SYNOPSIS
In 2005, the Centers for Disease Control and Prevention established the STD Surveillance Network (SSuN), a sentinel surveillance system comprising local, enhanced sexually transmitted disease (STD) surveillance systems that follow common protocols. The purpose of SSuN is to improve the capacity of national, state, and local STD programs to detect, monitor, and respond rapidly to trends in STDs through enhanced collection, reporting, analysis, visualization, and interpretation of clinical, behavioral, and geographic information obtained from a geographically diverse sample of individuals diagnosed with STDs. To demonstrate the utility of a national sentinel surveillance network, this article reviews the lessons learned from the first three years of SSuN, which, through its enhanced gonorrhea and genital warts sentinel surveillance projects, has proved to be a useful adjunct to routine STD surveillance in the U.S. that can be expanded into other areas of STD public health interest.
National surveillance for sexually transmitted diseases (STDs) is dependent on mandatory case reporting by laboratories and providers to U.S. states and territories. However, data derived from the case reporting system are limited and incomplete. First, only a few STDs (including syphilis, gonorrhea, and chlamydia) are reportable in all states. Second, the amount of information received by the Centers for Disease Control and Prevention (CDC) and available for national analysis is limited. For example, data transmitted from state and local health departments to CDC for gonorrhea cases contain no information on whether or which antibiotics were used for treatment. Also, no information about gender of sex partners is available, such that trends of STDs among men who have sex with men (MSM) cannot be obtained from national surveillance data. Third, case report data are notoriously incomplete. For example, race/ethnicity data were missing in more than 20% of reported gonorrhea and chlamydia cases in the U.S. in 2006.1 Fourth, to an unknown extent, case reporting is also hampered by underreporting, particularly for chlamydia, but for gonorrhea as well. As a result, case report data have limited utility in defining the epidemiology of STDs and in guiding STD control interventions, at both the national and local levels. Fifth, STD case reporting also suffers from reporting and analysis delays at the national level; thus, evolving trends may not be detected in a timely manner.
While surveys employing nationally representative sampling schemes have been used to augment information collected from case reporting, they are often insufficient in filling in the epidemiologic gaps, require large amounts of resources, and take considerable time to plan and implement. For example, the National Health and Nutrition Examination Survey and AddHealth have been instrumental in assessing the general population prevalence of genital herpes2 and chlamydia.3–5 However, these surveys are not designed to focus specifically on STDs and, thus, in-depth information on risk behaviors and STD history is not always available. Furthermore, these large national surveys cannot be readily or rapidly modified. Also, populations at high risk for STDs such as MSM and injection drug users, who are often the focus of STD control activities, may be underrepresented in these general population surveys such that it is difficult to arrive at adequate epidemiologic assessments for these populations. Finally, general population surveys have limited local usefulness, and they generally are unable to show geographic differences and are much less timely than case report data.
Prevalence monitoring activities such as the Infertility Prevention Project (IPP) have also been used to provide STD data at a national level. However, IPP data are limited in that data are available primarily from family planning clinics, but also some STD clinics, prenatal clinics, jails, and juvenile detention centers. The positivity data that result from this activity are subject to variation in the number of participating sites, screening practices, and test technology. Furthermore, there may be differences among sites in how and what data are collected.
Thus, to better inform public health interventions and to evaluate the impact of innovative approaches to preventing STDs, there is a need to obtain additional information on STDs, including morbidity, etiology, diagnosis, and treatment as well as sociodemographic and risk behavior information. There is a need to better understand STD syndromes that are not nationally reportable, such as pelvic inflammatory disease and urethritis. To identify emerging national trends, there is also a need for more timely information from local jurisdictions with populations at highest risk for STDs. Sentinel surveillance—defined as the systematic collection of clinical, epidemiologic, and behavioral data from a limited number of sites—is one way of providing needed STD data.
The Gonococcal Isolate Surveillance Project (GISP) is the prototype for STD sentinel surveillance in the U.S. and has proved useful both at the national and local levels. Because it has consistently collected information on the gender of infected men's sex partners, GISP has helped demonstrate the reemergence of gonorrhea among MSM.6 GISP also identified emerging resistance to fluoroquinolone antibiotics that led initially to changes in CDC's gonorrhea treatment recommendations for Hawaii and California7,8 and among MSM9,10 and subsequently to changes in treatment recommendations regardless of geography and sexual behavior.11
The reemergence of syphilis and increases of gonorrhea and other STDs among MSM reported from numerous venues and jurisdictions since the mid-1990s12–15 established the need for a simple, efficient system to track STD clinical and behavioral data from MSM at highest risk for STDs. Thus, CDC initiated the MSM Prevalence Monitoring Project and began collecting data on MSM visiting STD clinics and human immunodeficiency virus (HIV) care clinics in eight geographically diverse U.S. cities. Besides yielding important data for local use, the project addressed important gaps in STD information at the national level, such as the paucity of data on gonorrhea by anatomic site. MSM Prevalence Monitoring Project data have demonstrated the potential for substantial underdiagnosis of gonorrhea and chlamydia infections among MSM if routine testing is not conducted at all exposed anatomic sites.16 However, the MSM Prevalence Monitoring Project is currently an unfunded project relying on existing clinic resources, resulting in a wide variety of data collection practices. Furthermore, current data in the project are limited to MSM, thus limiting the project's ability to conduct comparative analyses.
Experiences with GISP and the MSM Prevalence Monitoring Project have demonstrated the utility of STD clinics as potential sites for the provision of other STD-related information as well. Until recently, though, data abstraction from clinical charts was a labor-intensive and, therefore, costly proposition. However, as clinics increasingly adopt electronic medical records, the data abstraction process has become considerably more efficient and can employ computer algorithms to abstract records with a high degree of specificity. Computer database queries allow for more frequent and varied analyses than are possible with manual data extraction.
In addition to STD clinic-based sentinel surveillance, population-based sampling is also useful for answering some STD questions. In 2005, 65% of syphilis cases, 73% of gonorrhea cases, and 88% of chlamydia cases reported nationally came from providers not associated with STD clinics.1 Thus, a system with the capacity to collect data from outside the STD clinic setting will also be important to guide STD prevention interventions.
To address these surveillance needs, in 2005 CDC established the STD Surveillance Network (SSuN), a sentinel surveillance system comprising local, enhanced STD surveillance systems that follow common protocols. The purpose of SSuN is to improve the capacity of national, state, and local STD programs to detect, monitor, and respond rapidly to trends in STDs through enhanced collection, reporting, analysis, visualization, and interpretation of clinical, behavioral, and geographic information obtained from a geographically diverse sample of individuals diagnosed with STDs.
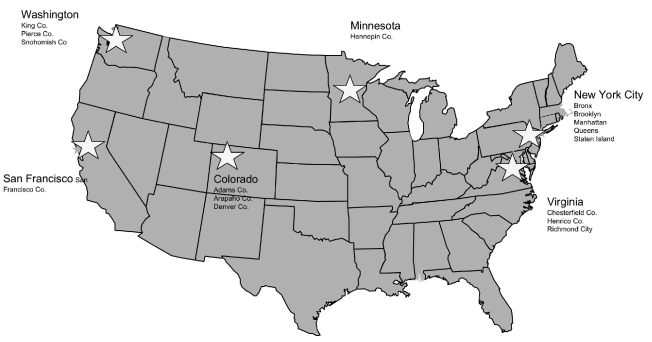
SSuN is composed of six collaborating sites in six geographically diverse areas, which together encompass 16 counties, boroughs, or independent cities: San Francisco (San Francisco County), Colorado (Adams, Arapaho, and Denver counties), Minnesota (Hennepin County), New York City (Bronx, Brooklyn, Manhattan, Queens, and Staten Island boroughs), Virginia (Chesterfield and Henrico counties, and the city of Richmond), and Washington state (King, Pierce, and Snohomish counties) Figure. SSuN collects data from 18 STD clinics operating in SSuN counties; each SSuN site has one participating STD clinic except for Virginia, which has four clinics, and New York City, which has 10 clinics. Data are abstracted from the clinics' medical records, which in some clinics are supplemented with self-administered patient surveys. In addition, population-level data are collected in 11 counties (all collaborating sites except New York) from systematically or randomly selected cases reported by all providers other than participating STD clinics. Periodically, all participating sites send de-identified datasets to CDC.
Figure.
Sites participating in the STD Surveillance Network
STD = sexually transmitted disease
Co. = county
To demonstrate the utility of a national sentinel surveillance network, this article reviews the lessons learned from the first three years of SSuN.
SSuN: INITIAL PROJECTS
During its first three years, SSuN has implemented two projects for which STD clinic-based data have been collected (enhanced gonorrhea surveillance and genital warts surveillance), and one project for which population-level data have been collected (enhanced gonorrhea surveillance in counties).
Enhanced gonorrhea surveillance in STD clinics
Because the paucity of complete and detailed gonorrhea data at the local and national level was seen as a major impediment to the development of a comprehensive gonorrhea control plan, SSuN selected enhanced gonorrhea surveillance as its first major project. Enhanced gonorrhea surveillance includes extensive data collection on demographics, risk behaviors, sexual behaviors, anatomic site of infection, and treatment. Participating sites collaborated with CDC to develop a common protocol stipulating which data elements would be collected, including mandatory and optional items. As part of this process, SSuN sites aimed to standardize data elements as much as possible, and STD clinics made changes to their medical records to accommodate standardization. For example, after much debate, it was decided to adhere to a three-month recall time frame for most behavioral indicators (e.g., number of sex partners), and changes in the sites' medical-records systems were made accordingly.
Data collection for enhanced gonorrhea surveillance in STD clinics started in April 2006 and is ongoing. These data are submitted to CDC every quarter and are analyzed and distributed as an internal newsletter to CDC and participating sites on a quarterly basis. An abstract based on these data was presented at the International Society for STD Research Conference in August 2007.17 Analyses found that the characteristics of STD clinic patients with gonorrhea differed greatly by gender and sexual behavior. Heterosexual men and women with gonorrhea tended to be younger and African American, and to have minimal risk behaviors other than more than one sex partner. In contrast, MSM with gonorrhea reported a greater number of sex partners, more sex with anonymous partners, more sex with partners met through the Internet, and more frequent drug use. Such data suggest two markedly different gonorrhea epidemics among heterosexuals and MSM.17
Enhanced gonorrhea surveillance in counties
Because STD clinic-based cases are likely to be different from cases reported from other sources (e.g., private physicians), enhanced gonorrhea surveillance on a sample of non-STD clinic cases is also being conducted in 11 counties surrounding the clinics (New York City does not participate in county surveillance). Data collection for enhanced gonorrhea surveillance in counties started in February 2006 and is ongoing. Staff at participating SSuN sites in Colorado, Minnesota, Virginia, and Washington interview the first individuals with reported cases of infection each calendar month until they have successfully interviewed 10 male and 10 female patients (for an annual total SSuN-interviewed sample size of 120 men and 120 women from each site). San Francisco samples county patients on a weekly basis, with adjustment for nonresponse, and Washington State interviews a random sample of people with reported cases of infection throughout each month. State or local health department personnel interview selected patients by phone or in person to obtain data on a set of variables similar to what is collected for STD clinic SSuN cases. In addition, data are collected on several socioeconomic characteristics, incarceration status of the patient, characteristics of the patient's most recent sexual partner, the type of facility where the patient was diagnosed, prior attendance at an STD clinic, and history of previous gonorrhea infection in the past three months and year.
Similar to the gonorrhea data from the STD clinics, data from enhanced gonorrhea surveillance in counties are analyzed and distributed as an internal newsletter to CDC and participating sites on a quarterly basis. Data from this activity have also been presented at national conferences.16–18 As also seen in the data from enhanced gonorrhea surveillance in STD clinics, one analysis found differing characteristics between heterosexuals and MSM with gonorrhea, as well as important racial/ethnic differences regarding where patients sought care for gonorrhea.18 Such data suggest that different interventions may be needed to reduce gonorrhea transmission among heterosexuals and MSM. Another analysis found that revised national gonorrhea treatment guidelines reduced overall fluoroquinolone use, but that the decreases varied widely by state, and greater decreases were seen among providers from STD and family planning clinics than among emergency room, hospital, and primary care providers. These findings suggest that novel strategies are needed to ensure that providers offer appropriate therapy for gonorrhea.19
Additional analyses have been planned to compare cases identified inside and outside of STD clinics, better understand racial/ethnic differences in care-seeking behavior, characterize patients with repeat gonorrhea infection, and eventually look at trends over time. These data can be used to drive local and national interventions to reduce gonorrhea infections as well as assist in the evaluation of implemented measures designed to reduce disease transmission.
Genital warts surveillance
Genital warts surveillance was initiated as an SSuN activity in August 2006 to provide data on clinic utilization for the diagnosis and treatment of genital warts in participating SSuN STD clinics. The principal aim of this component of SSuN is to provide data regarding the impact of the quadrivalent human papillomavirus (HPV) vaccine that protects against infection with HPV-6 and HPV-11 subtypes, the cause of most genital warts. Currently, the quadrivalent HPV vaccine has been recommended by the Advisory Committee on Immunization Practices for routine use among girls aged 11 to 12 years, with catch-up vaccination recommended for females aged 13 to 26 years.20 It is expected that this vaccine, which is estimated to have approximately 99% efficacy against genital warts, and possible future vaccines that include these subtypes may have considerable impact on the prevalence of genital warts and the clinical settings where genital warts are diagnosed and treated. Thus, surveillance in STD clinics may assist in demonstrating this impact and, indirectly, provide important data on the uptake and coverage of the HPV vaccine.
Data from genital wart surveillance in STD clinics continue to be collected and have been presented at CDC's Advisory Committee on Immunization Practices meetings as well as at the National STD Prevention Conference in March 2008.21 Preliminary data from August 2006 through December 2007 (a time in which the quadrivalent HPV vaccine was either not available or had not yet been widely administered in all of the participating areas and, thus, would not yet be expected to have an effect on genital wart incidence) suggest that genital wart visits account for 2% to 13% of all STD clinic visits. More than half of genital wart visits are due to recurrent or subsequent wart episodes, and approximately two-thirds of genital wart visits involved provider-administered treatments. These baseline data indicate that widespread population uptake of the quadrivalent HPV vaccine may potentially have a significant impact on STD clinic workload.
DISCUSSION
Limitations
While the SSuN project was initiated to overcome the limitations of traditional STD surveillance, sentinel surveillance has its own limitations. First, by its nature, sentinel surveillance in STD clinics is biased toward populations with specific demographics and risk characteristics, limiting the generalizability of the data. This limitation underscores the importance of sampling non-STD clinic patients in the same counties where the clinics are located (as is done in the SSuN project). Second, the relatively small number of clinic and county sites involved with the project to date limits the generalizability of SSuN data. Expansion of the number of SSuN sites to achieve a wide geographic representation is, therefore, a priority. Third, routine data collection in STD clinics for surveillance purposes is limited to what is clinically relevant and feasible in the context of standard patient care in the clinics. Thus, the collection of in-depth information regarding specific risk behaviors may not be possible without conducting additional surveys or appending research components to the existing surveillance structure.
Lessons learned and future prospects
During its relatively brief existence, SSuN has demonstrated utility in providing multisite data in the areas of enhanced gonorrhea and genital warts surveillance. Protocols, data systems, and lines of communication have been established that can be used as a platform for future sentinel surveillance activities. As the SSuN members gain experience working together in designing the system and interpreting the collectively obtained data, they can undertake additional projects with increasing ease, especially in STD clinic settings with electronic medical record systems and/or easily accessible electronic databases.
During the first phase of the SSuN project, we have learned a number of lessons. First, sentinel STD surveillance can be implemented even in an era of limited resources through use of existing systems. Second, surveillance systems can be designed to serve local needs and address gaps in national surveillance systems. Third, developing collaborative systems in different clinical and county environments is challenging, but it is possible to overcome such barriers to establish a core set of comparable data. Finally, surveillance systems need to be rigorous, yet flexible enough to address new and pressing public health needs.
Future prospects for SSuN might include: (1) expansion of activities to conduct integrated STD, HIV, hepatitis, and tuberculosis surveillance; (2) evaluation of STD clinical practices and interventions; (3) active surveillance of emerging conditions and pathogens; and (4) expansion of the network to additional geographic areas to improve the representativeness of SSuN data.
CONCLUSION
At a national level, SSuN data have been used to assist the development, implementation, and evaluation of STD prevention policies and programs. Locally, in addition to improved data for STD control programs, benefits of SSuN participation include improved capacity to conduct local data analysis, enhanced comparability of data with other clinical programs, opportunities to collaborate with colleagues who have similar interests, and the gratification of contributing to a program of national importance. It is anticipated that additional utility and benefits of this STD sentinel surveillance collaboration will continue to evolve and emerge in the future.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) Sexually transmitted disease surveillance, 2006. Atlanta: CDC: 2007. [cited 2009 Jul 14]. Also available from: URL: http://www.cdc.gov/std/stats06/toc2006.htm. [Google Scholar]
- 2.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 3.Datta SD, Sternberg M, Johnson RE, Berman S, Papp JR, McQuillan G, et al. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147:89–96. doi: 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Satterwhite CL, Joesoef MR, Datta SD, Weinstock H. Estimates of Chlamydia trachomatis infections among men: United States. Sex Transm Dis. 2008;35(11 Suppl):S3–7. doi: 10.1097/OLQ.0b013e31816b3219. [DOI] [PubMed] [Google Scholar]
- 5.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291(2):229–36. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 6.Fox KK, del Rio C, Holmes KK, Hook EW, 3rd, Judson FN, Knapp JS, et al. Gonorrhea in the HIV era: a reversal in trends among men who have sex with men. Am J Public Health. 2001;91:959–64. doi: 10.2105/ajph.91.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fluoroquinolone-resistance in Neisseria gonorrhoeae Hawaii, 1999 and decreased susceptibility to azithromycin in N.gonorrhoeae, Missouri, 1999. MMWR Morb Mortal Wkly Rep. 2000;49(37):833–7. [PubMed] [Google Scholar]
- 8.Increases in fluoroquinolone-resistant Neisseria gonorrhoeae—Hawaii and California, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(46):1041–4. [PubMed] [Google Scholar]
- 9.Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003 and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(16):335–8. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (US) Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006 [published erratum appears in MMWR Recomm Rep 2006;55(36):997] MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 11.Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56(14):332–6. [PubMed] [Google Scholar]
- 12.Resurgent bacterial sexually transmitted disease among men who have sex with men—King County Washington, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48(35):773–7. [PubMed] [Google Scholar]
- 13.Increases in unsafe sex and rectal gonorrhea among men who have sex with men—San Francisco California, 1994–1997. MMWR Morb Mortal Wkly Rep. 1999;48(3):45–8. [PubMed] [Google Scholar]
- 14.Rietmeijer CA, Patnaik JL, Judson FN, Douglas JM., Jr Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex Transm Dis. 2003;30:562–7. doi: 10.1097/00007435-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Stolte IG, Dukers NH, de Wit JB, Fennema JS, Coutinho RA. Increase in sexually transmitted infections among homosexual men in Amsterdam in relation to HAART. Sex Transm Infect. 2001;77:184–6. doi: 10.1136/sti.77.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahle KC, Helms DJ, Golden MR, Asbel LE, Cherneskie T, Gratzer B, et al. Missed gonorrhea infections by anatomic site among asymptomatic men who have sex with men attending U.S. STD clinics, 2002–2006; Presented at the 2008 National STD Prevention Conference; 2008 Mar 10–13; Chicago. [Google Scholar]
- 17.Newman L, Guled H, Donnely J, Kent C, Klingler EJ, Stenger MR, et al. Using sentinel surveillance to characterize patients with gonorrhea in the United States, 2006; Presented at the 17th Biennial Meeting of the International Society for STD Research; 2007 Jul 31; Seattle. [Google Scholar]
- 18.Newman LM, Ahrens K, Donnelly JA, Martins S, Stenger MR, Vasiliu OE, et al. Population-based gonorrhea surveillance through the STD Surveillance Network (SSuN); Presented at the 2008 National STD Prevention Conference; 2008 Mar 10–13; Chicago. [Google Scholar]
- 19.Dowell D, Peterman TA, Newman LM, Yee E, Weinstock H. Evaluation of the impact of revised treatment recommendations on the use of fluoroquinolones for gonorrhea treatment—United States, 2007; Presented at the 57th Annual Epidemic Intelligence Service Conference; 2008 Apr 14–18; Atlanta. [Google Scholar]
- 20.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 21.Markowitz LE, Newman LM, Datta SD, Hariri S, Dunne EF, Bartlett DL, et al. Monitoring the impact of the human papillomavirus (HPV) vaccine: the national perspective; Symposium at the 2008 National STD Prevention Conference; 2008 Mar 10–13; Chicago. [Google Scholar]