = 9) compared with controls (3.71 ± 0.30 Hz, n = 17, P < 0.01; A and D). The cumulative probability and the mean amplitude of mIPSCs were not different between control and dysplastic cortex (15.02 ± 1.14 pA in control; 13.9 ± 1.35 pA in dysplastic cortex; Fig. 4, C and E). We did not find any significant change in kinetic properties of mIPSCs between the two groups (Fig. 4B). The mean 10–90% rise time and decay time constant of mIPSCs were 1.87 ± 0.16 and 14.79 ± 1.26 ms, respectively, in control and 1.86 ± 0.18 and 13.73 ± 1.46 ms, respectively, in dysplastic cortex.
Fig. 4.
Miniature IPSC (mIPSC) frequency is reduced in FS interneurons in dysplastic cortex. A: representative mIPSCs recorded from FS interneurons in control and dysplastic cortex recorded in the presence of 1 μM TTX, 10 μM NBQX and 50 μM d-AP5. B: 30 consecutive individual mIPSCs, selected at random, from control (B1) and dysplastic cortex (B2) were superimposed and averaged, and 2 averaged traces were scaled to the same amplitude and superimposed (B2, inset), showing that there was no difference in mIPSC kinetics in FS interneurons from control and dysplastic cortex. C: cumulative probability curve showing an increase of interevent interval (left; Kolmogorov-Smirnov test, P < 0.01) and no difference in amplitude of mIPSCs (right; Kolmogorov-Smirnov test, P > 0.05) from the representative FS interneurons in dysplastic cortex (→) compared with control cortex (▴). D and E: pooled data showing a decrease of mIPSC frequency (D, P < 0.01)) and no difference in mIPSC amplitude (E) in FS interneurons from dysplastic cortex (n = 9) compared with control cortex (n = 17).
Ratio of excitatory-to-inhibitory spontaneous synaptic currents is decreased in dysplastic cortex
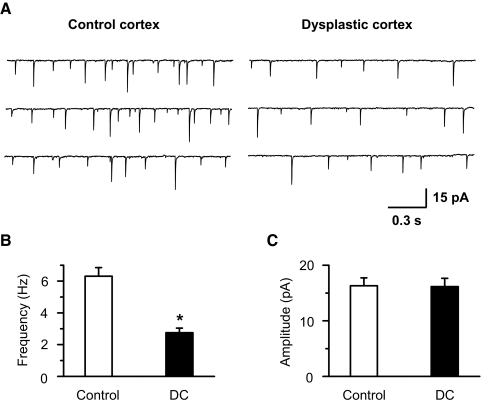
To determine whether the balance between excitatory and inhibitory synaptic transmission was altered in dysplastic cortex, sEPSCs, mEPSCs, sIPSCs, and mIPSCs were acquired from individual FS cells at a holding potential of – 40 mV (Figs. 5 A and 7A). At that potential, sIPSCs and mIPSCs appeared as outward currents while sEPSCs and mEPSCs were recorded as inward currents (Figs. 5A and 7A). Outward currents were completely blocked by 100 μM picrotoxin, a GABAA receptor antagonist, confirming that these currents were GABAergic IPSCs (Fig. 6). The mean frequency and amplitude of sEPSCs were 6.31 ± 0.54 Hz and 16.31 ± 1.42 pA, respectively, in control and 2.75 ± 0.29 Hz and 16.15 ± 1.49 pA, respectively, in dysplastic cortex in the presence of 100 μM picrotoxin (Fig. 6, B and C). Inward currents were block by 10 μM NBQX and 50 μM d-AP5, indicating that these currents were glutamatergic EPSCs (Figs. 3A and 4A). Quantitative analysis showed that the cumulative probability of the ratio of sEPSCs to sIPSCs frequency in dysplastic cortex was distinguishable from controls (Fig. 5B). The mean ratio of frequency of sEPSCs to sIPSCs in dysplastic cortex (0.78 ± 0.06, n = 14) was significantly decreased compared with control cortex (1.23 ± 0.08, n = 26, P < 0.01; Fig. 5, A and C), indicating that the balance between excitation and inhibition was shifted to favor inhibition over excitation in FS interneurons in dysplastic cortex. The ratio of sEPSC to sIPSC amplitude was no different between dysplastic and control cortex (1.12 ± 0.11 in control, 1.09 ± 0.09 in dysplastic cortex). The mean frequency and amplitude of sEPSCs and sIPSCs were also obtained. The mean frequency of both sEPSCs and sIPSCs was decreased in dysplastic cortex compared with controls while the amplitude showed no difference between two groups. The mean frequency of sEPSCs and sIPSCs were 5.75 ± 0.45 and 4.74 ± 0.36 Hz, respectively, in control (n = 26) and 2.38 ± 0.23 and 2.97 ± 0.24 Hz, respectively, in dysplastic cortex (n = 14; Fig. 5D). The mean amplitude of sEPSCs and sIPSCs were 15.76 ± 1.39 and 14.13 ± 1.23 pA, respectively, in control and 16.22 ± 1.45 and 14.72 ± 1.36 pA, respectively, in dysplastic cortex (Fig. 5E).
Fig. 5.
Ratio of sEPSC to sIPSC frequency is decreased in FS interneurons in dysplastic cortex. A: representative traces showing sEPSCs and sIPSCs recorded simultaneously at a holding potential of – 40 mV. B: cumulative probability curve showing an decrease of the ratio of sEPSC to sIPSC frequency (left; Kolmogorov-Smirnov test, P < 0.01) and no difference in amplitude (right; Kolmogorov-Smirnov test, P > 0.05) from representative FS interneurons in dysplastic cortex (→) compared with control cortex (▴). C: the ratio of sEPSC to sIPSC frequency was decreased (P < 0.01) in FS interneurons in dysplastic cortex while amplitude was unchanged compared with controls. D and E: group data showing that sEPSC and sIPSC frequency was decreased (D) in FS interneurons in dysplastic cortex. However, there was no difference in amplitude of sEPSCs and sIPSCs (E) in FS interneurons in dysplastic cortex compared with controls. Legend in E applies to C and D.
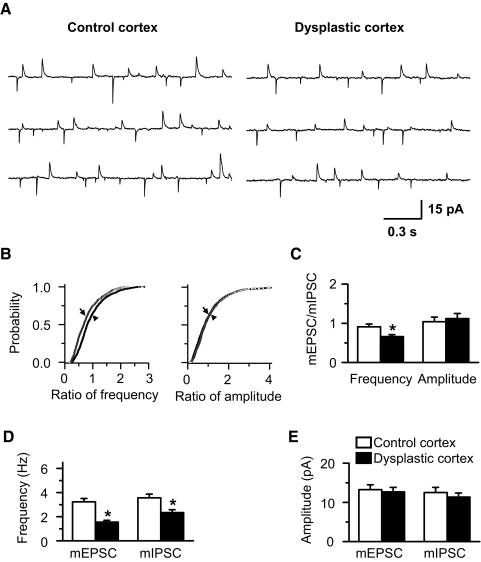
Fig. 7.
Ratio of mEPSC to mIPSC frequency is decreased in FS interneurons in dysplastic cortex. A: representative traces showing mEPSCs and mIPSCs recorded simultaneously at holding potential of – 40 mV in the presence of 1 μM TTX. B: cumulative probability curve showing a decrease of the ratio of mEPSCs to mIPSC frequency (left; Kolmogorov-Smirnov test, P < 0.01) and no difference in amplitude (right; Kolmogorov-Smirnov test, P > 0.05) from the representative FS interneurons in dysplastic cortex (→) compared with control cortex (▴). C: the ratio of mEPSC to mIPSC frequency was decreased (P < 0.01) in FS interneurons in dysplastic cortex while amplitude was unchanged compared with controls. D and E: group data showing that mEPSC and mIPSC frequency decreased (D, P < 0.01) while amplitude was unchanged in FS interneurons in dysplastic cortex compared with controls. Legend in E applies to C and D.
Fig. 6.
Frequency of spontaneous EPSCs is reduced in FS interneurons in dysplastic cortex. A: representative sEPSCs recorded from FS interneurons in control and dysplastic cortex. B and C: pooled data showing a decrease of sEPSC frequency (B, P < 0.01) and no difference in sEPSC amplitude (C) of FS interneurons from dysplastic cortex (n = 10) compared with control cortex (n = 13). Spontaneous EPSCs were recorded in the presence of 100 μM picrotoxin.
We next compared miniature synaptic currents in the presence of 1 μM TTX (Fig. 7 A). Similar to the spontaneous currents, we observed a significant difference in cumulative probability of the ratio of mEPSC to mIPSC frequency (Fig. 7B), and a decrease of the mean ratio of frequency of mEPSCs to mIPSC in dysplastic cortex (0.66 ± 0.05, n = 10) compared with controls (0.91 ± 0.07, n = 20, P < 0.01; Fig. 7C). This indicates that the ratio of mEPSC to mIPSC frequency is shifted toward inhibition in dysplastic cortex. The mean frequency of both mEPSCs and mIPSCs was decreased in dysplastic cortex. They were 3.23 ± 0.28 and 3.56 ± 0.31 Hz, respectively, in control (n = 20) and 1.55 ± 0.15 and 2.34 ± 0.24 Hz, respectively, in dysplastic cortex (n = 10; Fig. 7D). The amplitudes of mEPSCs and mIPSCs were similar between the two groups. They were 13.24 ± 1.25 and 12.53 ± 1.30 pA, respectively, in control, and 12.67 ± 1.16 and 11.34 ± 1.03 pA, respectively, in dysplastic cortex (Fig. 7E).
Short-term plasticity of evoked IPSCs is altered in dysplastic cortex
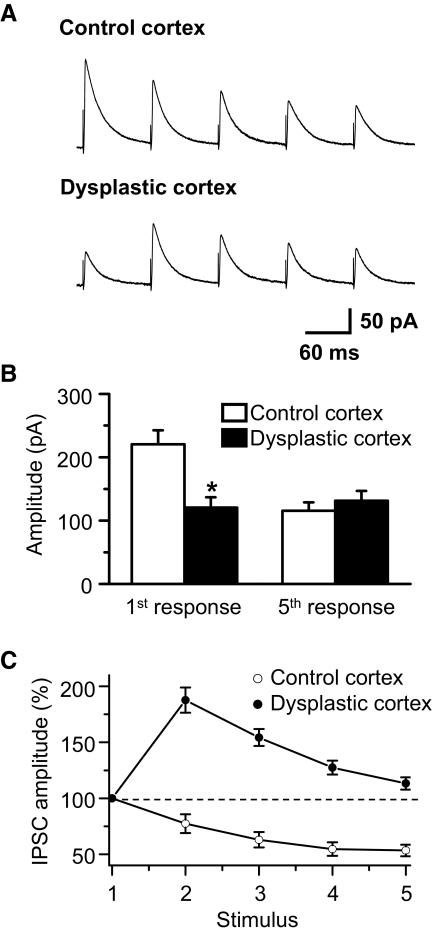
Synaptic plasticity, the change of synaptic strength in response to repetitive stimulation, is an important aspect of synaptic function. Short-term plasticity (STP), in the form of depression or facilitation, varies depending on cell type, cortical region, and stage of maturation. STP is largely dependent on presynaptic release probability. Synapses with high initial release probability demonstrate short-term depression while synapses with low initial release probability show facilitation (Stevens and Wang 1995). Alterations in release probability from GABAergic terminals on FS interneurons could affect the frequency of mIPSCs and contribute to the differences in dysplastic cortex described in the preceding text. Therefore we studied STP of stimulus-evoked IPSCs in FS cells in dysplastic and control cortex as a surrogate marker for release probability. As shown in Fig. 8 A, evoked IPSCs were outward currents at a holding potential of – 40 mV. The kinetic properties of evoked IPSCs were not different between dysplastic and control cortex. Mean rise time and decay time constants were 3.8 ± 0.4 and 45.7 ± 3.6 ms (n = 11), respectively, in control and 3.5 ± 0.3 and 43.7 ± 3.9 ms (n = 7), respectively, in dysplastic cortex. However, with similar stimulation intensity (244 ± 14 μA, n = 11 in control; 240 ± 18 μA, n = 7 in dysplastic cortex), the amplitude of the first response was significantly decreased in dysplastic cortex (115.7 ± 13.1 pA, n = 7) compared with controls (220.5 ± 22.1 pA, n = 11, P < 0.01, Fig. 8, A and B). In addition, control cortex demonstrated short-term depression while short-term facilitation was seen in dysplastic cortex (Fig. 8, A and C). In control FS interneurons, the second to fifth responses were depressed to 77.3 ± 8.4, 62.9 ± 6.9, 54.6 ± 6.1, and 53.4 ± 5.4% (n = 11), respectively, of the first response (Fig. 8, A and C). In dysplastic cortex, the second to fifth responses facilitated to 187.7 ± 11.2, 154.3 ± 7.6, 127.4 ± 6.6, and 113.2 ± 5.5% (n = 7), respectively, of the first response (Fig. 8, A and C). The amplitude of the fifth response was 131.4 ± 15.6 pA (n = 7) in dysplastic cortex and 120.6 ± 16.5 pA (n = 11, P > 0.05, Fig. 8B) in controls. These results indicate that STP of stimulus-evoked IPSCs was altered in FS interneurons from dysplastic cortex.
Fig. 8.
Short-term plasticity of stimulus-evoked IPSCs of FS interneurons is altered in dysplastic cortex. A: representative averaged traces (10 successive responses at 0.1 Hz) of evoked IPSCs in FS interneurons from control and dysplastic cortex showing depression and facilitation, respectively. A train of 5 pulses at 10 Hz was used. The stimulus strength was 200 μA for both cells. B: amplitude of the 1st response was decreased in dysplastic cortex (n = 7) compared with controls (n = 11, P < 0.01). There was no difference in the amplitude of the 5th response between groups. C: averaged data from all recorded cells showing relative evoked IPSC amplitude (IPSCn/IPSC1) demonstrating short-term facilitation in dysplastic cortex and short-term depression in controls.
DISCUSSION
This study is the first to examine inhibitory synaptic activity in FS interneurons in the irradiated rat model of CD. Layer IV FS interneurons were chosen for several reasons. FS cells are the most abundant subtype of interneuron in the neocortex (Cauli et al. 1997; Gonchar and Burkhalter 1997), and they can be unambiguously identified by intrinsic firing properties at the time of physiological testing (McCormick et al. 1985). Layer IV FS cells in the somatosensory cortex exert a powerful influence over the function of the barrels. They receive strong excitatory input from thalamic afferents and mediate powerful feed-forward inhibition in neighboring layer IV spiny neurons (Beierlein et al. 2003; Gibson et al. 1999; Sun et al. 2006). In vivo studies have shown that a small number of suspected FS interneurons provide synchronized inhibitory activity that significantly limits the time window for thalamocortical excitation of neighboring principal cells (Swadlow 2003; Swadlow et al. 1998). Most FS interneurons express PV (Gibson et al. 1999; Kawaguchi and Kubota 1998). In the current study, we found that the majority of FS cells that could be recovered for histological analysis were immunoreactive for PV in both control and dysplastic cortex. The remaining cells may not express PV or the PV may not have been present in detectable levels on postfixation analysis due to leaching of the protein from the cytoplasm into the recording pipette during the whole cell recordings.
We found that frequency of sIPSCs and mIPSCs was reduced in FS cells in dysplastic cortex while amplitude of both currents was unchanged. This suggests that inhibition is impaired in these cells as it is for layer V pyramidal cells (Zhu and Roper 2000) and heterotopic pyramidal cells (Chen and Roper 2003) in this model. The reduction in frequency of mIPSCs suggests a presynaptic locus for this impairment. There are two mechanisms that could contribute to this result: fewer GABAergic presynaptic terminals on the FS cells and decreased release probability in those terminals. The STP experiments were designed to address the issue of altered release probability. STP is largely determined by release probability in the presynaptic terminals with high release probability producing short-term depression and low release probability producing short-term facilitation (Stevens and Wang 1995). We found that IPSCs in control cortex showed short-term depression while IPSCs in dysplastic cortex showed short-term facilitation. This suggests that initial release probability is lower in GABAergic synapses on FS cells in dysplastic cortex. This could contribute partially or in total to the reduced mIPSC frequency in FS cells in dysplastic cortex. It does not, however, rule out a reduced number of GABAergic terminals on these cells. The known reduction in density and numbers of GABAergic interneurons in dysplastic cortex (Deukmedjian et al. 2004; Roper et al. 1999) would make reduced numbers of GABAergic terminals likely, regardless of the target cell. But the current data do not address this issue directly.
The current finding of altered STP in IPSCs in layer IV FS interneurons is different from IPSCs in layer V pyramidal cells where there was no difference in STP of IPSCs between control and dysplastic cortex (Zhu and Roper 2000). CD does affect STP of EPSCs in layer V pyramidal cells where, at 4 wk of age and older, dysplastic tissue showed short-term facilitation and control cortex showed short-term depression (Chen et al. 2007). This suggested preservation of immature synaptic properties in the excitatory terminals with an increased release probability. Reviewing all of these findings, it appears that the effect of CD on release probability and STP varies depending on the target cell, the type of presynaptic terminal, and the stage of development.
The key question of excitability in the FS interneurons involves the balance of excitatory and inhibitory synaptic inputs. Although our previous study demonstrated reduced frequency of sEPSCs and mEPSCs in layer IV/V FS cells (Xiang et al. 2006), the inhibitory half of the equation was never examined. The finding of reduced frequency of sEPSCs and mEPSCs in FS interneurons confirms findings from our previous study (Xiang et al. 2006). Actual values, both in controls and irradiated rats, were slightly higher in the current study. This is most likely due to the fact that the current experiments were performed at a higher recording temperature (30°C compared with 22°C). This change in recording temperature was made in an effort to characterize synaptic activity under more physiological conditions. As discussed in our previous paper, we feel that the reduced frequency of sEPSCs and mEPSCs reflects a reduction in the number of excitatory terminals on FS interneurons in dysplastic cortex.
The current study attempted to address the issue of balance directly by recording in conditions where both EPSCs and IPSCs could be recorded simultaneously from the same neuron. We found that although the frequency of both EPSCs and IPSCs was reduced, the EPSCs were reduced more than the IPSCs such that the ratio of EPSCs to IPSCs was lower in CD than control cortex. This was true for both spontaneous and miniature currents. We predict that this shift of synaptic activity to favor inhibition would likely make FS interneurons in dysplastic cortex less active and less likely to perform their role of dampening excitatory activity in the local circuit during periods of increased excitability. The expression of EPSC and IPSC frequency as a ratio is not meant to imply that one EPSC has the same physiological impact as one IPSC. In fact, excitatory postsynaptic potentials (EPSPs) from a single excitatory neuron can elicit action potentials in FS interneurons in somatosensory cortex (Sun et al. 2006) and area CA3 of the hippocampus (Miles 1990). The ratio is only used as a means of comparison between the experimental and control groups.
Although the basic impetus for studying inhibition in irradiated rat has been to examine a link with epilepsy, the relationship between inhibition and epileptogenesis appears to be quite complex and incompletely understood. The notion that interneurons serve only to dampen excitatory activity and maintain some degree of homeostasis of firing of excitatory cells is clearly incomplete and simplistic (Cossart et al. 2005). The role of interneurons in synchronizing firing of local neurons even suggests a possible pro-convulsant role. This is exemplified by certain types of bursting behavior that are mediated by interneurons (D'Antuono et al. 2004; Khalilov et al. 2003; Lopantsev and Avoli 1998). FS interneurons, in particular, appear to play an important role in the maintenance of physiologically important gamma oscillations (Bartos et al. 2007; Cobb et al. 1995) and depression of the strength of excitatory synapses on FS interneurons is an important step in the transition from gamma oscillations to epileptiform bursting in area CA3 of mouse hippocampus (Traub et al. 2005). Therefore, although it seems reasonable that loss of interneurons and shift of synaptic drive to favor inhibition in FS cells would contribute to a pro-epileptic state in CD, this hypothesis is currently untested.
Other models of epilepsy have demonstrated impairment of inhibition through a variety of mechanisms. Loss of hippocampal interneurons has been seen in human temporal lobe epilepsy (de Lanerolle et al. 1989) and animal models of the same (Cossart et al. 2001; Kobayashi and Buckmaster 2003; Kumar and Buckmaster 2006; Obenaus et al. 1993). Recordings from pyramidal cells in human CD have shown a reduction in the frequency of sIPSCs (Calcagnotto et al. 2005), very similar to that reported in irradiated rat (Zhu and Roper 2000). However, this study did not show a frank reduction in density of interneurons. Other histological studies have described reduced numbers of interneurons in human CD (Ferrer et al. 1994; Spreafico et al. 2000).
Studies that examine excitatory connectivity in interneurons in epilepsy models have been less common. In kainate-treated rats, interneurons in the stratum lacunosum/moleculare of CA1 showed reduced amplitude and conductance of stimulus-evoked EPSCs (Morin et al. 1998). Short-term depression of EPSCs was exaggerated in hilar interneurons in the pilocarpine model of temporal lobe epilepsy (Doherty and Dingledine 2001), and this was interpreted as a dynamic reduction in excitation. Excitatory drive was tested in interneurons of the entorhinal cortex in the pilocarpine model, but it did not appear to be altered (Kumar and Buckmaster 2006). The balance of excitatory to inhibitory activity was measured in layer V pyramidal cells in the paramicrogyral (PMG) cortex of rats treated with a perinatal freeze lesion to the cortex, a focal model of polymicrogyria (Jacobs and Prince 2005). These neurons received an excessive amount of inhibitory input, and analysis of IPSCs after pharmacologic blockade of glutamatergic receptors suggested that this was due to increased excitatory inputs onto the neighboring interneurons. This was postulated to be the result of rerouting of thalamic afferents into the PMG due to loss of targets in the microgyrus. Additional studies in layer IV of the PMG showed a reduced strength of inhibitory connections of layer IV FS cells onto neighboring spiny neurons, and this resulted in an increased susceptibility for epileptiform activity after thalamic stimulation (Sun et al. 2005).
Lis 1 +/− mice are a genetic model of human lissencephaly, a severe and diffuse type of CD, with demonstrated hyperexcitability in the hippocampus (Fleck et al. 2000). Recordings from area CA1 of the hippocampus in these animals show increased frequency of IPSCs in pyramidal cells and increased EPSCs and spontaneous firing in nearby FS interneurons (Jones and Baraban 2007). When inhibitory currents were recorded, FS interneurons showed a reduced frequency of mIPSCs in the mutant mice (Jones and Baraban 2009). Therefore hippocampal FS interneurons in Lis 1 +/− mice show synaptic changes that are different from CD in irradiated rats; i.e., they show increased excitatory activity and decreased inhibitory activity. These studies underscore the fact that there are many animal models and types of human CD and that epilepsy in CD is probably a final common pathway for a variety of pathogenetic mechanisms. To our knowledge, the current study is the only one to document an imbalance of excitatory to inhibitory input toward reduced excitation in identified interneurons in an animal model of CD.
This study expands our understanding of impaired inhibition in the irradiated rat model of CD. We have documented reduced numbers of cortical interneurons and a concomitant loss of inhibitory postsynaptic currents in large pyramidal cells in dysplastic cortex. We have also found a reduction in sEPSCs and mEPSCs in layer IV/V interneurons. The current results show that even though IPSCs are reduced in frequency in layer IV FS interneurons, the reduction in EPSCs is more pronounced. This results in a shift in the balance of EPSCs to IPSCs in favor of inhibition. This would further contribute to loss of inhibition in this model of CD. This raises the possibility that it may be involved in the pathophysiology of certain types of human CD as well.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-35651 to S. N. Roper and by the Edward Shedd Wells and Densch Foundations.
REFERENCES
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007 [DOI] [PubMed] [Google Scholar]
- Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, Dubinsky S, Newmark ME, Leibson C, So EL, Rocca WA. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 41: 342–351, 2000 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Tihan T, Barbaro NM, Baraban SC. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci 25: 9649–9657, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17: 3894–3906, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Roper SN. Reduction of spontaneous inhibitory synaptic activity in experimental heterotopic gray matter. J Neurophysiol 89: 150–158, 2003 [DOI] [PubMed] [Google Scholar]
- Chen HX, Xiang H, Roper SN. Impaired developmental switch of short-term plasticity in pyramidal cells of dysplastic cortex. Epilepsia 48: 141–148, 2007 [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378: 75–78, 1995 [DOI] [PubMed] [Google Scholar]
- Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci 28: 108–115, 2005 [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4: 52–62, 2001 [DOI] [PubMed] [Google Scholar]
- Cowen D, Geller LM. Long-term pathological effects of prenatal x-irradiation on the central nervous system of the rat. J Neuropathol Exp Neurol 19: 488–527, 1960 [DOI] [PubMed] [Google Scholar]
- D'Antuono M, Louvel J, Kohling R, Mattia D, Bernasconi A, Olivier A, Turak B, Devaux A, Pumain R, Avoli M. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain 127: 1626–1640, 2004 [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495: 387–395, 1989 [DOI] [PubMed] [Google Scholar]
- Deukmedjian AJ, King MA, Cuda C, Roper SN. The GABAergic system of the developing neocortex has a reduced capacity to recover from in utero injury in experimental cortical dysplasia. J Neuropathol Exp Neurol 63: 1265–1273, 2004 [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Reduced excitatory drive onto interneurons in the dentate gyrus after status epilepticus. J Neurosci 21: 2048–2057, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MA, DeRosa MJ, Curran JG, Secor DL, Cornford ME, Comair YG, Peacock WJ, Shields WD, Vinters HV. Neuropathologic findings in cortical resections (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol 83: 246–259, 1992 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Oliver B, Russi A, Casas R, Rivera R. Parvalbumin and calbindin-D28k immunocytochemistry in human neocortical epileptic foci. J Neurol Sci 123: 18–25, 1994 [DOI] [PubMed] [Google Scholar]
- Fleck MW, Hirotsune S, Gambello MJ, Phillips-Tansey E, Suares G, Mervis RF, Wynshaw-Boris A, McBain CJ. Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J Neurosci 20: 2439–2450, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7: 347–358, 1997 [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Prince DA. Excitatory and inhibitory postsynaptic currents in a rat model of epileptogenic microgyria. J Neurophysiol 93: 687–696, 2005 [DOI] [PubMed] [Google Scholar]
- Jones DL, Baraban SC. Characterization of inhibitory circuits in the malformed hippocampus of Lis1 mutant mice. J Neurophysiol 98: 2737–2746, 2007 [DOI] [PubMed] [Google Scholar]
- Jones DL, Baraban SC. Inhibitory inputs to hippocampal interneurons are reorganized in Lis1 mutant mice. J Neurophysiol 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically identified GABAergic cells in the rat frontal cortex. Neuroscience 85: 677–701, 1998 [DOI] [PubMed] [Google Scholar]
- Kellinghaus C, Kunieda T, Ying Z, Pan A, Luders HO, Najm IM. Severity of histopathologic abnormalities and in vivo epileptogenicity in the in utero radiation model of rats is dose dependent. Epilepsia 45: 583–591, 2004 [DOI] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci 6: 1079–1085, 2003 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci 23: 2440–2452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Najm I, Kunieda T, Perryman S, Yacubova K, Luders HO. Electroencephalographic characterization of an adult rat model of radiation-induced cortical dysplasia. Epilepsia 42: 1221–1227, 2001 [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci 26: 4613–4623, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 342: 314–319, 2000 [DOI] [PubMed] [Google Scholar]
- Lehohla M, Russell V, Kellaway L. NMDA-stimulated Ca2+ uptake into barrel cortex slices of spontaneously hypertensive rats. Metab Brain Dis 16: 133–141, 2001 [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophysiol 79: 352–360, 1998 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Riggs HE, Schwarz HP. Malformation of the adult brain (albino rat) resulting from prenatal irradiation. J Neuropathol Exp Neurol 15: 432–446; discussion, 446–437, 1956 [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea pig in vitro. J Physiol 428: 61–77, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Cell-specific alterations in synaptic properties of hippocampal CA1 interneurons after kainate treatment. J Neurophysiol 80: 2836–2847, 1998 [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13: 4470–4485, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y. Focal neuronal migration disorders and intractable partial epilepsy: results of surgical treatment. Ann Neurol 30: 750–757, 1991 [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Gloor P, Quesney F, Andermann E, Paglioli E, Paglioli-Neto E, Coutinho L, Leblanc R, Kim HI. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol 37: 476–487, 1995 [DOI] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of the cortical dysplasias. Neurology 62: S2–8, 2004 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates New York: Academic, 1986 [DOI] [PubMed] [Google Scholar]
- Porter BE, Judkins AR, Clancy RR, Duhaime A, Dlugos DJ, Golden JA. Dysplasia: a common finding in intractable pediatric temporal lobe epilepsy. Neurology 61: 365–368, 2003 [DOI] [PubMed] [Google Scholar]
- Roper SN. In utero irradiation of rats as a model of human cerebrocortical dysgenesis: a review. Epilepsy Res 32: 63–74, 1998 [DOI] [PubMed] [Google Scholar]
- Roper SN, Eisenschenk S, King MA. Reduced density of parvalbumin- and calbindin D28-immunoreactive neurons in experimental cortical dysplasia. Epilepsy Res 37: 63–71, 1999 [DOI] [PubMed] [Google Scholar]
- Roper SN, Gilmore RL, Houser CR. Experimentally induced disorders of neuronal migration produce an increased propensity for electrographic seizures in rats. Epilepsy Res 21: 205–219, 1995 [DOI] [PubMed] [Google Scholar]
- Roper SN, King MA, Abraham LA, Boillot MA. Disinhibited in vitro neocortical slices containing experimentally induced cortical dysplasia demonstrate hyperexcitability. Epilepsy Res 26: 443–449, 1997 [DOI] [PubMed] [Google Scholar]
- Spreafico R, Tassi L, Colombo N, Bramerio M, Galli C, Garbelli R, Ferrario A, Lo Russo G, Munari C. Inhibitory circuits in human dysplastic tissue. Epilepsia 41, Suppl 6: S168–173, 2000 [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron 14: 795–802, 1995 [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26: 1219–1230, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Reorganization of barrel circuits leads to thalamically evoked cortical epileptiform activity. Thalamus Relat Syst 3: 261–273, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex 13: 25–32, 2003 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol 79: 567–582, 1998 [DOI] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, Lebeau FE, Buhl EH, Garner H, Monyer H, Whittington MA. Transient depression of excitatory synapses on interneurons contributes to epileptiform bursts during gamma oscillations in the mouse hippocampal slice. J Neurophysiol 94: 1225–1235, 2005 [DOI] [PubMed] [Google Scholar]
- Xiang H, Chen HX, Yu XX, King MA, Roper SN. Reduced excitatory drive in interneurons in an animal model of cortical dysplasia. J Neurophysiol 96: 569–578, 2006 [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Roper SN. Reduced inhibition in an animal model of cortical dysplasia. J Neurosci 20: 8925–8931, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]