Abstract
Collaboration between QOPI and the NCCCP sites represents an evolution in the QOPI process, in which QOPI provides a metric for measuring quality and serves as a springboard for comprehensive quality improvement across independent but mutually committed practices.
Introduction
The science of cancer care has fundamentally been a data-driven process. Although the clinical significance of research findings and the applicability of results can be debated, there has historically been a system developed for measuring and reporting these types of outcomes to standardize the discourse. There are mutually acknowledged requirements that need to be exceeded to allow for acceptance of a given hypothesis, and a common language has evolved that allows for collaboration between researchers, clinicians, institutions, and policymakers.
Despite this well-respected and carefully crafted tradition in cancer research, the science of measuring the quality of cancer care practice has developed only recently. In response to what was perceived to be a deficiency in the monitoring and reporting of quality measures, the Institute of Medicine released a report in 1999 entitled Ensuring Quality Cancer Care.1 What preceded and shortly followed this report was the development of a series of clinical practice guidelines from multiple organizations both nationally and internationally.2–5 However, these guidelines were voluntary, and there was no effective way of tracking compliance in physician offices, where the majority of cancer care was provided. Data from disciplines other than oncology suggested that adherence to guidelines improved quality of care in other clinical areas. These studies were associated with some methodologic challenges,6,7 not the least of which were:
The shifting nature of standards of care
The validity of the guidelines themselves
The adequacy of the dissemination of guidelines to providers of care
The ability to have measurements systems reflect these guidelines
The acceptance by practitioners of both the guidelines and monitoring process
The efforts to enhance quality cancer care that followed have largely been elaborated by others in this issue of Journal of Oncology Practice. ASCO created the National Initiative on Cancer Care Quality in 2000, with the goal of developing measures to better understand the relationship between clinical performance and health outcomes in breast and colon cancers.8 At the same time, the National Cancer Institute (NCI) partnered with the American College of Surgeons and the National Quality Forum to develop voluntary consensus measures for breast cancer and colorectal cancer diagnosis and treatment in hospital-based cancer programs.9 ASCO, the National Comprehensive Cancer Network, and the American College of Surgeons then collaborated and standardized three breast cancer and four colorectal cancer measures. These measures were to be used in ascertaining the delivery of quality cancer care in physician office–based practice.10 However, the creation of these parameters did not provide a mechanism through which cancer care providers practicing in office-based settings could logistically, and in an economically acceptable fashion, assess and review their own performance. The development of ASCO's Quality Oncology Practice Initiative (QOPI) provided that opportunity. Using a computerized data entry system to record the results of medical record abstraction and adherence to a variety of quality indicators, the preliminary results of the program were reported in 2005.11 Analysis of data from practices in 2004 that participated in two data retrieval sessions and demonstrated an improvement in mean overall performance was proof of principle that such a methodology was acceptable to practitioners. The data also suggested the potential to not only document the provision of quality cancer care but also provide practice support and a metric to measure performance and track improvement over time. Entry into the program became available to the entire ASCO membership in 2006.12
Although QOPI provides a mechanism for self-assessment of quality at the practice level, the program has not been used in a collaborative fashion among practitioners to engineer quality improvement across multiple practices. As part of the quality of care initiative of the NCI Community Cancer Centers Program (NCCCP), QOPI was used as a metric to benchmark collaborating practices within the NCCCP organization and to then devise efforts to improve the provision of cancer care on the basis of best practices inferred from these data. Here, we describe the preliminary results of that collaboration.
The NCCCP Collaboration
In an effort to study the optimal methods for disseminating advances in cancer care to the community, the NCI created the NCCCP in 2007. The program studies how to adopt and expand components of optimal cancer care and research in community settings across the cancer care continuum, from prevention and screening to diagnosis and treatment to survivorship and end of life.13 The institutions competitively selected (Table 1) are expected to function synergistically, collaborating in the development of new network-wide projects and providing examples of their own best practices, from which the collective could benefit. The infrastructure of the program was developed to enhance the collaborative effort, with the creation of subcommittees composed of at least one representative from each pilot site. The pillars of focus for the NCCCP are:
Improving the quality of care
Reducing health care disparities
Increasing accrual to clinical trials
Optimizing biospecimen collection and retention
Enhancing survivorship programs
Integrating efforts related to information technology
Table 1.
NCI Community Cancer Centers Program Site and Practice Locations
| Institution | Site | Practice |
|---|---|---|
| Ascension Health, St Louis, MO | St Vincent Oncology Center, St Vincent Indianapolis Hospital, Indianapolis, IN | |
| Columbia St Mary's Cancer Center, Columbia St Mary's, Milwaukee, WI | John Burfein, MD, Charles Tiber, MD, Prospect Medical Commons, Milwaukee, WI | |
| Shivers Cancer Center, Brackenridge Hospital, Austin, TX | ||
| Billings Clinic, Billings Cancer Center, Billings, MT | James Burke, MD, Billings Cancer Center | |
| Helen F. Graham Cancer Center at Christiana Care, Christiana Hospital, Newark, DE | Steven Grubbs, MD, Medical Oncology Hematology Consultants, Newark, DE | |
| Timothy Wozniak, MD, Regional Hematology and Oncology, Newark, DE | ||
| Catholic Health Initiatives, Denver, CO | Penrose Cancer Center, Penrose–St Francis Health Services, Colorado Springs, CO | James Young, MD, Jason Huff, MD, Pikes Peak Cancer Specialists, Colorado Springs, CO |
| St Joseph Cancer Institute, St Joseph Medical Center, Towson, MD | ||
| Coordinated regional program in Nebraska sponsored by: | ||
| —Good Samaritan Cancer Center, Good Samaritan Hospital, Kearney, NE | ||
| —St Elizabeth Cancer Center, St Elizabeth Regional Medical Center, Lincoln, NE | ||
| —St Francis Cancer Treatment Center, St Francis Medical Center, Grand Island, NE | Mehmet Copur, MD, St Francis Cancer Treatment Center, Grand Island, NE | |
| Helen and Harry Gray Cancer Center, Hartford Hospital, Hartford, CT | Robert Siegel, MD, Jeffrey Kamradt, MD, Oncology Associates, Hartford, CT | |
| Our Lady of the Lake Cancer Center and Mary Bird Perkins Cancer Center, Our Lady of the Lake Regional Medical Center, Baton Rouge, LA | Bryan Bienvenu, MD, Frederic Billings, MD, David Hanson, MD, Judd Patten, MD, Derrick Spell, MD, Louisiana Hematology Oncology Associates, Baton Rouge, LA | |
| Sanford Cancer Center, Sanford University of South Dakota Medical Center, Sioux Falls, SD | ||
| Gibbs Regional Cancer Center, Spartanburg Regional Hospital, Spartanburg, SC | James Bearden, MD, Palmetto Hematology/Oncology Associates, Spartanburg, SC | |
| St Joseph's Hospital Cancer Center, St Joseph's Hospital, Orange, CA | Birbal Bhaskar, MD, Medical Oncology Care Associates, Orange, CA | |
| James Padova, MD, Hematology Oncology Medical Group of Orange County, Orange, CA | ||
| Nancy N. and J.C. Lewis Cancer Center and Research Pavilion, St Joseph's Candler, Savannah, GA |
Although each of these pillars has a subcommittee devoted to its analysis, program assessment, and implementation, none of the working groups exists in a vacuum. There is opportunity for individual subcommittees to coordinate related programs with those of another area of focus within the NCCCP.
The NCI and ASCO have recognized, since the beginning of the NCCCP, the complementary relationship between the measurement objectives of QOPI and the NCCCP quality improvement goals. In fact, ASCO staff participated in the initial kickoff meeting of the project and have been working with NCI/NCCCP staff ever since to build a strong oncology quality improvement focus in the pilot program. Organizationally, our group's QOPI project is an initiative of the NCCCP Quality of Care (QOC) Subcommittee. The concept behind the work of the committee is grounded in the collaborative model inherent in the NCCCP. As an initiative, the QOPI project is consistent with a variety of pre-existing partnerships sponsored by the NCI in its support of quality of care research.14 In addition, the mission of QOPI complements many of the other projects under evaluation by the NCCCP QOC Subcommittee, all of which, with varied methodologies, are geared toward the optimal and timely provision of evidence-based medicine in the community. Consistent with the modus operandi of the NCCCP, the goal of the organization's QOPI project is to measure the quality of care provided at respective institutions. Data are shared between pilot sites, best practices are identified, and new policies and procedures are collectively instituted across the institutions to address areas of deficiency. The degree of success afforded by these collaborative interventions can then be measured using QOPI metrics in subsequent rounds of data collection.
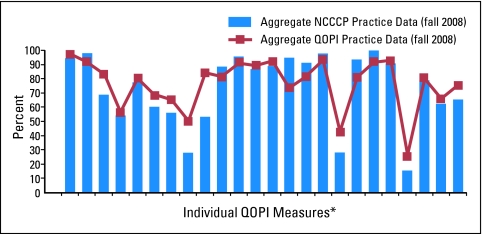
The feasibility of this effort hinges on the ability of the sites to review one another's data. QOPI historically has provided individual practices with its own data as well as the aggregated data from all participants for comparison. To preserve privacy and confidentiality, the agreements between ASCO and its constituent QOPI practices promise anonymity and prohibit data sharing. There was initially no mechanism for the sharing of data among and between individual institutions, as envisioned by the NCCCP program. ASCO helped resolve this issue by allowing the individual practices to identify themselves as NCCCP sites, thus creating a separate group category for data analysis that retains the anonymity of individual practices. In addition, each participating site signs a document allowing its identifiable data to go back to the NCCCP coordinating center, where the identifiers are maintained. Ultimately, each practice receives its own data in a manner similar to that of any other non-NCCCP QOPI institution. In addition, however, data as compiled in Figure 1 are sent to the NCCCP coordinating center for additional discussion and analysis with the NCCCP sites. Finally, the coordinating center maintains the identifiable individual performances of each site. When an opportunity arises to identify best practices, these specific data are released to the group in accordance with an understanding developed between the practices in the creation of this consortium. The aggregated national QOPI data from all institutions provide yet another opportunity for relative benchmarking.
Figure 1.
NCCCP/QOPI physician summary results for core measures (fall 2008). Each column corresponds to an individual performance indicator. (*) The QOPI core measures are not identified in this figure because this information has not been released for publication.
Results
The initiative began in fall 2007, and practices were given the opportunity to volunteer for enrollment in QOPI as part of the research collaborative. Before this, none of the NCCCP-affiliated practices had been part of QOPI. Because enrollment in QOPI had the potential to result in an unfunded financial and manpower commitment on the part of the practices, participation on a voluntary basis was deemed appropriate. Over the subsequent data retrieval cycles, participation gradually increased. In fall 2007, six medical oncology practices representing six NCCCP sites participated. In fall 2008, 11 practices representing 48 medical oncologists from 10 NCCCP sites were involved in data collection (Table 1). Initially, practices were allowed to pick their own QOPI modules for self-assessment in an effort to engender familiarity with the process. By fall 2008, participation in modules that had been mutually agreed on had become standard to allow for meaningful comparison of outcomes on a variety of measures.
Figure 1 is representative of the data obtained from the efforts of the consortium. The figure plots the aggregated data of the NCCCP sites (bar graph) versus the aggregated data from all QOPI sites (line graph) on the QOPI core measures. Without focusing on specific measures, this graph provides an opportunity to compare the NCCCP sites in aggregate with the overall QOPI data. Disparities in quality become evident immediately, as do the magnitude of differences. This level of assessment is analogous to the information that an individual practice would receive, subsequent to which, presumably, there would be an assessment of the various deficiencies and interventions planned to rectify them.
The NCCCP consortium adds another layer of analysis unavailable to the individual practice. Once a deficiency is identified, a more granular assessment of the data can be undertaken, in which the performance of individual participating NCCCP sites can be compared on measures of concern. As an example, the NCCCP as a group fared suboptimally in providing treatment summaries to patients at the conclusion of their care. Although the overall adherence to this measure by our group was 28%, a more focused data analysis indicated that our individual practices scored from 0% to 95% on this parameter. The highest scoring practice has become our internal expert and leader on this issue and has begun to advise us on the technical, logistic, and motivational requirements that contributed to its success. Thus, QOPI has become not only a means of measuring quality but also an impetus for the dissemination of best practices within our consortium. Future data collection and analysis will determine how effective these documentation and procedural improvements are when identified in one practice from one measurement period to other members of the NCCCP consortium.
Discussion
The collaboration of QOPI and the NCCCP sites represents an evolution in the use of the QOPI process. As used in this consortium, QOPI not only represents a metric for measuring quality but also serves as a springboard for comprehensive and collaborative quality improvement across independent but mutually committed practices. Ultimately, even the most precise measurement of quality is of limited benefit if it does not engender an equally robust effort to enhance the provision of quality cancer care.
Although the NCCCP was the unifying “glue” that brought this collaborative together, presumably others with similar goals might have the same success in creating such a quality working group. ASCO has been a willing partner in the creation and maintenance of our ongoing participation as a group by creating a separate designation for the NCCCP sites and allowing, with approval of the individual sites, the data sharing necessary to accomplish our goals. However, no program of this nature can move forward without the commitment of the constituent practices that make up the collective. The practices making up the NCCCP QOPI working group represent only a portion of the available NCCCP practices. Full participation would have undoubtedly added to the significance of our effort and the potential for enhanced quality improvement. There remain impediments for many practices that make QOPI participation difficult. The most notable of these reported by NCCCP-affiliated physicians are limited manpower and the absence of an electronic health record (EHR). Nevertheless, as pressure increases from professional organizations and insurers to monitor quality, as EHR systems become more commonplace in physician offices, and as QOPI becomes a certifying organization for practices,15 we believe participation in QOPI will only continue to increase, both within the NCCCP and for cancer care providers overall.
In an editorial16 written after publication of the preliminary QOPI findings,11 Dilts raised concerns about the variability of the adherence by practices to quality measures and described this as the “Achilles' heel in quality cancer care.” We respectfully disagree. Those of us in the NCCCP collaborative perceive this variability not as an impediment but rather as an opportunity. Our participation in QOPI has allowed us to raise the quality bar for all sites while striving to eliminate the variable adherence to quality measures seen within and between medical practices. We believe that such an undertaking is uniquely suited to the integrated, supportive, and mutually beneficial quality improvement effort created by the NCCCP in association with QOPI. Informally, the QOPI project has already resulted in the development of a mutually agreed on chemotherapy consent form, the discussion of physician credentialing for ongoing participation in institutional cancer programs, and the development of a treatment summary, as mentioned in this report.
An underappreciated benefit of any quality improvement collaborative is efficiency. In the spring 2009 data collection cycle, the size of practices enrolled in QOPI nationally varied from one to 111 physicians, with a mean of seven, indicating that the participating practices were more heavily skewed toward small and medium-sized organizations (Pamela Kadlubek, MPH, QOPI staff, ASCO, personal communication, July 2009). Of those participating, 64% identified themselves as private practitioners. No matter what the commitment of these individuals, the development of quality improvement projects for the more than 80 measures potentially assessed in QOPI is undoubtedly daunting. The demographics of the participating NCCCP sites mirror the national data. However, in creating our collaborative, we have leveraged the expertise of multiple institutions and individuals sharing best practices, and we are striving to implement quality improvement initiatives across all the institutions in an efficient manner. Additionally, such efforts have a ripple effect outside the QOC Subcommittee of the NCCCP, with some of the evolving innovations complementing the efforts of those working in both the Disparities and Survivorship Subcommittees. We believe such innovations are inherent to the synergistic relationships of the NCCCP and not necessarily as obtainable through the efforts of an individual practice, no matter how well intentioned. Blayney et al17 recently published the quality improvements made at the University of Michigan Comprehensive Cancer Center (consisting of 135 faculty physicians and 345 support staff members; Ann Arbor, MI) using QOPI over several cycles of data extraction. Although laudable, this experience does not provide a blueprint for individual community practices, which lack the resources, size, and varied physician expertise comprising a comprehensive cancer center. Although the use of QOPI has had a demonstrable effect on the quality of care provided by community practices,18 we believe that improvements could potentially be more substantial with consortia such as ours. As Horbar et al19 indicated, the nature of clinical practice can be insular, and collaboration among disciplines and others with varied experience is critical for making improvements.
Conclusion
In summary, we believe the innovative relationship between the NCCCP and QOPI can provide a model for other collectives in the arena of quality improvement. The NCCCP embodies a social, professional, and logistic structure that rewards integration and collaboration. QOPI complements this by providing a continually updated and evolving process by which our collaborative can both measure and work to improve the quality of cancer care in our institutions. Rather than the Babel that can arise from insular attempts at local quality improvements, QOPI provides a standard language that can cut across institutions and allow for a data-driven approach to the enhancement of quality of care.
Acknowledgment
We thank the 48 physicians from the NCCCP-affiliated QOPI practices for agreeing to participate in the national collaborative and share their QOPI data. We also thank Pam Kadlubek, MPH, and Kristen McNiff, MPH, at ASCO for their program and technical support of the NCCCP QOPI project and comments on an earlier draft of this manuscript.
Footnotes
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Hewitt ME, Simone JV. Washington, DC: National Academies Press; 1999. Ensuring Quality Cancer Care. [PubMed] [Google Scholar]
- 2.American Society of Clinical Oncology: Clinical practice guidelines. http://jco.ascopubs.org/misc/specialarticles.dtl.
- 3.National Comprehensive Cancer Network: Clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 4.Browman GP, Newman TE, Mohide EA, et al. Progress of clinical oncology guidelines development using the Practice Guidelines Development Cycle: The role of practitioner feedback. J Clin Oncol. 1998;16:1226–1231. doi: 10.1200/JCO.1998.16.3.1226. [DOI] [PubMed] [Google Scholar]
- 5.Burgers JS, Fervers B, Haugh M, et al. International assessment of the quality of clinical practice guidelines in oncology using the appraisal of guidelines and research and evaluation instrument. J Clin Oncol. 2004;22:2000–2007. doi: 10.1200/JCO.2004.06.157. [DOI] [PubMed] [Google Scholar]
- 6.Bach PB. Using practice guidelines to assess cancer care quality. J Clin Oncol. 2005;23:9041–9043. doi: 10.1200/JCO.2005.03.6111. [DOI] [PubMed] [Google Scholar]
- 7.Smith TJ, Hillner BE. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol. 2001;19:2886–2897. doi: 10.1200/JCO.2001.19.11.2886. [DOI] [PubMed] [Google Scholar]
- 8.National Quality Forum. Washington, DC: 2009. Jun 10, National Voluntary Consensus Standards for Quality of Cancer Care—Agency for Healthcare Research and Quality Contract 290-01-0002. [Google Scholar]
- 9.Schneider EC, Malin JL, Emanuel EJ. Developing a system to assess the quality of cancer care: ASCO's National Initiative on Cancer Care Quality. J Clin Oncol. 2004;22:2985–2991. doi: 10.1200/JCO.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 10.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol. 2008;26:3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 11.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: The Quality Oncology Practice Initiative. J Clin Oncol. 2005;23:6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 12.McNiff K. The Quality Oncology Practice Initiative: Assessing and improving care within the medical oncology practice. J Oncol Pract. 2006;2:26–29. doi: 10.1200/jop.2006.2.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MR, Clauser SB, Beveridge JM, et al. Translating scientific advances into the community setting: The National Cancer Institute Community Cancer Centers Program pilot. Oncol Issues. 2009;24:24–28. [Google Scholar]
- 14.Clauser SB. National Cancer Institute partnerships in quality-of-care research. Cancer Control. 2009;16:283–292. doi: 10.1177/107327480901600402. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Clinical Oncology. ASCO announces new national physician practice certification program to enhance the quality of cancer care in the U.S. http://www.asco.org/ASCOv2/Press+Center/Latest+News+Releases/General+News+Releases/ASCO+Announces+New+National+Physician+Practice+Certification+Program+to+Enhance+the+Quality+of+Cancer+Care+in+the+U.S.
- 16.Dilts DM. Practice variation: The Achilles' heel in quality cancer care. J Clin Oncol. 2005;23:5881–5882. doi: 10.1200/JCO.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Blayney DW, McNiff K, Hanauer D, et al. Implementation of Quality Oncology Practice Initiative at a university comprehensive cancer center. J Clin Oncol. 2009;27:3802–3807. doi: 10.1200/JCO.2008.21.6770. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26:1893–1898. doi: 10.1200/JCO.2007.14.2992. [DOI] [PubMed] [Google Scholar]
- 19.Horbar JD, Plsek PE, Leahy K, et al. NIC/Q 2000: Establishing habits for improvement in neonatal intensive care units. Pediatrics. 2003;111:e397–e410. [PubMed] [Google Scholar]